Introduction
Carcinoembryonic antigen-related cell adhesion
molecule 1 (CEACAM1), also known as CD66a is a key molecule in
several intracellular and intercellular signaling pathways, with
multiple functional and structural roles. It is expressed in some
normal cells (epithelial cells of large bowel, prostate, bile duct,
salivary ducts, endothelial cells, T lymphocytes) and also in
several tumors (melanoma, non-small cell lung carcinomas, breast,
large bowel, gastric, thyroid carcinoma) (1). CEACAM1 is a transmembrane protein that
includes an extracellular N-terminal variable domain and three
constant C2-like immunoglobulin domains (2). Its extracellular domain is responsible
for homophilic (CEACAM1-CEACAM1) and heterophilic intercellular
adhesion with CEA and T cell immunoglobulin and mucin domain
(3).
CEACAM1 expression in melanoma was studied as
diagnosis marker, as well as a prognosis and treatment efficacy
indicator (4,5). CEACAM1 expression has often been
described in the invading part of the tumor and associated with
increased melanoma cell invasion and migration and a poor prognosis
(4,6–8).
Although initially it was identified as a tumor suppressor, with
roles in apoptosis and tumorigenesis (9), CEACAM1 demonstrates involvement in a
mechanism used by tumor cells to evade the immune system,
especially cytotoxic lymphocyte attack (4). In melanoma, through homophilic
interactions, CEACAM1 inhibits natural killer (NK) cell activity,
as well as immune functions of tumor infiltrating lymphocytes
(TILs), such as cytotoxicity and IFN-γ release (3,4,6,10,11).
Some patients with melanoma have increased serum levels of CEACAM1,
described as a factor of poor prognosis, predicting response
failure to immunotherapy and tendency to early progression and
metastasis (6,12,13).
These serum levels can also be used in patient surveillance as
indicators for tumor progression and treatment response (6,12,13).
For the above reasons, CEACAM1 was identified as
potential therapeutic target in melanoma, and some monoclonal
antibodies against CEACAM1 were designed and studied in melanoma
immunotherapy (14).
Some studies compared CEACAM1 expression in various
melanocytic lesions and identified an increased expression
correlated with the severity and evolutive potential of the lesion:
CEACAM1 was negative in non-tumoral melanocytes, positive in very
few common nevi and increasingly more frequenty positive in
dysplastic nevi, thin melanomas, thick melanomas and melanoma
metastasis (10,15,16).
There are still only a few studies concerning
special characteristics of CEACAM1 expression in melanoma and their
correlations with other morphologic and immunohistochemical traits
of tumors. Especially, in thin melanomas the data can help in
stratifying patients according their risk to progression and poor
outcome, since there is a significant variability of these patients
evolution (17).
On the other hand, regression in melanoma is a
studied feature, with controversial biologic potential. It is
defined as decrease of the number of tumor cells accompanied by
stromal reaction (fibrosis, inflammatory infiltrate with variable
number of melanophages, capillaries ectasias), determined by the
immune response of the host (18,19).
Some studies credited regression with an aggressive comportment,
and, although it is not included in staging, there are opinions
supporting the idea that presence of histological confirmed
regression is an indicator for sentinel node biopsy in patients
with thin melanomas (17,18). Multiple efforts were made to identify
the best way to characterize regression in order to make it a
reproducible routine feature to be reported by pathologists
(20). Three types of regression
(complete, segmentary and partial) have been described with
different incidence and different impact on patient outcome
(20). Globally, regression is
present in about half of melanomas, being less frequent in thick
melanomas (20).
Similar lesions occur in benign melanocytic tumors
(‘halo nevi’) but there are evident morphologic differences with
areas of regression in melanoma beyond the atypical character of
the melanoma cells: distribution of the tumor cells and lymphocytes
and the lack of fibrosis in benign lesions (21).
Mechanisms of regression in melanoma are
multifactorial and still controversial. It is an inflammatory
immune-mediated pathway involving CD8-positive cytotoxic T
lymphocytes and NK cells (18).
Probably the trigger of regression is a melanocytic antigen
(22), destruction of tumor cells
being mediated by an inflammatory response of the host (18).
We studied CEACAM1 expression in regressing versus
non-regressing thin melanomas, knowing that the phenomenon of
regression represents a valuable model for understanding tumor
immunity (23).
Materials and methods
The retrospective study included 53 consecutive
cases of thin melanoma, 21 with regression and 32 without. We used
the cut-off value for thin melanoma of 2 mm, corresponding to stage
1 and 2 tumors. All the cases were diagnosed in Colentina
University Hospital, Department of Pathology in last two years,
using histopathological and immunohistochemical techniques.
Clinical and histological data were collected and all cases were
reexamined by two independent senior pathologists, using Nikon
Eclipse E200 microscope (Nikon Instruments Europe B.V., Amsterdam,
The Netherlands). Multiple features were analyzed: tumor subtype,
thickness, presence or absence of ulceration, mitotic index,
Breslow and Clark levels, CEACAM expression.
This study was performed on patients who previously
signed a written informed consent and it was approved by the Ethics
Committee of Colentina University Hospital. Slides used for
research purposes were sectioned after the final diagnosis was
signed and did not exhaust the biological material in paraffin
blocks, allowing further tests if needed.
All specimens were collected from skin surgical
resections of tumors. After surgical resection, samples were
immediately immersed in 10% buffered formalin, then automatically
processed using a Leica ASP300 S Fully Enclosed Tissue Processor
(Leica Biosystems, Newcastle, UK) and embedded in paraffin.
Multiple 3-µm sections were obtained for usual and special stains.
After examining hematoxylin and eosin slides, all cases were
subjected to appropriate immunohistochemical stains for diagnostic
confirmation. In all cases, the final diagnosis was malignant
melanoma with Breslow index <2 mm.
For this study, serial sections from the
formalin-fixed, paraffin-embedded blocks were obtained and used for
detection of CEACAM1 using three different isoforms of the marker
(AA 1-428, extracellular domain, rabbit, cat. no. ABIN1997563; AA
1-428, mouse, clone 8B6E2F4 cat. no. ABIN 1997555; and AA 1-468,
full length, mouse, clone 2F6, cat. no. ABIN513717; all from
antibodies-online GmbH, Aachen, Germany). Tissue thin sections (3
µm) were deparaffinized in xylene, rehydrated with increasing
dilutions of ethanol and then with water, and pretreated in a
steamer for 30 min in citrate buffer (10 mM, pH 6.0). Slides were
washed in Tris-buffered saline (TBS, pH 7.4) and endogenous
peroxidase activity was blocked by treatment with peroxidase block,
then, incubated with CEACAM1 isoforms (Table I).
 | Table I.Markers used in this study. |
Table I.
Markers used in this study.
| Epitope | Host | Clonality | Specificity | Supplier |
|---|
| AA 1–428 | Rabbit | Polyclonal | Human
CEACAM1/CD66 | antibodies-online
GmbH |
| AA 1–428 | Mouse |
Monoclonal | Human
CEACAM1/CD66 | antibodies-online
GmbH |
| AA 1–468 | Mouse |
Monoclonal | Human
CEACAM1/CD66 | antibodies-online
GmbH |
Then, slides were examined and membrane positivity
for CEACAM-1 was assessed in tumor cells. There were comparatively
analyzed tumor cells from areas of partial regression and areas
without regression in regressed melanomas and the most intensely
positive areas in non-regressed melanoma. Also, we assessed the
intensity of the expression in different areas of the tumors
(junctional, dermal, invasion front), and classified it on a
semi-quantitative scale: 0, absent; 1, weak positivity; 2, moderate
positivity; and 3, intense positivity.
The data were introduced into a data base and
statistically analyzed using Microsoft Excel and SPSS (IBM Corp.,
Released 2015, IBM SPSS Statistics for Windows, version 23.0,
Armonk, NY, USA). Welch's t-test and Pearson correlation test were
used. P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Of our samples, 52 cases were superficial spreading
melanomas and just one case (with regression) was an
acral-lentiginous melanoma. Also, just one case (regressed) had
ulceration, all the others lacked this feature.
Median Breslow index of tumors included in the study
was 0.688 mm (with values between 0.16 and 1.75 mm), higher in
non-regressed melanomas (0.770 mm) than in regressed ones (0.660
mm).
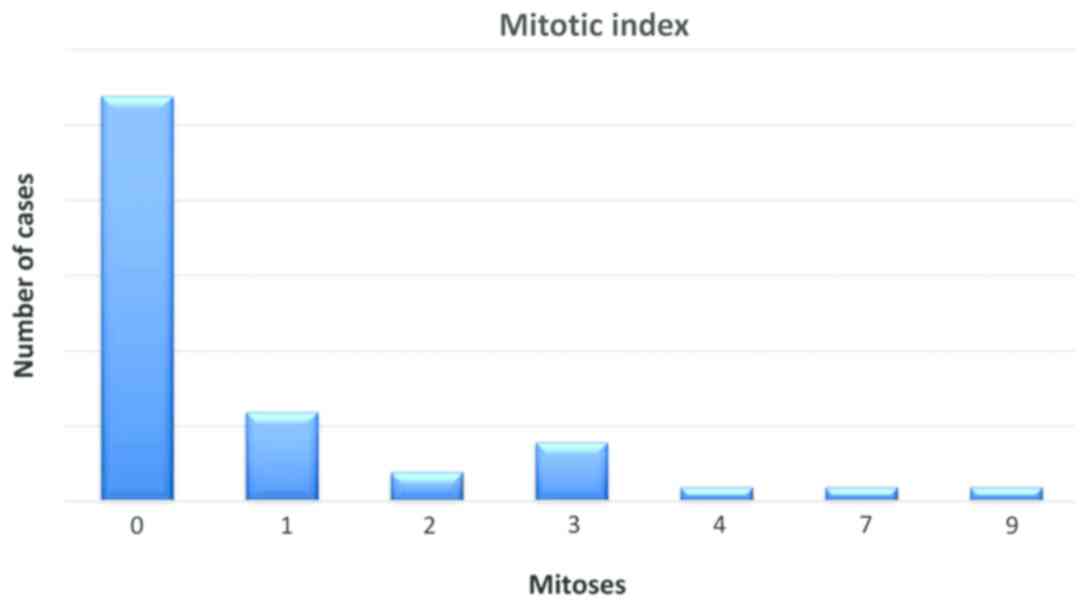
Mitotic index was evaluated by quantifying the
number of mitotic figures on one square millimeter, in hot spots,
the values ranged between 0 and 9 mitotic figures, with an average
value of 1 for the entire group (Fig.
1).
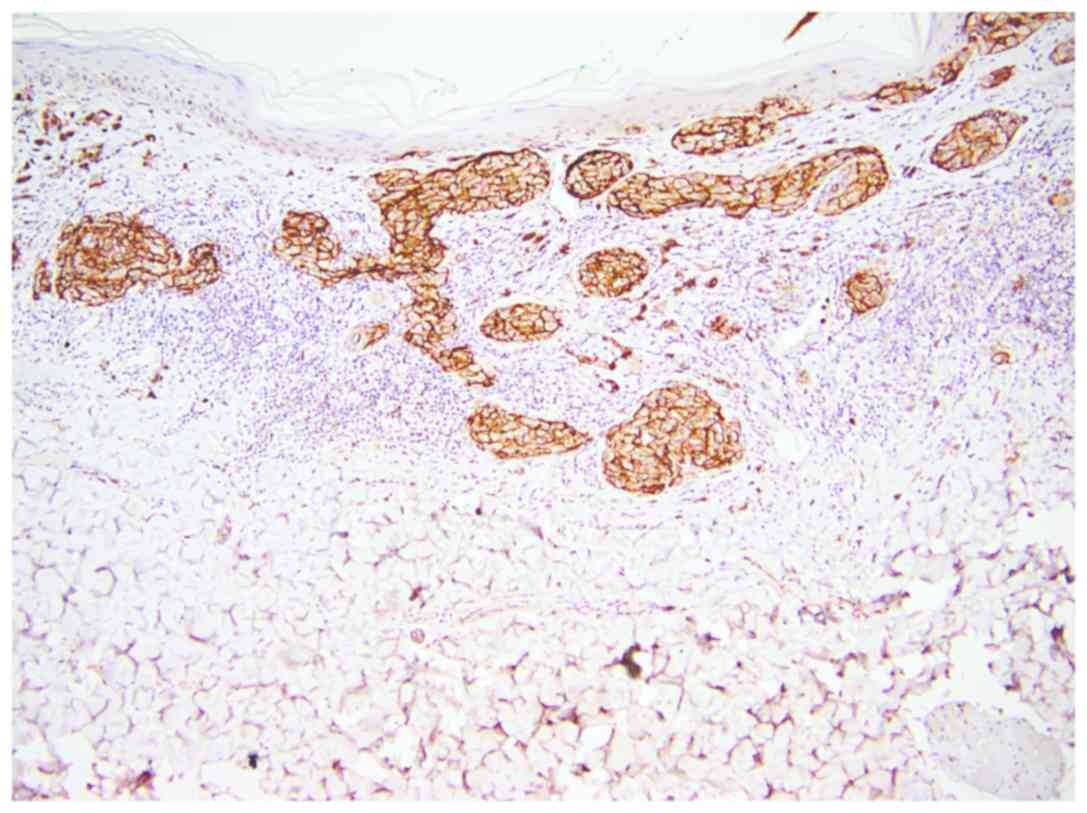
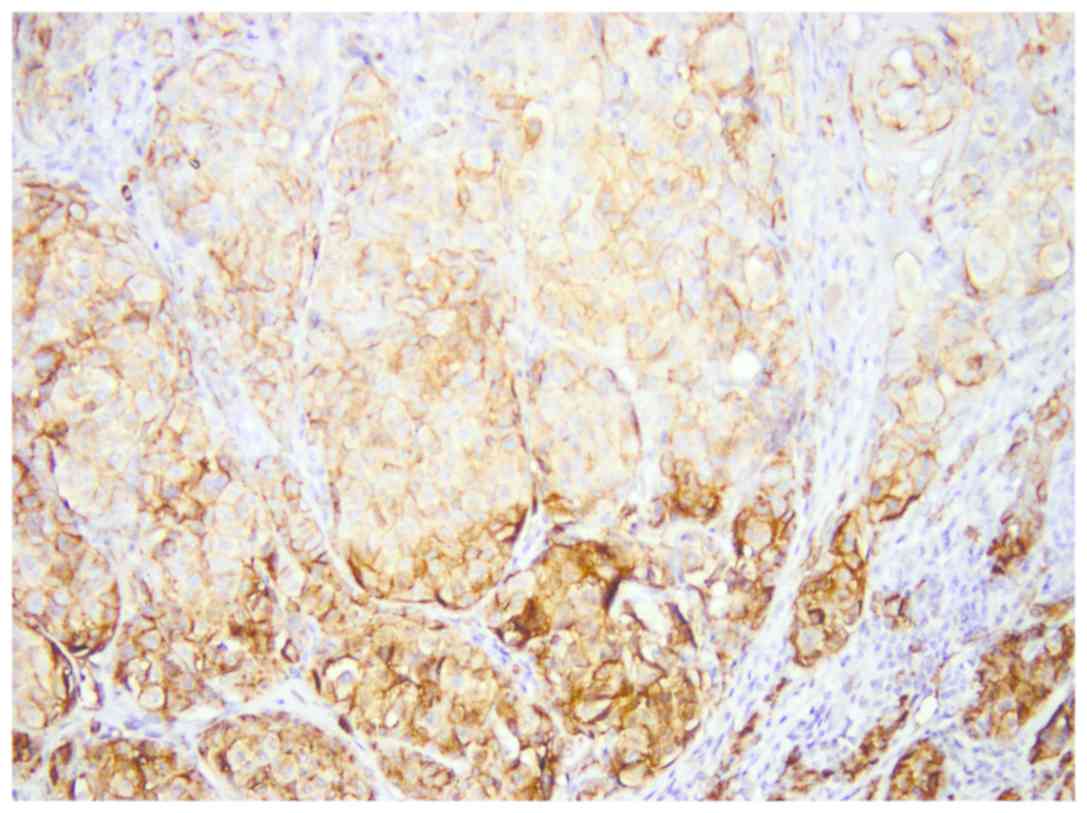
All three isoforms of CEACAM-1 had similar
reactivity. CEACAM-1 was positive in epithelial cells of sweat
ducts and sebaceous glands (used as positive internal control)
(Fig. 2).
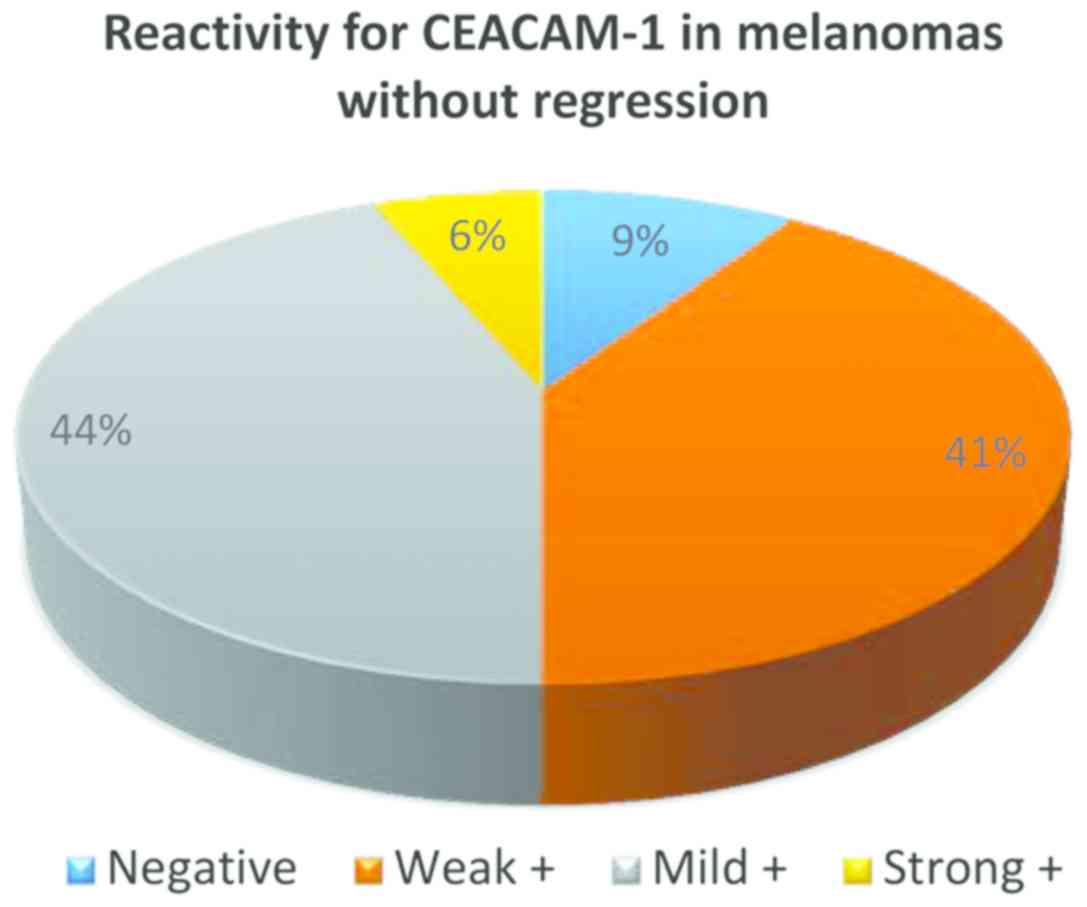
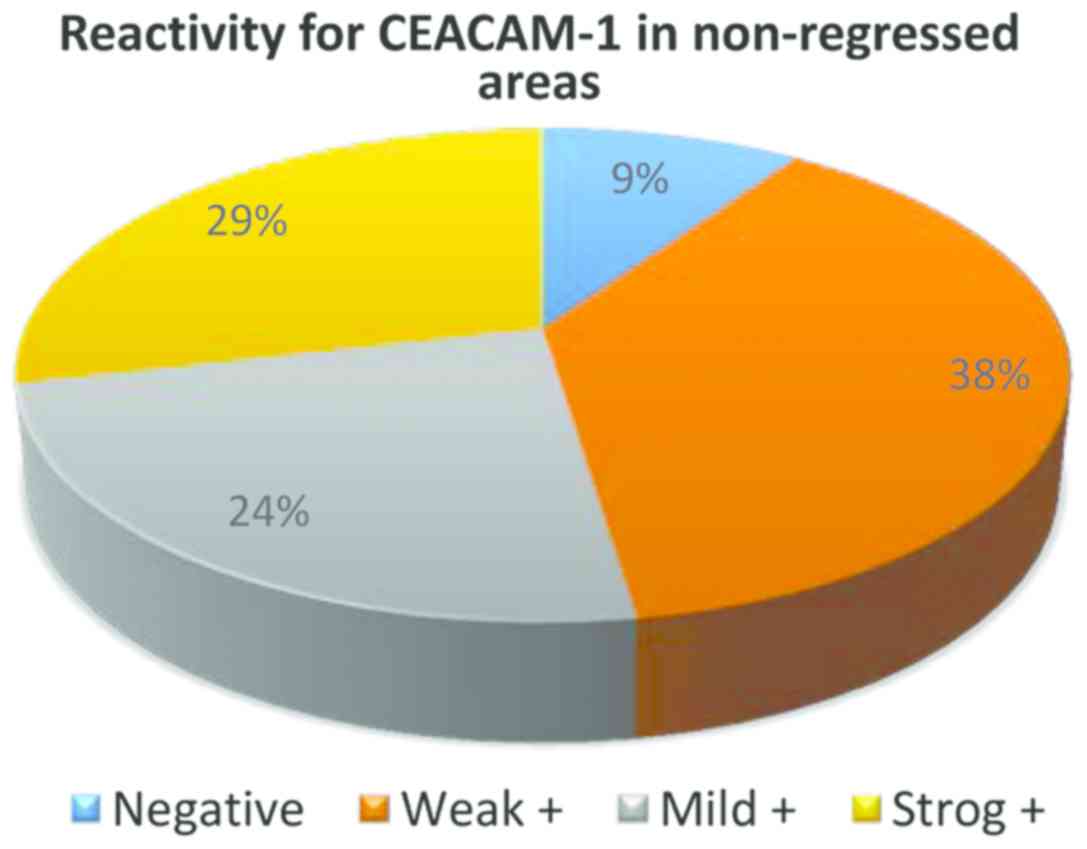
From the 32 cases of melanoma without regression,
only in 3 cases CEACAM-1 was negative in tumor cells, in all the
other cases membrane positivity was identified: weak (13 cases),
mild (14 cases) and strong (2 cases) (Fig. 3).
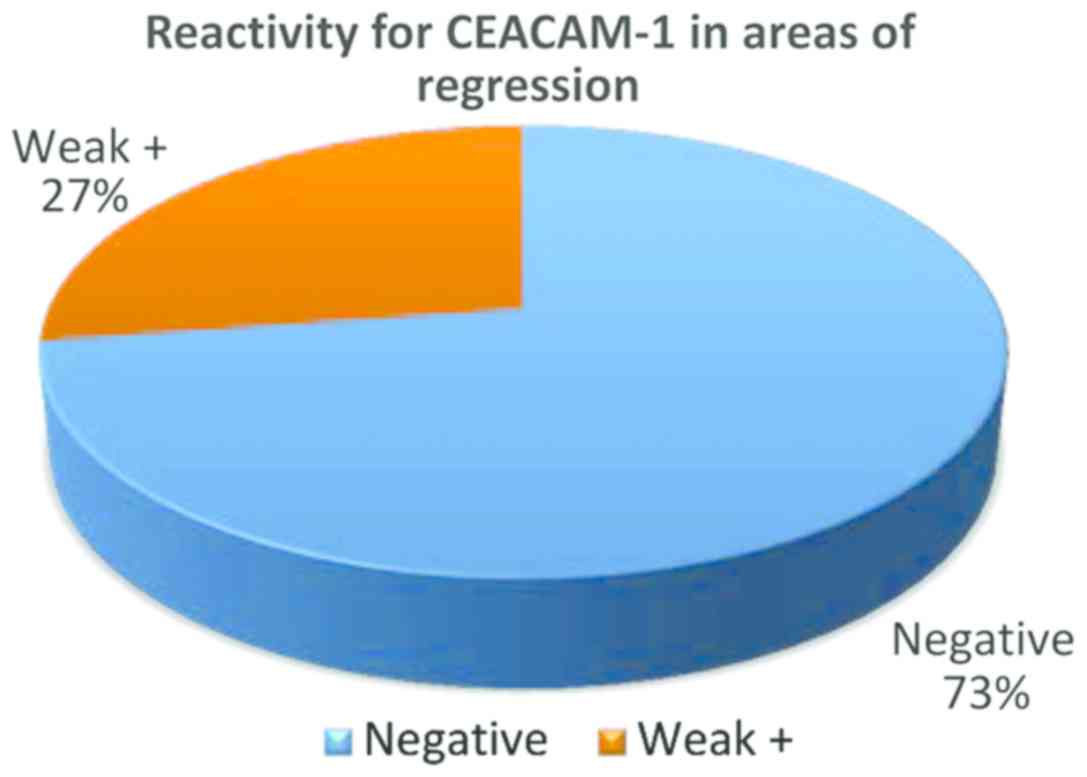
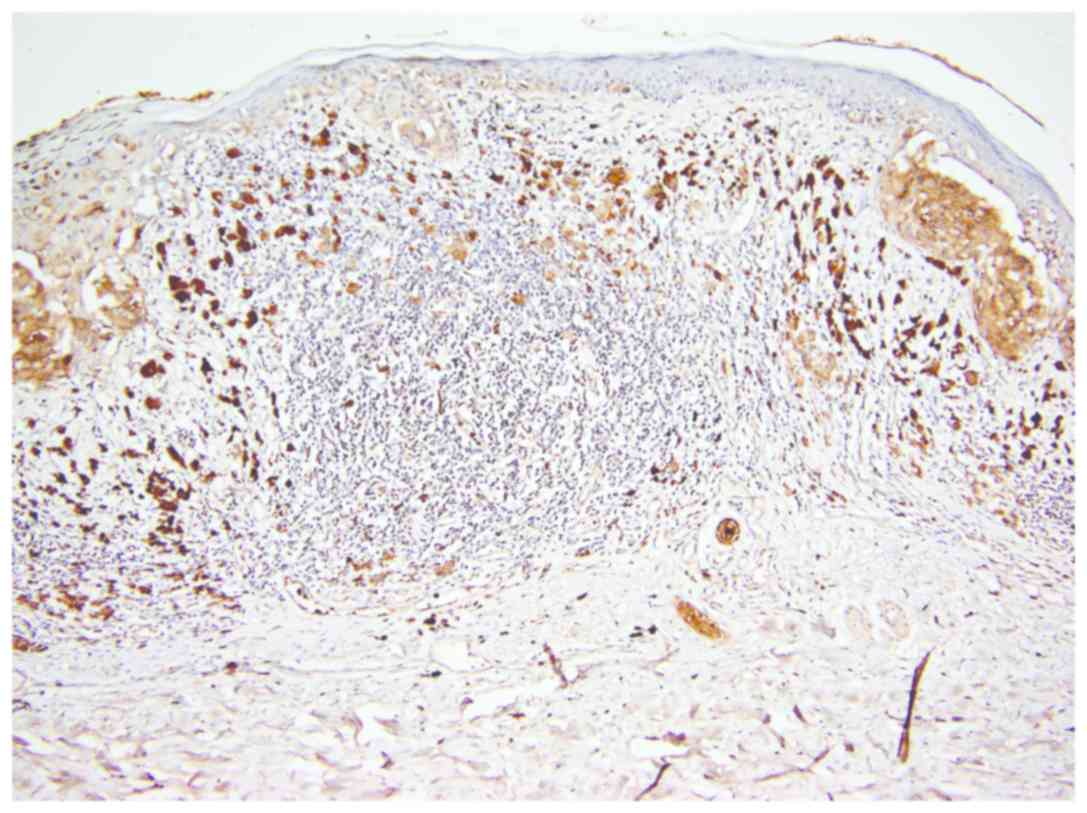
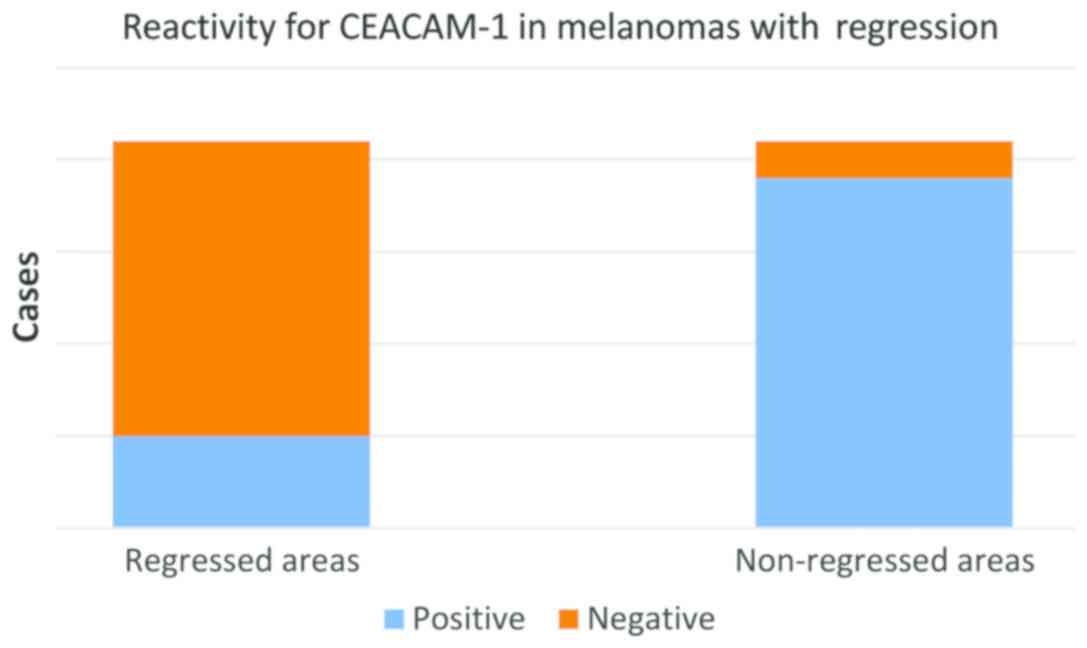
In the group of regressed melanomas, a significant
difference was observed between remaining (non-regressed) tumor
cells from regressed areas (Fig. 4)
and tumor cells from non-regressed areas (Fig. 5). While in regressed areas, tumor
cells were negative for CEACAM-1 in 16 cases and weakly positive in
only 5 cases, in the same group, in non-regressed areas, tumor
cells were positive in 19 cases and negative in only 2 cases
(Fig. 5).
Statistical correlations showed that the difference
of CEACAM-1 reactivity in tumor cells from regressed and
non-regressed area (Fig. 6) is
highly significant (t-test, P<0.0001) (Fig. 7).
Correlation between Breslow index and CEACAM-1
reactivity was significant only in regressed melanomas (Pearson
correlation test, P=0.22589). Practically, thicker tumors had
stronger global positivity for CEACAM-1 in all studied tumors, but
the correlation was not statistically significant in non-regressed
melanomas.
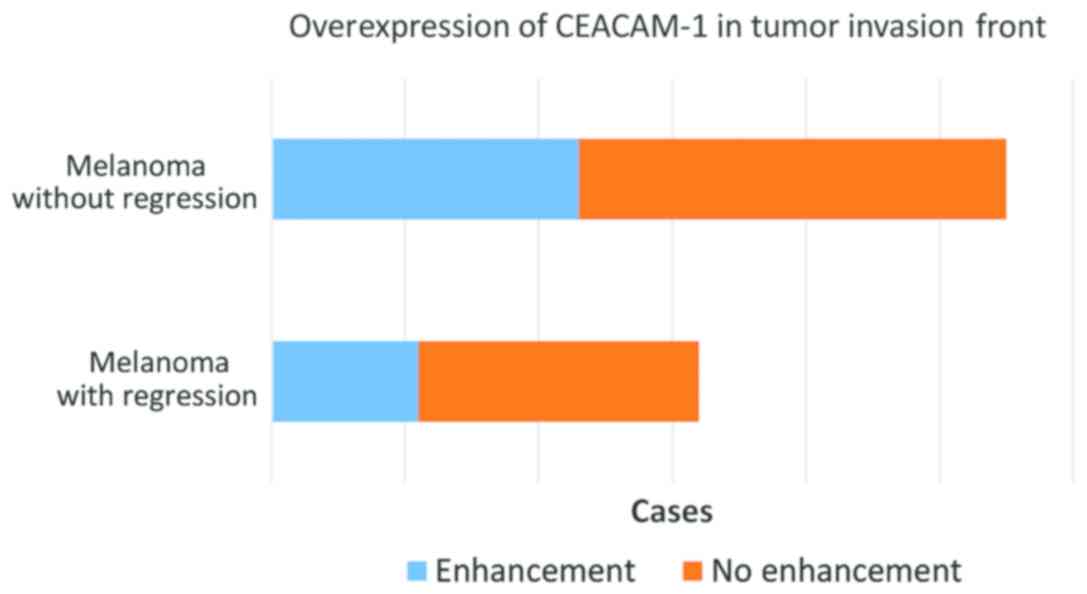
In 34 cases, representing approximately 64%, we
observed a stronger expression of CEACAM-1 in tumor cells from the
deep invasion front (Fig. 8). This
feature was observed both in regressed melanomas (11 out of 21) and
non-regressed tumors (23 out of 32) (Fig. 9).
Discussion
Superficial spreading melanoma is the most frequent
subtype and a less aggressive form of the tumor. Since it has a
long period of radial growth, without invasion, it is the most
probable tumor to be diagnosed as thin melanoma (24). In our study, the vast majority of
thin melanomas were superficial spreading type, with nodular growth
and invasion. Ulceration is also a rare feature in thin melanomas
but is considered a poor prognosis factor related with positive
sentinel node and significant risk of metastasis (25–27). In
our group only one tumor was ulcerated, a regressed superficial
spreading melanoma with high mitotic index (9) and Breslow level 1.4 mm, confirming the
aggressive potential of this proliferation. In this particular
tumor, CEACAM-1 was weakly positive in regressed areas and strongly
positive in non-regressed areas. Considering that CEACAM-1 is a
membrane protein that promotes invasion and metastasis in melanoma,
this characteristic confirms the fact that this tumor could have a
poor outcome (28).
Immunohistochemical testing for CEACAM-1 in melanomas could help
stratifying patients according to their risk to progression and
represents an interesting feature that can represent not only a
therapeutical target, but also a morphologic trait that helps
personalizing the treatment and surveillance in melanoma
patients.
CEACAM-1 is positive in the vast majority of cases
of melanoma, but our study identified a significant difference
between the intensity of reaction in various areas of the tumor.
Melanomas without regression and areas without regression from
regressed melanomas showed a similar profile of CEACAM-1
positivity, while melanomas with regression exhibited a very low
rate of CEACAM-1 positivity in remaining tumor cells form regressed
areas. This specific immunophenotype implies that these tumor cells
have special characteristics and lack a feature that confers their
counterparts from non-regressed areas with potential of invasion.
This observation raises two hypotheses: either regression affects
only some tumor cells, less aggressive, with special features, or
inflammatory cells and inflammatory mediators are inducing changes
in tumor cell phenotype and behaviour.
Some studies demonstrated that CEACAM-1 enhances
vascular neo-proliferation and reduces cell sensitivity to hypoxia,
mechanism involved in inflammatory-driven tumor regression
(29,30). These data suggest that CEACAM-1
defective cells are more sensitive to hypoxia and less capable of
stimulating tumor angiogenesis. Also, CEACAM-1 expression modulates
apoptosis (10). These
characteristics make tumor cells more sensitive to microenvironment
changes induced by inflammation and more prone to be destroyed by T
lymph cells and NK cells. Melanoma is a poor immunogenic tumor in
advanced stages leading to immune suppression and consequently to
immune tolerance by so-called ‘immunosculpting’ process (31,32).
On the other hand, our study confirms that CEACAM-1
is overexpressed in cells from the invasion front, in regressed and
non-regressed melanomas. This is related in all studies with a
decrease of cell-adhesion and a higher capacity of stromal invasion
(28,33). Overexpression of CEACAM-1 in front
invasion cells represents an important dysregulation of cell
adhesion, enhancing the transition from radial growth phase (with
good prognosis and reduced risk of metastasis) to vertical growth
phase (with high risk of metastasis and poor prognosis) (33,34).
CEACAM-1 may intervene in mechanisms involved in immune
surveillance escaping (35,36) and it is a possible candidate to be a
monitoring biomarker and a possible target; supplementary
investigations are necessary (3,37,38).
CEACAM1 is a valuable marker in melanoma that can be
used for a more complete description of tumor features related to
invasiveness and aggressive behavior. It is more intensely positive
in thick melanomas and in invasion front, indicating that
CEACAM1-positive cells have a higher potential of invasion and
metastasis.
Also, there is significant loss of CEACAM1
expression in melanoma cells from areas of regression indicating
that regression is not only the result of inflammation, but also of
some specific characteristics of some tumor cells that make them
more sensitive to the cytotoxic lymphocyte action.
Monoclonal antibodies against CEACAM1 can induce
loss of CEACAM1 expression in melanoma cells and enhance antitumor
effect of patients' immune system and has been evaluated in a phase
1 study for safety and tolerability (NCT02346955) completed in 2017
(https://clinicaltrials.gov/ct2/show/NCT02346955;
identification no. NCT02346955). Phase 2 clinical trials will
further evaluate the adequate doses. CEACAM1 is a promising
therapeutic target, since loss of expression in tumor cells seems
to stimulate regression and inhibit vertical growth and
invasion.
Acknowledgments
The authors would like to thank physicians from
Colentina Hospital and Dermato-oncology Excellence Centre
(Bucharest, Romania) for diagnosing, treating and monitoring the
patients with cutaneous melanoma included in this research.
Funding
This study was partially supported by grants of
Romanian Ministry of Research and Innovation, CCCDI-UEFISCDI,
project nos. 61PCCDI⁄2018 PN-III-P1-1.2-PCCDI-2017-0341 and
CNCS-UEFISCDI and 183/2017PN-III-P4-ID-PCE-2016-0641, within
PNCDI–III.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LN and SZ contributed to the concept and draft of
this study. LN, SZ, CP, MC and DI designed the study and
implemented each step of the research. LN, SZ, CP, MC, ABa, LJ, LS
and PS examined the archives and identified the cases included in
the study, examined the slides and collected pathological
information. RN, ABr and GT clinically diagnosed the patients,
obtained the informed consent, harvested tissue samples. All
authors participated in statistical analysis, finding and
interpreting the results, drafting the manuscript and revising it
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This research follows international and national
regulations in accordance with the Declaration of Helsinki. It was
approved by the Ethics Committee of Colentina University Hospital
(Bucharest, Romania). All patients signed an informed consent
before being included in this study. Their personal data were
maintained confidential during this research, only their medical
doctors (dermatologists and pathologists) having access to their
identity.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Fiori V, Magnani M and Cianfriglia M: The
expression and modulation of CEACAM1 and tumor cell transformation.
Ann Ist Super Sanita. 48:161–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beauchemin N, Draber P, Dveksler G, Gold
P, Gray-Owen S, Grunert F, Hammarström S, Holmes KV, Karlsson A,
Kuroki M, et al: Redefined nomenclature for members of the
carcinoembryonic antigen family. Exp Cell Res. 252:243–249. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dankner M, Gray-Owen SD, Huang YH,
Blumberg RS and Beauchemin N: CEACAM1 as a multi-purpose target for
cancer immunotherapy. OncoImmunology. 6:e13283362017.PubMed/NCBI
|
|
4
|
Neagu M: The immune system - a hidden
treasure for biomarker discovery in cutaneous melanoma. Adv Clin
Chem. 58:89–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sapoznik S, Ortenberg R, Schachter J and
Markel G: CEACAM1 in malignant melanoma: A diagnostic and
therapeutic target. Curr Top Med Chem. 12:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sivan S, Suzan F, Rona O, Tamar H, Vivian
B, Tamar P, Jacob S, Gal M and Michal L: Serum CEACAM1 correlates
with disease progression and survival in malignant melanoma
patients. Clin Dev Immunol. 2012:2905362012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thies A, Berlin A, Brunner G, Schulze HJ,
Moll I, Pfüller U, Wagener C, Schachner M, Altevogt P and
Schumacher U: Glycoconjugate profiling of primary melanoma and its
sentinel node and distant metastases: Implications for diagnosis
and pathophysiology of metastases. Cancer Lett. 248:68–80. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khatib N, Pe'er J, Ortenberg R, Schachter
J, Frenkel S, Markel G and Amer R: Carcinoembryonic antigen cell
adhesion molecule-1 (CEACAM1) in posterior uveal melanoma:
Correlation with clinical and histological survival markers. Invest
Ophthalmol Vis Sci. 52:9368–9372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nittka S, Günther J, Ebisch C,
Erbersdobler A and Neumaier M: The human tumor suppressor CEACAM1
modulates apoptosis and is implicated in early colorectal
tumorigenesis. Oncogene. 23:9306–9313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turcu G, Nedelcu RI, Ion DA, Brînzea A,
Cioplea MD, Jilaveanu LB and Zurac SA: CEACAM1: Expression and role
in melanocyte transformation. Dis Markers. 2016:94063192016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Markel G, Seidman R, Stern N, Cohen-Sinai
T, Izhaki O, Katz G, Besser M, Treves AJ, Blumberg RS, Loewenthal
R, et al: Inhibition of human tumor-infiltrating lymphocyte
effector functions by the homophilic carcinoembryonic cell adhesion
molecule 1 interactions. J Immunol. 177:6062–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ortenberg R, Sapoznik S, Zippel D,
Shapira-Frommer R, Itzhaki O, Kubi A, Zikich D, Besser MJ,
Schachter J and Markel G: Serum CEACAM1 elevation correlates with
melanoma progression and failure to respond to adoptive cell
transfer immunotherapy. J Immunol Res. 2015:9021372015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kluger HM, Hoyt K, Bacchiocchi A, Mayer T,
Kirsch J, Kluger Y, Sznol M, Ariyan S, Molinaro A and Halaban R:
Plasma markers for identifying patients with metastatic melanoma.
Clin Cancer Res. 17:2417–2425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ortenberg R, Sapir Y, Raz L, Hershkovitz
L, Ben Arav A, Sapoznik S, Barshack I, Avivi C, Berkun Y, Besser
MJ, et al: Novel immunotherapy for malignant melanoma with a
monoclonal antibody that blocks CEACAM1 homophilic interactions.
Mol Cancer Ther. 11:1300–1310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ebrahimnejad A, Streichert T, Nollau P,
Horst AK, Wagener C, Bamberger AM and Brümmer J: CEACAM1 enhances
invasion and migration of melanocytic and melanoma cells. Am J
Pathol. 165:1781–1787. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gambichler T, Grothe S, Rotterdam S,
Altmeyer P and Kreuter A: Protein expression of carcinoembryonic
antigen cell adhesion molecules in benign and malignant melanocytic
skin lesions. Am J Clin Pathol. 131:782–787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gimotty PA and Guerry D: Prognostication
in thin cutaneous melanomas. Arch Pathol Lab Med. 134:1758–1763.
2010.PubMed/NCBI
|
|
18
|
Aung PP, Nagarajan P and Prieto VG:
Regression in primary cutaneous melanoma: Etiopathogenesis and
clinical significance. Lab Invest. 97:657–668. 2017. View Article : Google Scholar
|
|
19
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zurac S, Negroiu G, Petrescu S, Andrei R,
Tebeica T, Popp C, Musţată R, Neagu M, Constantin C, Solovan C, et
al: Spectrum of morphologic alterations of regression in cutaneous
melanoma - potential for improving disease prognosis. Rom J Intern
Med. 50:145–153. 2012.PubMed/NCBI
|
|
21
|
Nedelcu RI, Zurac SA, Brînzea A, Cioplea
MD, Turcu G, Popescu R, Popescu CM and Ion DA: Morphological
features of melanocytic tumors with depigmented halo: Review of the
literature and personal results. Rom J Morphol Embryol. 56 (Suppl
2):659–663. 2015.PubMed/NCBI
|
|
22
|
Saleh FH, Crotty KA, Hersey P and Menzies
SW: Primary melanoma tumour regression associated with an immune
response to the tumour-associated antigen melan-A/MART-1. Int J
Cancer. 94:551–557. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LM, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non-melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shashanka R and Smitha BR: Head and neck
melanoma. ISRN Surg. 2012:9483022012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rubinstein JC, Han G, Jackson L, Bulloch
K, Ariyan S, Narayan D, Rothberg BG and Han D: Regression in thin
melanoma is associated with nodal recurrence after a negative
sentinel node biopsy. Cancer Med. 5:2832–2840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diaconeasa A, Boda D, Solovan C, Enescu
DM, Vîlcea AM and Zurac S: Histopathologic features of Spitzoid
lesions in different age groups. Rom J Morphol Embryol. 54:51–62.
2013.PubMed/NCBI
|
|
27
|
Blendea A, Georgescu CV, Ţolea I,
Brănişteanu DE and Costache A: An unusual cutaneous malignant
melanoma arised de novo: A case report. Rom J Morphol Embryol.
56:1217–1221. 2015.PubMed/NCBI
|
|
28
|
Ortenberg R, Galore-Haskel G, Greenberg I,
Zamlin B, Sapoznik S, Greenberg E, Barshack I, Avivi C, Feiler Y,
Zan-Bar I, et al: CEACAM1 promotes melanoma cell growth through
Sox-2. Neoplasia. 16:451–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ludewig P, Flachsbarth K, Wegscheid C,
Tiegs G, Richard G, Wagener C, Bartsch U and Horst AK: CEACAM1
confers resistance toward oxygen-induced vessel damage in a mouse
model of retinopathy of prematurity. Invest Ophthalmol Vis Sci.
55:7950–7960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horst AK, Ito WD, Dabelstein J, Schumacher
U, Sander H, Turbide C, Brümmer J, Meinertz T, Beauchemin N and
Wagener C: Carcinoembryonic antigen-related cell adhesion molecule
1 modulates vascular remodeling in vitro and in vivo. J Clin
Invest. 116:1596–1605. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bulman A, Neagu M and Constantin C:
Immunomics in skin cancer - improvement in diagnosis, prognosis and
therapy monitoring. Curr Proteomics. 10:202–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013. View Article : Google Scholar
|
|
33
|
Thies A, Moll I, Berger J, Wagener C,
Brümmer J, Schulze HJ, Brunner G and Schumacher U: CEACAM1
expression in cutaneous malignant melanoma predicts the development
of metastatic disease. J Clin Oncol. 20:2530–2536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Caruntu C, Boda D, Constantin C, Caruntu A
and Neagu M: Catecholamines increase in vitro proliferation of
murine B16F10 melanoma cells. Acta Endocrinol (Copenh). 10:545–558.
2014.
|
|
35
|
Markel G, Seidman R, Cohen Y, Besser MJ,
Sinai TC, Treves AJ, Orenstein A, Berger R and Schachter J: Dynamic
expression of protective CEACAM1 on melanoma cells during specific
immune attack. Immunology. 126:186–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Markel G, Ortenberg R, Seidman R, Sapoznik
S, Koren-Morag N, Besser MJ, Bar J, Shapira R, Kubi A, Nardini G,
et al: Systemic dysregulation of CEACAM1 in melanoma patients.
Cancer Immunol Immunother. 59:215–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Neagu M, Constantin C and Tanase C:
Immune-related biomarkers for diagnosis/prognosis and therapy
monitoring of cutaneous melanoma. Expert Rev Mol Diagn. 10:897–919.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neagu M, Caruntu C, Constantin C, Boda D,
Zurac S, Spandidos DA and Tsatsakis AM: Chemically induced skin
carcinogenesis: Updates in experimental models (Review). Oncol Rep.
35:2516–2528. 2016. View Article : Google Scholar : PubMed/NCBI
|