Introduction
Sequential therapy is the mainstay of treatment for
metastatic renal cell carcinoma. Targeted therapy using tyrosine
kinase inhibitors (TKIs) and mammalian target of rapamycin
inhibitors has played an important role in sequential therapy
(1). Inactivation of the von
Hippel-Lindau (VHL) tumor suppressor protein is one characteristic
of clear cell renal carcinoma. Loss of VHL gene is recognized in
60–70% of clear cell renal carcinoma (2,3).
Inactivation of VHL gene results in the up-regulation of
hypoxia-inducible factor 1-α and the growth factor signaling,
including vascular endothelial growth factor (VEGF),
platelet-derived growth factor, and transforming growth factor-a.
These factors play a key role in angiogenesis. Various multikinase
inhibitors have been developed for the treatment of renal cancer
(4).
Angiogenesis is the most important factor for the
growing of renal cell carcinoma (RCC) (5). VEGF has been recognized as playing an
important role in angiogenesis (6,7). In RCC,
high expression of VEGF and its receptor have been detected
(8).
The two TKIs, axitinib and pazopanib, were approved
for advanced RCC. VEGFR and PDGFR are presented as the main targets
of both TKIs. Axitinib has a higher potency and selectivity against
VEGFRs, especially VRGFR-2 (9,10).
Pazopanib targets other receptors, including C-kit, fibroblast
growth factor receptors and colony-stimulating factor-1 receptor
(11). Recently, the association
between TKIs and tumor immunity has been reported. Both drugs can
also augment antitumor immunity through reducing MDSCs which is one
of immune suppressor cells (12,13).
Recently, anti-programmed death-1 (PD-1) antibody,
an immune checkpoint inhibitor (ICI), was approved for the
treatment of metastatic renal cell carcinoma (14). PD-1 is expressed on T cells (15). Programmed death-1 ligand-1 (PD-L1) on
cancer cells delivers an inhibitory signal to T cells through PD-1
(16). Anti-PD-1 antibody blocks the
interaction between PD-L1 and PD-1, thereby activating the T cells
against the cancer cells (14,17).
There was some difficulty initially in determining the best
sequence for administering these drugs, with some studies
suggesting that ICI might impact the response to subsequent
cytotoxic agents (18,19). In this report, we describe two cases
in which sensitivity to TKIs was restored after immunotherapy with
an anti-PD-1 antibody. These findings corroborate the hypothesis
that ICI impacts the response to subsequently administered
cytotoxic agents.
Case report
Case 1
A 56-year-old Japanese woman with multiple lung
nodules on chest X-ray was referred to our hospital in 2012.
Computed tomography (CT) disclosed bilateral renal tumors and
bilateral adrenal, hepatic, lung, and lymph node metastases. Based
on a biopsy of a renal tumor, RCC was diagnosed (cT3N2M1 stage IV).
Her Karnofsky performance status was 100. The peripheral neutrophil
and platelet counts, serum lactate dehydrogenase (LDH), and
corrected calcium (cCa) values were normal. Based on both the
Memorial Sloan-Kettering Cancer Center (MSKCC) and the
International Metastatic RCC Database Consortium (IMDC) risk
classifications, sequential targeted therapy was performed. She
received five lines of targeted therapies from 2012 to 2016 in the
following order: Sorafenib, sunitinib, temsirolimus, axitinib, and
pazopanib. Each drug was stopped due to tumor progression on CT
with the exception of pazopanib. Pazopanib was stopped due to the
adverse effect of anemia. The administration period was 2, 2, 3, 36
and 3 months, respectively. The best response achieved was stable
disease (SD), progressive disease (PD), PD, partial response (PR),
and SD, for sorafenib, sunitinib, temsirolimus, axitinib and
pazopanib, respectively. At the end of the fifth line therapy,
multiple lung and lymph node metastases had disappeared completely
while the bilateral renal tumors and adrenal and hepatic metastases
were still visible on CT.
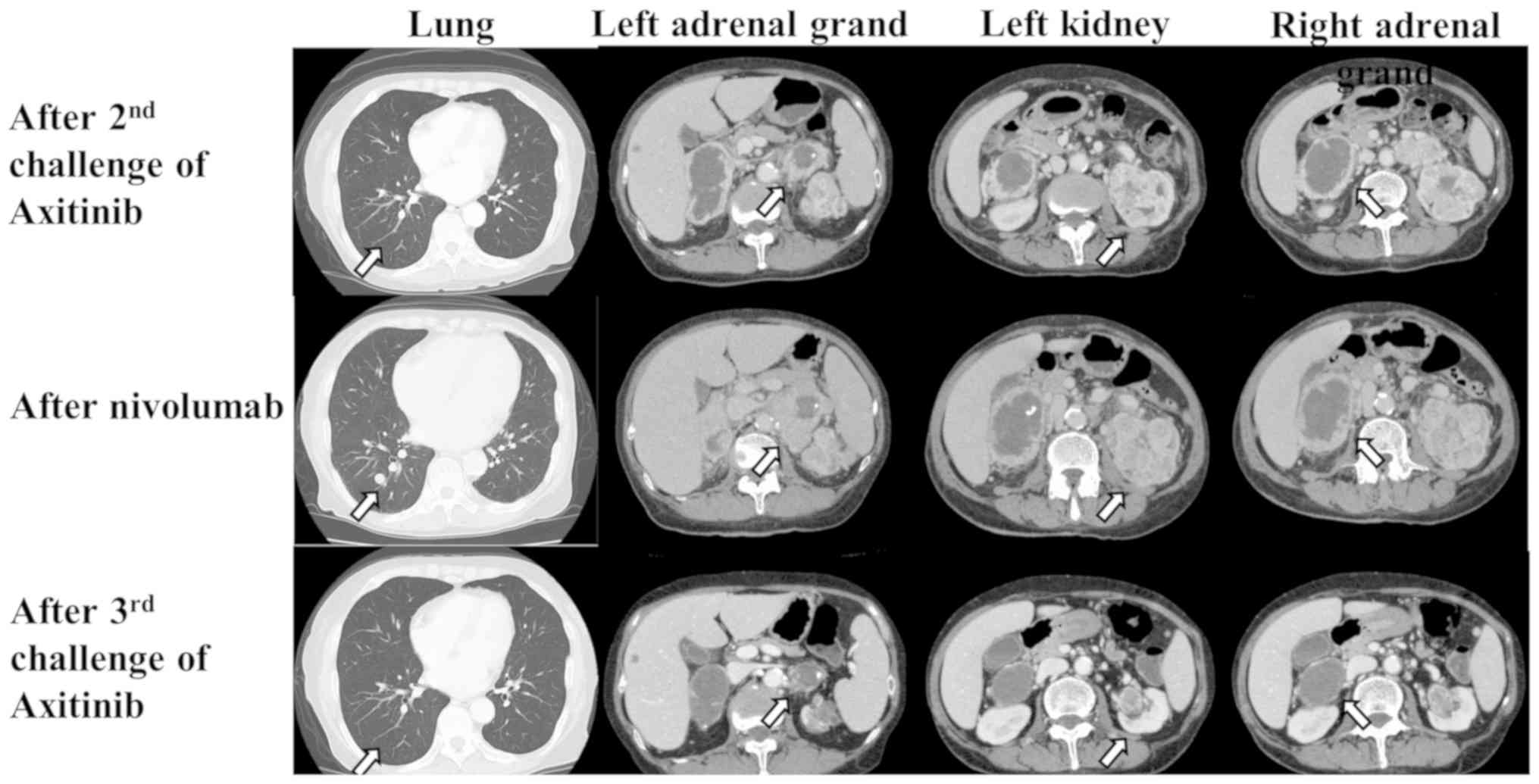
As sixth line therapy, an axitinib rechallenge was
administered in 2016 because this drug was the most effective among
those previously used. Although the response after the second
axitinib challenge was SD for eight months, the primary left renal
tumor and bilateral adrenal metastases showed enlargement on CT
(Fig. 1).
As the seventh line of therapy, immunotherapy with
nivolumab was administered in 2017. After 16 administrations, the
left renal tumor and bilateral adrenal metastases enlarged, and
multiple lung metastases recurred (Fig.
1).
As the eighth line therapy, a third axitinib
challenge was administered. One month later, the left renal tumor
and multiple lung and bilateral adrenal metastases showed slight
shrinkage. Surprisingly, six months later, multiple lung and
bilateral adrenal metastases showed clear shrinkage (Fig. 1). Fourteen months after a third
axitinib challenge, the metastases remained stable in their
shrunken state.
Case 2
A 64-year-old Japanese man with a left renal tumor
detected by ultrasonography was referred to our hospital in 2008.
Based on the radiological findings, renal cell carcinoma was
diagnosed (cT2N0M0, Stage II). He received a left radical
nephrectomy. A pathological examination revealed clear cell
carcinoma, Fuhrman Grade 2 and pT2. Three years later, multiple
lung metastases were visible on CT. His Karnofsky performance
status was 100. The peripheral neutrophil and platelet counts and
the serum LDH and cCa values were normal. Based on the MSKCC and
IMDC risk classifications, sequential targeted therapy was
performed. The patient received two lines of targeted therapy in
the order of sunitinib and axitinib from 2011 to 2016. Each drug
was stopped due to tumor progression on CT. The administration
period was 24 and 36 months, and the best response achieved was SD
for both sunitinib and axitinib.
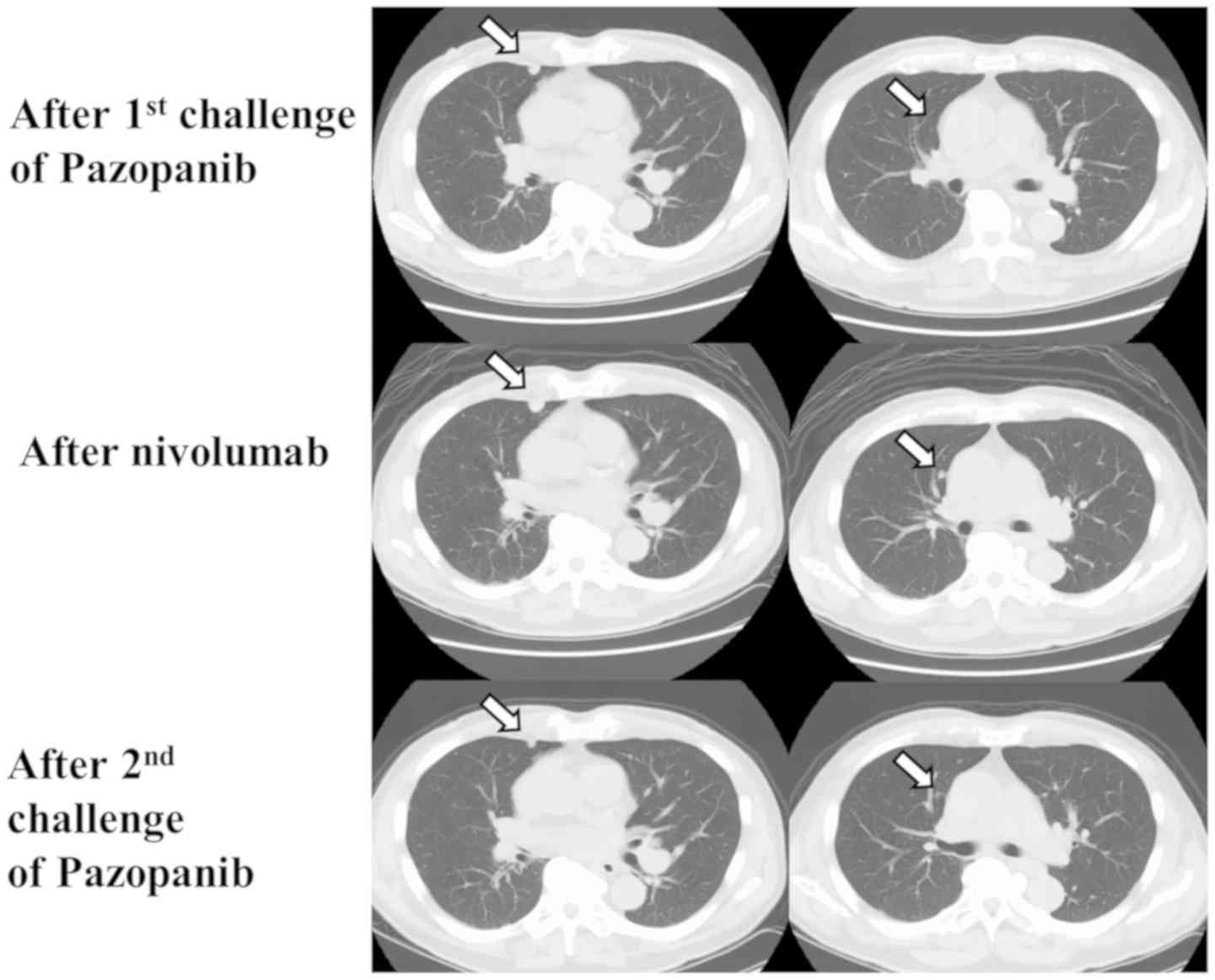
After the second line therapy, CT showed that
multiple lung, lymph node, pancreas, bone, and muscle metastases
had developed (Fig. 2). As the third
line therapy, pazopanib was administered in 2016. Three months
later, the lung metastases slightly decreased in volume, and the
response was SD. However, the lung, lymph node, and muscle
metastases enlarged eight months later (Fig. 2).
As fourth line therapy, immunotherapy with nivolumab
was administered in 2017. CT showed that after five
administrations, new metastatic nodules had developed in the
lung.
As fifth line therapy, a second pazopanib challenge
was administered. Four months later, some of the lung metastases
had shrunk, and the response was SD (Fig. 2). Eight months after a second
pazopanib challenge, the metastases enlarged slightly from their
shrunken state.
Discussion
We demonstrated two cases of metastatic
TKI-refractory renal cell carcinoma which regained sensitivity to
TKI after immunotherapy with nivolumab. Surprisingly, in Case 1,
the third axitinib challenge after nivolumab caused dramatic tumor
shrinkage although the second axitinib challenge immediately before
nivolumab administration had no effect. The lack of T cell number
of activation level in PMBCs for evaluation of ICI effect as a
potential limitation of the study.
Recently, the hypothesis that ICI can impact the
patient's response to subsequently cytotoxic agents was advanced
(18,19). In a phase III trial, nivolumab was
able to improve overall survival to a greater extent than in the
control but was unable to improve progression-free survival
(20–22). Trials of a combination therapy with
ICI and a cytotoxic agent have shown good results (23,24).
These studies revealed that a combination of ICI and cytotoxic
agents had a synergistic effect. These findings are supported by
the fact that the response to cytotoxic agents or targeted drugs
improved after nivolumab therapy. Recently, some studies have
reported findings supporting this hypothesis. In lung cancer,
docetaxel after ICI had a stronger effect than docetaxel alone
(18,19). Albiges et al (25) also reported that in target therapy
for RCC, ICI may impact the response to subsequent therapy. These
reports suffered from the limitation of being retrospective. Our
report, however, was able to corroborate this hypothesis.
Our findings strongly suggest that ICI affected
cytotoxic agents and target drugs via the same mechanism. There are
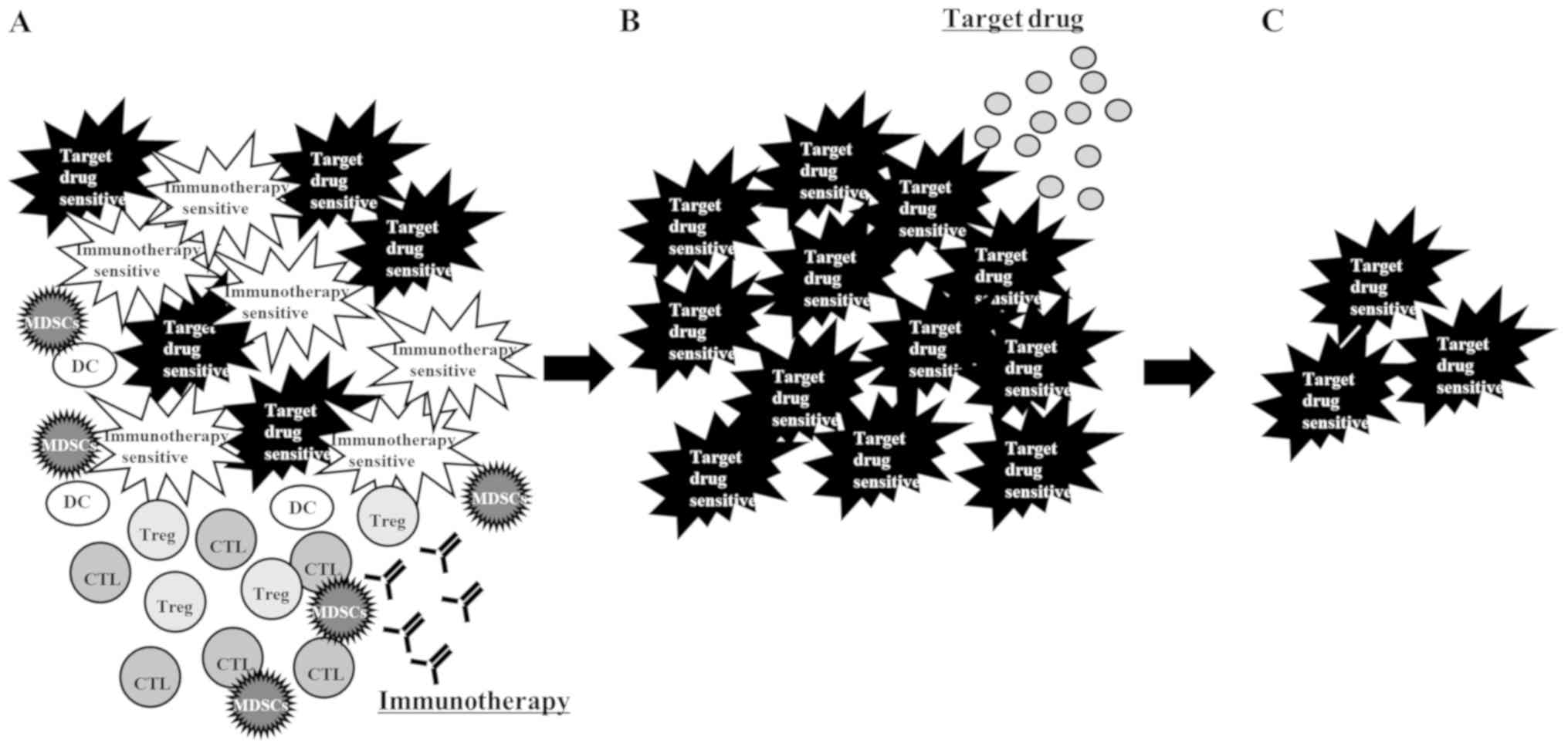
four possible explanations for the effectiveness of a rechallenge
with a TKI after ICI: First, the presence of a heterogeneous cancer
cell population (26) consisting of
those sensitive to immunotherapy and those sensitive to TKI.
Immunotherapy killed only the immunotherapy-sensitive cancer cells,
leaving the TKI-sensitive cells to grow. Radiological examination
apparently induced disease progression. Rechallenge with a TKI
shrank the tumors consisting mostly of TKI-sensitive cells
(Fig. 3); second, some patients
treated with ICIs experienced an initial increase in the size of
their tumors, confirmed by biopsy as inflammatory cell infiltrates
or necrosis, which was followed by a decreased tumor burden. These
delayed clinical responses were observed in patients with melanoma,
sarcoma or bladder, breast, colorectal, esophageal, gastric, head
and neck, lung, pancreatoduodenal, ovarian, renal cell or uterine
cancer. In all these cases, an increase in the total tumor burden
was later followed by tumor regression. These findings were
classified as pseudoprogression merely due to nivolumab, and the
rechallenge with the TKI was considered not to have had any effect,
i.e., only a delayed response to ICI was observed (27,28).
Fujimoto have reported that 14 (3%) patients with non-small cell
lung cancer showed pseudoprogression of 542 patients who received
nivolumab monotherapy. Pseudoprogression was uncommon, and the
response duration in patients with pseudoprogression was shorter
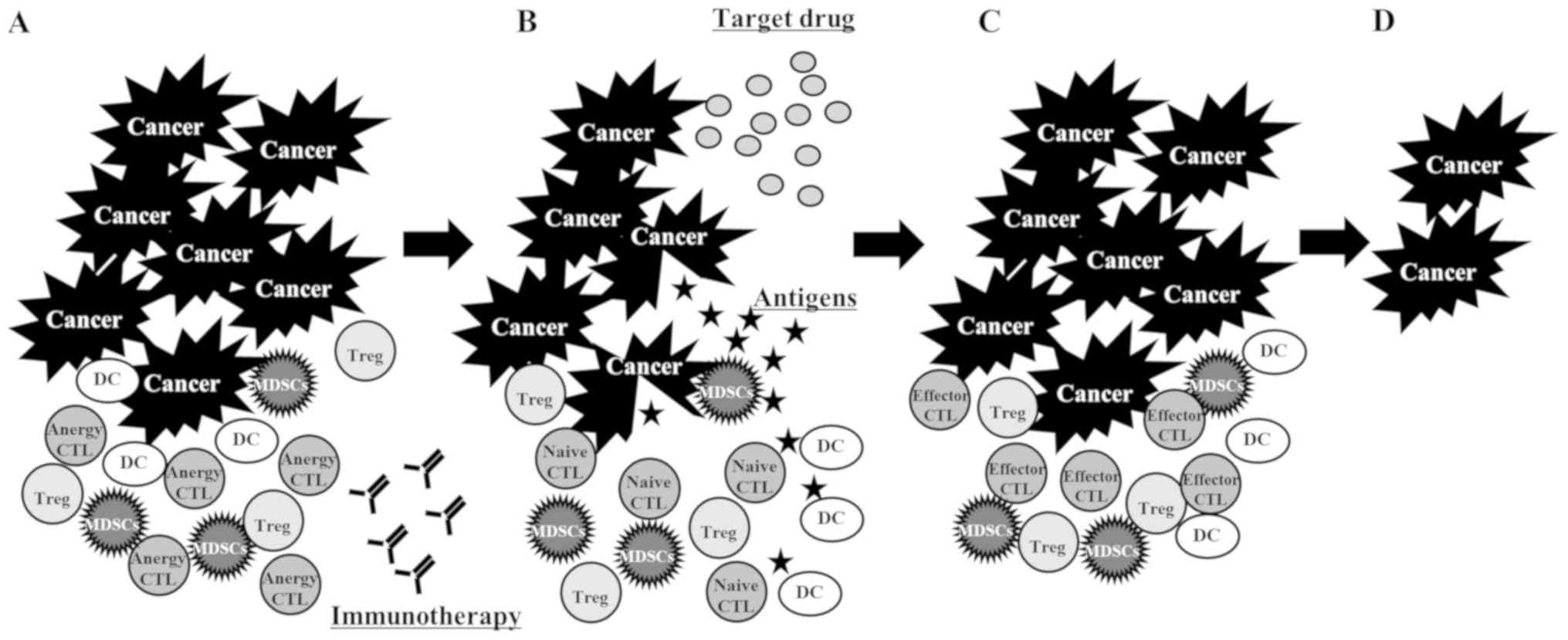
than that in patients with typical response (29); third, the PD-L1 and PD-1 interaction
was able to control T cell anergy, which the ICI reversed (30). The administration of ICI resolved the
anergic state of the CTLs. These naïve CTLs were primed with
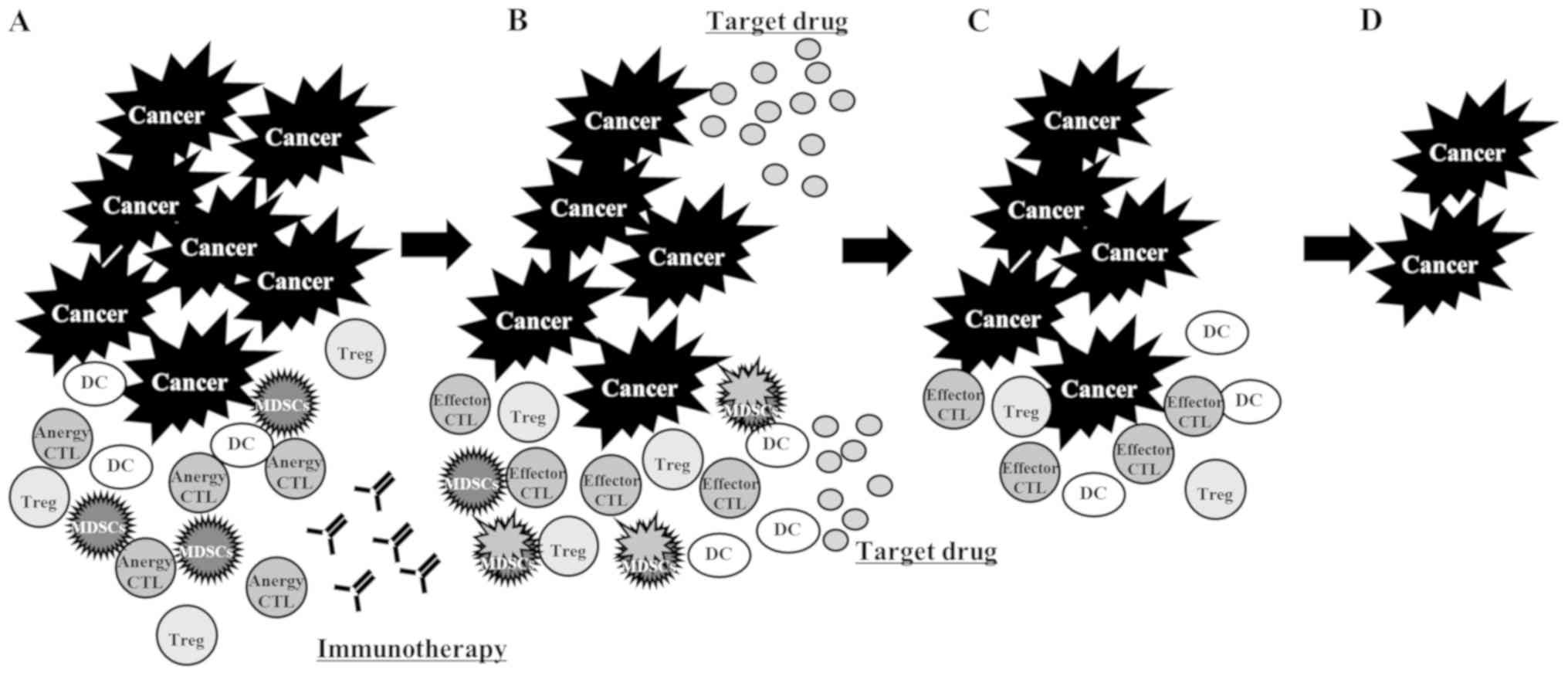
antigens released from the tumor cells killed by the TKI (Fig. 4); and fourth, the administration of
ICI resolved the anergic state of the CTLs, but these CTLs were
suppressed by myeloid-derived suppressor cells (MDSCs). The CTLs
were activated through the reduction of MDSCs by the TKI (31) (Fig.
5).
To conclude, sequential therapy is recommended for
metastatic RCC (1). However, the
optimal sequence of drug administration in sequential therapy is
controversial. Recently, the effect of a combination therapy of ICI
and a cytotoxic agent was tested with good results (12,13).
However, the optimal drug for combination with ICI is still
uncertain. Clarifying the mechanism underlying this phenomenon is
the first step to choosing the best drug and sequence of drug
administration in sequential therapy.
Acknowledgements
Not applicable.
Funding
Data analysis and interpretation of this study was
supported by JSPS KAKENHI Grant Number 17K11169 to T. Azuma.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TA and HK collaborated in the conception and design
of the study. TA, TS, SH, UY, FN, and IT collected the data and
designed the analysis. TA analyzed the data. All authors were
involved in writing the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lambea J, Anido U, Etxániz O, Flores L,
Montesa Á, Sepúlveda JM and Esteban E: The wide experience of the
sequential therapy for patients with metastatic renal cell
carcinoma. Curr Oncol Rep. 18:662016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rathmell WK and Chen S: VHL inactivation
in renal cell carcinoma: Implications for diagnosis, prognosis and
treatment. Expert Rev Anticancer Ther. 8:63–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gnarra JR, Tory K, Weng Y, Schmidt L, Wei
MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al: Mutations of the
VHL tumour suppressor gene in renal carcinoma. Nat Genet. 7:85–90.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of clear cell renal cell
carcinoma. Nature. 499:43–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Folkman J: The role of angiogenesis in
tumor growth. Semin Cancer Biol. 3:65–71. 1992.PubMed/NCBI
|
|
6
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomisawa M, Tokunaga T, Oshika Y, Tsuchida
T, Fukushima Y, Sato H, Kijima H, Yamazaki H, Ueyama Y, Tamaoki N
and Nakamura M: Expression pattern of vascular endothelial growth
factor isoform is closely correlated with tumour stage and
vascularisation in renal cell carcinoma. Eur J Cancer. 35:133–137.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhargava P and Robinson MO: Development of
second-generation VEGFR tyrosine kinase inhibitors: Current status.
Curr Oncol Rep. 13:103–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu-Lowe DD, Zou HY, Grazzini ML, Hallin
ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu
EY, et al: Nonclinical antiangiogenesis and antitumor activities of
axitinib (AG-013736), an oral, potent, and selective inhibitor of
vascular endothelial growth factor receptor tyrosine kinases 1, 2,
3. Clin Cancer Res. 14:7272–7283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan H, Cai P, Li Q, Wang W, Sun Y, Xu Q
and Gu Y: Axitinib augments antitumor activity in renal cell
carcinoma via STAT3-dependent reversal of myeloid-derived
suppressor cell accumulation. Biomed Pharmacother. 68:751–756.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pal SK, Hossain DM, Zhang Q, Frankel PH,
Jones JO, Carmichael C, Ruel C, Lau C and Kortylewski M: Pazopanib
as third line therapy for metastatic renal cell carcinoma: Clinical
efficacy and temporal analysis of cytokine profile. J Urol.
193:1114–1121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Azuma T, Yao S, Zhu G, Flies AS, Flies SJ
and Chen L: B7-H1 is a ubiquitous antiapoptotic receptor on cancer
cells. Blood. 111:3635–3643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schvartsman G, Peng SA, Bis G, Lee JJ,
Benveniste MFK, Zhang J, Roarty EB, Lacerda L, Swisher S, Heymach
JV, et al: Response rates to single-agent chemotherapy after
exposure to immune checkpoint inhibitors in advanced non-small cell
lung cancer. Lung Cancer. 112:90–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ogawara D, Soda H, Iwasaki K, Suyama T,
Taniguchi H, Fukuda Y and Mukae H: Remarkable response of
nivolumab-refractory lung cancer to salvage chemotherapy. Thorac
Cancer. 9:175–180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Atkins MB, Plimack ER, Puzanov I, Fishman
MN, McDermott DF, Cho DC, Vaishampayan U, George S, Olencki TE,
Tarazi JC, et al: Axitinib in combination with pembrolizumab in
patients with advanced renal cell cancer: A non-randomised,
open-label, dose-finding, and dose-expansion phase 1b trial. Lancet
Oncol. 19:405–415. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Albiges L, Fay AP, Xie W, Krajewski K,
McDermott DF, Heng DY, Dariane C, DeVelasco G, Lester R, Escudier B
and Choueiri TK: Efficacy of targeted therapies after PD-1/PD-L1
blockade in metastatic renal cell carcinoma. Eur J Cancer.
51:2580–2586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen L, Azuma T, Yu W, Zheng X, Luo L and
Chen L: B7-H1 maintains the polyclonal T cell response by
protecting dendritic cells from cytotoxic T lymphocyte destruction.
Proc Natl Acad Sci USA. 115:3126–3131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiou VL and Burotto M: Pseudoprogression
and immune-related response in solid tumors. J Clin Oncol.
33:3541–3543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujimoto D, Yoshioka H, Kataoka Y,
Morimoto T, Hata T, Kim YH, Tomii K, Ishida T, Hirabayashi M, Hara
S, et al: Pseudoprogression in previously treated patients with
non-small cell lung cancer who received nivolumab monotherapy. J
Thorac Oncol. Nov 20–2018.(Epub ahead of print). View Article : Google Scholar
|
|
30
|
Tsushima F, Yao S, Shin T, Flies A, Flies
S, Xu H, Tamada K, Pardoll DM and Chen L: Interaction between B7-H1
and PD-1 determines initiation and reversal of T-cell anergy.
Blood. 110:180–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ko JS, Zea AH, Rini BI, Ireland JL, Elson
P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, et al:
Sunitinib mediates reversal of myeloid-derived suppressor cell
accumulation in renal cell carcinoma patients. Clin Cancer Res.
15:2148–2157. 2009. View Article : Google Scholar : PubMed/NCBI
|