Introduction
Approximately 70% of patients with renal cell
carcinoma (RCC) are diagnosed with localized RCC, and incidental
detection of asymptomatic RCC is increasing with the widespread use
of ultrasonography and computed tomography (CT) (1). Localized RCCs are treated by radical
nephrectomy or partial nephrectomy. After complete surgical
resection for localized RCC, 20 to 30% of patients progress to
metastatic disease (2). The
prognosis for patients with RCC is primarily dependent on disease
stage, and patients with a high TNM stage have a poorer prognosis.
Once RCC has metastasized, the 5-year survival rate is <10%
(3). The identification of
prognostic markers would be useful to prevent localized RCC
recurrence after surgery.
RCC is an immunogenic tumor and pathological
specimens contain large numbers of tumor-infiltrating lymphocytes
(TILs) (4). RCC can impair host
antitumor immunity (5–7). Cancer cells express tumor-specific
aberrant antigens and evade immune detection to survive by inducing
immunosuppression or deriving survival signals from
tumor-infiltrating immune cells (8,9). Various
cells and cytokines are involved in the immune escape of tumor
cells. For instance, regulatory T cells (Tregs) and the B7 family
are associated with tumor immune escape. Tregs play an important
role in maintaining the stability of the immune system and tumor
immune tolerance. Forkhead box p3 (Foxp3) is a specific
transcription factor expressed in Tregs (10). The B7 family is composed of
cell-surface proteins that regulate immune responses by delivering
co-stimulatory or co-inhibitory signals through their ligands.
The correlation of Foxp3-positive cells or B7 family
with patient clinicopathological features have been investigated in
many cancers, including RCC (11–13).
Tissue samples or blood samples only have tended to be used, with
few studies examining both sample types. Furthermore, CD276 can
increases the secretion of interferon-gamma (IFNγ) by activated T
cells (14), and Foxp3 products
interleukin (IL)-10, transforming growth factor-β (TGF-β), and
tumor necrosis factor-alpha (TNFα) (15). Although the correlation between TILs
and associated cytokines has been reported, there is no consensus
on their interactions.
Postoperative recurrence of RCC after radical
surgery mostly depends on the presence of micrometastasis
preoperatively. Here, we hypothesized the possible correlation of
preoperative topical and systemic immunoreactions with the risk of
micrometastasis. To evaluate topical and systemic preoperative
immunoreactions, we investigated the correlation between the
profile of TILs and preoperative serum cytokine levels. The aim of
this study was to identify non-invasive and preoperative markers
that could be valuable to predict the recurrence of localized clear
cell RCC (ccRCC) after radical nephrectomy.
Materials and methods
Patients selection and data
collection
Eighty seven patients who underwent radical
nephrectomy for clinically localized ccRCC between January 2009 and
December 2014 were included in this retrospective study. All
patients underwent preoperative whole body computed tomography (CT)
for tumor staging. The clinical stage was determined according to
the TNM classification (16).
Clinicopathological variables and laboratory data were extracted
from medical records. The SSIGN score, which is an outcome
prediction model for patients with ccRCC based on pathological
stage, tumor size, nuclear grade, and necrosis (17), was evaluated as one of the
clinicopathological variables. The histopathological review was
conducted by an experienced uropathologist to determine the T
category and Fuhrman grade (18), as
well as the presence of necrosis, sarcomatoid variant, and
lymphovascular invasion (LVI). All subjects gave their written
informed consent for inclusion before they participated in the
study. The study was conducted in accordance with the Declaration
of Helsinki, and the protocol was approved by the Ethics Committee
of Nara Medical University (Nara, China) (Project identification
code: 1630, accepted: August 21, 2017).
Immunohistochemistry (IHC)
staining
Resected tissue specimens were fixed in formalin,
embedded in paraffin, and then subjected to IHC staining for the
cell surface and immunological markers CD4, CD8, CD80 (B7-1), CD86
(B7-2), CD276 (B7-H3), and Foxp3 (a Treg marker). Paraffin blocks
were cut and placed on Superfrost Plus microslides (Thermo Fisher
Scientific, Inc., Yokohama, Japan). Sections were deparaffinized
and citric acid buffer (pH 6.0) antigen retrieval was carried out
with autoclaving. IHC staining was performed using the Histofine
ABC kit (Nichirei Biosciences, Tokyo, Japan) according to the
manufacturer's instructions. Briefly, slides were incubated
overnight at 4°C with monoclonal antibodies against CD4 (clone
4B12, ready-to-use; Nichirei Biosciences), CD8 (clone C8/144B,
ready-to-use; Nichirei Biosciences), CD80 (clone EPR1157 (2), 1:500 dilution; Abcam, Cambridge, UK),
CD86 (clone EP1158Y, 1:500 dilution; Abcam), CD276 (clone 6A1,
1:1000 dilution; Abcam), and Foxp3 (clone 236A/E7, 1:500 dilution;
Abcam). The slides were counterstained with Mayer's hematoxylin,
dehydrated, and sealed with a cover slide.
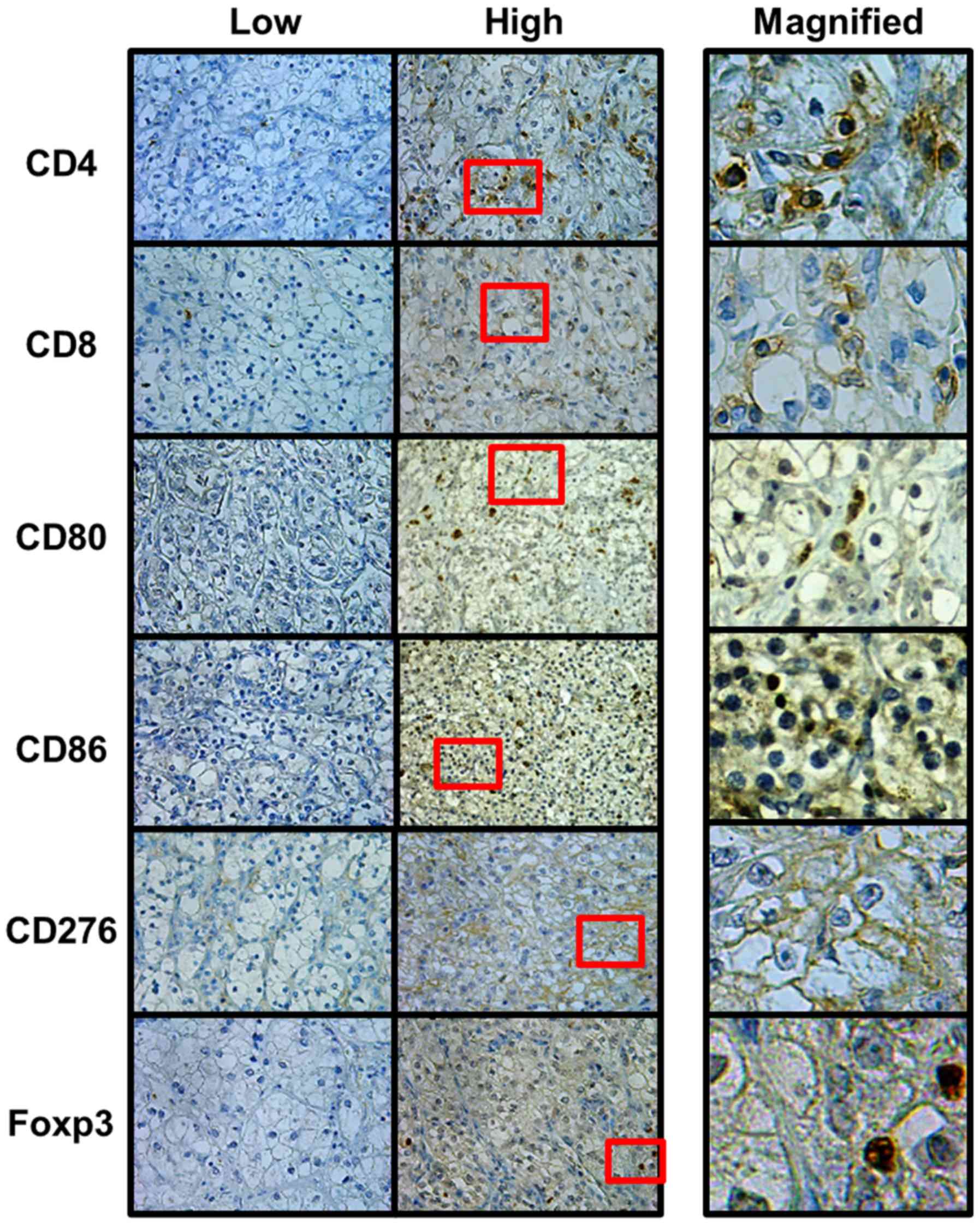
Expression of markers
All stained tissue samples were evaluated by two
investigators (KO and YI) without knowledge of the patients'
clinical records. One sample was divided into four randomly
selected tumor areas at ×400 magnification. The percentage of
stained cells was calculated by taking the mean of percentage
calculated by dividing a positive cell count by the total cell
count of positive cell count and negative cell count. Specimens
were classified into two groups (low and high) based on the
staining population. The cutoff level of CD4 and CD8 was set at
20%, and the cutoff level of CD80, CD86, CD276, and Foxp3 was set
at 10% (13,19). The expression of Foxp3 was evaluated
with the nucleus of tumor cells exhibiting immunoreactivity, and
the expressions of other markers were evaluated with the cell
membrane (Fig. 1).
Measurement of serum cytokines
Serum was collected from every patient in tubes
before the operation and centrifuged at 1,000 × g for 15 min. The
supernatant was recovered and stored at −80°C until analysis. Based
on the result of the IHC analysis, six cytokines (IL-6, IL-10,
IL-17, IFN-γ, TNF-α, and TGF-β) were selected. Their profiles in
preoperative sera were determined using the following ELISA kits:
human IL-6 (950.030.096, Diaclone SAS, Besancon, France), human
IL-10 (950.060.096, Diaclone SAS), human IL-17A (850.940.096,
Diaclone SAS), TNF-α (950.090.096, Diaclone SAS), IFN-γ
(950.000.096, Diaclone SAS), and TGF-β1 (DB100B, R&D Systems,
Minneapolis, MN, USA). A microplate reader (Tecan Systems Inc., San
Jose, CA, USA) was used to measure the absorbance at 450 nm.
Statistical analyses
The statistical analyses were performed with SPSS
for Windows (version 20.0; IBM, Corp., Armonk, NY, USA). Figure
plotting was performed using GraphPad Prism 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA). The correlations between the counts of
TILs and clinicopathological characteristics were analyzed using
the Mann-Whitney U
test, Chi-square test, or Fisher's exact test as appropriate. The
correlations between the counts of TILs and serum levels of
cytokines were analyzed using the Mann-Whitney U test. Disease-free
survival (DFS) was used as an endpoint, and Kaplan-Meier survival
curves were plotted and compared using the log-rank test for
univariate analysis. The Cox regression model was used for
multivariate DFS analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The median follow-up period after radical
nephrectomy for the DFS analysis was 39.9 months (range, 4–93
months). During the follow-up period, 22 patients (25.3%)
experienced recurrence, which was defined as local recurrence or
metastasis involving lymph node, bone, lung, or other sites. The
median follow-up period after radical nephrectomy for the overall
survival analysis was 48.8 months (range 6–100 months). During the
follow-up period, 14 patients (16.1%) died.
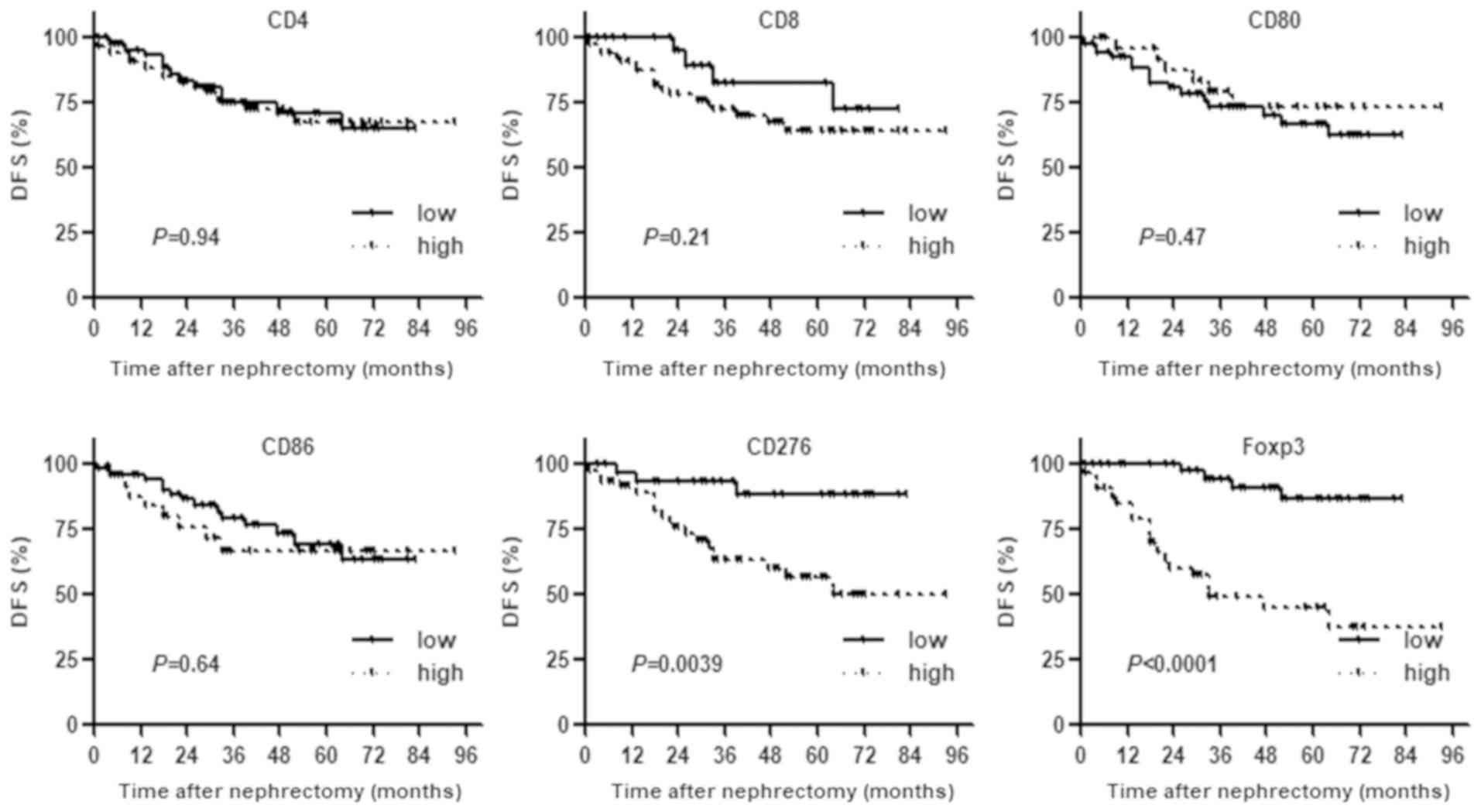
Expression of TIL counts and clinical
course
Patients were classified into low and high groups
based on staining (Fig. 1).
Univariate analysis with Kaplan-Meier curves and log-rank test
analysis showed that patients with high counts of CD276+
or Foxp3+ TILs had a significantly higher risk of
recurrence after nephrectomy compared with patients with low counts
of CD276+ or Foxp3+ TILs. With regard to the
other markers, there were no significant differences in DFS between
the two groups (Fig. 2).
Correlation between CD276 or Foxp3
counts and clinicopathological variables
The baseline clinicopathological variables for the
87 cases and their association with the counts of CD276+
or Foxp3+ TILs are summarized in Table I. Patients with high counts of
CD276+ TIL had a significantly high pT stage and were
linked with the presence of LVI compared to patients in the low
group (Table I). Moreover, patients
with high counts of the Foxp3+ TIL had a significantly
high pT stage and high SSIGN score compared to patients in the low
group.
 | Table I.Characteristics of clear cell renal
cell carcinoma patients in dependent of CD276 and Foxp3
expression. |
Table I.
Characteristics of clear cell renal
cell carcinoma patients in dependent of CD276 and Foxp3
expression.
|
|
| CD276 | Foxp3 |
|---|
|
|
|
|
|
|---|
| Variables | Total n | Low | High | P-value | Low | High | P-value |
|---|
| n | 87 | 36 | 51 |
| 53 | 34 |
|
| Sex |
|
|
| 0.89a |
|
| 0.92a |
|
Male | 67 | 28 | 39 |
| 41 | 26 |
|
|
Female | 20 | 8 | 12 |
| 12 | 8 |
|
| Age, median
(range), years | 67 | 68.5 (36–87) | 66.0 (39–85) | 0.96b | 67 (36–87) | 67.5 (42–85) | 0.61b |
| MSKCC |
|
|
| 0.60a |
|
| 0.60a |
|
Good | 44 | 17 | 27 |
| 28 |
|
|
|
Intermediate/poor | 43 | 19 | 24 |
| 25 | 18 |
|
| pT category |
|
|
| 0.0021a |
|
| 0.01a |
| T1 | 32 | 21 | 11 |
| 26 | 6 |
|
| T2 | 3 | 1 | 2 |
| 2 | 1 |
|
|
T3/4 | 52 | 14 | 38 |
| 25 | 27 |
|
| SSIGN score |
|
|
| 0.065a |
|
| 0.0083a |
| ≤4 | 58 | 28 | 30 |
| 41 | 17 |
|
|
>4 | 29 | 8 | 21 |
| 12 | 17 |
|
| LVI |
|
|
| 0.0039a |
|
| 0.91a |
|
LVI- | 42 | 24 | 18 |
| 33 | 9 |
|
|
LVI+ | 45 | 12 | 33 |
| 20 | 25 |
|
Prognostic factors of recurrence after
radical nephrectomy
Cox univariate analyses showed that high pT stage,
high SSIGN score, high counts of CD276+ TILs, and high
counts of Foxp3+ TILs were factors for poor prognosis
for recurrence (Table II).
Multivariate analyses showed that high counts of the
CD276+ and Foxp3+ TILs were independent
factors for poor prognosis due to recurrence.
 | Table II.Univariate and multivariate analyses
for disease-free survival of clinicopathological variables in
patients with clear cell renal cell carcinoma. |
Table II.
Univariate and multivariate analyses
for disease-free survival of clinicopathological variables in
patients with clear cell renal cell carcinoma.
|
| Disease-free
survival |
|---|
|
|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
| 0.93 |
|
|
|
|
Male | 1 |
|
|
|
|
|
|
Female | 0.96 | 0.36–2.58 |
|
|
|
|
| Age, years |
|
| 0.73 |
|
|
|
|
<70 | 1 |
|
|
|
|
|
|
≥70 | 1.16 | 0.50–2.73 |
|
|
|
|
| MSKCC |
|
| 0.26 |
|
|
|
|
Good | 1 |
|
|
|
|
|
|
Intermediate/poor | 1.69 | 0.68–4.18 |
|
|
|
|
| pT category |
|
| 0.02 |
|
| 0.16 |
|
≤T2 | 1 |
|
|
|
|
|
| T3 | 2.74 | 1.17–6.42 |
| 0.32 | 0.069–1.54 |
|
| SSIGN score |
|
| 0.0023 |
|
| 0.30 |
| ≤4 | 1 |
|
|
|
|
|
|
>4 | 4.19 | 1.67–10.54 |
| 1.65 | 0.64–4.26 |
|
| LVI |
|
| 0.13 |
|
|
|
| − | 1 |
|
|
|
|
|
| + | 1.92 | 0.83–4.44 |
|
|
|
|
| CD276
expression |
|
| 0.0039 |
|
| 0.033 |
|
Low | 1 |
|
|
|
|
|
|
High | 3.48 | 1.49–8.12 |
| 3.76 | 1.12–12.67 |
|
| Foxp3
expression |
|
| <0.0001 |
|
| 0.006 |
|
Low | 1 |
|
|
|
|
|
|
High | 8.05 | 3.33–19.44 |
| 2.92 | 1.35–6.31 |
|
Correlation between TIL counts and
preoperative serum level of cytokines
Based on the result of the immunohistochemical
analysis, we focused on the association between
CD276/Foxp3-positive lymphocytes and the circulating cytokines.
Relationships with various cytokines have been reported in
CD276/Foxp3-positive lymphocytes, where CD276 increases IFNγ
secretion by activated T cells (15)
as well as Foxp3 products IL-10, TGF-β, and TNFα (16). Furthermore, levels of IL6 and IL17,
which were associated with Th17, were also assessed because the
balance of Th17 and Treg can be skewed in patients with RCC
(20). Therefore, putative cytokines
including IL-6, IL-10, IL-17, IFN-γ, TNF-α, and TGF-β were selected
and their levels in the preoperative serum were measured. Patients
with high counts of CD276+ TILs had significantly high
serum levels of TNF-α and IFN-γ compared to patients in the low
group. Patients with high counts of Foxp3+ TILs had a
significantly high serum level of TGF-β1 compared to patients in
the low group (Table III).
 | Table III.Correlation between the peritumoral
immune associated antigens (CD276 and Foxp3) and preoperative serum
levels of cytokines. |
Table III.
Correlation between the peritumoral
immune associated antigens (CD276 and Foxp3) and preoperative serum
levels of cytokines.
|
| CD276 | Foxp3 |
|---|
|
|
|
|
|---|
| Variables | Low | High | P-value | Low | High | P-value |
|---|
| Total n | 36 | 51 | – | 53 | 34 | – |
| TNFα, median
(range) | 0.024
(0.016–0.072) | 0.058
(0.016–0.49) | 0.03 | 0.049
(0.016–0.49) | 0.036
(0.016–0.16) | 0.19 |
| TGFβ, median
(range) | 0.066
(0.15–1.19) | 0.065
(0.098–1.18) | 0.57 | 0.61
(0.15–1.18) | 0.71
(0.098–1.19) | 0.02 |
| IFNγ, median
(range) | 0.031
(0.018–0.070) | 0.034
(0.015–0.082) | 0.04 | 0.031
(0.015–0.078) | 0.034
(0.018–0.082) | 0.32 |
| IL-6, median
(range) | 0.71
(0.071–3.80) | 0.66
(0.071–3.82) | 0.23 | 0.66
(0.071–3.82) | 0.71
(0.073–3.80) | 0.38 |
| IL-10, median
(range) | 0.11
(0.023–0.88) | 0.018
(0.019–3.33) | 0.39 | 0.11
(0.019–1.43) | 0.20
(0.023–3.33) | 0.18 |
| IL-17, median
(range) | 0.11
(0.025–1.28) | 0.12
(0.025–1.06) | 0.82 | 0.090
(0.025–0.80) | 0.15
(0.025–1.28) | 0.95 |
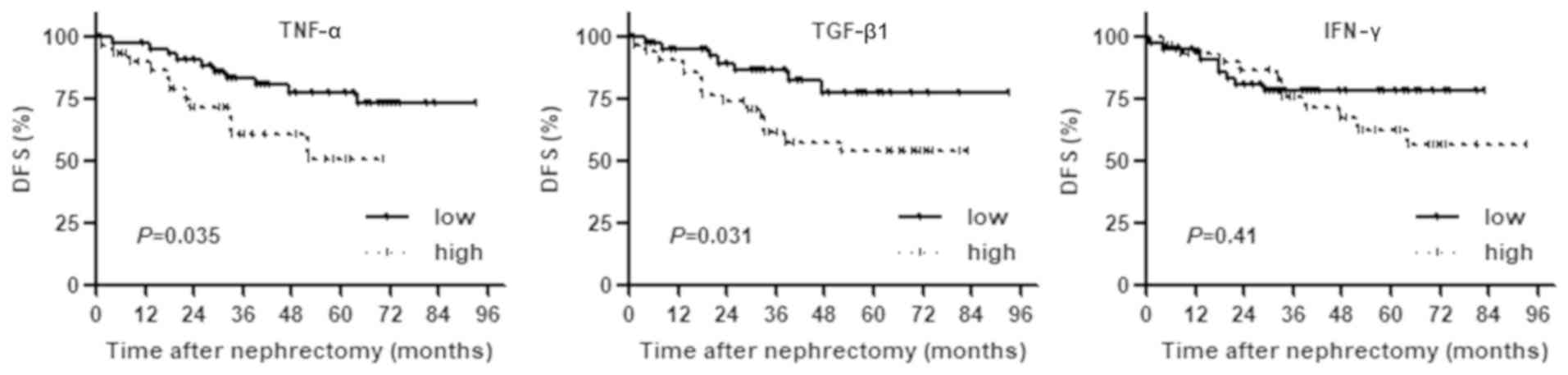
Prognostic value of preoperative serum
level of TNF-α, IFN-γ, and TGF-β1
To identify preoperative blood-based tests useful in
predicting prognosis, the association of TNF-α, TGF-β1, and IFN-γ
with prognosis was analyzed, since these three cytokines have been
linked with the expression of CD276+ or
Foxp3+ TILs. Kaplan-Meier analysis revealed that
patients with high serum levels of TNF-α and TGF-β1 had a
significantly higher risk of recurrence after nephrectomy compared
to patients with low serum levels. There was no significant
difference in IFN-γ between the two groups (Fig. 3).
Discussion
In this study, we found that high counts of
CD276+ and Foxp3+ in tumor tissues were
independent factors for the poor prognosis of visceral metastasis
or local recurrence after radical nephrectomy for localized ccRCC.
Moreover, we found that high preoperative serum levels of TNF-α and
TGF-β1 were also factors for poor prognosis.
Foxp3 belongs to the transcription factor Forkhead
box family and is a master gene of Treg differentiation and a
specific transcription factor expressed in Tregs. Foxp3 exists in
the tumor microenvironment and is a negative factor that controls
antitumor immunity by disrupting T cell increase (10). It has also been reported that the
expression of tumor-infiltrating Foxp3-positive lymphocytes was
associated with poor prognosis in several cancers, including lung
(21), breast (22), liver (23), pancreatic (24), ovarian (25), and kidney cancer (26).
CD276 is a member of the B7 family, which comprises
cell-surface proteins that regulate immune responses by delivering
co-stimulatory or co-inhibitory signals through their ligands.
B7-H3 is one of the most recently described members of the B7
family, so its function and binding partners have not yet been
elucidated. Tumor-infiltrating CD276-positive lymphocytes have been
associated with poor prognosis in several cancers, including lung
(27), colon (28), and kidney cancer (29).
In this study, high counts of Foxp3+ TILs
were associated with pT stage and SSIGN score, and high counts of
CD276+ TILs were associated with pT stage and LVI. The
SSIGN score is an outcome prediction model for patients with ccRCC
treated with radical nephrectomy. The score is based on
pathological stage, tumor size, nuclear grade, and necrosis
(18). LVI, pT stage, and SSIGN
score are important prognostic factors of RCC, but they cannot be
evaluated preoperatively. Therefore, we focused on preoperative
serum levels of several cytokines, which have been reported to be
correlated with the recruitment of CD276+ cells and
Foxp3+ cells.
The relationships between Tregs or CD276 and several
cytokines have been reported previously. von Boehmer et al
(30) reported that Tregs have
several modes of suppressive action at their disposal that may
depend on the microenvironment in which the suppressor cells are
activated, and may be differentially used to suppress different
forms of immunopathology. The secreted factors, such as IL-10 (an
inhibitor for dendritic cells) and TGF-β1 (which directly act on T
cells) participate in the suppressive action. B7-H3 reportedly
costimulates the proliferation of both CD4+ and
CD8+ T cells, enhances the induction of cytotoxic T
cells, and selectively stimulates IFNγ production in the presence
of T cell receptor signaling (14).
In contrast, inclusion of antisense B7-H3 oligonucleotides
decreases the expression of B7-H3 on dendritic cells and inhibits
IFNγ production by dendritic cell-stimulated allogeneic T cells.
The over-expression of B7-H3 and B7-H4 induce T cells to secrete
TGF-β1 and the immunosuppressive cytokines IL-2, IL-6, and IL-17
(31). The authors concluded that
TGF-β1 leads to T cell-mediated tumor evasion through the increased
expression of B7-H3 and B7-H4.
In this study, high counts of CD276+ TILs
were linked with high levels of TNF-α and IFN γ in the preoperative
serum, and high counts of Foxp3+ TILs were linked with
the preoperative high serum level of TGF-β1. These three cytokines
were compared with the clinical course as candidate prognosis
predictors. High serum levels of TNF-α and TGF-β1 were
significantly correlated with the higher risk of recurrence.
One possible scenario is that tumor cells increase
the production of TNF-α and TGF-β1 to help tumor cells progress.
Tumor cells may increase the expression of B7-H3 and promote
differentiation from T cells to Tregs. As a result, the production
of TNF-α and TGF-β1 are increased, which may support the immune
escape and progression of tumor cells.
Moreover, based on the present results, it can be
suggested that in patients with high serum levels of TNF-α and
TGF-β1, the topical immunoreaction in the tumor site might have
some kind of influence on systemic immunoreactions preoperatively,
leading to poor prognosis in patients. Thus, preoperative serum
levels of TNF-α and TGF-β could be good candidate risk
stratification biomarkers of localized ccRCC.
Some limitations exist in this study. First, this
study was a retrospective design, had a relatively small number of
cases, and the follow-up period was short. The findings need
further validation in forthcoming studies in prospective controlled
large sampled clinical trials. Secondly, we described one possible
scenario for the progression of tumor cells, but we did not inspect
these results in animal or cell experiments. In addition, other
types of TILs and cytokines were not evaluated. Further studies
should inspect the processes via in vivo or in vitro
studies, and evaluate other types of TILs and cytokines.
In conclusion, our findings have demonstrated the
possible association of topical intratumoral immunoreaction and
systemic immune status in patients with localized RCC. The topical
induction of the CD276+ and Foxp3+ TILs was
suggested to be linked with high levels of serum TNF-α and IFN-γ.
Preoperative serum levels of TNF-α and TGF-β could be simple and
non-invasive biomarkers for risk stratification before radical
surgery.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
KI, MM and KF contributed to the design of study and
writing of the manuscript. KO, SH, YM, DG, YI, and SO conducted the
molecular biology studies. YN, SA and NT performed the statistical
tests. MM and KF assisted with the writing of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Nara Medical University
(Nara, China) approved this protocol. (Project identification code:
1630, accepted: August 21, 2017). All subjects gave their written
informed consent for inclusion before they participated in the
study.
Patient consent for publication
All subjects gave their informed consent for
inclusion before they participated in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rini B, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eggener SE, Yossepowitch O, Pettus JA,
Snyder ME, Motzer RJ and Russo P: Renal cell carcinoma recurrence
after nephrectomy for localized disease: Predicting survival from
time of recurrence. J Clin Oncol. 24:3101–3106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miyake M, Kuwada M, Hori S, Morizawa Y,
Tatsumi Y, Anai S, Hosokawa Y, Hayashi Y, Tomioka A, Otani T, et
al: The best objective response of target lesions and the incidence
of treatment-related hypertension are associated with the survival
of patients with metastatic renal cell carcinoma treated with
sunitinib: A Japanese retrospective study. BMC Res Notes. 9:792016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bromwich EJ, McArdie PA, Canna K, McMillan
DC, McNicol AM, Brown M and Aitchison M: The relationship between
T-lymphocyte infiltration, stage, tumor grade and survival in
patients undergoing curative surgery for renal cell cancer. Br J
Cancer. 89:1906–1908. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Webster WS, Lohse CM, Thompson RH, Dong H,
Frigola X, Dicks DL, Sengupta S, Frank I, Leibovich BC, Blute ML,
et al: Mononuclear cell infiltration in clear-cell renal cell
carcinoma independently predicts patient survival. Cancer.
107:46–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uzzo RG, Rayman P, Kolenko V, Clark PE,
Bloom T, Ward AM, Molto L, Tannenbaum C, Worford LJ, Bukowski R, et
al: Mechanisms of apoptosis in T cells from patients with renal
cell carcinoma. Clin Cancer Res. 5:1219–1229. 1999.PubMed/NCBI
|
|
7
|
Rayman P, Wesa AK, Richmond AL, Das T,
Biswas K, Raval G, Storkus WJ, Tannenbaum C, Novick A, Bukowski R
and Finke J: Effect of renal cell carcinomas on the development of
type 1 T-cell responses. Clin Cancer Res. 10:6360S–6366S. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2016. View Article : Google Scholar
|
|
9
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 311:1565–1570. 2011. View Article : Google Scholar
|
|
10
|
Maxilloux AW and Young MR: Regulatory
T-cell trafficking: From thymic development to tumor-induced immune
suppression. Crit Rev Immunol. 30:435–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liotta F, Gacci M, Frosali F, Querci V,
Vittori G, Lapini A, Santarlasci V, Semi S, Cosmi L, Maggi L, et
al: Frequency of regulatory T cells in peripheral blood and in
tumour-infiltrating lymphocytes correlates with poor prognosis in
renal cell carcinoma. BJU Int. 107:1500–1506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye Z, Zheng Z, Li X, Zhu Y, Zhong Z, Peng
L and Wu Y: B7-H3 overexpression predicts poor survival of cancer
patients: A meta-analysis. Cell Physiol Biochem. 39:1568–1580.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukuda T, Kamai T, Masuda A, Nukui A, Abe
H, Arai K and Yoshida K: Higher preoperative serum levels of PD-L1
and B7-H4 are associated with invasive and metastatic potential and
predictable for poor response to VEGF-targeted therapy and
unfavorable prognosis of renal cell carcinoma. Cancer Med.
5:1810–1820. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chapoval AI, Ni J, Lau JS, Wilcox RA,
Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K and Chen L:
B7-H3: A costimulatory molecule for T cell activation and IFN-gamma
production. Nat Immunol. 2:269–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anthony LD, Richard ML and Miranda R:
Immunity: The immune response in infectious and inflammatory
disease. New Sci Press. 3872007.
|
|
16
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: International union against cancer. UICC TNM classification of
malignant tumors. 7th. Wiley-Liss; pp. 255–257. 2009
|
|
17
|
Frank I, Blute ML, Cheville JC, Lohse CM,
Weaver AL and Zincke H: An outcome prediction model for patients
with clear cell renal cell carcinoma treated with radical
nephrectomy based on tumor stage, size, grade and necrosis: The
SSIGN score. J Urol. 168:2395–2400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fuhman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thompson RH, Gillett MD, Cheville JC,
Lohse CM, Dong H, Webster WS, Chen L, Zincke H, Blute ML, Leibovich
BC and Kwon ED: Costimulatory molecule B7-H1 in primary and
metastatic clear cell renal cell carcinoma. Cancer. 15:2084–2091.
2005. View Article : Google Scholar
|
|
20
|
Li L, Yang C, Zhao Z, Xu B, Zheng M, Zhang
C, Min Z, Guo J and Rong R: Skewed T-helper (Th)1/2- and Th17/T
regulatory-cell balances in patients with renal cell carcinoma. Mol
Med Rep. 11:947–953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Petersen RP, Campa MJ, Sperlazza J, Conlon
D, Joshi MB, Harpole DH Jr and Patz EF Jr: Tumor infiltrating
Foxp3+ regulatory T-cells are associated with recurrence
in pathologic stage I NSCLC patients. Cancer. 107:2866–2872. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bates GJ, Fox SB, Han C, Leek RD, Garcia
JF, Hamis AL and Banham AH: Quantification of regulatory T cells
enables the identification of high-risk breast cancer patients and
those at risk of late relapse. J Clin Oncol. 24:5373–5380. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B,
Zhang Z, Yang H, Zhang H, Zhou C, et al: Increased regulatory T
cells correlate with CD8 T-cell impairment and poor survival in
hepatocellular carcinoma patients. Gastroenterology. 132:2328–2339.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hiraoka N, Onozato K, Kosuge T and
Hirohashi S: Prelavence of Foxp3+ regulatory cells
increases during the progression of pancreatic ductal
adenocarcinoma and its premalignant lesions. Clin Cancer Res.
12:5423–5434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Polimeno M, Napolitano M, Costantini S,
Portella L, Esposito A, Capone F, Guerriero E, Trotta A, Zanotta S,
Pucci L, et al: Regulatory T cells, interleukin (IL)-6, IL-8,
vascular endothelial growth factor (VEGF), CXCL10, CXCL11,
epidermal growth factor (EGF) and hepatocyte growth factor (HGF) as
surrogate markers of host immunity in patients with renal cell
carcinoma. BJU Int. 112:686–696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R,
Lefvert AK and Wang X: B7-H3 and B7-H4 expression in non-small-cell
lung cancer. Lung Cancer. 53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M,
Tan Y, Wang HT, Lu BF and Zhang XG: Clinical significance and
regulation of the costimulatory molecule B7-H3 in human colorectal
carcinoma. Cancer Immunol Immunother. 59:1163–1171. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Crispen PL, Sheinin Y, Roth TJ, Lohse CM,
Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich
BC, et al: Tumor cell and tumor vasculature expression of B7-H3
predict survival in clear cell renal cell carcinoma. Clin Cancer
Res. 14:5150–5157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
von Boehmer H: Mechanism of suppression by
suppressor T cells. Nat Immunol. 6:338–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou X, Mao Y, Zhu J, Meng F, Chen Q, Tao
L, Li R, Fu F, Liu C, Hu Y, et al: TGF-β1 promotes colorectal
cancer immune escape by elevating B7-H3 and B7-H4 via the
miR-155/miR-143 axis. Oncotarget. 7:67196–67211. 2016.PubMed/NCBI
|