Introduction
Pancreatic cancer is one of the most lethal cancer
types, with a 5-year survival rate of 8% according to American
Cancer Society Statistics in 2017 (1). The median overall survival time of
patient with pancreatic cancer is only 5.0–7.2 months, according to
statistics from France published in 2011 (2). The prognosis for patients with
pancreatic cancer is poor due to this cancer type frequently being
diagnosed at an advanced stage due to rapid progression, with
limited symptoms at early stages meaning surgery is applicable only
to a minority of patients (<20%), but also because it is
resistant to chemotherapeutic agents and radiation therapy
(3). The current first-line
chemotherapeutic drug, gemcitabine, for pancreatic cancer treatment
remains unsatisfactory (2).
Therefore, there is an urgent requirement for novel therapeutic
approaches and drug combinations to provide a beneficial treatment
for patients with pancreatic cancer.
Isochorismatase domain-containing protein 1 (ISOC1)
may have putative isochorismatase activity, catalyzing the
conversion of isochorismate into 2,3-dihydroxy-2,3-dihydrobenzoate
and pyruvate via hydrolysis (4).
Aside from its putative isochorismatase activity, the precise
function of ISOC1 in human pancreatic cancer has not been fully
elucidated. A number of recently published papers, using
high-throughput techniques, including genetic sequencing and
proteomic mass spectrometry, revealed that ISOC1 was one novel
target that is positively regulated by estrogen in breast cancer,
negatively regulated by microRNA-130a in neutrophil development and
is a new peroxisomal constituent in human liver peroxisomes
(5–8). Collectively, these recent observations
indicate that ISOC1 may have important functions in ontogenesis,
and that it is worthy of an in-depth investigation.
In the present study, the pancreatic cancer cell
lines, SW 1990 and PANC-1, were used to investigate the potential
role of ISOC1 in pancreatic cancer. ISOC1 expression was inhibited
with short hairpin (sh)ISOC1 in order to determine its effects on
cell growth, proliferation, apoptosis, migration and invasion
following ISOC1 knockdown. The observations of the present study
helped to improve our understanding of the role of ISOC1 in
pancreatic cancer.
Materials and methods
Reagents
The Rabbit polyclonal ISOC1 antibody anti-human
ISOC1 was purchased from Abcam (#ab118245; Cambridge, UK).
Anti-GAPDH (#sc-32233), peroxidase-conjugated antirabbit IgG
(#sc-2004) and anti-mouse IgG (#sc-2005) were purchased from
Santa-Cruz Biotechnology, Inc. (Dallas, TX, USA). RPMI 1640 medium
with L-Gultamine (#11875-093), fetal bovine serum (FBS; #10100-147)
and Lipofectamine® 2000 (#11668019) were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Penicillin-streptomycin (#1107440001) and Giemsa staining solution
(#32884) were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany).
Cell lines and cell culture
Human pancreatic carcinoma SW 1990, PANC-1 and
AsPC-1 cells and normal pancreatic duct epithelial (HPDE) cells
were purchased from American Type Culture Collection (Manassas, VA,
USA). These cell lines were maintained at 37°C in RPMI-1640 medium
supplemented with 10% (v/v) heat-inactivated FBS, penicillin (100
IU/ml) and streptomycin (100 µg/ml).
Construction of ISOC1 knockdown
lentivirus
According to the sequence of ISOC1 (NM_016048),
shISOC1 and control shRNA (shCtrl) were designed. The sequences are
as follows: shISOC1:
5′-CCGGCCATTTGAGTACCAGCATTTACTCGAGTAAATGCTGGTACTCAAATGGTTTTT-3′;
and shCtrl:
5′-CCGGCCTTCTCCGAACGTGTCACGTCTCGAGTAAATGCTGGTACTCAAATGGTTTTT-3′.
The two shRNAs (100 ng/µl) were inserted into the plasmid GV115
(U6-MCS-CMV-EGFP; Shanghai GeneChem Co., Ltd., Shanghai, China).
Recombinant lentiviruses were generated by transfection of 293T
cells (Cell Bank of Chinese Academy of Science, Shanghai, China),
in a 10 cm dish at 80% confluency, with 20 µg GV115 plasmid and the
helper plasmids, including 15 µg Helper 1.0 and 15 µg Helper 2.0
(Shanghai GeneChem Co., Ltd.) using Lipofectamine® 2000.
After 48 h of transfection, viral supernatants were collected,
centrifuged at 10,000 × g at 4°C to remove cell debris and then
filtered through 0.45 µm polyvinylidene fluoride membranes.
Lentivirus titer (TU/ml) was measured using HIV p24 antigen ELISA
kit 2.0 (#0801002; ZeptoMetrix, Buffalo, NY, USA). SW 1990 and
PANC-1 cells were infected with shISOC1 lentivirus or control virus
at multiplicity of infection of 2 using polybrene (#H9268;
Sigma-Aldrich; Merck KGaA). At 72 h after infection, green
fluorescent protein (GFP) expression was observed and the achieved
infection efficiency was 80%, and the expression of ISOC1 was
analyzed by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blotting, according to the
subsequent protocols.
MTT assay
To test directly whether ISOC1 inhibition would
affect cell viability and proliferation, an MTT assay was performed
to compare the effects of decreased ISOC1 expression on cell
numbers. SW 1990 and PANC-1 cells were transfected with shISOC1
lentivirus or control virus at a multiplicity of infection of 2
using polybrene. Stably-transfected cells were seeded into a
96-well plate (2×103 cells/well) at 37°C for 16 h, and
after cell adhesion, 20 µl MTT (5 mg/ml; Genview, Houston, TX, USA;
#JT343) was added and cultured at 37°C for 4 h. Subsequently, the
media was completely removed and 100 µl dimethyl sulfoxide was
added and optical density 490 was determined on a microplate
reader. The viability of the cells was assessed by measuring the
absorbance at 490 nm using a Microplate Absorbance Reader (Tecan
Group, Ltd., Männedorf, Switzerland; #M2009PR). Each experiment was
repeated three times.
Western blot analysis
Total protein was extracted from cell lines by
incubating with 2X Lysis Buffer [100 mM Tris-HCl (pH 6.8), 4% SDS,
20% glycerol and 2% mercaptoethanol] on ice for 10 min and
sonication 5 sec each time for 4 times (200 W with 2 sec interval).
Total protein was measured through the bicinchoninic acid method
and separated by 10% SDS-PAGE. A total of 30 µg proteins were run
on gels and transferred to polyvinyl difluoride membranes. The
membranes were blocked in 5% bovine serum albumin (Gibco; Thermo
Fisher Scientific, Inc.; #37525) at room temperature for 1 hand
then probed with the primary antibodies overnight at 4°C. The
membranes were incubated with appropriate secondary antibodies
(1:2,000 dilution), either peroxidase-conjugated anti-mouse IgG or
anti-rabbit IgG at room temperature for 1 h. GAPDH was used as a
loading control. Subsequently, the antigen-antibody complexes were
detected by using an enhanced chemiluminescent blotting analysis
system.
RT-qPCR
Total RNA was extracted from cells using a
SuperfecTRI, Total RNA Isolation reagent (Shanghai Pufei
Biotechnology Co., Ltd., Shanghai, China; #3101-100). cDNA was
transcribed from 1 µg total RNA using a Promega M-MLV kit (Promega
Corporation, Madison, WI, USA; #M1705), according to the
manufacturer's protocol. cDNA was used as the template for qPCR
detection with primers and SYBR® Master Mixture on
LightCycler480 (Roche Diagnostics, Basel, Switzerland). The primers
for ISOC1 are forward, 5′-CGACATGCACCGCAAATTCG-3′, and reverse,
5′-TGAGCTGGATCTGCAACGG-3′. The primers for GAPDH are forward,
5′-TGACTTCAACAGCGACACCCA-3′, and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The qPCR was performed with the
following conditions: i) Hold at 95°C for 30 sec, ii) step-PCR for
40 cycles of 95°C for 5 sec and 60°C for 30 sec; and iii)
dissociation at 95°C for 15 sec, 60°C for 30 sec and 95°C for 15
sec. The expression of the ISOC1 gene, and endogenous control gene
GAPDH, was detected and analyzed. The relative mRNA expression
level of ISOC1 in each sample was calculated using the comparative
expression level 2−∆∆Cq method (9). All experiments were conducted in
triplicate for each data point.
ISOC1 mRNA level analysis from The
Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx)
Portal
RNA sequencing results of pancreatic adenocarcinoma
were downloaded from TCGA (http://www.cbioportal.org/datasets). RNA sequencing
results of normal pancreas tissue were downloaded from GTEx
(phs000424.v6.p1; ftp://ftp.ncbi.nlm.nih.gov/dbgap/studies/phs000424).
ISOC1 sequencing results were extracted, normalized and
integrated as transcripts per million.
Matrigel invasion assay
The Matrigel invasion assay was performed in a
24-well plate Transwell system (Corning Inc. Corning; NY, USA;
#07-200-537). The Transwell inserts were coated with 100 µl
Matrigel and incubated at 37°C for 30 min. A total of
5×103 SW 1990 cells were harvested and resuspended in
100 µl RPMI-1640 medium to the upper chamber of the Transwell
system. The lower chamber was infused with 100 µl RPMI-1640 with
30% FBS. The Transwell system was incubated at 37°C for 20 h, and
then the gel and cells in the upper chamber were cleared. Following
40% formalin fixation at room temperature for 15 min, the membrane
was stained with Giemsa staining solution for 3–5 min at room
temperature. Phase contrast images were captured and the cells on
the lower side of the membrane were counted in 6 random visual
fields under a ×20 objective lens of an inverted microscope (CKX41,
Olympus Corporation, Tokyo, Japan).
Celigo counting assay
SW 1990 and PANC-1 cells were transfected with
shISOC1 lentivirus or control virus at a multiplicity of infection
of 2 using polybrene. A total of 15×102 cells/well were
seeded in RPMI-1640 medium in a 96 well plate. The GFP-expressing
cell number was counted with a Celigo image cytometer once a day at
37°C (Nexcelom Bioscience, Lawrence, MA, USA) for 5 days.
Annexin V assay
Apoptosis of SW 1990 cells was detected using an
Annexin V-APC staining kit (eBioscience; Thermo Fisher Scientific,
Inc.; #88-8007) after lentivirus infection at multiplicity of
infection of 2 using polybrene for 6 and 8 days. Cells
(5×105 cells/dish) were cultured at 37°C in 10-cm dishes
to reach 80% confluency, and then they were harvested, washed twice
with PBS and stained with 10 µl Annexin V-APC at 37°C for 10–15
min. Subsequently, cells were kept on ice in the dark and subjected
to apoptosis analysis with a flow cytometer (EMD Millipore; #Guava
easyCyte HT; Billerica, MA, USA). Data were analyzed with Guava
Suite 3.3 software (EMD Millipore).
Caspase-Glo 3/7 Assay
SW 1990 and PANC-1 cells were transfected with
shISOC1 lentivirus or control virus at a multiplicity of infection
of 2 using polybrene. Caspase 3/7 activity in SW 1990 and PANC-1
cells following shCtrl or shISOC1 treatment was detected using a
Caspase-Glo 3/7 kit (Promega Corporation). A total of
1×104 cells infected with shCtrl or shISOC1 were seeded
in 96-well plates. After 3 days growth at 37°C, 100 ml Caspase 3/7
reagent were added to each well, mixed and incubated for 1 h at
room temperature. Luminescence was measured using a M2000 Infinite
Pro instrument (Tecan Group, Ltd.). Results of Caspase 3/7 activity
were expressed as percentage of the negative control.
Migration assay
A total of 3×104 cells were seeded in 96
well plate and incubated at 37°C for 16 h to achieve 90%
confluency. A 96 wounding replicator (VP scientific, Inc.;
#VP408FH; San Diego, CA, USA) was used to scratch a monolayer
across the surface of the well. Subsequently, the well was gently
washed with RPMI-1640 medium to remove the detached cells. The
cells were then incubated with fresh RPMI-1640 medium (containing
0.5% FBS) at 37°C for 24 h. Photos were captured at 0, 8 and 24 h.
The wound area was analyzed and measured with Image J 1.51 software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as mean ± standard deviation from
three independent experiments. The statistical analyses were
performed using the GraphPad Prism (Version 6.0; GraphPad Software,
Inc., La Jolla, CA, USA). Student's t-test was used for comparison
between two groups. Two-way ANOVA with Bonferroni post-hoc test
were used to compare the differences among multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ISOC1 is expressed in human pancreatic
adenocarcinoma tissues and cell lines
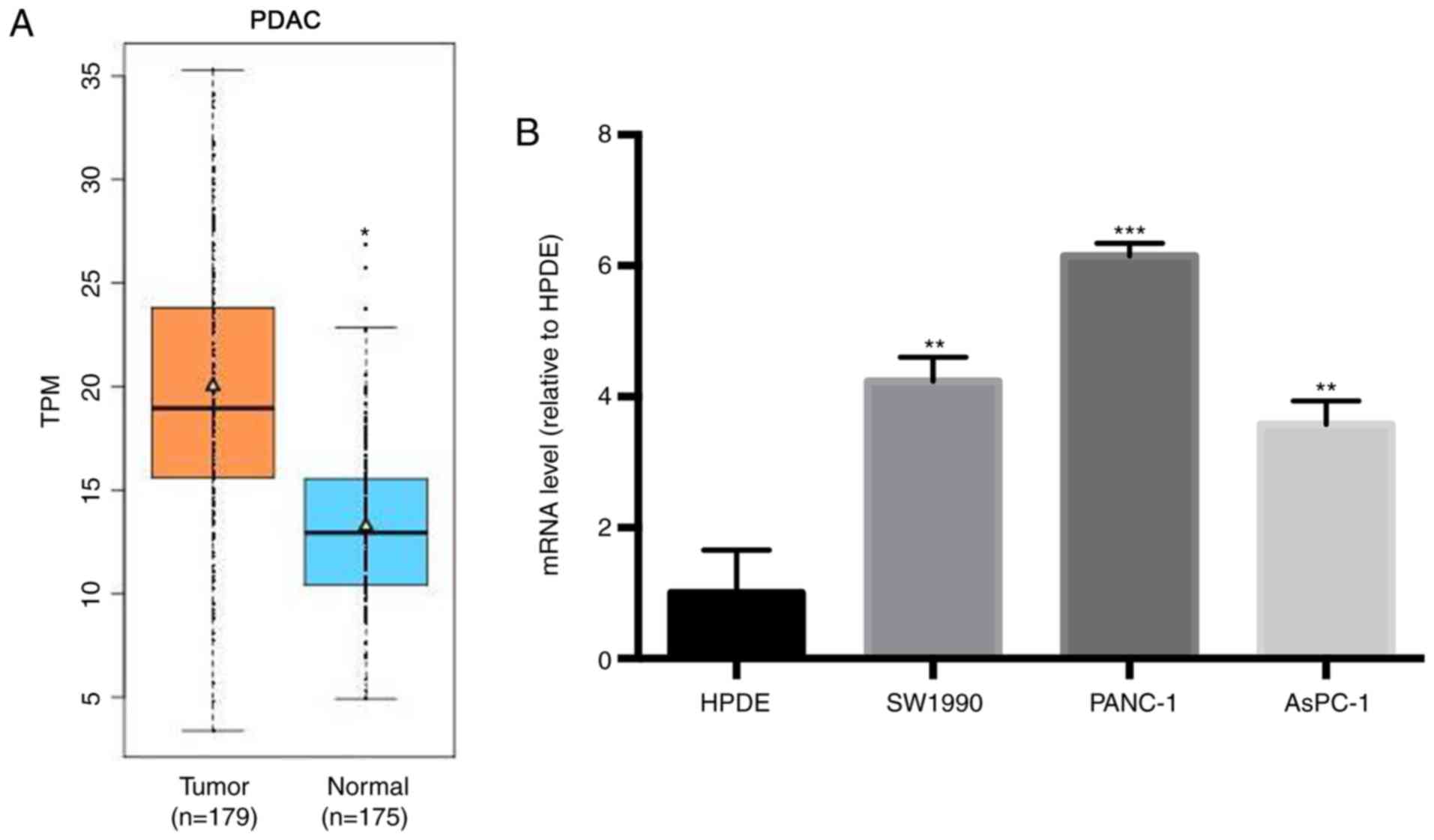
The expression levels of ISOC1 mRNA in 179
pancreatic adenocarcinoma (PDAC) tissues and 4 normal pancreatic
tissues were downloaded from TCGA database. The expression levels
of ISOC1 mRNA from a further 171 normal pancreatic tissues were
downloaded from the GTEx portal database. The mRNA expression
levels of ISOC1 from PDAC and normal pancreatic tissues is plotted
in the boxplot format in Fig. 1A. As
depicted in Fig. 1A, the level of
ISOC1 mRNA in PDAC tissues was significantly increased, compared
with normal pancreatic tissues. Subsequently, the expression level
of ISOC1 mRNA was assessed in the human PDAC cell lines, SW 1990,
PANC-1 and AsPC-1, and in the normal cell line, HPED. RT-PCR
analysis revealed that ISOC1 mRNA was expressed in all three human
PDAC cell lines (Fig. 1B).
Furthermore, the ISOC1 mRNA levels in PDAC cells were significantly
increased, compared with normal cells (P<0.01). Therefore,
elevated expression levels of ISOC1 may exert an important role in
the pathogenesis of human PDAC.
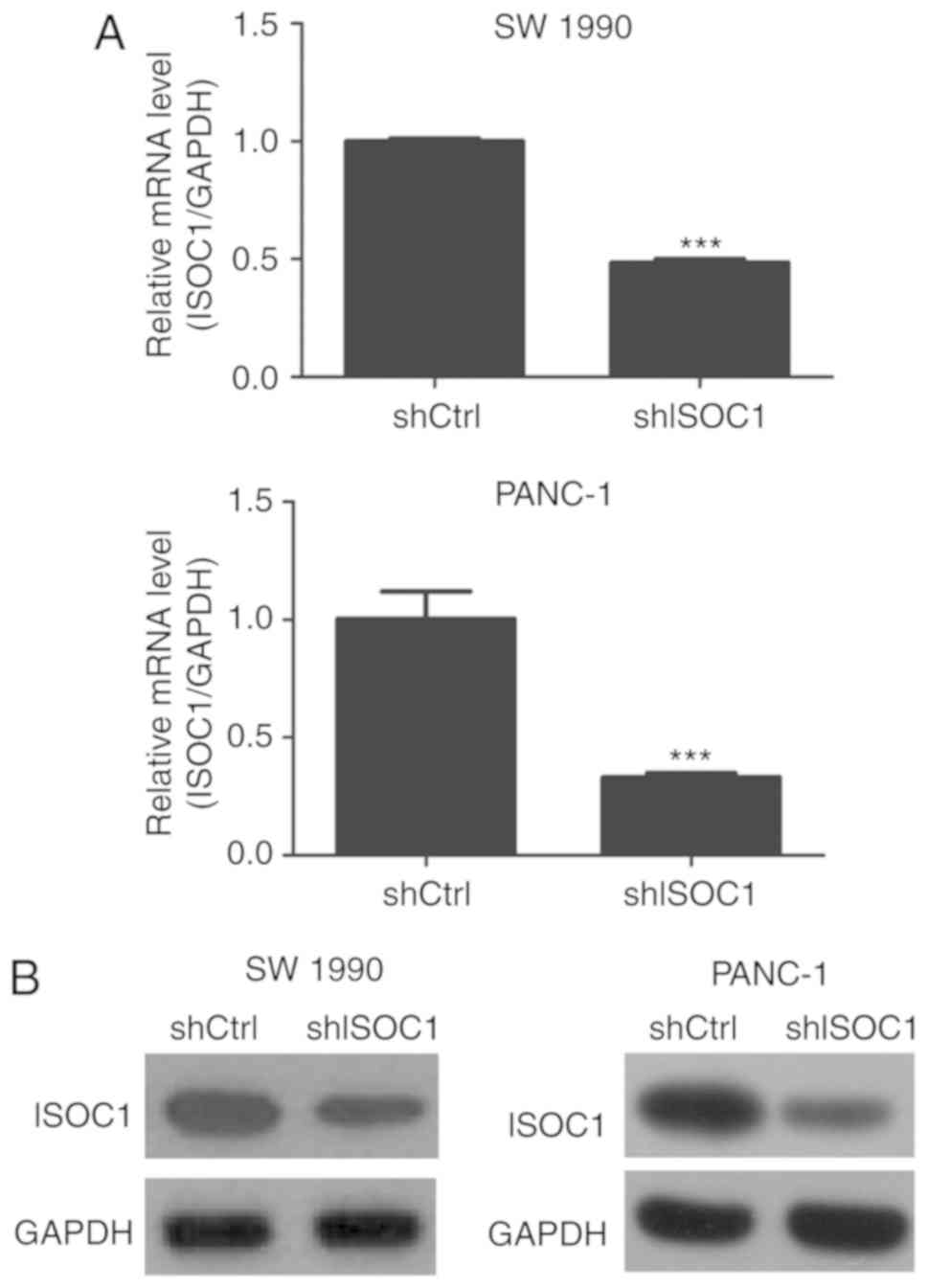
Successful knockdown of ISOC1 in SW
1990 and PANC-1
To investigate the function of ISOC1 in pancreatic
cancer, the expression of ISOC1 was inhibited by infection with
shISOC1 in the SW 1990 and PANC-1 PDAC cell lines. The inhibition
efficiency was confirmed by examining the mRNA level of ISOC1 using
RT-qPCR, and the protein expression level of ISOC1 by western
blotting, in shISOC1- and shCtrl-treated cell lines. The majority
of the cells revealed GFPpositive expression, indicating a high
infection efficiency. Furthermore, the mRNA levels of ISOC1 were
significantly downregulated in shISOC1-treated cells, compared with
the shCtrl group (Fig. 2A).
Consistently, treatment with shISOC1 decreased the protein level of
ISOC1 in the SW 1990 and PANC-1 cell lines (Fig. 2B). Collectively, these observations
confirmed that ISOC1 silencing in SW 1990 and PANC-1 cell lines had
been successfully accomplished.
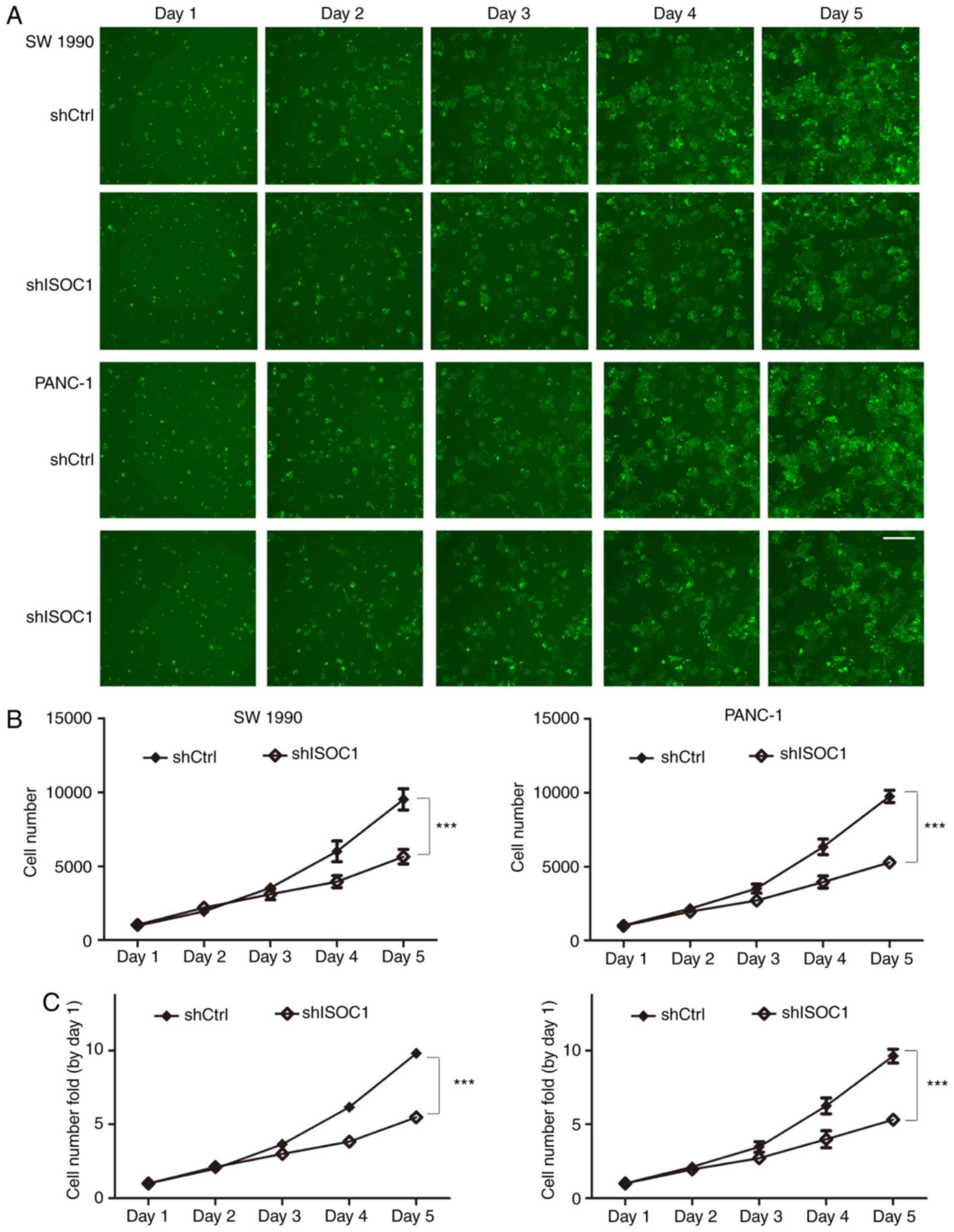
Inhibition of cell proliferation by
shISOC1
The potential effects of ISOC1 on cell proliferation
were examined in SW 1990 and PANC-1 cells by Celigo cell counting
and MTT assays. The same numbers of SW 1990 and PANC-1 cells (1,500
cells/well) from the shISCO1 and shCtrl groups were seeded into
96-well plates, and cell numbers were continuously counted using a
Celigo plate reader over the course of the following 5 days.
Cellular images and the cell number plots are depicted in Fig. 3A and B, respectively. The results
indicated that SW 1990 and PANC-1 cells infected with shISOC1
presented a significantly reduced proliferative rate, compared with
the control group (Fig. 3B and C).
The MTT assay revealed similar results (Fig. 3D and E). During the 5 days of growth,
the proliferation rates of the SW 1990 and PANC-1 cells were
significantly inhibited by ISOC1 knockdown, compared with the
control group. The aforementioned results revealed that ISOC1 may
serve an important role in pancreatic cancer cell
proliferation.
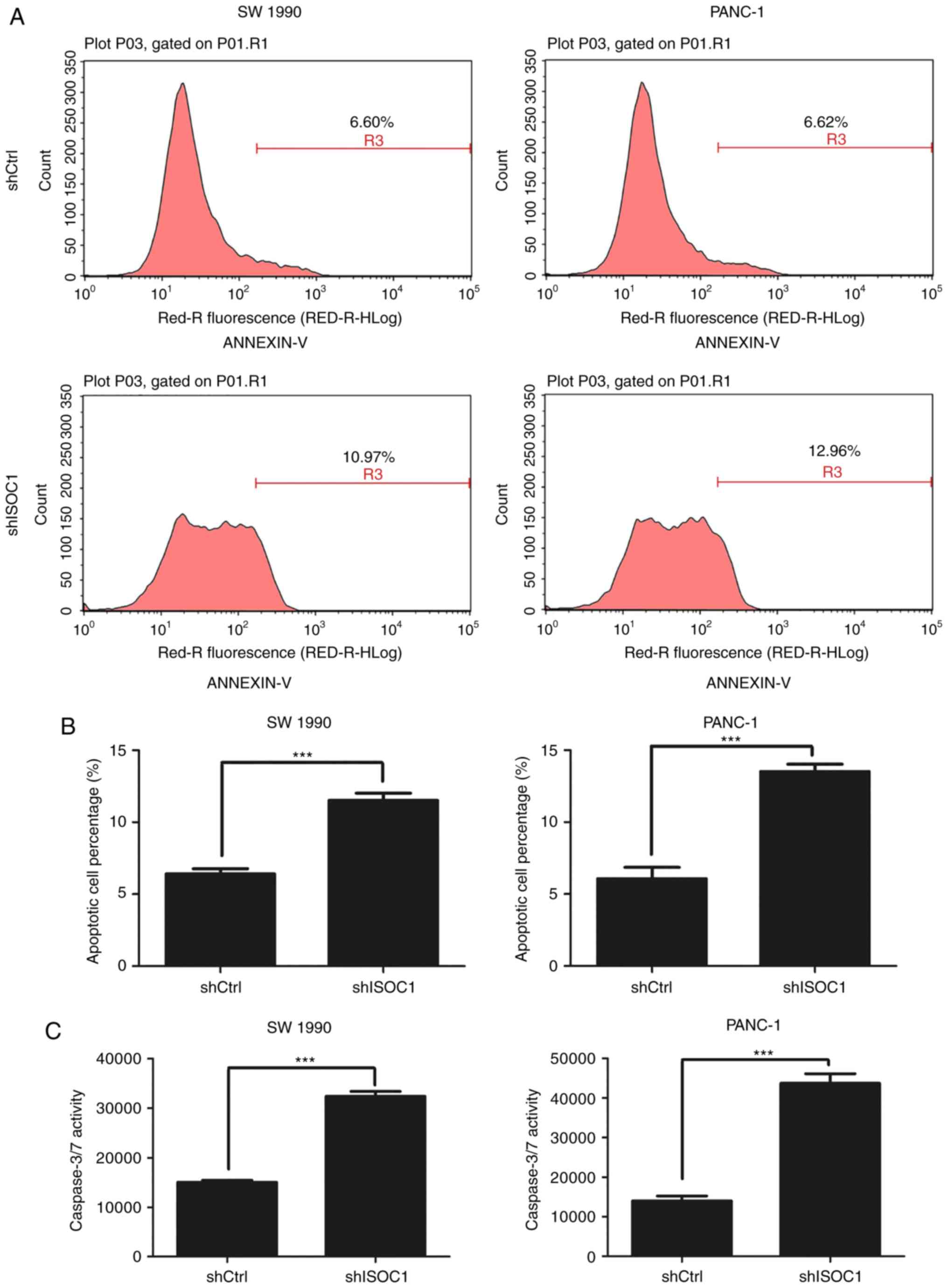
Knockdown of ISOC1 induces cell
apoptosis
To determine the possible underlying mechanism of
inhibition of cell proliferation, apoptosis was analyzed using
Annexin V-staining flow cytometry and caspase3/7 assay. As depicted
in Fig. 4A and B, the percentages of
apoptotic cells in shISOC1-infected SW 1990 and PANC1 cells were
significantly increased, compared with that in the shCtrl group. In
agreement with the flow cytometry data, caspase 3/7 activity was
significantly increased in shISOC1-infected cells, compared with
that in the shCtrl group (Fig. 4C).
These results indicated that knockdown of ISOC1 inhibited cell
proliferation through induction of cell apoptosis.
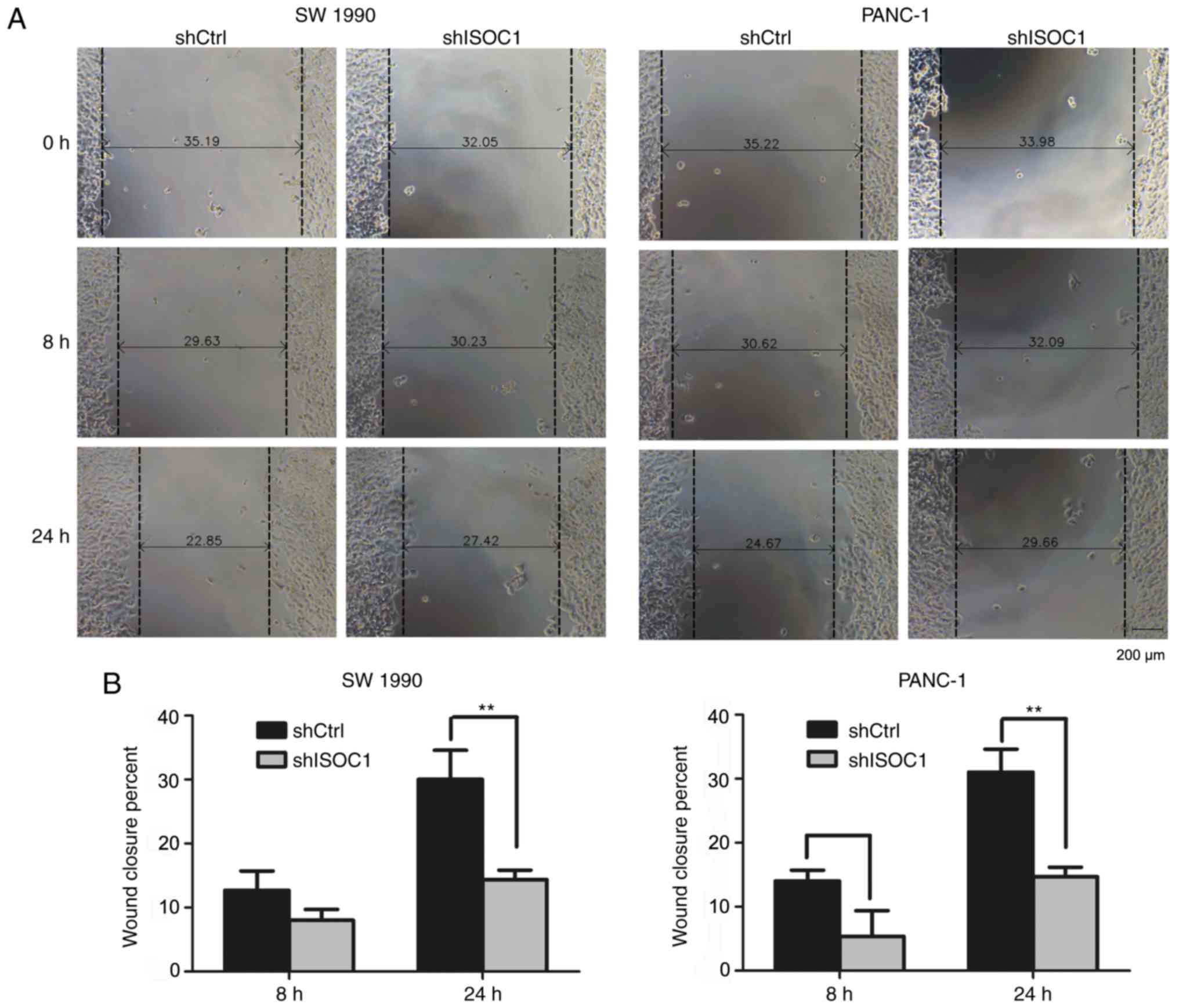
Knockdown of ISOC1 impairs cell
migration and invasion ability
In addition to the inhibition of cell proliferation
by ISOC1 knockdown, the effects of cell migration (up to 24 h) and
invasion (16 h) were also investigated in a shorter observational
window, compared with the MTT assay (5 days). The same numbers of
SW 1990 and PANC-1 cells (3×104) infected with shISOC1
and shCtrl were used for both assays. In the migration assay,
significantly reduced migration rates were identified in the
shISOC1 group, compared with the shCtrl group at 24 h (P<0.01;
Fig. 5A and B). Consistently,
significantly fewer cells invaded into the lower side of the
chamber in the shISOC1 group, compared with the shCtrl group
(Fig. 5C and D) after 16 h of
incubation. Collectively, these results indicated that ISCO1
inhibition impaired the migration and invasive abilities of the
pancreatic cells.
Discussion
In the present study, it was determined that the
ISOC1 mRNA level is significantly increased in PDAC tissues,
compared with normal pancreatic tissues, and the same trend was
observed in PDAC cell lines, compared with normal pancreatic cells.
Furthermore, the shISOC1-infected PDAC cell lines, SW 1990 and
PANC-1, were demonstrated to have a reduced level of ISOC1
expression, compared with the respective control cell lines. Cell
growth and proliferation was indicated to be significantly
inhibited following ISOC1 knockdown. Furthermore, this inhibition
of the cell growth and proliferation was determined to be
associated with cell apoptosis. Finally, ISOC1 inhibition also
resulted in an impairment of cell migration and invasion. To the
best of our knowledge, this is the first study to have revealed how
ISOC1 is involved in pancreatic cancer growth and migration.
Pancreatic cancer is notoriously difficult to detect
early and to cure. Current therapeutic options for patients are
limited and unsatisfactory (2). The
observations of the present study demonstrated that ISOC1 may be a
new potential intervention target in pancreatic cancer. Currently,
it has not been established whether the effects of knockdown of
ISOC1 on pancreatic cancer cells are dependent on, or otherwise
associated with, the putative isochorismatase enzyme activity of
ISOC1. Recently reported potential isochorismatase inhibitors,
including suberoylanilide hydroxamic acid (10) and cinnamic acid (11), may help to address the previous
question. Notably, ISOC2, an ISOC1 homolog, was determined to
directly interact with the tumor suppressor p16INK4a,
and to negatively regulate p16INK4a (12). Overexpressed ISOC2 inhibited the
expression of p16INK4a. Our hypothesis is that ISOC1 may
perform an action on p16INK4a similar to that of ISOC2,
and that this may be the mechanism utilized by ISCO1 in terms of
its role in tumor progression.
Previous studies revealed that ISOC1 was regulated
by estrogen in the breast cancer cell line, MCF-7 (7,8).
Furthermore, small interfering RNA knockdown of ISOC1 inhibits the
growth of MCF-7 cells, which indicated that ISOC1 is involved in
the growth of breast cancer cells (7). These results are consistent with those
in the present study. This indicates that the positive regulation
of ISOC1 on cancer cell proliferation may not be limited to
pancreatic cancer and breast cancer, but may be a more widespread
phenomenon in different types of cancer. It would be worthwhile to
test the validity of this hypothesis. The previous study revealed
that, in breast cancer, inhibition of ISOC1 results in a small
decrease in the migration ability of the cells, although this
difference was not observed to be significant (7). However, in the present study, it was
determined that knockdown of ISOC1 did significantly decrease the
migration and invasive ability of the pancreatic cancer cells.
These different effects elicited by ISOC1 knockdown may be due to
the different cancer context.
In conclusion, in the present study, the function of
ISOC1 in pancreatic cancer was examined and it was determined that
silencing ISOC1 expression induced apoptosis and suppressed cell
proliferation in pancreatic cancer. These results indicated that
ISOC1 could be a potential therapeutic target for the treatment of
pancreatic cancer. However, further studies are required to fully
elucidate the regulatory mechanisms of ISOC1 in pancreatic
tumorigenesis.
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science
Foundation (grant no. 81300350) and by Science and Technology
Commission Foundation of Shanghai Municipality (grant no.
16411950404).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XW proposed the hypothesis and designed the study.
LC performed the MTT and apoptosis assays, analyzed the data and
wrote the article. YZ, MT and ZL performed the cell wound healing
and Transwell assays, and analyzed the data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 2017. American Cancer Society. (Atlantla, GA).
2017.
|
|
2
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Eng J Med. 364:1817–1825. 2011.
View Article : Google Scholar
|
|
3
|
Hidalgo M, Cascinu S, Kleeff J, Labianca
R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL and Heinemann V:
Addressing the challenges of pancreatic cancer: Future directions
for improving outcomes. Pancreatology. 15:8–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walsh CT, Liu J, Rusnak F and Sakaitani M:
Molecular studies on enzymes in chorismate metabolism and the
enterobactin biosynthetic-pathway. Chem Rev. 90:1105–1129. 1990.
View Article : Google Scholar
|
|
5
|
Gronemeyer T, Wiese S, Ofman R, Bunse C,
Pawlas M, Hayen H, Eisenacher M, Stephan C, Meyer HE, Waterham HR,
et al: The proteome of human liver peroxisomes: Identification of
five new peroxisomal constituents by a label-free quantitative
proteomics survey. PLoS One. 8:e573952013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pedersen CC, Refsgaard JC, Ostergaard O,
Jensen LJ, Heegaard NH, Borregaard N and Cowland JB: Impact of
microRNA-130a on the neutrophil proteome. BMC Immunol. 16:702015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaga R, Ikeda K, Boele J, Horie-Inoue K,
Takayama K, Urano T, Kaida K, Carninci P, Kawai J, Hayashizaki Y,
et al: Systemic identification of estrogen-regulated genes in
breast cancer cells through cap analysis of gene expression
mapping. Biochem Biophys Res Commun. 447:531–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rezaul K, Thumar JK, Lundgren DH, Eng JK,
Claffey KP, Wilson L and Han DK: Differential protein expression
profiles in estrogen receptor-positive and -negative breast cancer
tissues using label-free quantitative proteomics. Genes Cancer.
1:251–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fischer JJ, Michaelis S, Schrey AK, Diehl
A, Graebner OY, Ungewiss J, Horzowski S, Glinski M, Kroll F, Dreger
M and Koester H: SAHA capture compound-A novel tool for the
profiling of histone deacetylases and the identification of
additional vorinostat binders. Proteomics. 11:4096–4104. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hubrich F, Mordhorst S and Andexer JN:
Cinnamic acid derivatives as inhibitors for chorismatases and
isochorismatases. Bioorg Med Chem Lett. 23:1477–1481. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang XY, Shi ZC, Wang W, Bai J, Chen Z,
Xu J, Zhang D and Fu S: Identification and characterization of a
novel protein ISOC2 that interacts with p16(INK4a). Biochem Biophys
Res Commun. 361:287–293. 2007. View Article : Google Scholar : PubMed/NCBI
|