Introduction
An estimated 338,000 new cases of kidney cancer are
diagnosed annually, and 143,000 patients die each year worldwide
(1). Renal cell carcinoma (RCC)
accounts for approximately 90% of kidney cancer cases, and half of
the patients eventually develop metastatic disease (2). In the case of metastatic RCC (mRCC),
remission remains exceptionally infrequent (3).
Several systemic pharmacotherapies, which target
vascular endothelial growth factor (VEGF) and mammalian target of
rapamycin complex 1 (mTORC1), have prolonged the survival of
patients with mRCC for the past decade. Most recently, the
immuno-oncology (I-O) drug nivolumab was developed and established
as a treatment for mRCCs that are resistant to VEGF-targeted agents
(3). A guideline by the European
Association of Urology recommends nivolumab and VEGF-targeted
agents, including axitinib and cabozantinib, as candidates for
second line treatment (4). However,
no indicators are clinically available to determine whether to
administer nivolumab, axitinib, or cabozantinib. The development of
I-O drugs has further stimulated the interest in predictive
biomarkers.
The most common RCC subtype is clear cell RCC
(ccRCC; 70–75%). Approximately 90% of ccRCCs harbor inactivation of
both copies of the von Hippel Lindau (VHL) tumor suppressor gene
(5). Loss of function of VHL protein
(pVHL) leads to the accumulation of hypoxia inducible factors
(HIFs), which promote the transcription of numerous genes,
including VEGF (6). Therefore, VEGF
could rationally be a therapeutic target in mRCC treatment, and is
the most frequently targeted molecule in clinical treatment. HIFs
also interact with mTORC1, which promotes their stability and
translation of HIF mRNAs. mTORC1 is a central crossroad of many
intracellular signaling pathways. It can be regulated upstream by
growth factors (GFs), the phosphoinositide 3-kinase (PI3K)/protein
kinase B (Akt) pathway, and the nutrient/5′ adenosine
monophosphate-activated protein kinase (AMPK) pathway, and it
regulates downstream pathways including those involving stabilizing
HIFs and those promoting cap-dependent translation through the
phosphorylation of S6 kinase and eukaryotic translation initiation
factor 4E-binding protein 1 (4EBP1) (7,8). Since
pVHL also suppressed Akt like HIFs, Akt/mTORC1 pathway is presumed
to be activated in ccRCC (9).
In a number of in vivo and in vitro
cancer cell line studies, aberrant activation of the
Akt/mTORC1/4EBP1 pathways contributed to tumor growth, cell
survival, angiogenesis, and metastasis. 4EBP1 binds and suppresses
eukaryotic initiation factor 4E (eIF4E). Phosphoryltion of 4EBP1
promotes to dissociate eIF4E/4EBP1 assembly, which leads to
eIF4E-dependent translation initiation (7). In RCC cell line studies, inhibition of
mTORC1 suppressed tumor growth, cell survival, angiogenesis, and
metastasis (10,11). Furthermore, our previous studies
demonstrated that activation of the PI3K/Akt/mTORC1 pathway
enhanced resistance to VEGF-targeted agents in RCC cell lines
(12,13). Resistance to the VEGF-targeted agent
sunitinib is correlated with phosphatase and tensin homolog deleted
from chromosome 10 (PTEN) expression, and restoration of PTEN
expression restores sensitivity to sunitinib (12). Akt activation by low-density
lipoprotein (LDL) addition in RCC cell lines counteracts the
anti-tumor effects of the VEGF-targeted agents sunitinib and
sorafenib (13). In adition, we have
previously reported that high levels of 4EBP1/eIF4E activeation
predict higher recurrence rate (14).
Hence, we hypothesized that increased
phosphorylation of 4EBP1 could cause progression of metastasis in
non-mRCC patients and precipitate resistance to VEGF-targeted
agents in mRCC patients. As expected, our results showed that
non-mRCC patients with high phosphorylation ratio had a shorter
disease-free interval (DFI). However, lack of 4EBP1 phosphorylation
correlated with worse cause-specific survival (CSS) in mRCC patient
cohort, contrary to our expectations.
Materials and methods
Patients
We retrospectively collected information on patient
and tumor characteristics, pathological data, recurrence,
treatments, response, and survival from hospital's electronic
database and from patients' medical records in Yamagata University
Hospital and hospitals where the patients had been followed up. The
date of data collection was December 2017.
We retrospectively analyzed two different cohorts.
The first cohort consisted of 254 non-mRCC patients who underwent
radical nephrectomy or nephron sparing surgery in the Yamagata
University Hospital between 2003 and 2010. All patients were
diagnosed using chest and abdominal computer tomography before
surgery, and patients with lymph node metastases, or distant
metastases at surgery were excluded from the non-mRCC cohort. We
included only clear cell RCC into the non-mRCC cohort. Patients who
received adjuvant interferon-alpha treatment after primary surgery
were included if they had no metastatic lesions at surgery.
The second cohort consisted of 60 mRCC patients with
available pre-treatment primary tumor tissues and distinct clinical
outcomes who underwent systemic therapy for mRCC in the Yamagata
University Hospital between 2008 and 2015.
Immunohistochemistry
The expression of total 4EBP1 (t4EBP1) and p4EBP1
were retrospectively evaluated by immunohistochemistry (IHC) as
described. A monoclonal anti-4EBP1 and anti-p4EBP1 (Thr37/46) (Cell
Signaling Technology, Osaka, Japan) were used. The primary tumors
were fixed in 10% buffered formalin and embedded in paraffin. A
3-µm-thick paraffin section was mounted on silanized glass slides
(Dako Cytomation, Tokyo, Japan). After deparaffination and
rehydration, epitopes were reactivated by autoclaving the sections
in 10 mM citric acid buffer (pH 6.0) for 10 min. The slides were
incubated with the primary antibody overnight at 4°C in a moist
chamber. After washing with phosphate buffered saline, the bound
antibody was detected by the peroxidase method using the Histofine
simple stain MAZ-PO (Nichirei, Tokyo, Japan). The staining reaction
was developed by diaminobenzidine in the presence of
H2O2. Nuclear counterstaining was performed
using hematoxylin. Positive and negative controls were included in
each staining series.
Two investigators (HK and TN), who were both blinded
to the patient data, evaluated the expression of t4EBP1 and p4EBP1
in tumor cells was determined (Fig.
1A).
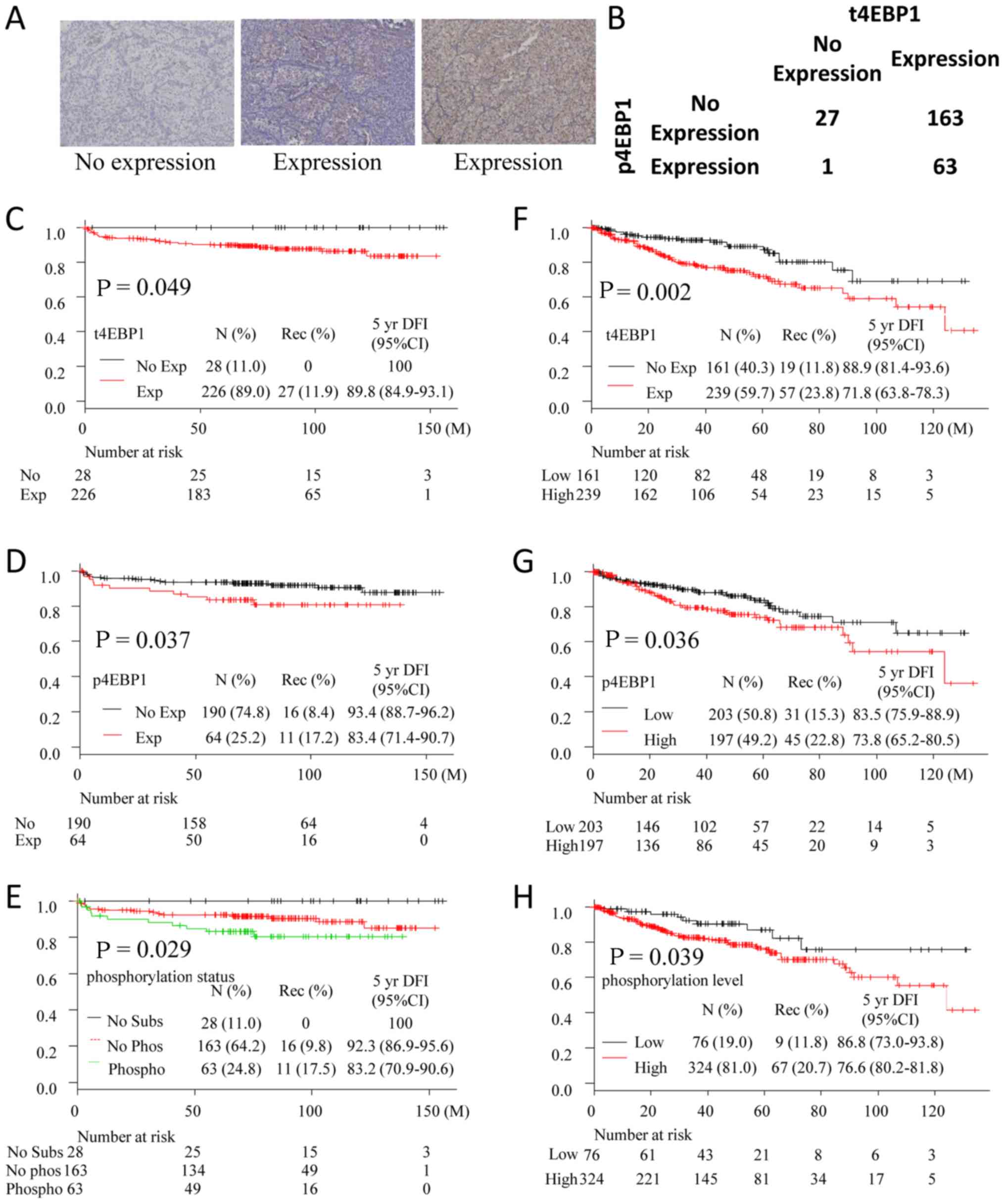 | Figure 1.(A) Representative sample of no
p4EBP1 expression and p4EBP1 expression. (B) Distribution of
patients with t4EBP1 and p4EBP1. (C-E) Kaplan-Meier curves for
disease-free survival in non-mRCC patients in Yamagata University
(C, divided by t4EBP1 expression; D, divided by p4EBP1 expression;
and E, divided by phosphorylation status). (F-H) Kaplan-Meier
curves for disease free survival in non-mRCC patients in TCGA
cohort (F, divided by t4EBP1 expression; G, divided by p4EBP1
expression; and H, divided by phosphorylation status). No subs, no
t4EBP1 expression patients; no Phos, no p4EBP1 expression patients;
phospho, p4EBP1 expression patients; t4EBP1, total eukaryotic
translation initiation factor 4E-bidnding protein 1; p4EBP1,
phosphorylated eukaryotic translation initiation factor 4E-binding
protein 1; mRCC, metastatic renal cell carcinoma. |
Statistical analysis
In the non-mRCC cohort, the endpoint of interest was
DFI from primary surgery to the date of metastatic diagnosis or
last follow-up. Firstly, DFIs which were categorized by expression
score of t4EBP1, p4EBP1, and 4EBP1 phosphorylation status were
overviewed. The phosphorylation status was defined as following: No
Substrate; no expression of t4EBP1, No Phosphorylation; expression
of t4EBP1 and no expression of p4EBP1, Phosphrylation; expression
of t4EBP1 and p4EBP1. Then 4EBP1 phosphorylation status, sex, age
(<55 vs. 56–60 vs. 61–65 vs. 66–70 vs. 71–75 vs. 76–80 vs.
>80 years), age (≤80 vs. >80 years), laterality, pT stage
(pT1a vs. pT1b-4), pathological grade (grade 1–2 vs. 3–4), and v (−
vs. +). The factors that significantly related to DFI in univariate
analyses were entered into multivariate analyses.
In the mRCC cohort, the endpoint of interest was CSS
from the date of metastatic diagnosis to the date of death or last
follow-up. Firstly, CSSs which were categorized by expression of
p4EBP1 were overviewed. Then univariate analyses were undertaken
with the patients categorized by age (≤60 vs. 61–70 vs. >70
years), age (≤60 vs. >60 years), sex, Memorial Sloan Kettering
Cancer Center (MSKCC) risk group (favorable vs. intermediate vs.
poor risk group), MSKCC risk group (favorable vs. intermediate or
poor risk group), subtype (clear cell vs. papillary vs. chromophobe
vs. collecting duct cell vs. mixed vs. Xp11.2 translocation vs.
unclassified vs. other), subtype (clear cell vs. non-clear cell),
pathological grade (1 vs. 2. vs. 3. vs. 4), and pathological grade
(1–3 vs. 4). Then the factors that statistically related to CSS in
the univariate analyses and p4EBP1 expression (no expression vs.
expression) were entered into multivariate analyses. Factors that
were divided over two categories were excluded as candidates in
multivariate analysis.
Finally, we investigated the correlations of p4EBP1
expression with best objective response (BOR) in first line
therapy, first VEGF-targeted therapy, first mTORC1 inhibitor
treatment, and treatment with each VEGF-targeted agent (sunitinib,
sorafenib, and axitinib). BOR was determined by Response Evaluation
Criteria in Solid Tumor version 1.1 (RECIST v1.1) (15). Complete response (CR) and partial
response were determined as clinical response. In cases where
patients had no target lesion, such as bone metastasis, that had
undergone radiotherapy, and where patients changed regimens before
evaluation of BOR, they were defined as ‘unevaluable’.
T classification and pathological grade were
determined according to the 2016 World Health Organization
classification (2). MSKCC risk group
was determined according to a report by Motzer et al
(16). Univariate analyses were
calculated using the Kaplan-Meier method, with the significance
determined using log-rank test. Multivariate analyses were
calculated using the Cox proportional hazard model with step-wise
regression procedures. Distribution was analyzed using the Fisher's
exact test. All statistical analyses were two-sided with a
significant level of 0.05, and were performed using a free
statistical software package R version 3.3.1.
Validation study using TCGA
database
To validate the value as prognostic indicator, we
collected clinical data and p4EBP1 protein level of Kidney Renal
Clear Cel Carcinoma in the Cancer Genome Atlas (TCGA; http://cancergenome.nih.gov/) via cBioPortal
(Http://www.cbioportal.org/, accessed on
28 June 2018). TCGA patients who were diagnosed M0 at pathological
diagnosis, were divided into two groups with high or low expression
level using the following cutoff level for each protein; −0.1 for
t4EBP1 and 0 for p4EBP1. Then we calculated DFI using the
Kaplan-Meier method and compared DFI with the significance
determined using log-rank test. M1 patients were divided into two
groups with high or low p4EBP1 expression using a cutoff level of
−0.7, and calculated and compared OS.
Results
Non-mRCC cohort
Baseline characteristics in the
non-mRCC cohort
The median age at primary surgery in 254 non-mRCC
patients was 64.5 years (range: 28–86 years). Twenty-seven patients
had recurrent RCC lesions during the follow-up period, and 227 did
not. The median follow-up period was 7.11 years [95% confidential
interval (CI) 6.71–7.52 years] as estimated by the Kaplan-Meier
method. Other baseline features are shown in Table I.
 | Table I.Baseline characteristics and DFR in
the non-metastatic renal cell carcinoma cohort. |
Table I.
Baseline characteristics and DFR in
the non-metastatic renal cell carcinoma cohort.
| Factor | Number (%) | Number of
recurrence (%) | % DFR at 5 year
(95% CI) | P-value for
DFIa |
|---|
| All | 254 | 27 (10.6) | 90.9
(86.5–93.9) |
|
| Age |
|
|
|
|
|
≤55 | 57 (22.4) | 4 (7.0) | 92.9
(82.2–97.3) | 0.285 |
|
56–60 | 52 (20.5) | 9 (17.3) | 89.8
(77.1–95.6) |
|
|
61–65 | 23 (9.1) | 2 (8.7) | 91.3
(69.5–97.8) |
|
|
66–70 | 42 (16.5) | 3 (7.1) | 94.7
(80.6–98.7) |
|
|
71–75 | 44 (17.3) | 4 (9.1) | 90.7
(77.0–96.4) |
|
|
76–80 | 23 (9.1) | 2 (8.7) | 90.2
(66.2–97.5) |
|
|
80< | 13 (5.1) | 3 (23.1) | 75.0
(40.8–91.2) |
|
|
≤80 | 241 (94.9) | 24 (10.0) | 91.7
(87.4–94.7) | 0.0451 |
|
>80 | 13 (5.1) | 3 (23.1) | 75.0
(40.8–91.2) |
|
| Sex |
|
|
|
|
|
Male | 157 (61.8) | 17 (10.8) | 92.1
(6.5–95.4) | 0.959 |
|
Female | 97 (38.2) | 10 (10.3) | 89.0
(80.4–93.9) |
|
| Laterality |
|
|
|
|
|
Right | 139 (54.7) | 18 (12.9) | 90.2
(83.8–94.2) | 0.201 |
|
Left | 115 (45.3) | 9 (7.8) | 91.7
(84.6–95.6) |
|
| pT |
|
|
|
|
| 1a | 158 (62.2) | 3 (1.9) | 98.6
(94.6–99.7) | <0.001 |
| 1b | 48 (18.9) | 8 (16.7) | 86.8
(72.9–93.9) |
|
| 2a | 10 (3.9) | 4 (40.0) | 80.0
(40.9–94.6) |
|
| 2b | 6 (2.4) | 2 (33.3) | 66.7
(19.5–90.4) |
|
| 3a | 19 (7.5) | 4 (21.1) | 87.3
(51.9–91.3) |
|
| 3b | 9 (3.5) | 4 (44.4) | 50.0
(15.2–77.5) |
|
| 3c | 2 (0.8) | 1 (50.0) | 0 (N.A-N.A) |
|
| 4 | 2 (0.8) | 1 (50.0) | 50.0
(35.4–0.6) |
|
| 1a | 158 (62.2) | 3 (1.3) | 98.6
(94.6–99.7) | <0.001 |
|
1b-4 | 96 (37.8) | 24 (25.0) | 77.8
(67.7–85.1) |
|
| Grade |
|
|
|
|
| 1 | 113 (44.5) | 6 (5.3) | 95.2
(88.9–98.0) | <0.001 |
| 2 | 110 (44.3) | 11 (10.0) | 93.3
(86.4–96.7) |
|
| 3 | 24 (9.4) | 8 (33.3) | 65.2
(42.3–80.8) |
|
| 4 | 7 (2.8) | 2 (28.6) | 71.4
(25.8–92.0) |
|
| 1 or
2 | 223 (87.8) | 17 (7.6) | 94.3
(90.1–96.7) | <0.001 |
| 3 or
4 | 31 (12.2) | 10 (32.3) | 65.8
(45.7–80.0) |
|
| Vessel
invasion |
|
|
|
|
| − | 224 (88.2) | 18 (8.0) | 93.4
(89.1–96.1) | <0.001 |
| + | 30 (11.8) | 9 (30.0) | 71.3
(50.6–84.5) |
|
Expression of t4EBP1 and p4EBP1 in the
non-mRCC cohort
Total of 226 and 64 tumors expressed t4EBP1 and
p4EBP1, respectively. Almost all tumors (except one tumor) without
t4EBP1 expression did not express p4EBP1 as expected (Fig. 1B). While 11.9% of patients with
t4EBP1 expression had recurrent disease during follow-up period, no
patients without t4EBP1 had a recurrent disease (P=0.049) (Fig. 1C and Table
I). Patients with p4EBP1 expression had shorter DFI (P=0.037)
(Fig. 1D and Table I). When patients were divided into
three groups (no substrate; no expression of t4EBP1, no
phosphorylation; expression of t4EBP1 and no expression of p4EBP1,
Phosphorylation; expression of t4EBP1 and p4EBP1), patients in no
phosphorylation group had statistically worse prognosis (P=0.029)
(Fig. 1E).
Correlation of clinical factors and
4EBP1 phosphorylation status with DFI in the non-mRCC cohort
Table I shows the
correlations of clinical factors with DFI in the non-mRCC cohort,
analyzed with univariate and multivariate methods. Patients with
age over 80 years, pT1b to 4, grade, grade 3 or 4 disease, and v+
had statistically shorter DFI by univariate analyses (P=0.045,
<0.001, <0.001 and <0.001). In the resulting univariate
analyses, six factors-age over 80 years, pT1b to 4, grade 3 or 4
disease, v+, t4EBP1 expression, and p4EBP1 expression-were included
in the multivariate analysis. Expression of p4EBP1, pT1b to 4, and
grade 3 or 4 disease were independent factors associated with
shorter DFI in the non-mRCC cohort by multivariate analysis
(Table II).
 | Table II.Multivariate analyses for
disease-free interval in non-metastatic renal cell carcinoma cohort
(N=254). |
Table II.
Multivariate analyses for
disease-free interval in non-metastatic renal cell carcinoma cohort
(N=254).
|
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Factor | Number (%) | Number of
recurrence (%) | % DFR at 5 years
(95% CI) | P-value for
DFIa | HR (95% CI) for
DFIa | P-value for
DFIa |
|---|
| Age |
|
≤80 | 241 (94.9) | 24 (10.0) | 91.7
(87.4–94.7) | 0.0451 | Withdrawn
stepwise |
|
|
80< | 13 (5.1) | 3 (23.1) | 75.0
(40.8–91.2) |
|
|
|
| pT |
| 1a | 158 (62.2) | 3 (1.3) | 98.6
(94.6–99.7) | <0.001 |
|
|
|
1b-4 | 96 (37.8) | 24 (25.0) | 77.8
(67.7–85.1) |
| 14.79
(4.40–49.75) | <0.001 |
| Grade |
| 1 or
2 | 223 (87.8) | 17 (7.6) | 94.3
(90.1–96.7) | <0.001 |
|
|
| 3 or
4 | 31 (12.2) | 10 (32.3) | 65.8
(45.7–80.0) |
| 4.24
(1.88–9.61) | <0.001 |
| v |
| − | 224 (88.2) | 18 (8.0) | 93.4
(89.1–96.1) | <0.001 | Withdrawn
stepwise |
|
| + | 30 (11.8) | 9 (30.0) | 71.3
(50.6–84.5) |
|
|
|
| t4EBP1 |
| No
expression | 28 (11.0) | 0 | 100 | 0.049 | Withdrawn
stepwise |
|
|
Expression | 226 (89.0) | 27 (11.9) | 89.8
(84.9–93.1) |
|
|
|
| p4EBP1 |
| No
expression | 190 (74.8) | 16 (8.4) | 93.4
(88.7–96.2) | 0.037 |
|
|
|
Expression | 64 (25.2) | 11 (17.2) | 83.4
(71.4–90.7) |
| 2.77
(1.13–6.78) | 0.026 |
Validation study using TCGA database
with regards to non-Mrcc
To validate the value of p4EBP1 as biomarker for DFI
in non-mRCC patients, we analyzed M0 patients in TCGA database.
Patients with high expression of t4EBP1, p4EBP1, and
phosphorylation level had shorter DFI (P=0.002, Fig. 1F; P=0.036, Fig. 1G; P=0.039, Fig. 1H, respectively). These results
corresponded with our study.
mRCC cohort
Baseline characteristics in the mRCC
cohort
The median age at primary surgery in the 60-patient
mRCC cohort was 64 years (range: 34–81 years). At the last
follow-up, 26 patients were alive, 32 had died due to RCC, and two
had died from RCC-unrelated causes. The median follow-up period was
4.96 years (95% CI; 3.97–6.78 years) as estimated by the
Kaplan-Meier method. Other baseline features are shown in Table III. At the commencement of
treatment, 27 patients had metastases. The median duration from
diagnosis of RCC to start of treatment was 30.8 months (range:
0.9–285.8 months).
 | Table III.Univariate and multivariate analyses
for cause-specific survival in metastatic renal cell carcinoma
cohort. |
Table III.
Univariate and multivariate analyses
for cause-specific survival in metastatic renal cell carcinoma
cohort.
|
|
|
| Univariate
(N=60) | Multivariate
(N=41) |
|---|
|
|
|
|
|
|
|---|
| Factor | N. (%) | RCC death (%) | Median CSS, year
(95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age |
|
≤60 | 19 (31.7) | 10 (52.6) | 7.66 (2.69-NA) | 0.270 |
|
|
|
61–70 | 24 (40.0) | 11 (45.8) | 4.73 (2.08-NA) |
|
|
|
|
>70 | 17 (28.3) | 11 (64.7) | 4.23 (1.29-NA) |
|
|
|
|
≤60 | 19 (31.7) | 10 (52.6) | 7.66 (2.69-NA) | 0.110 |
|
|
|
>60 | 41 (68.3) | 22 (53.7) | 4.73
(3.89–6.14) |
|
|
|
| Sex |
|
Male | 47 (78.3) | 24 (51.1) | 5.07
(4.07–6.17) | 0.994 |
|
|
|
Female | 13 (21.7) | 8 (61.5) | 2.69 (1.29-NA) |
|
|
|
| MSKCC risk
group |
|
Favorable | 22 (38.1) | 6 (23.8) | 8.03 (4.71-NA) | 0.017 |
|
|
|
Intermediate | 28 (50.9) | 19 (67.9) | 3.89
(1.60–6.14) |
|
|
|
|
Poor | 6
(10.9) | 4 (66.7) | 2.58 (0.15-NA) |
|
|
|
|
Unknown | 4 |
|
Favorable | 22 (38.1) | 6 (23.8) | 8.03 (4.71-NA) | 0.005 | Withdrawn by
stepwise |
| Int. or
poor | 34 (61.8) | 23 (67.6) | 3.89
(1.60–4.73) |
|
|
|
| Subtype |
|
Clear | 56 (93.3) | 28 (50.0) | 5.07
(4.07–8.03) | <0.001 |
|
|
|
Papillary | 1 (1.7) | 1 (100) | 1.29 |
|
|
|
|
Mixed | 1 (1.7) | 1 (100) | 2.20 |
|
|
|
|
Xp11.2 | 1 (1.7) | 1 (100) | 7.66 |
|
|
|
|
Other | 1 (1.7) | 1 (100) | 0.15 |
|
|
|
|
Clear | 56 (93.3) | 28 (50.0) | 5.07
(4.07–8.03) | 0.076 | Withdrawn by
stepwise |
|
Non-clear | 4 | 4 (100) | 1.74 (0.15-NA) |
|
|
|
| Grade |
| 1 | 4
(9.5) | 2 (50.0) | 4.73 (4.71-NA) | 0.002 |
|
|
| 2 | 18 (42.9) | 10 (55.6) | 4.34 (2.69-NA) |
|
|
|
| 3 | 15 (35.7) | 7 (46.7) | 4.07 (2.08-NA) |
|
|
|
| 4 | 5
(11.9) | 4 (80.0) | 1.60 (9.15-NA) |
|
|
|
| Unknown | 18 |
|
1–3 | 37 (88.1) | 19 (51.4) | 4.71
(3.89–6.17) | <0.001 | 1 |
|
| 4 | 5
(11.9) | 4 (80.0) | 1.60 (9.15-NA) |
| 10.72
(2.80–41.00) | <0.001 |
| t4EBP1 |
| No
expression | 0 |
|
Expression | 60 | 32 (53.3) | 5.07
(3.90–7.66) |
|
|
|
| p4EBP1 |
| No
expression | 8
(13.3) | 5 (62.5) | 2.69 (0.39-NA) | 0.023 | 1 |
|
|
Expression | 52 (86.7) | 27 (51.9) | 5.14
(4.34–7.66) |
| 0.210
(0.052–0.845) | 0.028 |
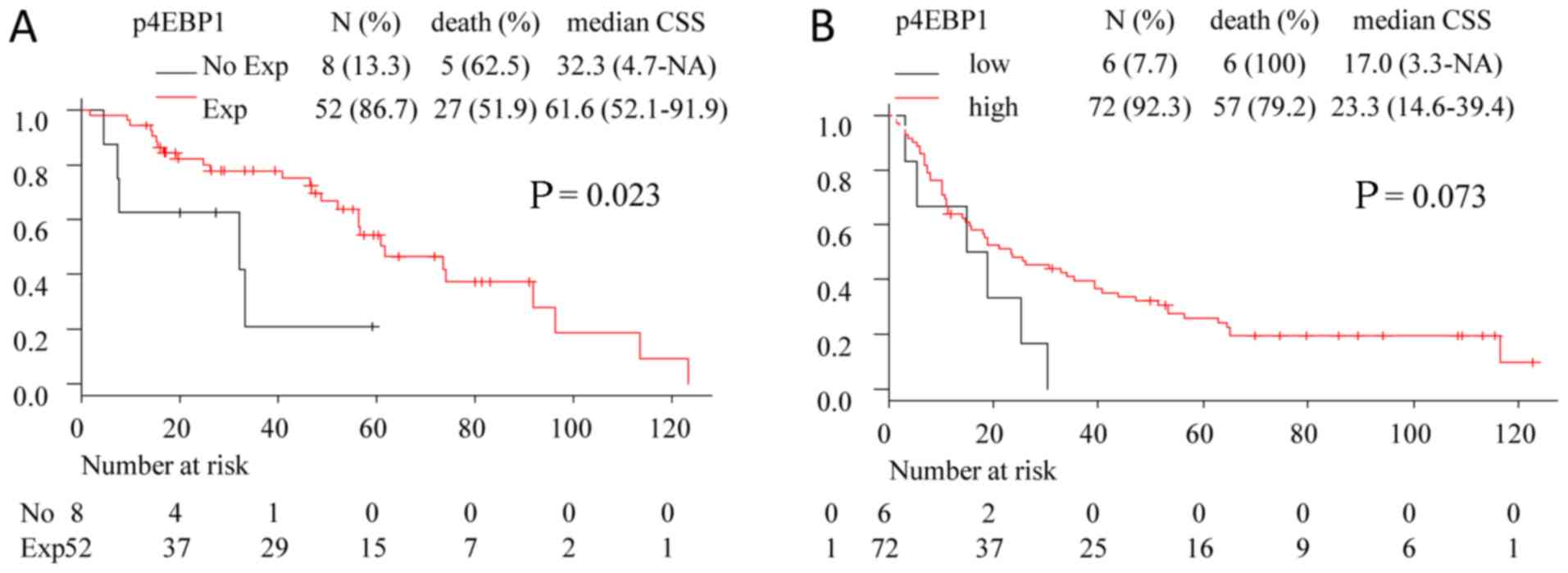
Expression of t4EBP1 and p4EBP1 in the
mRCC cohort
In the mRCC cohort, all tumors showed t4EBP1
expression. Patients with p4EBP1 expression had relatively longer
survival (Table III and Fig. 2A). The distributions of patients with
p4EBP1 expression was not statistically different by MSKCC risk
group, pathological grade, or subtype (P=0.760, 0.560 and >0.99,
respectively). Patients without p4EBP1 expression did not belong to
the MSKCC poor risk group and grade 4, and all had ccRCC (Table SI).
Correlation of clinical factors and
p4EBP1 expression with CSS in the mRCC cohort
Table II shows the
correlations of clinical factors and p4EBP1 expression with CSS,
analyzed with univariate and multivariate methods. Patients in the
intermediate or poor MSKCC risk group, those with non-clear cell
subtype, grade 4, and no p4EBP1 expression had statistically worse
CSS by univariate analyses (P=0.005, 0.076, <0.001 and 0.023,
respectively). No p4EBP1 expression and grade 4 were independently
predictive of worse CSS in the mRCC cohort (Table III).
Validation study using TCGA database
with regards to mRCC
To validate the value of p4EBP1 as biomarker for DFI
in mRCC patients, we analyzed M1 patients in TCGA database.
Patients with high expression of p4EBP1 had numerically longer OS,
but it was not statistically different (P=0.073) (Fig. 2B).
Correlation of p4EBP1 expression with
best overall response to each treatment
Finally, we investigated the correlation of p4EBP1
expression with BOR in first line, first VEGF-targeted agent, first
mTORC1 inhibitor, and sunitinib, sorafenib, and axitinib
treatments. In patients showing no p4EBP1 expression, no treatments
other than sorafenib induced clinical response. Only one of these
patients achieved CR with sorafenib (Table IV).
 | Table IV.Optimal objective response of each
medication. |
Table IV.
Optimal objective response of each
medication.
| Variable | Complete
response | Partial
response | Stable disease | Progression
disease | Unevaluable |
|---|
| First line
treatment |
| No
expression | 0 | 0 | 5 | 3 | 0 |
|
Expression | 0 | 5 | 19 | 17 | 11 |
| First VEGF targeted
drug |
| No
expression | 0 | 0 | 1 | 3 | 3 |
|
Expression | 0 | 6 | 16 | 10 | 3 |
| First mTORC1
inhibitor |
| No
expression | 0 | 0 | 1 | 1 |
|
|
Expression | 0 | 1 | 5 | 6 |
|
| Sunitinib |
| No
expression | 0 | 0 | 1 | 4 | 1 |
|
Expression | 0 | 4 | 9 | 11 | 9 |
| Sorafenib |
| No
expression | 1 | 0 | 0 | 2 | 2 |
|
Expression | 0 | 3 | 5 | 9 | 6 |
| Axitinib |
| No
expression | 0 | 0 | 0 | 3 | 1 |
|
Expression | 0 | 2 | 10 | 7 | 2 |
Discussion
Firstly our study have elucidated that both t4EBP1
and p4EBP1 expression predict recurrence after nephrctomy,
especially p4EBP1 is an independent predictor (Fig. 1 and Table
II). Several previous reports have mentioned that high
phosphorylation of 4EBP1 indicates poor prognosis in prostate,
colon, ovarian, and breast cancer, as well as ccRCC and Xp11.2
translocated RCC (11,17–20). Our
findings and the validation results regarding to p4EBP1 in the
non-mRCC cohort agree with the previous studies indicated in other
cancers. One supposed reason for the result is that phosphorylation
of 4EBP1 promotes to dissociate 4EBP1/eIF4E assembly (7,8).
Actually, we previously elucidated that high levels of 4EBP1/eIF4E
activation predicts RCC recurrence (14). Another reason is the consequence of
Akt/mTORC1 pathway activation. Akt/mTORC1 pathway activates another
molecules including S6K (7,8).
While p4EBP1 predictably indicates high RCC
recurrence, t4EBP1 was contrary to our expectations. Since 4EBP1 is
a suppressor of eIF4E which promotes cap-dependent translation,
cell proliferation, and metastasis (8), we had assumed that patients without
t4EBP1 expression could have frequently developed recurrence. A
possible reason for this unexpected result is compensation by other
molecules such as fragile X mental retardation protein (FMRP). FMRP
binds eIF4E and inhibits its function (8). This eIF4E inhibition by FMRP is
reported to suppress cancer cell proliferation and metastasis
(21). If low t4EBP1 expression
induces compensatory FMRP activation, t4EBP1 expression could be
compatible with high RCC recurrence rate. More detailed work is
necessary to resolve this issue.
The patients in the mRCC cohort with expression of
p4EBP1 showed longer survival than those without it in univariate
and multivariate analyses, on the contrary to the non-mRCC cohort
(Fig. 2 and Table II). To our knowledge, no other
studies have evaluated the correlation between p4EBP1 and OS in
mRCC. Besides, some reports evaluated p4EBP1 expression as a
predictor for drug effectiveness. One report evaluated that genetic
knock-down of 4EBP1 increased susceptibility for sorafenib,
sunitinib, and everolimus. Furthermore, they mentioned that p4EBP1
predicted worse PFS in sunitinib, but did not in sorafenib.
Regarding to everolimus, patients with low p4EBP1 expression had
relatively worse PFS, which did not show statistical difference
(22). Our results also showed that
p4EBP1 expression indicates effectiveness for both mTORC1
inhibitors and VEGF-targeted agents, except for sorafenib (Table III). Li et al also
demonstrated that phosphorylation of mTOR and ribosomal protein S6,
which is downstream of mTORC1, were associated with statistically
longer PFS in 18 patients treated with mTORC1 inhibitor (23). Thir and our results indicate that
p4EBP1 expression could be an indicator for response to mTORC1
inhibitors and VEGF-targeted agents, except sorafenib. In addition,
patients having no expression of p4EBP1 might be preferred
candidates for sorafenib or new treatment strategies, such as I-O
drugs. Since these studies, including ours, were based on a small
number of patients with highly miscellaneous backgrounds, a larger
cohort or direct comparison are required to confirm a predictive
biomarker for drug effectiveness.
We initially hypothesized that p4EBP1 expression
could have indicated resistance to VEGF-targeted agents, because
our previous cell line studies showed that RCC cells with activated
PI3K/Akt/mTORC1 are resistant to sunitinib and sorafenib (12,13). We
have several theories for this contrary result. Firstly, 4EBP1 is a
substrate of not only mTORC1 but also other kinases. For instance,
we previously demonstrated that glycogen synthase kinase-3 directly
phosphorylates 4EBP1 (11).
Secondly, mTORC1 is activated by the nutrient/AMPK pathway in
addition to the GFs/PI3K/Akt pathway (7). Thirdly, factors other than 4EBP1 can
also regulate eIF4E like FMRP (8).
Lastly, VEGF-targeted agents could have both anti-tumor and
anti-angiogenic effects (12,24). The
serum level of clinically available sorafenib suppresses RCC cell
proliferation in in vitro studies, while sunitinib does not
suppress cell proliferation at clinical serum levels (13). Sorafenib not only targets VEGF but
also Raf kinase, which is a molecule in another major pathway
activated by GFs (25). These
findings appear to indicate that sorafenib, in particular,
possesses anti-tumor action, while the anti-tumor mechanism of
sunitinib is weak. In addition, mTORC1 activation facilitates VEGF
expression. Since phosphorylation of p4EBP1 could partially
represent mTORC1 activation, it is reasonable to suppose that
VEGF-targeted agents and mTORC1 inhibitors exhibited no effect in
patients who showed no p4EBP1 expression, and these patients had a
shorter CSS as a result.
This study had several limitations. First, it was a
retrospective study. Second, these cohorts had incomplete data. We
were unable to determine MSKCC risk group in 5 of 60 patients in
the mRCC cohort because of insufficient data. Third, the non-mRCC
cohort was too small, especially when we analyzed the efficacy of
each medication. Fourth, since some of the medications are part of
sequential therapy, the tumors could have been affected by prior
medications. Last, IHC was performed on primary lesions, and these
pathological findings would be different from those of metastatic
lesions.
In summary, t4EBP1 and p4EBP1 expression correlated
with de novo metastasis. In contrast, patients with
metastatic disease who showed no p4EBP1 expression had shorter CSS.
This could be a predictive biomarker for the clinical efficacy of
mTORC1 inhibitors and VEGF-targeted agents, other than
sorafenib.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Department of
Urology, Yamagata University Faculty of Medicine.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SN, OI, HI, ToK, PM, VMK and NT participated in the
design of the present study. SN and OI drafted the manuscript and
figures, and performed statistical analyses. TaK and MiY assessed
the pathological features. HI and HF performed immunohistochemistry
procedures. HidK and TN assessed the immunohistochemistry data.
MaY, AY, YK, TS, HN, HisK, and TY collected data. ToK and NT
participated in the coordination and helped with drafting the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yamagata University Faculty of Medicine (approval no. 421, 2016).
The methods were carried out in accordance with the approved
guidelines. The need for consent to participate in this study has
been waived by the same institutional review board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
4EBP1
|
eukaryotic translation initiation
factor 4E-binding protein 1
|
|
AMPK
|
5′ adenosine monophosphate-activated
protein kinase
|
|
BOR
|
best objective response
|
|
CSS
|
cause-specific survival
|
|
DFI
|
disease free interval
|
|
eIF4E
|
eukaryotic initiation factor
|
|
FMRP
|
fragile X mental retardation
protein
|
|
GFs
|
growth factors
|
|
IHC
|
immunohistochemistry
|
|
I-O drug
|
immune-oncology drug
|
|
LDL
|
low-density lipoprotein
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MNKs
|
mitogen-activated protein kinase
interacting serine/threonine kinases
|
|
mRCC
|
metastatic renal cell carcinoma
|
|
MSKCC risk group
|
Memorial Sloan Kettering Cancer Center
risk group
|
|
mTORC1
|
mammalian target of rapamycin complex
1
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
PTEN
|
phosphatase and tensin homolog deleted
from chromosome 10
|
|
RCC
|
renal cell carcinoma
|
|
RESCIST v1.1
|
Response Evaluation Criteria in Solid
Tumor version 1.1
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greef B and Eisen T: Medical treatment of
renal cancer: New horizons. Br J Cancer. 115:505–516. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Powles T, Staehler M, Ljungberg B,
Bensalah K, Canfield SE, Dabestani S, Giles R, Hofmann F, Hora M,
Kuczyk MA, et al: Updated EAU guidelines for clear cell renal
cancer patients who fail VEGF targeted therapy. Eur Urol. 69:4–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato Y, Yoshizato T, Shiraishi Y, Maekawa
S, Okuno Y, Kamura T, Shimamura T, Sato-Otsubo A, Nagae G, Suzuki
H, et al: Integrated molecular analysis of clear-cell renal cell
carcinoma. Nat Genet. 45:860–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schödel J, Grampp S, Maher ER, Moch H,
Ratcliffe PJ, Russo P and Mole DR: Hypoxia, hypoxia-inducible
transcription factors and renal cancer. Eur Urol. 69:646–657. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pópulo H, Lopes JM and Soares P: The mTOR
signalling pathway in human cancer. Int J Mol Sci. 13:1886–1918.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bramham CR, Jensen KB and Proud CG: Tuning
specific translation in cancer metastasis and synaptic memory:
Control at the MNK-eIF4E axis. Trends Biochem Sci. 41:847–858.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo J, Chakraborty AA, Liu P, Gan W, Zheng
X, Inuzuka H, Wang B, Zhang J, Zhang L, Yuan M, et al: pVHL
suppresses kinase activity of Akt in a
proline-hydroxylation-dependent manner. Science. 353:929–932. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luan FL, Ding R, Sharma VK, Chon WJ,
Lagman M and Suthanthiran M: Rapamycin is an effective inhibitor of
human renal cancer metastasis. Kidney Int. 63:917–926. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Achermann C, Stenner F and Rothschild SI:
Treatment, outcome and prognostic factors in renal cell carcinoma-a
single center study (2000–2010). J Cancer. 7:921–927. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Makhov PB, Golovine K, Kutikov A, Teper E,
Canter DJ, Simhan J, Uzzo RG and Kolenko VM: Modulation of Akt/mTOR
signaling overcomes sunitinib resistance in renal and prostate
cancer cells. Mol Cancer Ther. 11:1510–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naito S, Makhov P, Astsaturov I, Golovine
K, Tulin A, Kutikov A, Uzzo RG and Kolenko VM: LDL cholesterol
counteracts the antitumour effect of tyrosine kinase inhibitors
against renal cell carcinoma. Br J Cancer. 116:1203–1207. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ichiyanagi O, Naito S, Ito H, Kabasawa T,
Narisawa T, Kanno H, Kurota Y, Kurokawa M, Fukuhara H, Sakurai T,
et al: Levels of 4EBP1/eIF4E activation in renal cell carcinoma
could differentially predict its early and late recurrence. Clin
Genitourin Cancer. 16:e1029–e1058. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Armengol G, Rojo F, Castellvi J, Iglesias
C, Cuatrecasas M, Pons B, Baselga J and Ramón y Cajal S: 4E-binding
protein 1: A key molecular ‘funnel factor’ in human cancer with
clinical implications. Cancer Res. 67:7551–7555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
No JH, Jeon YT, Park IA, Kim YB, Kim JW,
Park NH, Kang SB, Han JY, Lim JM and Song YS: Activation of mTOR
signaling pathway associated with adverse prognostic factors of
epithelial ovarian cancer. Gynecol Oncol. 121:8–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coleman LJ, Peter MB, Teall TJ, Brannan
RA, Hanby AM, Honarpisheh H, Shaaban AM, Smith L, Speirs V,
Verghese ET, et al: Combined analysis of eIF4E and 4E-binding
protein expression predicts breast cancer survival and estimates
eIF4E activity. Br J Cancer. 100:1393–1399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Campbell L, Jasani B, Griffiths DF and
Gumbleton M: Phospho-4e-BP1 and eIF4E overexpression
synergistically drives disease progression in clinically confined
clear cell renal cell carcinoma. Am J Cancer Res. 5:2838–2848.
2015.PubMed/NCBI
|
|
21
|
Joshi S and Platanias LC: Mnk kinase
pathway: Cellular functions and biological outcomes. World J Biol
Chem. 5:321–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakai Y, Miyake M, Morizawa Y, Hori S,
Tatsumi Y, Anai S, Onishi S, Tanaka N and Fujimoto K: Potential
biomarkers for the therapeutic efficacy of sorafenib, sunitinib and
everolimus. Oncol Rep. 37:227–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beuselinck B, Vano YA, Oudard S, Wolter P,
De Smet R, Depoorter L, Teghom C, Karadimou A, Zucman-Rossi J,
Debruyne PR, et al: Prognostic impact of baseline serum C-reactive
protein in patients with metastatic renal cell carcinoma (RCC)
treated with sunitinib. BJU Int. 114:81–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xin H, Zhang C, Herrmann A, Du Y, Figlin R
and Yu H: Sunitinib inhibition of Stat3 induces renal cell
carcinoma tumor cell apoptosis and reduces immunosuppressive cells.
Cancer Res. 69:2506–2513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|