Introduction
Gastric cancer is the fourth most common cancer and
the second leading cause of cancer-associated mortality worldwide.
Every year, ~1 million individuals develop gastric cancer and
>700,000 succumb to the disease (1). It is estimated that ~50% of these
patients are from China, and the majority have been diagnosed at
locally advanced or advanced stage (2,3).
Radiotherapy has been an important component of adjuvant treatment
for locally advanced gastric cancer. The upper abdomen, the
radiotherapy target region, is one of the regions influenced most
by respiration. The displacement of organs due to respiration is
the main source of uncertainty in the volume of the radiation
target. A study by Kim et al (4) measured the displacement of upper
abdominal organs during respiration, revealing that the
displacement of the liver, kidneys and spleen ranged between 8.9
and 13.0 mm. Another study by Hallman et al (5) quantified respiration-induced motion of
clinical target volume (CTV) in patients with liver and pancreatic
cancer, demonstrating that the mean distance of motion in these
patients was 9.7 and 5.0 mm, respectively. These studies indicated
that the target volume varied during respiration depending on the
target location. The introduction of novel radiation technologies,
including intensity-modulated radiotherapy (IMRT), has led to the
decrease of the radiation toxicity in normal tissue, but the
problem of target motion induced by breathing remains.
Since the treatment targets in gastric cancer
adjuvant radiation include several subsites with different
respiratory motion amplitudes, the characteristics of the target
motion of all subsites should be considered in the process of
treatment planning. However, little is known about the detailed
displacement pattern of the target volume in gastric cancer. To
guarantee effective dose coverage of the target volume, a wide
margin has to be added onto the CTV in order to account for the
displacement induced by respiration, increasing the radiation
toxicity of organs at risk (OARs).
Previously, significant toxicity associated with
adjuvant chemoradiation has been a serious concern (6). To ensure safety during radiation,
compromises on the coverage and dosage of the target in the final
treatment plan have to be made in numerous cases. Although accurate
radiotherapy has become increasingly widely implemented, there is
an increasing risk of underdosing sections of the target area.
Therefore, accurate radiotherapy requires more precisely delineated
areas of the target and normal organ tissue, increasing the demand
for accurately describing the displacement pattern of regions of
interest (ROIs).
The 4-dimensional computed tomography (4D-CT)
technique is one of the most reliable approaches to accurately
describe the displacement caused by respiration during radiation
(7). The technique has been widely
used in accurate thoracic radiotherapy, particularly in lung cancer
treatment (8–10). Nearly all of the associated studies
reached the conclusion that a more uniform dose distribution in the
target volume and a smaller internal target volume (ITV) can be
obtained through the application of 4D-CT. The majority of the
applications of 4D-CT in upper abdominal radiotherapy are from
research on liver and pancreatic cancer (11). There are currently no reports on the
application of 4D-CT in the treatment planning of gastric
cancer.
The present study was designed to examine the
displacement patterns of the ROIs associated with target volume in
the gastric cancer adjuvant radiation based on 4D-CT, and to
investigate its value in treatment planning. Based on the analysis
of ROI displacement patterns by 4D-CT, a recommendation of
asymmetric expanding margins to account for target displacement in
radiation for gastric cancer is proposed. The feasibility of using
an easier and more reliable way to acquire accurate
ITV3D based on the displacement data from 4D-CT is also
investigated.
Patients and methods
Samples and clinical data
A total of 10 patients with gastric adenocarcinoma
receiving adjuvant radiotherapy were enrolled in this study between
November and December 2013. The median age was 58 (range, 49–67).
The study protocol was approved by the Ethics Committee of Zhongnan
Hospital (Wuhan, China; approval no. 2013018) and informed consent
was obtained from all participants. The main characteristics of the
patients are listed in Table I.
 | Table I.Main characteristics of the
patients. |
Table I.
Main characteristics of the
patients.
| Patient no. | Sex | Age, years | Primary tumor
location | Surgery
pattern | Staging (AJCC
6th) |
|---|
| 1 | Male | 60 | Proximal | D1 | pT3N2M0 |
| 2 | Male | 62 | Proximal | D2 | pT4bN3M0 |
| 3 | Female | 61 | Distal | D2 | pT4aN3aM0 |
| 4 | Male | 49 | Distal | D2 | pT2N1M0 |
| 5 | Male | 67 | Body and
proximal | D1 | pT3N+M0 |
| 6 | Male | 57 | Distal | D2 | pT4bN2M0 |
| 7 | Male | 49 | Distal | D2 | pT4NxM0 |
| 8 | Female | 58 | Proximal | D2 | pT3N2M0 |
| 9 | Male | 57 | Distal and
body | D2 | pT2N3M0 |
| 10 | Female | 60 | Body | D2 | pT3N1M0 |
The inclusion criteria were as follows: i)
Histologically confirmed gastric adenocarcinoma, curative
gastrostomy with D2 lymph node dissection and R0 resection; ii)
patients aged between 18 and 75 years at diagnosis; iii) stage T3
to T4 and/or N1 to N3 [2010 American Joint Committee on Cancer
(AJCC) staging system 7th edition (11)]; iv) Eastern Cooperative Oncology
Group (ECOG) performance status of 0–2; and v) adequate bone marrow
function (hemoglobin ≥90 g/l, neutrophil count
≥1.5×109/l, platelet count ≥100×109/l);
adequate liver function (serum bilirubin ≤1.5× upper limit of
normal (ULN), aspartate aminotransferase (AST), and/or alanine
aminotransferase (ALT) ≤3×ULN); and adequate renal function (serum
creatinine ≤0.106 mmol/l, and calculated creatinine clearance ≤50
ml/min).
The primary exclusion criteria included the
following: i) Receiving neoadjuvant treatment; ii) hepatic, renal,
pulmonary or cardiac dysfunction; iii) severe comorbidities, such
as uncontrollable diabetes mellitus, uncontrollable hypertension,
myocardial infarction or unstable angina pectoris within 6 months
of surgery; iv) severe postoperative complications, such as
anastomotic fistula and pancreatic fistula; and v) diagnosis with
another carcinoma before operation.
CT data acquisition
The patients were immobilized in a supine position
with a vacuum foam pad and were advised to breathe normally.
Pressure sensors (AZ-733V; Anzai Medical Co., Ltd., Tokyo, Japan)
were fixed on the upper abdomen to record respiration signals. CT
data were acquired using multi-slice large aperture CT (Somatom
Sensation 64; Siemens Healthineers, Erlangen, Germany). The
respiration cycle was spilt into 10 phases according to the signal
received by the sensors. The phases included: EX0% (beginning of
exhalation), EX25%, EX50% (middle of exhalation), EX75%, EX100%
(end of exhalation), IN0% (beginning of inhalation), IN25%, IN50%
(middle of inhalation), IN75% and IN100% (end of inhalation). Every
patient underwent a regular 3D-CT scan, followed by a 4D-CT scan.
CT data were integrated with the respiration cycle data with the
MIM software (version 6.0; MIM Software Inc,. Cleveland, OH, USA).
Overall, 10 series of CT images were reconstructed for each phase
of respiration. All CT data were transferred to the Oncentra
brachytherapy planning system (version 4.1; Elekta Instrument AB,
Stockholm, Sweden). The CT image at EX50% was treated as baseline
and the CT images from the remaining respiration phases were fused
and reconstructed.
Definition of ROIs
Based on recurrence patterns and lymphatic drainage
regulations, 3D anatomy markers were drawn up for target volume
delineation in the postoperative radiation of patients with gastric
cancer. The radiation target volume of the regional lymph nodes
(LNs) was delineated according to the consensus of adjuvant
radiation for gastric adenocarcinoma in the Hubei Cancer of
Zhongnan Hospital.
CTV
The CTV is composed of 3 areas: Tumor bed,
anastomosis and draining LN basin. The CTV3D was
delineated upon simulation CT. The CTV4D was generated
by combining the CTV of all 10 aforementioned breathing phases. In
the present study, 7 representative ROIs were selected as the focus
for displacements analysis. These were as follows: Anastomotic
staples, station no. 9 LNs (LNs around the celiac artery), no. 10
LNs (splenic hilar LNs), no. 12P LNs (hepatoduodenal ligament LNs),
no. 13 LNs (LNs on the posterior surface of the pancreatic head),
no. 14V LNs (LNs along the superior mesenteric vein) and no. 16a2
LNs (LNs around the abdominal aorta between the upper margin of the
celiac trunk and the lower margin of the left renal vein). The LN
stations were defined according to the Japanese Gastric Cancer
Association (12). The ROIs in the
present study were composed of the main area of the CTV for
adjuvant radiation of gastric cancer. Each ROI was delineated
separately.
ITV
ITV was mainly used to account for the target
displacement attributed to breathing and organ movements during
radiation. In this study, the ITV4D was defined to be
equal to CTV4D. ITV3D was generated by adding
a margin of 1 cm radial, 1.5 cm distal and 1 cm proximal to the
CTV, according to the recommendation of the European Organization
for Research and Treatment of Cancer (EORTC) (13). For ITV3Dcal, a margin
recommended based on the present displacement analysis was used, as
described next.
Planning target volume (PTV)
The PTV encompassed ITV plus a setup error. The
setup error is set as 5 mm in Zhongnan Hospital (evaluated by cone
beam CT). The PTV4D was calculated by applying an
expansion of 5 mm on the ITV4D. For the PTV3D
and PTV3Dcal, the linear sum of all errors is an
overestimate of the total errors in in the majority of cases.
According to the 62nd Report of the International Commission on
Radiation Units and Measurements (14), the PTV3D and
PTV3Dcal were calculated based on the formula: PTV=CTV +
(IM2 + SM2)1/2, where IM refers to
the internal margin and SM is the setup margin.
Surgery
All patients had previously undergone curative
resection with D2 LN dissection (12). This procedure involved the resection
of the perigastric LNs, the left gastric artery, the common hepatic
artery, the celiac artery, the splenic hilum and the splenic artery
LNs. Pathological evaluation was performed on ≥15 LNs.
Treatment plans
All treatment plans were developed using the
Oncentra treatment plan system. In total, 3 sets of treatment plans
using IMRT technology were designed for each patient:
Plan3D, Plan3Dcal and Plan4D. The
prescription dose of radiation was 45 Gy in 25 fractions, and 7–9
coplanar and/or non-coplanar beams were used. The plans were
optimized to obtain satisfactory dose coverage, with ≥95% of the
PTV receiving the prescribed dose. The treatment plans were
delivered with 6-MV photon beams.
Plan evaluation and dose
limitation
A dose-volume histogram was used to analyze the
coverage of the target volume, where VN was the volume of the ROI
that received ≥N Gy, Dmax was the maximum dose
administered to the ROI, and Dmean was the mean dose.
The main dose limitations for OARs were as follows: Liver,
V30<50%; kidney, Dmean<20 Gy or V20<30% and
V5<65% for both; remnant gastric, V40<50% (no hot spot on it)
and Dmax<54 Gy; spinal cord, V45<0.03
cm3; intestinal (delineated upon simulation CT from 2 cm
above PVT in cephalic-caudal direction), Dmean<30 Gy,
V50<10% (no hot spots on it) and Dmax<54 Gy. For
patients with proximal gastric cancer, a dose limitation of
V30<30% for the heart and V20<20% for the lungs was
applied.
Displacement of ROIs
The displacement was measured in three directions:
Left-right (LR) direction, anterior-posterior (AP) direction and
cephalic-caudal (CC) direction. In order to compare the magnitudes
of displacement in the different directions in 3D, a displacement
vector (DV) was defined to quantify the displacement of all ROIs,
calculated with the following formula: DV=(ΔX2 +
ΔY2 + ΔZ2)1/2, where ΔX, ΔY and ΔZ
represent the maximum displacement distances on the x-, y- and
z-axis, respectively.
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 software (IBM Corp., Armonk, NY, USA). Analysis of
variance was used for comparison among groups and variables.
Multiple comparisons between the groups were performed using SNK
method. P≤0.05 was considered to indicate a statistically
significant difference. The results are presented as the mean ±
standard deviation. The 95% confidence interval (CI) of the
displacement was calculated with the following formula: CI (95%)
=X- ± S * zα/2, where X- refers to mean, S
refers to standard deviation, zα/2=2.262.
Results
Displacement of ROIs
The displacement ranged widely for various ROIs. In
the same direction, the anastomosis staples exhibited the largest
displacement magnitude on the LR direction (X-axis; 5.5±2.5 mm),
while no. 12p LNs went through the largest displacement on the AP
direction (Y-axis; 5.3±2.7 mm) and the cephalic-caudal (CC)
direction (Z-axis; 7.8±3.6 mm). A significant difference in the DV
was observed among the 7 ROIs (P<0.001). The no. 12p LNs had the
largest DV, while the no. 16a2 LNs had the smallest (Table II).
 | Table II.Displacement vectors of regions of
interest for the 10 patients (mean ± standard deviation). |
Table II.
Displacement vectors of regions of
interest for the 10 patients (mean ± standard deviation).
|
| Displacement
dimension, mm |
|
|---|
|
|
|
|
|---|
| Region of
interest | Left-right (x) | Anterior-posterior
(y) | Cephalic-caudal
(z) | Displacement
vector |
|---|
| No. 9 LNs | 2.2±1.2 | 3.8±2.2 | 4.2±2.2 | 9.6±1.8 |
| No. 10 LNs | 3.3±2.5 | 4.2±2.3 | 7.6±3.1 | 14.1±2.4 |
| No. 12P LNs | 3.4±2.1 | 5.3±2.7 | 7.8±3.6 | 14.6±1.6 |
| No. 13 LNs | 2.6±1.8 | 4.9±2.1 | 7.1±2.9 | 12.8±1.7 |
| No. 14V LNs | 2.9±2.4 | 4.6±2.4 | 5.4±3.1 | 12.5±2.3 |
| No. 16a2 LNs | 1.7±1.1 | 3.1±1.5 | 4.7±2.2 | 9.1±1.1 |
| Surgical
staples | 5.5±2.5 | 3.0±1.8 | 6.7±3.6 | 13.5±3.7 |
Recommended margin for expanding the
CTV
The 95% confidence interval (CI) was obtained based
on the data of the displacement of the ROIs. The
PTV3Dcal was calculated according to the displacement
analysis data by expanding the margins of the CTV3D,
according to the formula PTV3Dcal=CTV + (IM2
+ SM2)1/2, IM was generated by taking the
upper limit value, and SM was equal to 5 mm. Thus, the recommended
safety margins for ROIs were obtained and are presented in Table III.
 | Table III.Recommended safety margins for the
regions of interest. |
Table III.
Recommended safety margins for the
regions of interest.
|
| Margin dimension,
mm |
|---|
|
|
|
|---|
| Region of
interest | Left-right (x) | Anterior-posterior
(y) | Cephalic-caudal
(z) |
|---|
| No. 9 LNs | 7.0 | 8.5 | 9.0 |
| No. 10 LNs | 8.5 | 9.0 | 12.5 |
| No. 12P LNs | 8.0 | 10.0 | 13.0 |
| No. 13 LNs | 7.5 | 9.0 | 11.5 |
| No. 14V LNs | 8.0 | 9.0 | 10.0 |
| No. 16 LNs | 7.0 | 8.0 | 9.0 |
| Surgical
staples | 10.5 | 8.0 | 11.5 |
Comparison of the PTVs
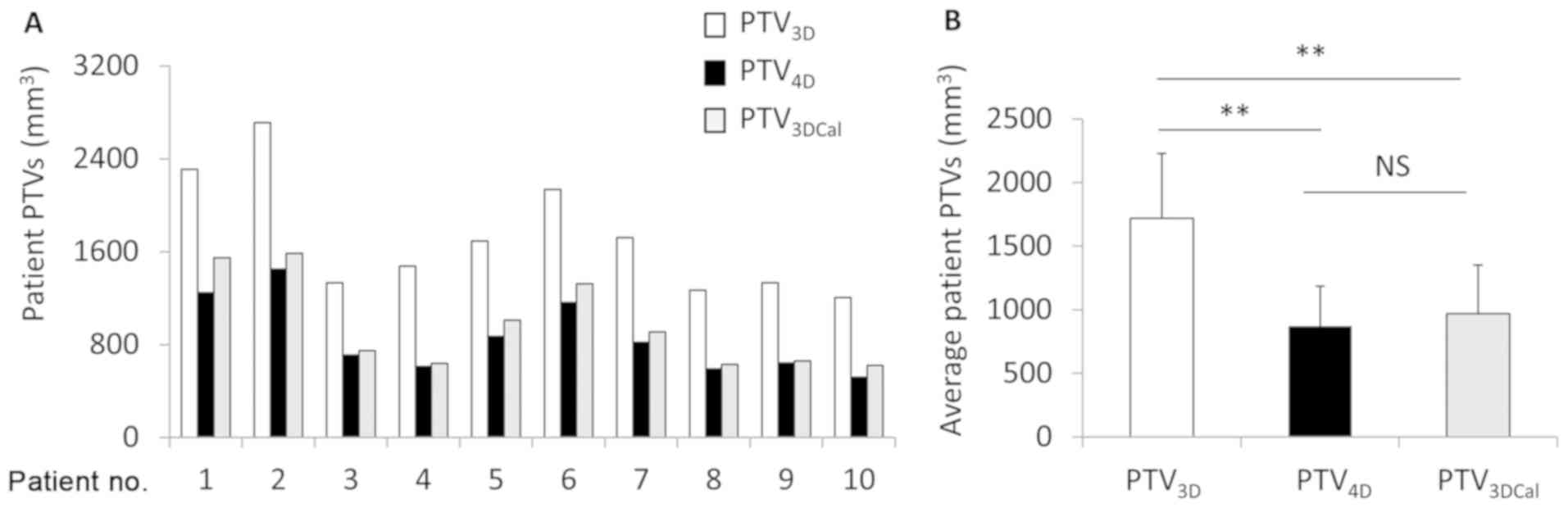
The 3 types of PTV were compared in each patient
(Fig. 1A). The volume of
PTV4D had no significant difference compared with that
of PTV3Dcal (868.87±318.18 vs. 967.87±384.81
cm3; P=0.538). The PTV3D (1719.04±509.35) was
the largest, significantly so compared with the PTV3Dcal
or PTV4D (P<0.001; Fig.
1B). All PTV3Dcal and PTV4D were included
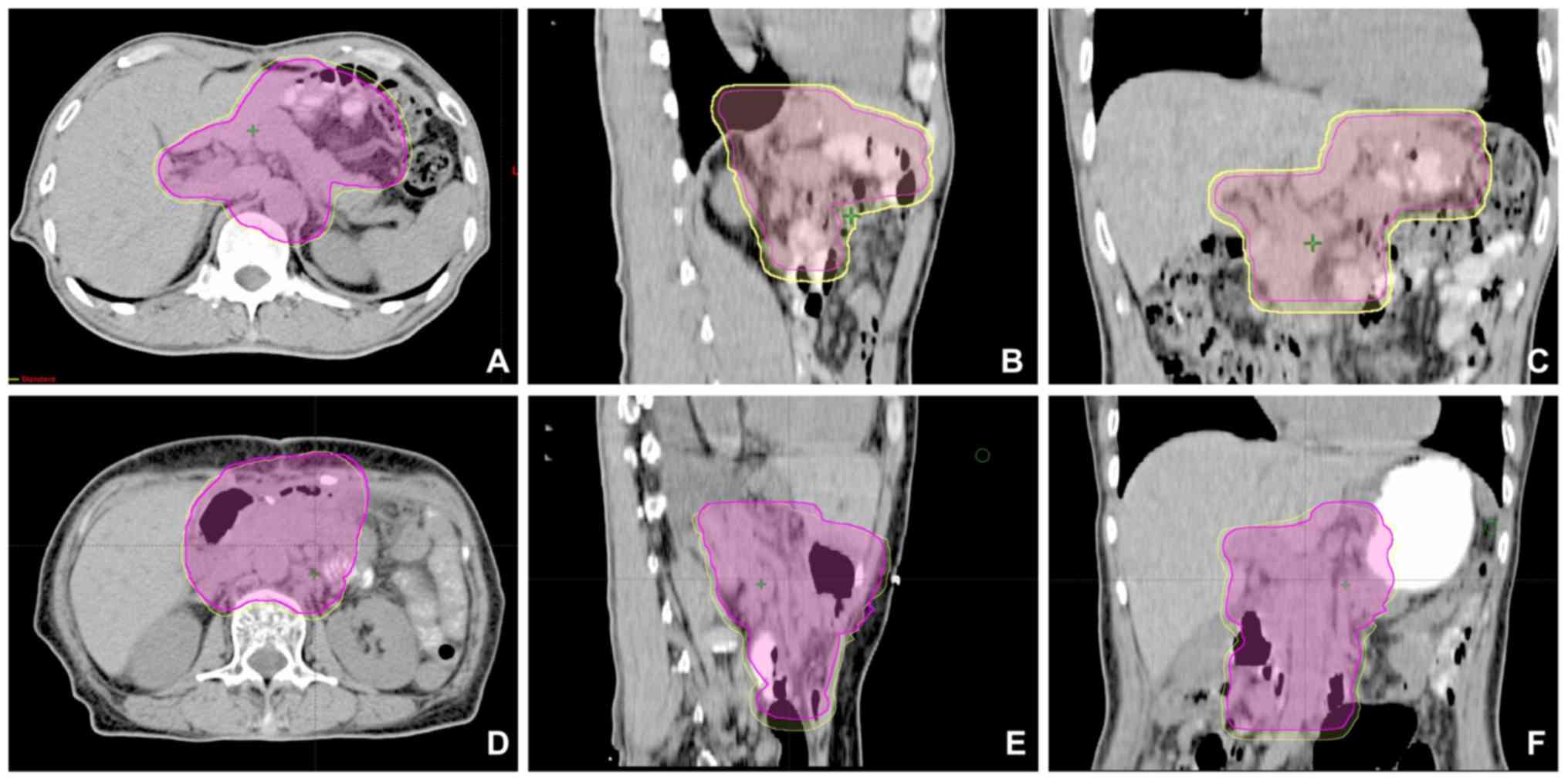
within the PTV3D. For the majority of patients (8/10),
the PTV4D was completely encompassed in the
PTV3DCal (Fig. 2A-C),
while the PTV3Dcal in certain sections was smaller than
the PTV4D in 2 out of 10 patients (Fig. 2D-F). However, the PTV3D
was larger than the PTV4D in all patients.
Dosage analysis in the PTVs
The 3 treatment plans (Plan3D,
Plan4D and Plan3Dcal) for all patients were
compared. Following the analysis of the DVH and a slice-by-slice
examination, no difference in the coverage and distribution was
detected in the 3 types of treatment plan for all patients.
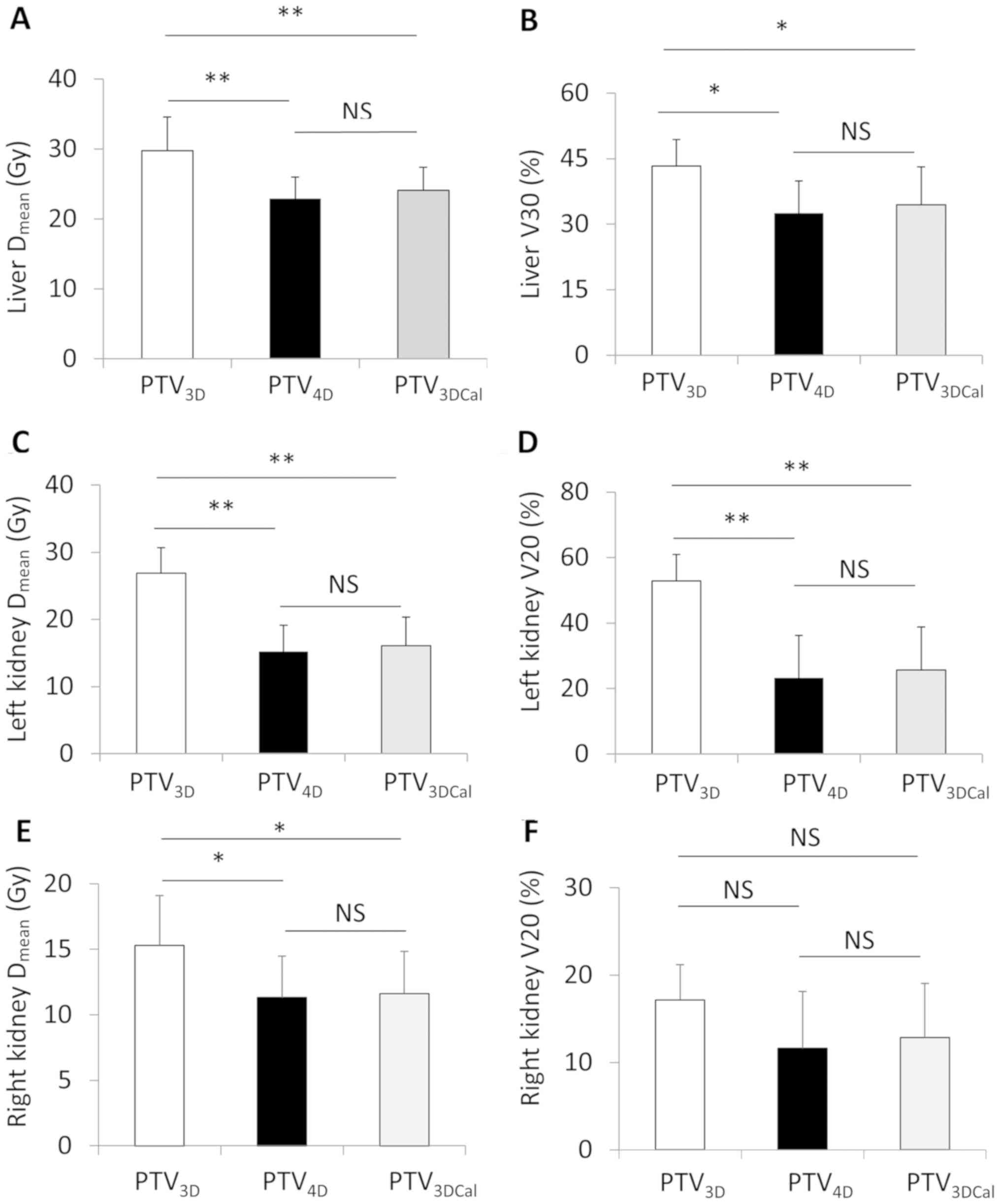
Dose of radiation on the OARs
The radiation doses on the OARs in the 3 types of
treatment plan were compared. The Dmean and V30 of the
liver showed no significant difference between the
PTV3Dcal and the PTV4D treatment plans. The
Dmean and V30 of the liver were significantly increased
in the PTV3D treatment plan compared with those in
PTV4D and PTV3Dcal treatment plans. The
Dmean of the kidney (right and left) were significantly
lower in PTV4D and PTV3Dcal compared with
these in PTV3D treatment plan. However, the V20 of the
right kidney showed no difference when PTV3D was
compared with PTV4D or PTV3Dcal (Fig. 3).
Discussion
The main findings of the present study were as
follows: i) Different displacement magnitudes and directions were
described for each ROI in the adjuvant radiation treatment of
patients with gastric cancer, implying that a uniform margin
expansion for all target areas is not optimal for the generation of
the PTV; ii) the PTV4D was smaller than the
PTV3D; iii) the treatment plan of PTV4D was
beneficial for the protection of the liver and left kidney; and iv)
regarding the PTV and protection of OARs, Plan3Dcal lay
between Plan4D and Plan3D, with a
satisfactory PTV coverage, despite demonstrating certain PTV
underestimation in ROIs with a large displacement range compared
with that of Plan4D.
While a number of previous studies have addressed
the displacement mode of upper abdominal organs or surgical staples
(4,5,15), the
present study, to the best of our knowledge, described for the
first time the displacement mode of radiotherapy-associated
lymphatic drainage stations of gastric cancer. The present findings
revealed that different subsites of the target volume had different
patterns of displacement, according to their anatomical positions.
Generally, the displacement magnitude was much larger in the CC
direction. The LN regions adjacent to the hepatic portal and
splenic hilum exhibited a larger displacement in the CC direction,
as they are located next to organs affected by the motion of
breathing. In addition to the displacement in the CC direction, the
anastomosis staple also demonstrated a large displacement in the LR
direction, which may be due to the peristalsis of the stomach.
Certain ROIs, including no. 9 and no. 12 LNs, exhibited much
smaller displacement than anticipated, as they are located in the
peritoneum and are fixed by the surrounding ligament. These
findings should be taken under consideration in the process of
expanding the margins of CTV to generate the PTV, as those areas
with large displacement magnitude could be administered an
insufficient radiation dosage, and wide margins should be
considered to account for any displacement. However, for the areas
with smaller displacement, a smaller margin is sufficient to cover
the target volume and this could lead to a decrease in radiation
toxicity.
The ITV was used to account for variations induced
by the target displacement and deformation. These variations
generally consist of two types, the interfractional and
intrafractional variations. Interfractional variations comprise the
changes in organ volume, including bowel/stomach variations, and
weight, while the sources of intrafractional variations mainly come
from respiration and peristalsis. Of all variations, the
displacement induced by breathing is the main source of uncertainty
in the adjuvant radiation for gastric cancer. As the main ROIs in
the present study were the draining LN regions, which are less
influenced by organ filling or weight changes, the interfractional
variations were ignored, and the combination of CTV4D
was defined as ITV4D.
In the dosimetric analysis, no differences were
observed in the PTV coverage and distribution among the three
treatment plans, while the volume of PTV4D was the
smallest among the 3 sets of treatment plans. As similar studies on
gastric cancer are few in number, only certain studies addressing
other upper abdominal tumors could be reviewed. Matoba et al
(16) compared treatment plans based
on 4D- and 3D-CT in gastric lymphoma, and concluded that
Plan4D was as effective as Plan3D and could
minimize the exposure of the OARs to radiation. Xi et al
(17) reached a similar conclusion
regarding the radiation treatment of liver cancer. It is worth
noting that, although the PTV4D is significantly smaller
than the PTV3D and PTV3DCal, it is closer to
the PTV3DCal than to the PTV3D. The
difference between PTV4D and PTV3D is >800
cm3, while that between PTV4D and
PTV3Dcal is <100 cm3, indicating that the
PTV3Dcal also has potential in protecting the normal
organs and tissues from radiation. Furthermore, the present study
confirmed that the expansion margin according to the recommendation
of the EORTC was sufficient to cover the displacement induced by
respiration in all patients. However, the margin may be considered
too large in certain target areas, where PTV3Dcal and
PTV4D are significantly superior to PTV3D in
protecting normal tissues.
The results of the present study demonstrate that
4D-CT has an advantage over traditional 3D-CT with regard to
obtaining a more accurate target volume in treatment planning.
However, treatment planning based on 4D-CT has certain limitations.
4D-CT demands higher requirements for equipment and personnel
training. Furthermore, the delineation of the CTV on all phases of
the CT image and treatment planning is more time-consuming than
that in regular treatment planning. We hope to develop a novel
method for performing treatment planning, based on 3D-CT, which
retains the advantage of a smaller and more accurate target volume.
Based on the analysis of the subsite target displacement patterns
of 4D-CT, a non-uniform expansion of the CTV can be calculated and
applied to generate the PTV3Dcal. This integrates the
main advantage of treatment planning based on 3D-CT and 4D-CT. In
the present study, the feasibility of the application of
PTV3Dcal in treatment planning was investigated. The
main result agrees with the hypothesis that the PTV3Dcal
can decrease the PTV3D and provide better protection for
OARs compared with the PTV3D.
However, in an analysis of target coverage of
PTV3Dcal compared with PTV4D, 2 out of 10
patients experienced target underdosing. The upper 95% CI
limitation of target displacement was used as the recommended
expansion of PTV and an estimated 5% target underdosing was
observed. The slice-by-slice dose coverage analysis revealed that
the portion of PTV4D receiving an insufficient radiation
dosage was small. To reach a balance between efficacy and toxicity,
a 5% PTV4D underdose may be considered acceptable. The
investigation into PTV3Dcal is one of the most important
aspects of the present study, as it has provided a novel approach
to delineation of a more accurate target volume, although certain
improvements are necessary. A more accurate target displacement
calculation based on a larger sample population is required, and
the establishment of a more optimized mathematical model is
required in order to integrate all the expansion margins.
Overall, the present study has described the
detailed displacement mode of the main region of the CTV, in
particular the displacement mode of the lymphatic drainage area, in
the 4D-CT-based adjuvant radiation treatment of gastric cancer. A
non-uniform expansion of CTV margins accounting for the
displacement due to respiration is recommended, based on the
displacement analysis data of ROIs. The PTV4D and
PTV3Dcal are significantly smaller than the
PTV3D and the treatment plans based on these are
beneficial in protecting OARs. The PTV3Dcal treatment
planning procedure may provide a novel, simple approach to
delineation of a more accurate target volume, although certain
further improvements are required.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Funds of China (grant nos. 81172129 and
81472798).
Availability of data and materials
The datasets used or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JP and JG performed the research and wrote the
manuscript. JM and XW collected and analysis data. HX collected and
analysis the clinical data; FZ designed the project and final
approved the manuscript. JD and YZ analysis part data, and reviewed
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Zhongnan Hospital (Wuhan, China) (approval no.
2013018) and written informed consent was obtained from all
participants.
Patient consent for publication
All patients enrolled in the present study provided
written consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YS, Park SH, Ahn SD, Lee JE, Choi EK,
Lee SW, Shin SS, Yoon SM and Kim JH: Differences in abdominal organ
movement between supine and prone positions measured using
four-dimensional computed tomography. Radiother Oncol. 85:424–428.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hallman JL, Mori S, Sharp GC, Lu HM, Hong
TS and Chen GT: A four-dimensional computed tomography analysis of
multiorgan abdominal motion. Int J Radiat Oncol Biol Phys.
83:435–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macdonald JS, Smalley SR, Benedetti J,
Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA,
Gunderson LL, Jessup JM and Martenson JA: Chemoradiotherapy after
surgery compared with surgery alone for adenocarcinoma of the
stomach or gastroesophageal junction. N Engl J Med. 345:725–730.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang SB: Radiotherapy of mobile tumors.
Semin Radiat Oncol. 16:239–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Ruysscher D, Faivre-Finn C, Nestle U,
Hurkmans CW, Le Péchoux C, Price A and Senan S: European
organisation for research and treatment of cancer recommendations
for planning and delivery of high-dose, high-precision radiotherapy
for lung cancer. J Clin Oncol. 28:5301–5310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li FX, Li JB, Zhang YJ, Liu TH, Tian SY,
Xu M, Shang DP and Ma CS: Comparison of the planning target volume
based on three-dimensional CT and four-dimensional CT images of
non-small-cell lung cancer. Radiother Oncol. 99:176–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Hayes S, Paskalev K, Jin L,
Buyyounouski MK, Ma CC and Feigenberg S: Dosimetric comparison of
stereotactic body radiotherapy using 4D CT and multiphase CT images
for treatment planning of lung cancer: Evaluation of the impact on
daily dose coverage. Radiother Oncol. 91:314–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang JW, Brown JG, Mauch PM and Ng AK:
Four-dimensional versus 3-dimensional computed tomographic planning
for gastric mucosa associated lymphoid tissue lymphoma. Pract
Radiat Oncol. 3:124–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matzinger O, Gerber E, Bernstein Z,
Maingon P, Haustermans K, Bosset JF, Gulyban A, Poortmans P,
Collette L and Kuten A: EORTC-ROG expert opinion: Radiotherapy
volume and treatment guidelines for neoadjuvant radiation of
adenocarcinomas of the gastroesophageal junction and the stomach.
Radiother Oncol. 92:164–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
International Commission on Radiation
Units and Measurements I: Prescribing, recording, and reporting
photon beam therapy (Supplement to ICRU Report 50). ICRU Report.
62:1999.
|
|
15
|
Yamashita H, Okuma K, Takahashi W, Sakumi
A, Haga A, Ino K, Akahane M, Ohtomo K and Nakagawa K:
Four-dimensional measurement of the displacement of metal clips or
postoperative surgical staples during 320-multislice computed
tomography scanning of gastric cancer. Radiat Oncol. 7:1372012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matoba M, Oota K, Toyoda I, Kitadate M,
Watanabe N and Tonami H: Usefulness of 4D-CT for radiation
treatment planning of gastric MZBCL/MALT. J Radiat Res. 53:333–337.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xi M, Liu MZ, Li QQ, Cai L, Zhang L and Hu
YH: Analysis of abdominal organ motion using four-dimensional CT.
Ai Zheng. 28:989–993. 2009.(In Chinese). PubMed/NCBI
|