Introduction
Diffuse large B-cell lymphoma (DLBCL) is considered
to be the most common subtype of non-Hodgkin lymphoma globally
(1). In adults, DLBCL accountedfor
30–40% of all cases of non-Hodgkin lymphoma worldwide until 2014
(2). Although significant advances
have been made during the last few years in the treatment of DLBCL,
particularly with immunochemotherapy, approximately one third of
cases remain fatal according to a recent research in the United
States in 2016, frequently due to chemotherapy resistance (3,4).
Therefore, continued investigations into novel therapeutic
strategies are required.
Bortezomib is a proteasome inhibitor, a novel class
of drugs that have antitumor activity, primarily through inhibition
of the nuclear factor (NF)-κB pathway. Additionally, it has been
approved clinically for treatment of multiple myeloma and mantle
cell lymphoma (5). Furthermore, a
number of clinical trials have demonstrated that bortezomib has
promising activity in patients with relapsed/refractory DLBCL
(6–8). However, it may induce the expression of
certain anti-apoptotic proteins, including heat shock protein 90
(9) and the antiapoptic Bcl-2 family
member Mcl-1 (10), that could limit
its antitumor efficacy. It has been demonstrated that B-cell
lymphoma-2-associated athanogene 3 (BAG3), an anti-apoptotic
molecule, is induced by proteasome inhibitors in various cancer
cells, and BAG3 knockdown by small interfering RNA sensitizes
cancer cells to proteasome inhibitor-induced apoptosis (11).
BAG3, also known as CAIR-1 or Bis, is a member of
the BAG protein family. It contains a conserved domain and binds
the ATPase domain of heat shock protein 70 (12). BAG3 mediates protein delivery to the
proteasome, modulates apoptosis and serves a role in the processes
of cell adhesion and migration (13). Evidence has indicated that BAG3
expression is upregulated in a number of cancer cell lines
(14–20), including thyroid carcinoma,
pancreatic cancer, prostate cancer, leukemic cells, ovarian cancer,
neuroblastoma and glioblastoma. As reported, BAG3 acts as a
pro-survival and anti-apoptotic protein in different cancer cells,
and it underlies resistance to chemotherapy through decreasing the
level of apoptosis (14,15,18).
Additionally, inhibition of BAG3 expression could potentiate the
effectiveness of chemotherapy (21),
indicating that BAG3 is a candidate therapeutic target of human
cancer.
The phosphatidylinositol 3-kinase (PI3K)/RAC-α
serine/threonine-protein kinase (AKT) pathway is constitutively
activated in a number of lymphoid malignancy types, primarily by
phosphorylation (22,23). It has been implicated as serving
crucial roles in the activation of growth and anti-apoptotic
pathways (24). Overexpression of
phosphorylated (p)-AKT is associated with a poor outcome in DLBCL
(22,25). Thus, the PI3K/AKT signaling pathway
may represent a promising target for therapeutic intervention in
DLBCL.
A number of studies reported that BAG3 may be
induced by proteasome inhibitors, but this has not been
investigated in DLBCL cell lines (26–28). It
has been demonstrated that the anticancer effect of bortezomib is
enhanced by PI3K/AKT pathway inhibitors in a number of tumor types,
including myelodysplastic syndrome (29), hepatocellular carcinoma (30) and melanoma (31), however, this also has not been
investigated in DLBCL. The present study therefore aimed to
investigate whether proteasome inhibitors induce BAG3 in DLBCL cell
lines, whether there is a synergistic anticancer effect between
proteasome inhibitors and PI3K/AKT pathway inhibitors in DLBCL cell
lines, and whether the synergy effect was due to the decreased
expression of the anti-apoptotic protein BAG3. In the present
study, it was demonstrated that the PI3K/AKT inhibitor LY294002
significantly suppressed the induction of BAG3 by proteasome
inhibitors in DLBCL cell lines. It was further observed that
inhibition of the PI3K/AKT pathway decreased the level of
proliferation and increased the level of apoptosis induced by
proteasome inhibitors. These results indicated that inhibition of
the PI3K/AKT pathway could enhance sensitivity of DLBCL cells to
proteasome inhibitors, at least partially, by suppression of BAG3
expression.
Materials and methods
Antibodies and reagents
Anti-BAG3 (dilution, 1:5,000; cat. no. ab92309),
anti-AKT1 (dilution, 1:8,000; cat. no. ab32505) and anti-p-AKT1
(dilution, 1:8,000; cat. no. ab81283) were purchased from Abcam
(Cambridge, UK). PI3K inhibitor LY294002 was obtained from Abcam.
Proteasome inhibitor MG132 was obtained from Abmole Bioscience,
Inc. (Houston, TX, USA). Proteasome inhibitor bortezomib was
obtained from Xian-Janssen Pharmaceutical Ltd. (Shaanxi,
China).
Cell culture
The human DLBCL cell lines LY1 and LY8 were provided
by Professor B. Hilda Ye (Albert Einstein College of Medicine, New
York, NY, USA), and were cultured in Iscove's modified Dulbecco's
medium (IMDM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% (vol/vol) fetal bovine serum (HyClone,
Logan, UT, USA) at 37°C in a humidified atmosphere containing 5%
CO2. To analyze the effects of LY294002, MG132 and
bortezomib on cell growth and expression of BAG3, LY1 and LY8 cells
were cultured in the presence or absence of the drugs for 24 h at
37°C, the working concentrations of LY294002 were 2.5, 5, 10, 20
and 40 µM, the working concentrations ofbortezomib were 5, 10, 20,
40 and 80 nM, and the working concentrations of MG132 were 0.5, 1,
2, 4 and 8 µM. For experiments with LY294002 and proteasome
inhibitor (bortezomib and MG132) treatments, LY1 and LY8 cells were
pre-treated with LY294002 for 1 h prior to exposure to proteasome
inhibitors.
Western blotting
Total protein was extracted by
radioimmunoprecipitation assay buffer (Shenergy Biocolor Ltd.,
Shanghai, China), 1% phenylmethylsulfonyl fluoride and 1%
phosphatase inhibitor. Protein concentrations were assessed using a
Bicinchoninic Acid assay prior to loading the samples. Equal
amounts of 40 µg totalprotein was separated via 8% SDS-PAGE and
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA). Membranes were blocked at room temperature for
1 h with 5% defatted milk dissolved in tris-buffered saline
containing 0.05% Tween-20 (TBST), and then membranes were incubated
overnight at 4°C with primary antibodies against BAG3 (dilution,
1:5,000; cat. no. ab92309; Abcam), AKT1 (dilution, 1:8,000; cat.
no. ab32505; Abcam) and pAKT1 (dilution, 1:8,000; cat. no. ab81283;
Abcam). Membranes were washed with TBST followed by hybridized at
room temperature for 1 h with the anti-rabbit and anti-mouse IgG
horseradish peroxidase (HRP)-conjugated secondary antibodies
(dilution, 1:1,000; cat. no. SPN-9001 and SPN-9002 respectively;
OriGene Technologies, Inc., Beijing, China). Protein bandswere
detected using an Enhanced Chemiluminescence Detection kit (EMD
Millipore). GAPDH (dilution, 1:1,000; cat. no. TA-08; OriGene
Technologies, Inc.) was used as the endogenous control.
Assessment of cell viability
The antiproliferative effects of LY294002 and
proteasome inhibitors, alone or in combination, were determined
using a Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology, Haimen, China). Briefly, cells (1×105)
were incubated in IMDM for 24 h at 37°C in a humidified atmosphere
containing 5% CO2, in triplicate, in a 96-well plate.
LY294002 (0, 2.5, 5, 10, 20 and 40 µM), bortezomib (0, 5, 10, 20,
40 and 80 nM) and MG132 (0, 0.5, 1, 2, 4 and 8 µM) were then added
to the cultures. After 24 h incubation at 37°C, 10 µl CCK-8 was
added to each well. After 24 h incubation at 37°C in a humidified
atmosphere containing 5% CO2, the absorbance at a
wavelength of 450 nm was measured. The percentage cell viability
was calculated as follows: (A in experimental group - A in blank
group/A in control group - A in blank group) ×1 00%.
Flow cytometric analysis
LY1 and LY8 cells were treated with LY294002 (10
µM), or together with bortezomib (40 nM) for 24 h at 37°C, and then
cells were harvested and the percentage apoptosis was measured by
flow cytometry. Briefly, an aliquot of 1×105 cells was
incubated with Annexin V-phycoerythrin and 7-amino-actinomycin D
(7-AAD) for 15 min at room temperature in the dark, according to
the manufacturer's protocols (BD Biosciences; Becton, Dickinson and
Company, Franklin Lakes, NJ, USA). Subsequently, cells were
immediately analyzed with a FACSCalibur flow cytometer (BD
Biosciences; Becton, Dickinson and Company). The data were analyzed
with FlowJo version 7.6 software (Tree Star, Inc., Ashland, OR,
USA).
Statistical analysis
The software used for statistical analysis was SPSS
for windows (version 17.0; SPSS, Inc., Chicago, IL, USA).
Experiments were repeated at least three times. Data are expressed
as the mean ± standard deviation. The half-maximal inhibitory
concentration (IC50) values were analyzed with linear regression.
Comparisons between groups of control and experimental were
performed using one-way analysis of variance, and post hoc analysis
was conducted with Fisher's least significant difference test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of proteasome inhibitors on
the expression of BAG3 in DLBCL cell lines
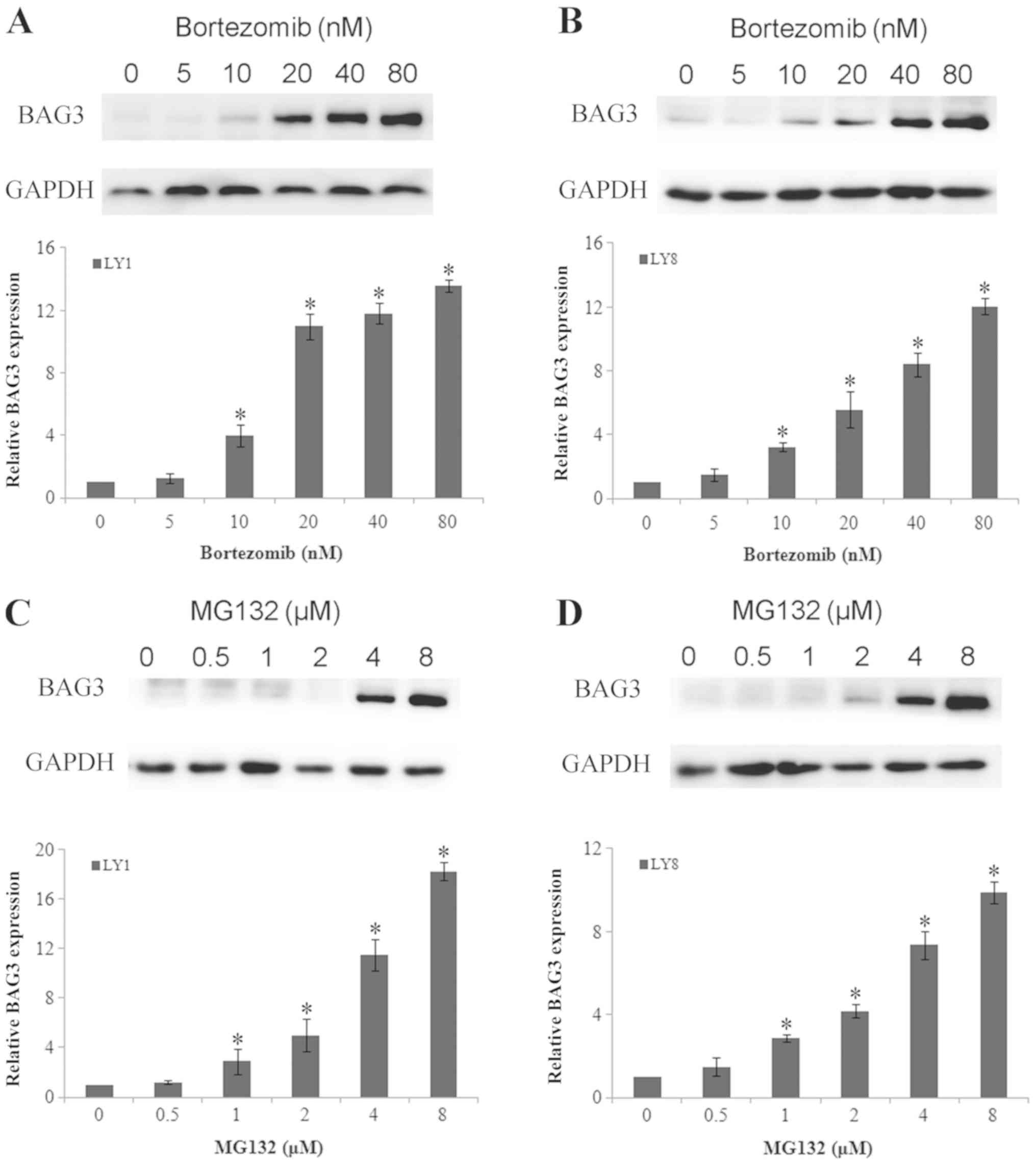
To investigate the effect of proteasome inhibitors
on the expression of BAG3 in DLBCL cell lines, LY1 and LY8 cells
were cultured in the presence or absence of different
concentrations of bortezomib and MG132 for 24 h. Bortezomib and
MG132 are two different proteasome inhibitors, and the working
concentration ranged from 5–80 nM for bortezomib and 0.5–8 µM for
MG132. As depicted in Fig. 1, in the
LY1 and LY8 cell lines, the BAG3 protein was significantly induced
upon exposure to bortezomib (10, 20, 40 and 80 nM) and MG132 (1, 2,
4 and 8 µM) in a dose-dependent manner. It was observed that LY1
and LY8 cells exhibited a higher expression of BAG3 upon exposure
to bortezomib (10, 20, 40 and 80 nM) and MG132 (1, 2, 4 and 8 µM)
compared with the control groups (P<0.05).
Effects of proteasome inhibitors and
LY294002 on cell viability
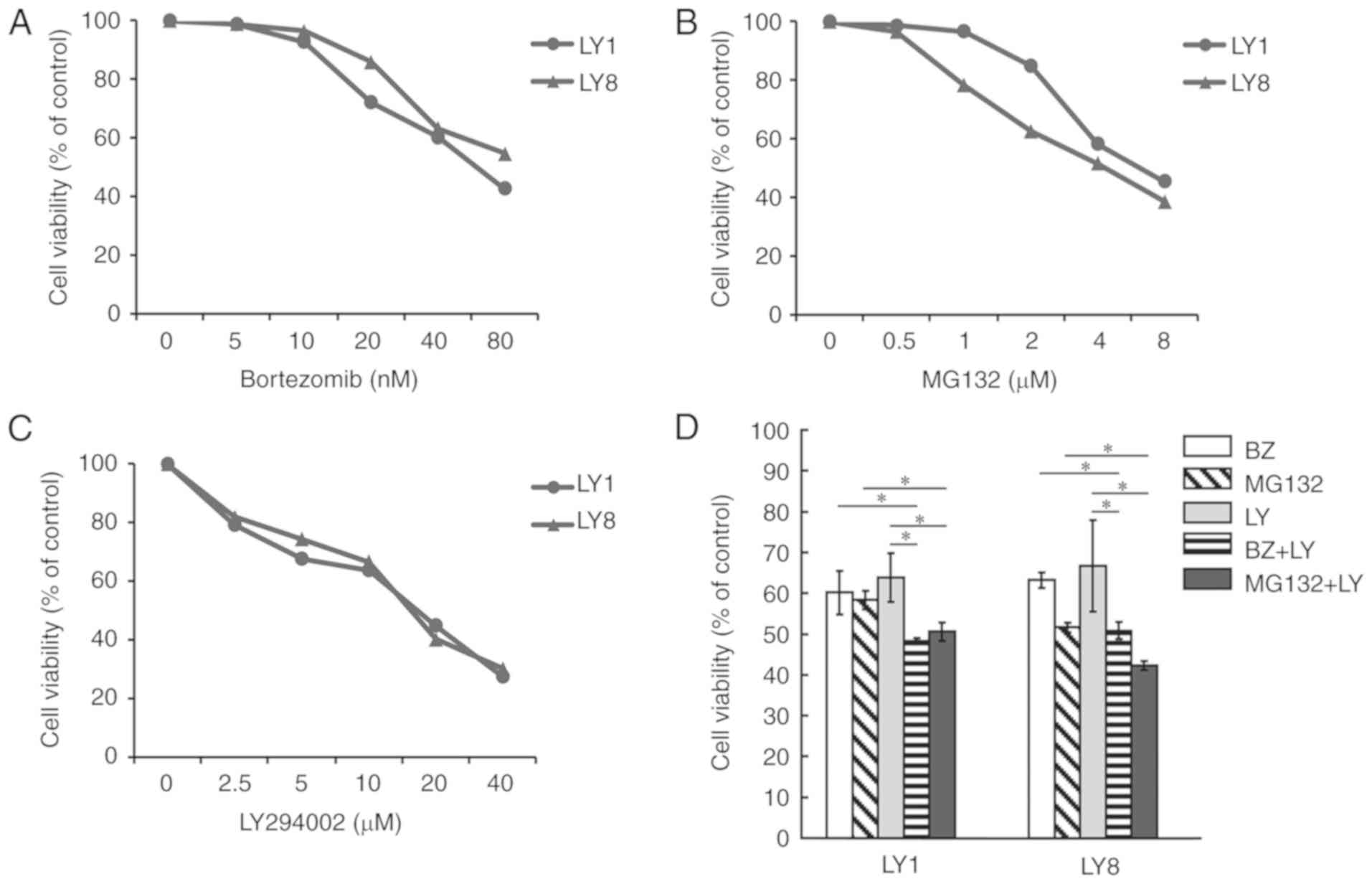
Subsequently, it was determined whether the
proteasome inhibitors and PI3K/AKT pathway inhibitor affect the
DLBCL cell viability, and whether they have synergistic effects on
cell death. The survival rates of LY1 and LY8 cells were measured
with a CCK-8 assay. Fig. 2A-C
depicts that cell viability was inhibited by bortezomib, MG132 and
LY294002 in a concentration-dependent manner. The IC50 of
bortezomib, MG132 and LY294002 in LY1 cells were 45 nM, 4.5 and 12
µM, respectively. In LY8 cells, the IC50 of bortezomib, MG132 and
LY294002 were 42 nM, 4.5 and 13 µM, respectively. Therefore, 40 nM
bortezomib, 4 µM MG132 and 10 µM LY294002 were selected as the
working concentrations for the following experiments. The PI3K/AKT
pathway inhibitor LY294002 significantly decreased cell viability
when used in combination with bortezomib and MG132 compared
withseparate treatments (Fig. 2D;
P<0.05), indicating that the PI3K/AKT pathway has a role in the
tolerance of DLBCL cells to proteasome inhibitors.
Role of PI3K/AKT inhibitor in
proteasome inhibitor-induced BAG3 expression
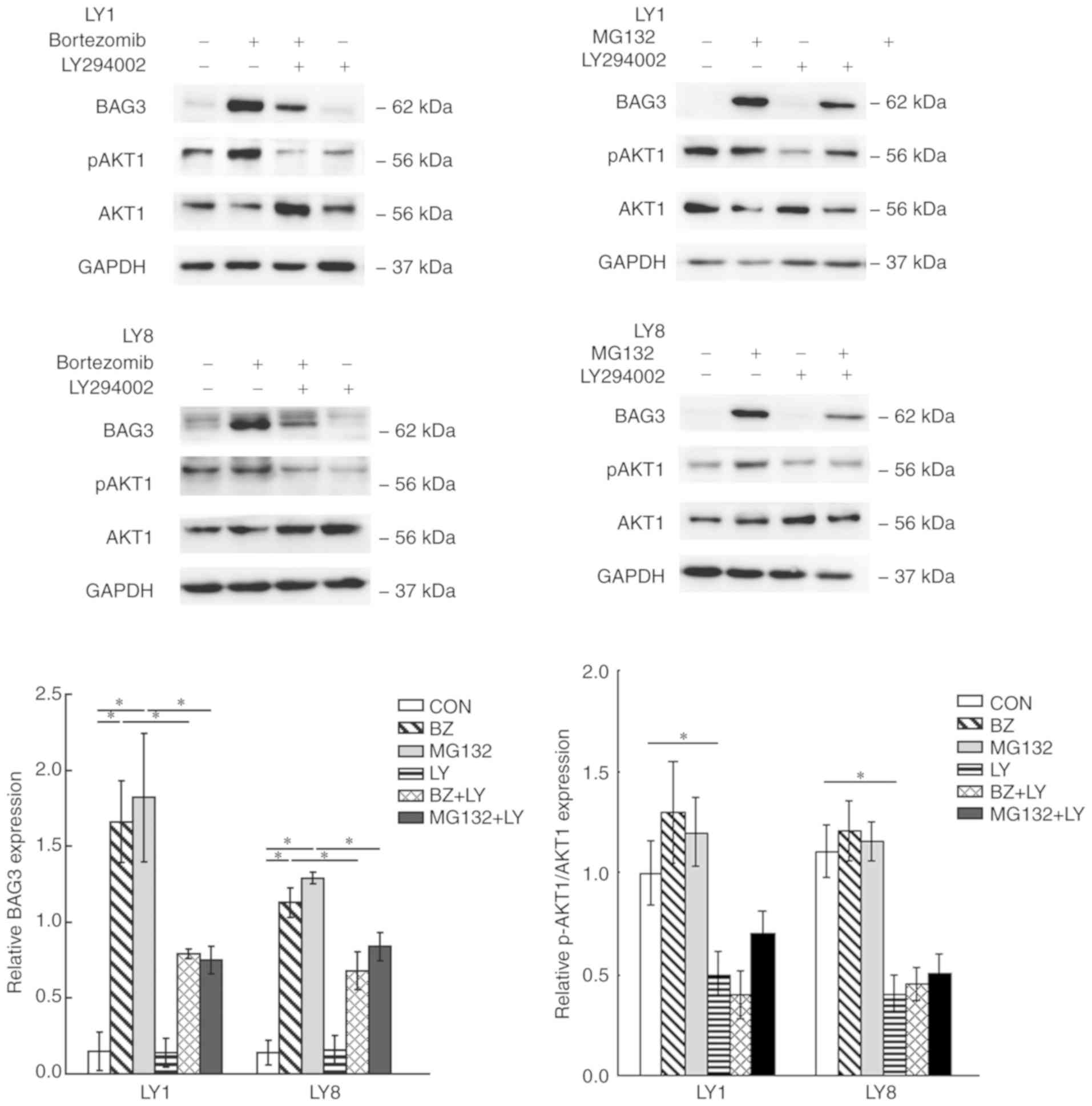
To investigate whether the PI3K/AKT pathway was
involved in the proteasome inhibitor-induced BAG3 expression in
DLBCL cell lines, and to confirm whether the decreased cell
viability effect of LY294002 with proteasome inhibitors was
associated with the expression of the anti-apoptotic protein BAG3,
the expression level of BAG3 was detected in LY1 and LY8 cells,
which were pre-treated with LY294002 and then co-cultured with
proteasome inhibitors for 24 h. The AKT1 and p-AKT1 protein levels
were detected by western blot analysis to represent the activation
state of the PI3K/AKT pathway. The results of western blot analysis
demonstrated that LY294002 notably suppressed the expression of
BAG3 induced by proteasome inhibitors (P<0.05). The treatment
with the proteasome inhibitors demonstrated no significant effect
on relative p-AKT1 protein expression levels, compared with the
controls, whereas pre-treatment with LY294002 markedly reduced
p-AKT1 protein levels (Fig. 3). The
data indicated that the PI3K/AKT pathway was involved in the
induction of BAG3 expression by proteasome inhibitors in DLBCL cell
lines.
Bortezomib-induced apoptosis is
promoted by LY294002
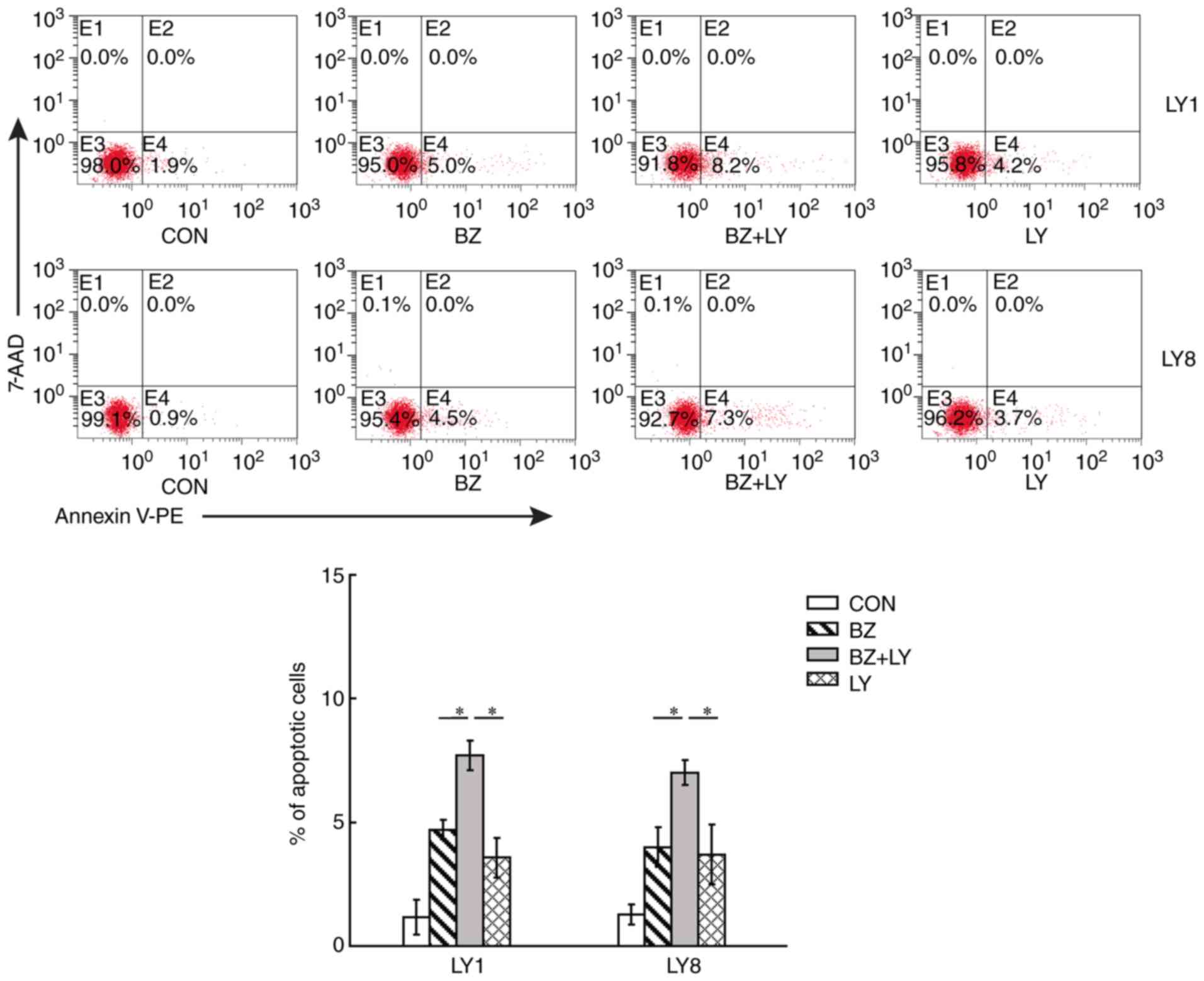
Subsequently, it was investigated whether PI3K/AKT
inhibitor LY294002 affected the apoptotic response of DLBCL cells
to bortezomib by flow cytometric analysis. Cells were incubated
with LY294002 (10 µM) for 1 h prior to exposure to bortezomib (40
nM), and then LY1 and LY8 cells were collected after 24 h to detect
the apoptotic rate. The results are presented in Fig. 4. The levels of apoptosis were
elevated in LY1 and LY8 cells when treated with LY294002 in
combination with bortezomib, compared with the controls and the
single treatment groups (P<0.05). In the bortezomib treated
group, the apoptotic rates of LY1 and LY8 cells were 4.70±0.36 and
4.00±0.78%, respectively, in the LY294002 treated group, the
apoptotic rates of LY1 and LY8 cells were 3.57±2.51 and 3.73±1.20%,
respectively, and the percentage of apoptotic cells in
bortezomib+LY294002 group reached 7.70±0.56 (LY1) and 7.00±0.49%
(LY8). The results indicated the sensitizing effects of LY294002 on
the apoptotic responses of DLBCL cells to bortezomib.
Discussion
In the present study, it was observed that
proteasome inhibitors activated the expression of BAG3 in DLBCL
cells. The present data demonstrated that LY294002, a specific
inhibitor of the PI3K/AKT pathway, significantly reduced BAG3
expression induced by proteasome inhibitors in DLBCL cells. The
decreased expression of BAG3 was associated with reduced cell
viability and increased apoptotic rate. Blockage of the PI3K/AKT
pathway not only inhibited BAG3 expression, but also contributed to
sensitivity of DLBCL cells to proteasome inhibitors. These data
indicated that targeting the PI3K/AKT pathway could overcome
resistance of DLBCL cells to proteasome inhibitors, partially
through downregulation of BAG3 expression.
Proteasome inhibitors are emerging as a promising
class of chemotherapeutic agents in the treatment of a variety of
cancer types, including multiple myeloma, mantle cell lymphoma,
acute myeloid leukaemia, myelodysplastic symdrome, non-small cell
lung cancer and breast cancer (32–36).
Their anticancer activities are performed through a number of
cellular mechanisms, including induction of apoptosis, interference
with cell cycle progression and inhibition of angiogenesis
(36,37). For DLBCL, data demonstrated that
proteasome inhibitors, including bortezomib, inhibit constitutive
NF-κB activity and result in inhibition of cell proliferation via
the induction of apoptosis in the activated DLBCL cells (38). However, in clinical trials, it has
been observed that bortezomib alone has no significant notably
increased response and median overall survival in activated B
cell-like (ABC) DLBCL, compared with germinal center B cell-like
(GCB) DLBCL (39). The results
indicated that a number of unknown mechanisms could induce
chemotherapy resistance of GBC DLBCL to proteasome inhibitors.
In DLBCL, a synergistic effect of proteasome
inhibitors combined with a number of novel agents has been
demonstrated, including mechanistic target of rapamycin kinase
inhibitors (40), the Bruton
tyrosine kinase inhibitor (41) and
the pan-histone deacetylase inhibitor (42). In multiple tumor models, including
glioblastoma and mantle cell lymphoma, the anticancer ability of
bortezomib has been demonstrated to be enhanced by combination with
PI3K pathway inhibitors (43–46). For
the first time, the synergy effect of bortezomib in combination
with the PI3K/AKT pathway inhibitor LY294002 in DLBCL cells was
elucidated.
Accumulating evidence indicated that BAG3, an
anti-apoptotic protein, could be induced by proteasome inhibitors
and may be responsible for the resistance to chemotherapy in
various cell lines (14–20). It has been reported that bortezomib
upregulates BAG3 expression in leukemic cells and BAG3 gene
silencing notably potentiates the apoptosis level of this drug
(21). Downregulation of BAG3 has
been observed to increase cell death in response to proteasome
inhibition in various cancer cells (21,27,28).
Collectively, these reports indicatedthat BAG3 induction is an
unwanted molecular consequence of utilizing proteasome inhibitors
to combat the malignant diseases, and thus may represent a
potential therapeutic target for treating cancer with proteasome
inhibitors.
In the present study, the mechanisms of
chemoresistance of GCB DLBCL to proteasome inhibitors in the GCB
DLBCL cell lines LY1 and LY8 was investigated, with the primary
focus on the sensitizing effect of PI3K/AKT pathway inhibitor
LY294002 to the proteasome inhibitors, and the association between
the synergy effect and the expression level of the anti-apoptotic
protein BAG3. Other investigators demonstrated the molecular
mechanism of BAG3 upregulation induced by proteasome inhibitors
(26–28,47). It
has been reported that heat shock transcription factor 1 is
involved in BAG3 induction by proteasome inhibitor MG132 (26,47). The
c-Jun N-terminal kinase pathway has been reported to be associated
with the protective response against proteasome inhibition, by
mediating induction of BAG3 (28).
In rhabdomyosarcoma cells, NF-κB-inducing kinase (NIK) is critical
in mediating NF-κB activation and BAG3 induction upon co-treatment
of cytoplasmic HDAC inhibitor and proteasome inhibitor (27). The present study revealed for the
first time that inhibition of the PI3K/AKT signaling pathway
enhanced sensitivity of DLBCL cells to proteasome inhibitors by
suppression of BAG3 expression.
In the present study, there are various
shortcomings. In addition to the conclusions drawn, it was
determined that proteasome inhibitors have a tendency to activate
the PI3K signaling pathway, but the result was not statistically
significant. The results demonstrated that the two inhibitors had a
synergistic effect that was statistically significant, but the
synergistic effect was not notably pronounced. It is possible that
alternative signaling pathways, including those involved in
autophagy, may have been activated in response to combined
treatment and may explain the observed reduction in cell viability.
The exact mechanism by which BAG3 participates in the proteasome
inhibitor induced expression and the PI3K signaling pathway
requires further investigation.
In conclusion, the present data demonstrated that
the PI3K/AKT pathway was associated with the activation of BAG3
expression in DLBCL cells and was involved in the protective
response against proteasome inhibition. These results may have
notable impact on the development of combined targeted approaches
in DLBCL. Targeting BAG3 or the PI3K/AKT pathway combined with
bortezomib may have an exciting anticancer effect for patients with
GCB DLBCL. Additionally, animal, preclinical and clinical studies
are required to validate the present results in future studies.
Acknowledgements
Not applicable.
Funding
The present study was partly supported by the
National Natural Science Foundation (grant nos. 81473486 and
81270598), the Technology Development Projects of Shandong Province
(grant no. 2014GSF118021), the Program of Shandong Medical Leading
Talent, and the Taishan Scholar Foundation of Shandong
Province.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TY and XW designed and guided the study. TY
conducted all of the experiments. TY wrote and revised the
manuscript. FZ, XZ, YL, YZ, and YX analyzed the obtained data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
BAG3
|
B-cell lymphoma-2-associated
athanogene 3
|
References
|
1
|
A clinical evaluation of the International
Lymphoma Study Group classification of non-Hodgkin's lymphoma. The
non-hodgkin's lymphoma classification project. Blood. 89:3909–3918.
1997.
|
|
2
|
McGuire S: World cancer report 2014.
(Geneva, Switzerland). World health organization, International
agency for research on cancer, WHO Press 2015. Adv Nutr. 7:418–419.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sarkozy C and Sehn LH: Management of
relapsed/refractory DLBCL. Best Pract Res Clin Haematol.
31:209–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teras LR, DeSantis CE, Cerhan JR, Morton
LM, Jemal A and Flowers CR: 2016 US lymphoid malignancy statistics
by World Health Organization subtypes. CA Cancer J Clin. Sep
12–2016.(Epub ahead of print). doi: 10.3322/caac.21357. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma A and Bhimji SS: Bortezomib.
StatPearls [Internet]. Treasure Island (FL) StatPearls Publishing.
Oct 27–2018.
|
|
6
|
Camicia R, Winkler HC and Hassa PO: Novel
drug targets for personalized precision medicine in
relapsed/refractory diffuse large B-cell lymphoma: A comprehensive
review. Mol Cancer. 14:2072015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yazbeck V, Shafer D, Perkins EB, Coppola
D, Sokol L, Richards KL, Shea T, Ruan J, Parekh S, Strair R, et al:
A phase II trial of bortezomib and vorinostat in mantle cell
lymphoma and diffuse large B-cell lymphoma. Clin Lymphoma Myeloma
Leuk. 18:569–575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Evens AM, Rosen ST, Helenowski I, Kline J,
Larsen A, Colvin J, Winter JN, van Besien KM, Gordon LI and Smith
SM: A phase I/II trial of bortezomib combined concurrently with
gemcitabine for relapsed or refractory DLBCL and peripheral T-cell
lymphomas. Br J Haematol. 163:55–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitsiades CS, Mitsiades NS, McMullan CJ,
Poulaki V, Kung AL, Davies FE, Morgan G, Akiyama M, Shringarpure R,
Munshi NC, et al: Antimyeloma activity of heat shock protein-90
inhibition. Blood. 107:1092–1100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nencioni A, Hua F, Dillon CP, Yokoo R,
Scheiermann C, Cardone MH, Barbieri E, Rocco I, Garuti A,
Wesselborg S, et al: Evidence for a protective role of Mcl-1 in
proteasome inhibitor-induced apoptosis. Blood. 105:3255–3262. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HQ, Liu HM, Zhang HY, Guan Y and Du
ZX: Transcriptional upregulation of BAG3 upon proteasome
inhibition. Biochem Biophys Res Commun. 365:381–385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sondermann H, Scheufler C, Schneider C,
Hohfeld J, Hartl FU and Moarefi I: Structure of a Bag/Hsc70
complex: Convergent functional evolution of Hsp70 nucleotide
exchange factors. Science. 291:1553–1557. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi H, Xu H, Li Z, Zhen Y, Wang B, Huo S,
Xiao R and Xu Z: BAG3 regulates cell proliferation, migration, and
invasion in human colorectal cancer. Tumour Biol. 37:5591–5597.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu H, Wu W, Fu Y, Shen W, Miao K, Hong M,
Xu W, Young KH, Liu P and Li J: Overexpressed BAG3 is a potential
therapeutic target in chronic lymphocytic leukemia. Ann Hematol.
93:425–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Festa M, Del Valle L, Khalili K, Franco R,
Scognamiglio G, Graziano V, De Laurenzi V, Turco MC and Rosati A:
BAG3 protein is overexpressed in human glioblastoma and is a
potential target for therapy. Am J Pathol. 178:2504–2512. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gentilella A and Khalili K: BAG3
expression in glioblastoma cells promotes accumulation of
ubiquitinated clients in an Hsp70-dependent manner. J Biol Chem.
286:9205–9215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao Q, Ozawa F, Friess H, Zimmermann A,
Takayama S, Reed JC, Kleeff J and Büchler MW: The anti-apoptotic
protein BAG-3 is overexpressed in pancreatic cancer and induced by
heat stress in pancreatic cancer cell lines. FEBS Lett.
503:151–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Romano MF, Festa M, Petrella A, Rosati A,
Pascale M, Bisogni R, Poggi V, Kohn EC, Venuta S, Turco MC, et al:
BAG3 protein regulates cell survival in childhood acute
lymphoblastic leukemia cells. Cancer Biol Ther. 2:508–510. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiappetta G, Ammirante M, Basile A,
Rosati A, Festa M, Monaco M, Vuttariello E, Pasquinelli R, Arra C,
Zerilli M, et al: The antiapoptotic protein BAG3 is expressed in
thyroid carcinomas and modulates apoptosis mediated by tumor
necrosis factor-related apoptosis-inducing ligand. J Clin
Endocrinol Metab. 92:1159–1163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Staibano S, Mascolo M, Di Benedetto M,
Vecchione ML, Ilardi G, Di Lorenzo G, Autorino R, Salerno V, Morena
A, Rocco A, et al: BAG3 protein delocalisation in prostate
carcinoma. Tumour Biol. 31:461–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu P, Xu B, Li J and Lu H: BAG3 gene
silencing sensitizes leukemic cells to Bortezomib-induced
apoptosis. FEBS Lett. 583:401–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uddin S, Hussain AR, Siraj AK, Manogaran
PS, Al-Jomah NA, Moorji A, Atizado V, Al-Dayel F, Belgaumi A,
El-Solh H, et al: Role of phosphatidylinositol 3′-kinase/AKT
pathway in diffuse large B-cell lymphoma survival. Blood.
108:4178–4186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang X, Jiang Y, Feng L, Chen H, Zhen C,
Ding M and Wang X: Blockade of PI3K/AKT pathway enhances
sensitivity of Raji cells to chemotherapy through down-regulation
of HSP70. Cancer Cell Int. 13:482013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
West KA, Castillo SS and Dennis PA:
Activation of the PI3K/Akt pathway and chemotherapeutic resistance.
Drug Resist Updat. 5:234–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hasselblom S, Hansson U, Olsson M, Torén
L, Bergström A, Nilsson-Ehle H and Andersson PO: High
immunohistochemical expression of p-AKT predicts inferior survival
in patients with diffuse large B-cell lymphoma treated with
immunochemotherapy. Br J Haematol. 149:560–568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du ZX, Zhang HY, Meng X, Gao YY, Zou RL,
Liu BQ, Guan Y and Wang HQ: Proteasome inhibitor MG132 induces BAG3
expression through activation of heat shock factor 1. J Cell
Physiol. 218:631–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rapino F, Abhari BA, Jung M and Fulda S:
NIK is required for NF-κB-mediated induction of BAG3 upon
inhibition of constitutive protein degradation pathways. Cell Death
Dis. 6:e16922015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang HQ, Liu BQ, Gao YY, Meng X, Guan Y,
Zhang HY and Du ZX: Inhibition of the JNK signalling pathway
enhances proteasome inhibitor-induced apoptosis of kidney cancer
cells by suppression of BAG3 expression. Br J Pharmacol.
158:1405–1412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang J, Ding T, Yang M, Liu H, Sun X and
Jin J: Antitumor activity and drug interactions of proteasome
inhibitor Bortezomib in human high-risk myelodysplastic syndrome
cells. Int J Hematol. 93:482–493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen KF, Yeh PY, Hsu C, Hsu CH, Lu YS,
Hsieh HP, Chen PJ and Cheng AL: Bortezomib overcomes tumor necrosis
factor-related apoptosis-inducing ligand resistance in
hepatocellular carcinoma cells in part through the inhibition of
the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem.
284:11121–11133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yeramian A, Sorolla A, Velasco A,
Santacana M, Dolcet X, Valls J, Abal L, Moreno S, Egido R, Casanova
JM, et al: Inhibition of activated receptor tyrosine kinases by
Sunitinib induces growth arrest and sensitizes melanoma cells to
Bortezomib by blocking Akt pathway. Int J Cancer. 130:967–978.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ruggeri B, Miknyoczki S, Dorsey B and Hui
AM: The development and pharmacology of proteasome inhibitors for
the management and treatment of cancer. Adv Pharmacol. 57:91–135.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Crawford LJ, Walker B and Irvine AE:
Proteasome inhibitors: A therapeutic strategy for haematological
malignancy. Front Biosci. 13:4285–4296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park DJ and Lenz HJ: The role of
proteasome inhibitors in solid tumors. Ann Med. 36:296–303. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jones MD, Liu JC, Barthel TK, Hussain S,
Lovria E, Cheng D, Schoonmaker JA, Mulay S, Ayers DC, Bouxsein ML,
et al: A proteasome inhibitor, bortezomib, inhibits breast cancer
growth and reduces osteolysis by downregulating metastatic genes.
Clin Cancer Res. 16:4978–4989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Manasanch EE and Orlowski RZ: Proteasome
inhibitors in cancer therapy. Nat Rev Clin Oncol. 14:417–433. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Voorhees PM and Orlowski RZ: The
proteasome and proteasome inhibitors in cancer therapy. Annu Rev
Pharmacol Toxicol. 46:189–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bu R, Hussain AR, Al-Obaisi KA, Ahmed M,
Uddin S and Al-Kuraya KS: Bortezomib inhibits proteasomal
degradation of IκBα and induces mitochondrial dependent apoptosis
in activated B-cell diffuse large B-cell lymphoma. Leuk Lymphoma.
55:415–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dunleavy K, Pittaluga S, Czuczman MS, Dave
SS, Wright G, Grant N, Shovlin M, Jaffe ES, Janik JE, Staudt LM, et
al: Differential efficacy of bortezomib plus chemotherapy within
molecular subtypes of diffuse large B-cell lymphoma. Blood.
113:6069–6076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wright JJ: Combination therapy of
bortezomib with novel targeted agents: an emerging treatment
strategy. Clin Cancer Res. 16:4094–4104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dasmahapatra G, Patel H, Dent P, Fisher
RI, Friedberg J and Grant S: The Bruton tyrosine kinase (BTK)
inhibitor PCI-32765 synergistically increases proteasome inhibitor
activity in diffuse large-B cell lymphoma (DLBCL) and mantle cell
lymphoma (MCL) cells sensitive or resistant to bortezomib. Br J
Haematol. 161:43–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fenske TS, Shah NM, Kim KM, Saha S, Zhang
C, Baim AE, Farnen JP, Onitilo AA, Blank JH, Ahuja H, et al: A
phase 2 study of weekly temsirolimus and bortezomib for relapsed or
refractory B-cell non-Hodgkin lymphoma: A wisconsin oncology
network study. Cancer. 121:3465–3471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu W, Chen Y, Xiang R, Xu W, Wang Y, Tong
J, Zhang N, Wu Y and Yan H: Novel phosphatidylinositol 3-kinase
inhibitor BKM120 enhances the sensitivity of multiple myeloma to
bortezomib and overcomes resistance. Leuk Lymphoma. 58:428–437.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qu FL, Xia B, Li SX, Tian C, Yang HL, Li
Q, Wang YF, Yu Y and Zhang YZ: Synergistic suppression of the PI3K
inhibitor CAL-101 with bortezomib on mantle cell lymphoma growth.
Cancer Biol Med. 12:401–408. 2015.PubMed/NCBI
|
|
45
|
Lin L, Gaut D, Hu K, Yan H, Yin D and
Koeffler HP: Dual targeting of glioblastoma multiforme with a
proteasome inhibitor (Velcade) and a phosphatidylinositol 3-kinase
inhibitor (ZSTK474). Int J Oncol. 44:557–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim A, Park S, Lee JE, Jang WS, Lee SJ,
Kang HJ and Lee SS: The dual PI3K and mTOR inhibitor NVP-BEZ235
exhibits anti-proliferative activity and overcomes bortezomib
resistance in mantle cell lymphoma cells. Leuk Res. 36:912–920.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Franceschelli S, Rosati A, Lerose R, De
Nicola S, Turco MC and Pascale M: Bag3 gene expression is regulated
by heat shock factor 1. J Cell Physiol. 215:575–577. 2008.
View Article : Google Scholar : PubMed/NCBI
|