Introduction
Differentiated thyroid carcinoma (DTC), which mainly
includes cancers of papillary and follicular histologies, is the
most common form of thyroid cancer (1) DTC typically has a favorable prognosis,
with an overall 10-year survival rate above 90%. As reported by
epidemiological studies, the incidence of thyroid cancer has been
on the increase in previous decades (2). The vast majority (87%) of thyroid
cancers detected in the last 15 years were diagnosed as small DTC
(sDTC), which is defined as tumors ≤2 cm in their largest diameter
(3,4). Thus, defining the appropriate treatment
and management strategies for patients with early stage DTC,
especially sDTC, is necessary.
In the 6th edition of the Union for International
Cancer Control (UICC)/American Joint Committee on Cancer (AJCC)
(5–7), the tumor, node, metastases (TNM)
staging system defined intrathyroidal tumors, with the largest
diameter being ≤2 cm, as T1. Previous findings have suggested that
tumors >1 cm have a worse prognosis (8). Thus, the 7th AJCC TNM staging system
made a subdivision of T1 tumors into T1a (≤1 cm) and T1b (1–2 cm)
(9). Furthermore, the 8th AJCC TNM
staging system continued to use T1a/T1b (10). The 2015 American Thyroid Association
guidelines recommended different therapeutic management for the two
DTC subgroups (11). However, in
other studies it was emphasized that smaller DTCs did not indicate
better prognosis, and that there was no difference between the two
subgroups (12–14). Due to the inconsistent conclusions of
the previous respective studies and a lack of prospective studies,
whether it is clinically beneficial to subdivide sDTCs remains
controversial.
The aim of the present study was to investigate
whether the subgroups (group S1, defined as tumors ≤1 cm and
group S2, defined as tumors >1 cm and ≤2 cm) are
distinguishable based on patient prognosis.
Materials and methods
Search strategy and study
selection
PRISMA-P (Preferred Reporting Items for Systematic
review and Meta-Analysis Protocols) was referred in the process of
our meta-analysis (15). A
comprehensive literature search for studies published before
September 2018 was performed in the PubMed and Web of Science
databases. We used the following keywords as the search algorithm:
(‘differentiated thyroid carcinoma’ OR ‘DTC’) AND (‘follow up’ OR
‘result’ OR ‘prognosis’ OR ‘death’ OR ‘recurrence’) AND (‘tumor
size’ OR ‘T1’) AND ‘patients’. All of the reference lists from the
main articles were inspected for additional eligible studies.
Studies were considered eligible if they met the
following inclusion criteria: i) The original publication was in
English; ii) all enrolled patients received surgery without other
forms of treatments (including radiotherapy, chemotherapy and
targeted therapy); iii) studies were about primary tumors; iv)
baseline characteristics of enrolled patients were described in
detail (including sex, age, and treatment); v) studies provided
information regarding recurrence and death events in relation to
clinicopathological factors of the patients.
First, abstracts of all identified citations were
screened, and those not meeting the inclusion criteria were
excluded. Subsequently, the full texts of the remaining articles
were rescreened, and studies were excluded if they met the
following exclusion criteria: i) Small sample size (≤20); ii)
studies lacking prognosis or recurrence data classified by tumor
size; iii) articles that only offered the relative information of
one group; iv) tumor sizes were measured using imaging data,
instead of in surgery or pathological specimen.
Data extraction and quality
assessment
According to the inclusion/exclusion criteria, 21
studies were included in the current meta-analysis. We extracted
the following data from each study: the first author's name, year
of publication, study location, number of patients included, study
design, period of follow-up, sex composition, therapeutic method,
and odds ratios (ORs) with their 95% confidence intervals (95%
CIs), resulting from univariate analysis (either published or
derived from reported data).
Study quality was scored using the Newcastle-Ottawa
Scale (NOS) (16). The NOS is
frequently used for non-random studies, such as cohort studies. The
maximum score for a cohort study was 9, and studies scoring between
5 and 9 are generally considered high quality. The quality scores
of the 21 studies ranged from 7 to 9, and all were considered
adequate for inclusion in the meta-analysis.
Ethics approval
The present study was approved by the Human Ethics
Committee/Institutional Review Board of Fudan University Shanghai
Cancer Center (Shanghai, China).
Statistical analysis
The meta-analysis was performed using STATA version
12.0 program (StataCorp, College Station, TX, USA) to facilitate
the pooling of results across studies. The final results are
expressed as an OR (odds ratio) and its 95% confidence interval
(CI). Heterogeneity in each study was assessed using χ2
tests (Q-value and P-value) and I2 measures.
Significant heterogeneity was defined as a χ2 test with
P<0.05 or I2 >50%. Random-effect models
(Mantel-Haenszel and Der Simonnian-Laird methods) were then used
for primary analyses in datasets with significant heterogeneity.
Primary datasets without significant heterogeneity were analyzed
using fixed-effect models (Mantel-Haenszel method). If there was
significant heterogeneity and >10 studies included, we accounted
for statistical heterogeneity by meta-regression analysis.
Publication biases were assessed by Begg's test in each
meta-analysis, and we assumed publication bias was present if
P<0.05.
Results
Baseline study and patient
characteristics
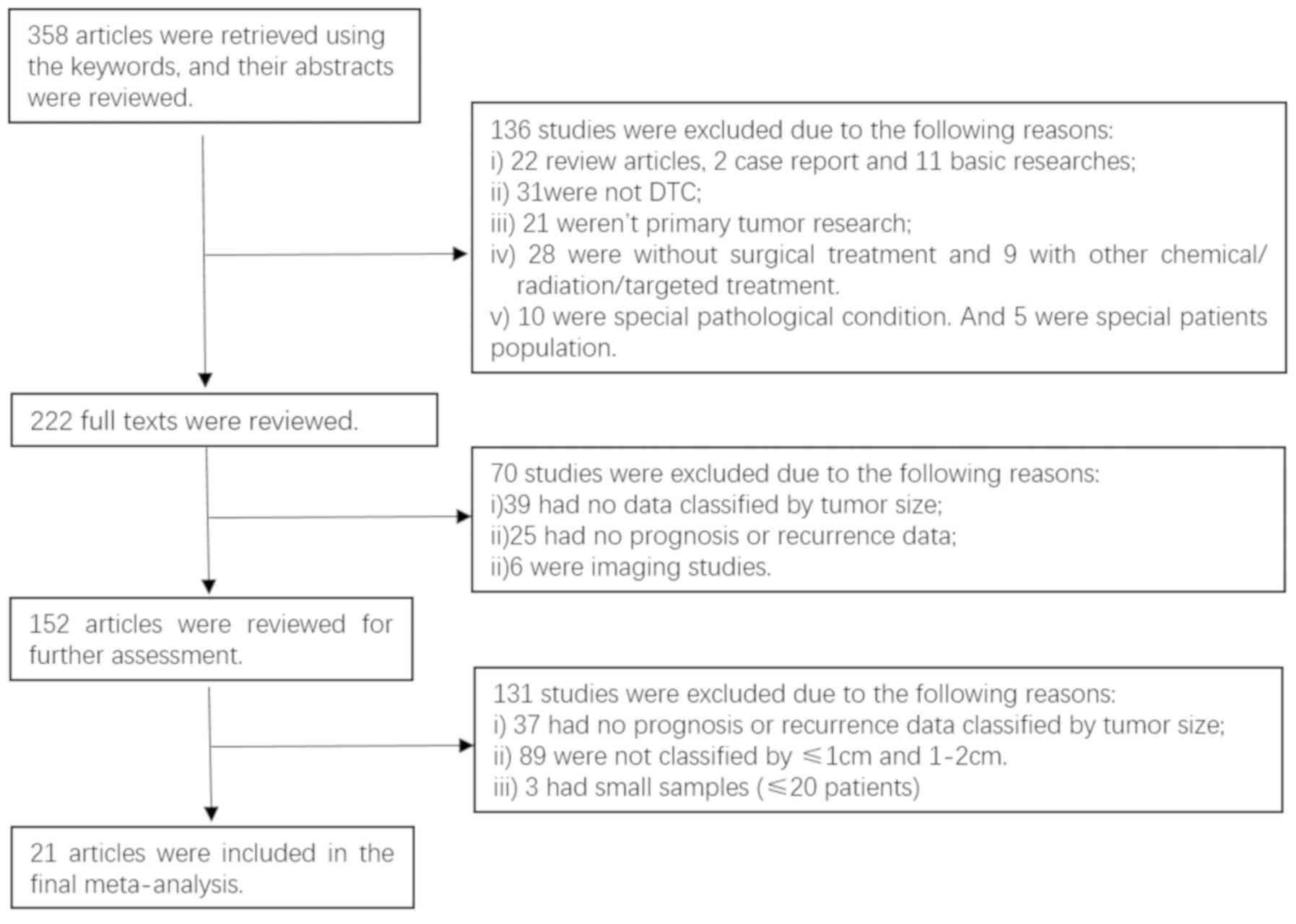
The process of selecting studies according to the
inclusion/exclusion criteria is shown in Fig. 1, whereby 21 studies were included in
the final meta-analysis (5–8, 12–14,
17–30). The principal characteristics of the
21 studies are shown in Table I. In
total 219,291 patients were involved, and the mean age of the
enrolled patients in each study was similar. All 21 studies were
retrospective cohort studies published between 2004 and 2017,
except one prospective study. The follow-up time ranged from 3.6 to
14.1 years. The main treatment was total thyroidectomy (TT) or near
total thyroidectomy (nTT) with postoperative radioiodine as
selective adjuvant therapy.
 | Table I.Baseline characteristics of the
studies included. |
Table I.
Baseline characteristics of the
studies included.
| First author | Region | Year | Sample | Histology | Studya | Follow-up
(years) | Mean age
(years) |
Surgeryb | RAI | Quality score | (Refs.) |
|---|
| Pellegriti | Italy | 2004 | 299 | PTC | R | 3.8 | 41.9 | nTT,TT | 30.40% | 9 | (18) |
| Barbaro | Italy | 2005 | 91 | PTC | R | None | No detail | TT | No detail | 7 | (19) |
| Reddy | USA | 2006 | 551 | DTC | R | 3.6 | No detail | TT or sTT | No detail | 7 | (20) |
| Bilimoria | USA | 2007 | 28,016 | PTC | R | 5.8 | 43 | TT,lobectomy | 51.30% | 7 | (8) |
| Rossi | Italy | 2008 | 426 | DTC | R | 5.4 | 53 | nTT,TT | 95.80% | 9 | (14) |
| Krämer | Germany
Austria | 2010 | 221 | DTC | P | 6.3 | 47.7 | TT | all | 8 | (21) |
| Ito | Japan | 2010 | 2,638 | PTC | R | 7.6 | 51.1 | TT, sTT,
lobectomy | 0.10% | 8 | (17) |
| Nixon | USA | 2011 | 707 | DTC | R | 8.2 | No detail | TT,sTT,
lobectomy | 26% | 7 | (22) |
| Ibrahimpasic | USA | 2012 | 200 | PTC | R | 5.2 | No detail | TT | 40.1% | 8 | (23) |
| Avram | USA | 2013 | 116 | DTC | R | None | 47 | TT, lobectomy | 3.40% | 9 | (24) |
| Momesso | Brazil | 2014 | 176 | DTC | R | 14.1 | 40.2 | TT+sTT | 57.3% | 9 | (26) |
| Han | Korea | 2014 | 176 | DTC | R | 7.2 | 50.1 | TT | all | 8 | (27) |
| Wang | USA | 2014 | 1,522 | DTC | R | 3.8 | 48 | TT,sTT,
lobectomy | 6% | 9 | (13) |
| Smith | England | 2015 | 35 | PTC | R | 6 | No detail | TT, lobectomy | 51.40% | 6 | (25) |
| Baek | Korea | 2015 | 659 | PTC | R | None | 45.9 | TT,sTT,
lobectomy | No detail | 9 | (28) |
| Nilubol | USA | 2015 | 30,184 | PTC | R | 4.5 | No detail | TT,sTT,
lobectomy | 47.10% | 8 | (5) |
| Vrachimis | Germany | 2015 | 221 | DTC | R | 7.8 | 47,7 | TT | all | 8 | (6) |
| Chereau | France | 2016 | 2,518 | PTC | R | 6.5 | 50 | TT, lobectomy | 49.60% | 9 | (12) |
| Anderson | USA | 2016 | 149,912 | DTC | R | None | 50.6 | TT, lobectomy | 37.60% | 9 | (7) |
| Sun | China | 2017 | 269 | DTC | R | None | No detail | TT,lobectomy | No detail | 7 | (29) |
| Aydin Buyruk | Turkey | 2017 | 354 | PTC | R | None | No detail | TT | No detail | 8 | (30) |
All the NOS scores of the eligible studies were
greater than 5 for the 9 questions, with an average of 8.05 (range,
7–9), indicating good quality for meta-analysis. The included
studies were all cohort studies with definitive controls for
selection, yielding the good scores.
Association between tumor size and
recurrence in DTC
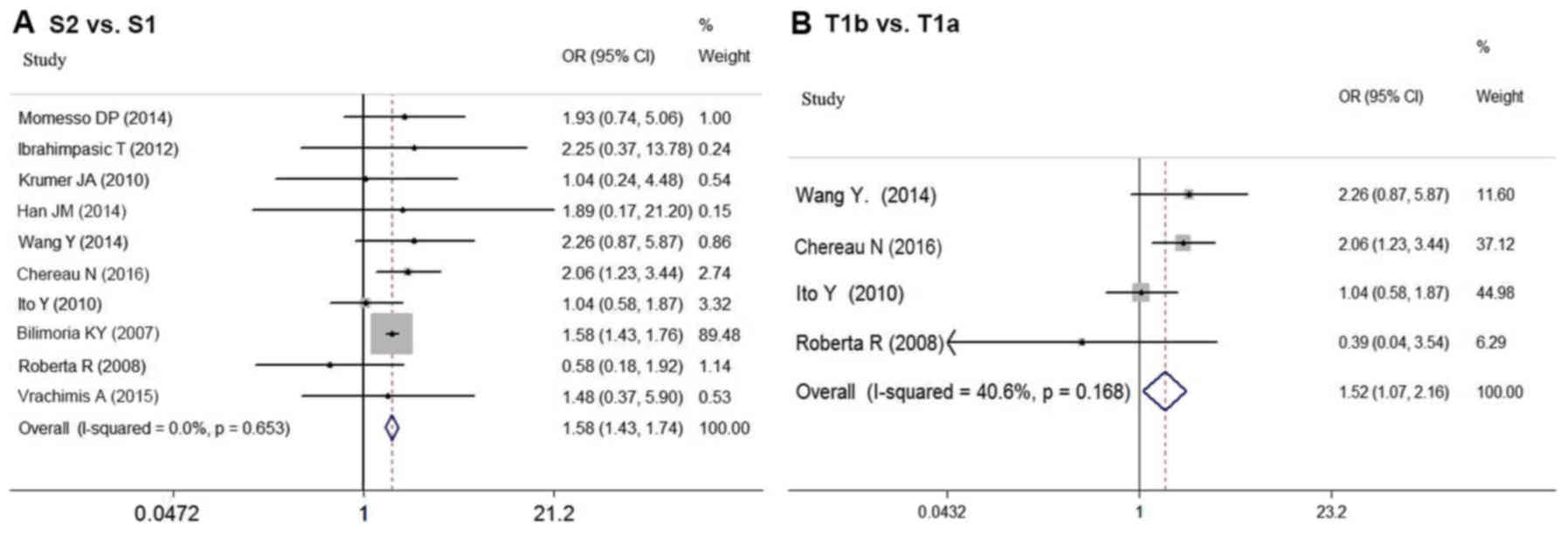
As shown in Fig. 2A,
the risk of recurrence for DTC patients based on tumor size was
compared in 10 studies (6,8,12–14,17,21,23,26–27).
In the meta-analysis, larger tumor size (S2 vs. S1) was
associated with higher risk of postoperative recurrence in sDTC
patients (OR=1.575; 95% CI=1.428–1.738; P<0.05). No
significant statistical heterogeneity was detected among these
studies (P=0.653; I2=0.0%).
The prognostic influence of tumor size is partly
attributable to its association with more aggressive histologic
features such as extrathyroidal extension (ETE), rather than the
impact of size itself. Thus, our analysis compared the influence of
T1a and T1b on recurrence based on the data from four studies
(12–14,17). In
the meta-analysis, T1b also indicated higher risk of recurrence
than T1a (OR=1.520; 95% CI=1.072–2.155; P<0.05) (Fig. 2B). There was no significant
statistical heterogeneity among these studies (P=0.168,
I2=40.6%).
Association between tumor size and
survival in DTC
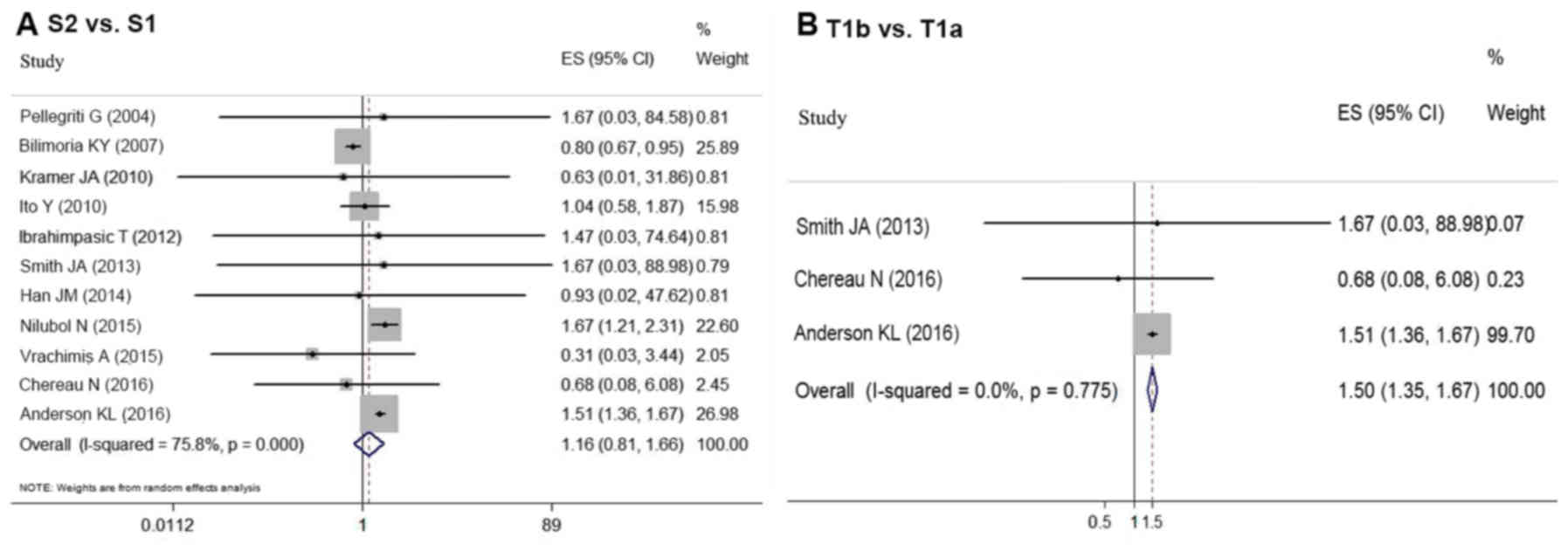
Eleven articles provided survival data for groups
S1 and S2 (5–8,12,17,18,21,23,25,27).
Due to the favorable prognosis of DTC, there was no end-point
mortality data in 5 studies. Based on the random-effect
meta-analysis, tumor size (S1 vs. S2) had no association
with survival in DTC patients (OR=1.160, 95% CI=0.810–1.662;
P=0.448) (Fig. 3A). However,
significant statistical heterogeneity was detected among these
studies (P<0.05, I2=75.8%). Thus, a
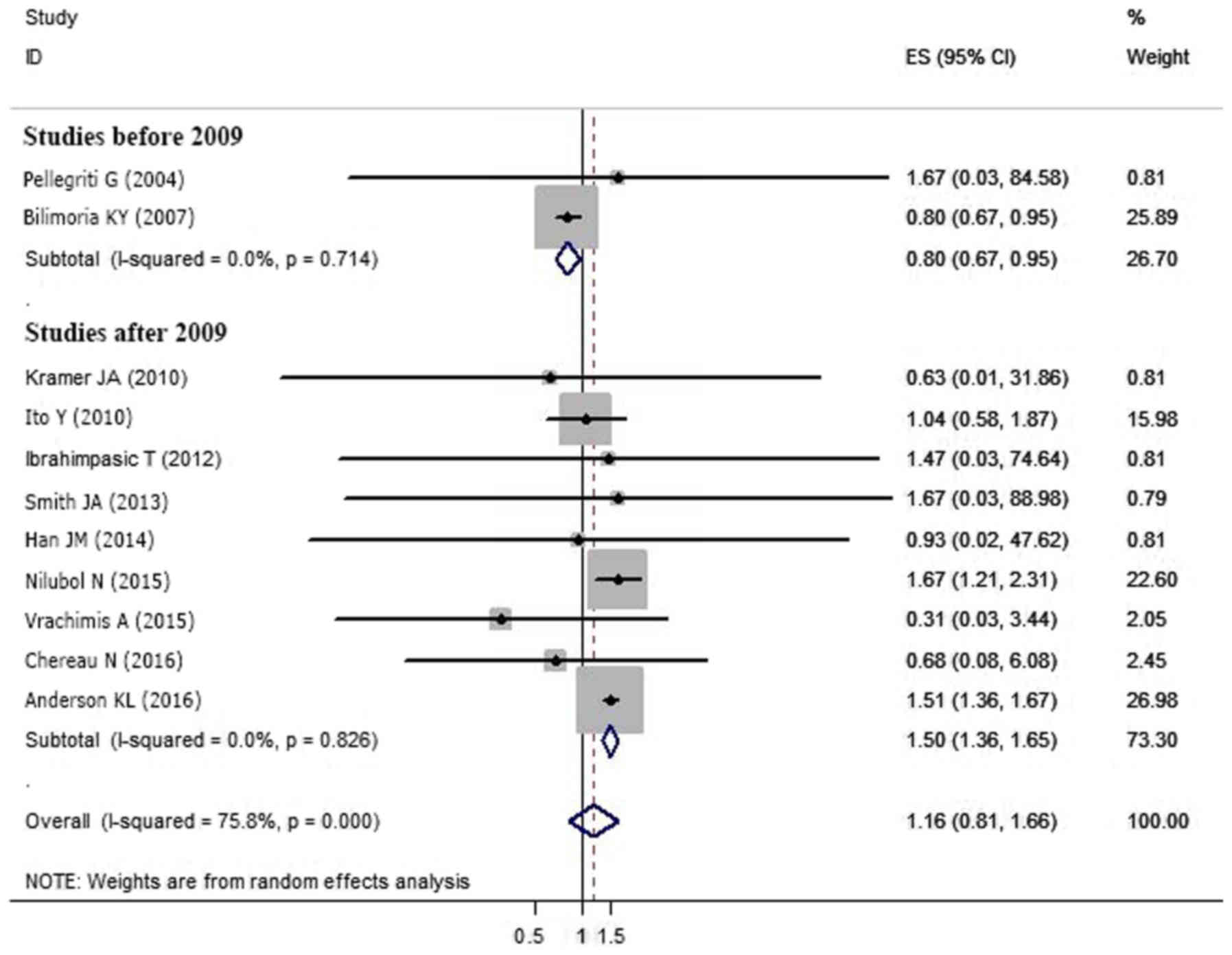
meta-regression analysis was performed to explore the sources of
heterogeneity. Study location (P=0.49), cohort size
(P=0.286), follow-up period (P=0.282), patient age
(P=0.131) and sex composition (P=0.866) were not the
sources of heterogeneity. However, the year of publication
potentially caused statistical heterogeneity (P<0.05).
Subgroup analysis of the nine studies published after 2009
(5–7,12,17,21,23,25,27)
indicated group S2 had a worse prognosis compared with
S1 (OR=1.498, 95%CI=1.357–1.653; P<0.05). However,
an analysis of the two studies conducted prior to 2009 showed the
opposite conclusion (OR=0.800; 95% CI=0.670–0.955;
P<0.05) (8,18) (Table
II; Fig. 4). There was no
significant statistical heterogeneity among the studies when
divided by publication date (before 2009, P=0.714,
I2=0.0%; after 2009, P=0.826,
I2=0.0%).
 | Table II.Subgroup analysis of association
between tumor size and prognosis. |
Table II.
Subgroup analysis of association
between tumor size and prognosis.
| Factor | Standard | No. | Q-value | P-value | I2 | OR | 95% CI |
|---|
| Year | Before
2009 | 2 | 0.13 | 0.014 | 0.0% | 0.800 | 0.670–0.955 |
|
| After 2009 | 9 | 4.33 | 0.000 | 0.0% | 1.498 | 1.357–1.653 |
Aggressive histological features can also affect
prognosis; therefore, T stage was analyzed independently to
determine whether T1b impacted postoperative survival compared with
T1a. The meta-analysis of the data from three studies (7,12,25)
revealed that T1b indicated a worse prognosis compared with T1a
(OR=1.504, 95% CI=1.353–1.672; P<0.05) (Fig. 3B). No statistical heterogeneity
(P=0.775, I2=0.0%) was detected.
Association between tumor size and
prognosis in PTC
Considering that different histologies (papillary
thyroid, follicular thyroid and Hürthle cell cancer) indicate
different prognoses, we independently analyzed the influence of the
two groups (S1 vs. S2) on the prognosis of PTC
patients. The meta-analysis based on four studies (8,12,17,23)
revealed that larger tumor size (S2) increased the risk of
recurrence for sPTC patients compared with S1 (OR=1.580, 95%
CI=1.430–1.747; P<0.05), and there was no statistical
heterogeneity between these studies (P=0.337,
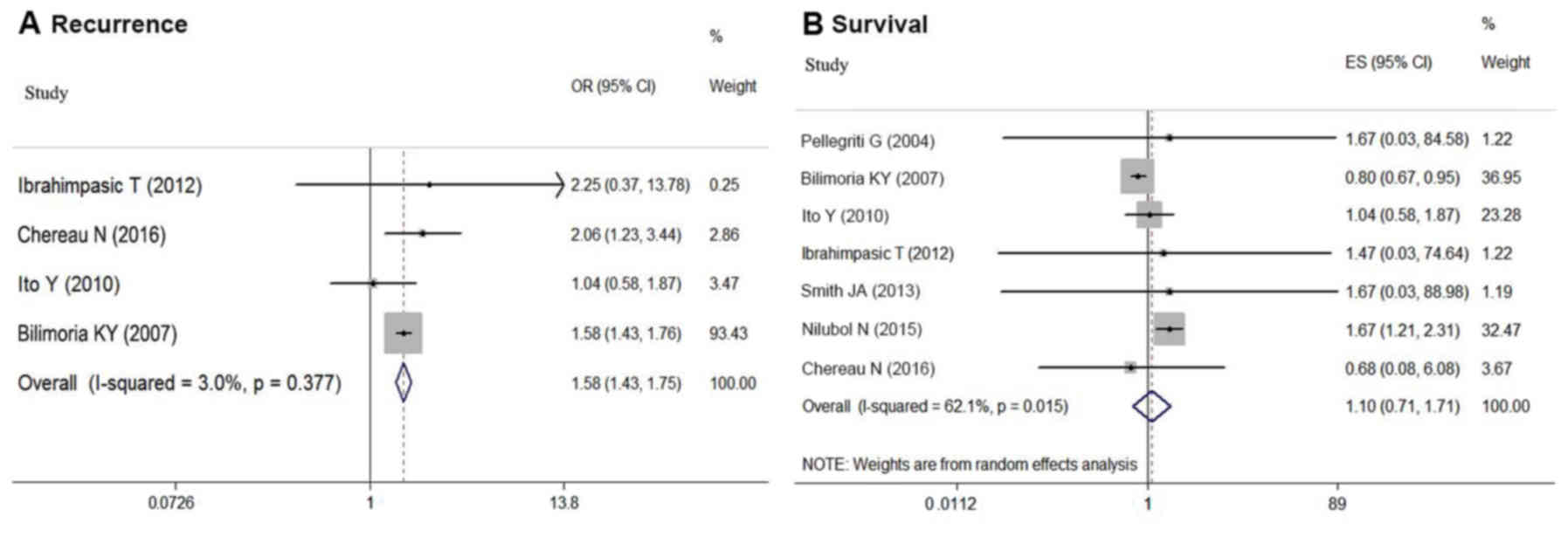
I2=3.0%) (Fig. 5A). Due
to the significant heterogeneity (P=0.015,
I2=62.1%), the random effect model was used for the
remaining seven studies (5,8,12,17,18,23,25)
to explore the subgroup effect on survival. The analysis revealed
that there was no statistical difference between the two groups
(S2 vs. S1) in survival (OR=1.101, 95% CI=0.708–1.712;
P=0.668) (Fig. 5B).
Tumor size and aggressive histologic
features in DTC
The relationship between tumor size and aggressive
histological features was also analyzed. Multifocality, ETE,
bilaterality, vascular invasion, lymph node metastases and distant
metastases were compared in eight (12–14,18,19,24,25,28),
five (14,18,19,22,27),
three (12,18,28),
four (7,12,13,18),
eleven (6,7,12,14,18–20,24,28–30)
and five (7,12,14,18,24)
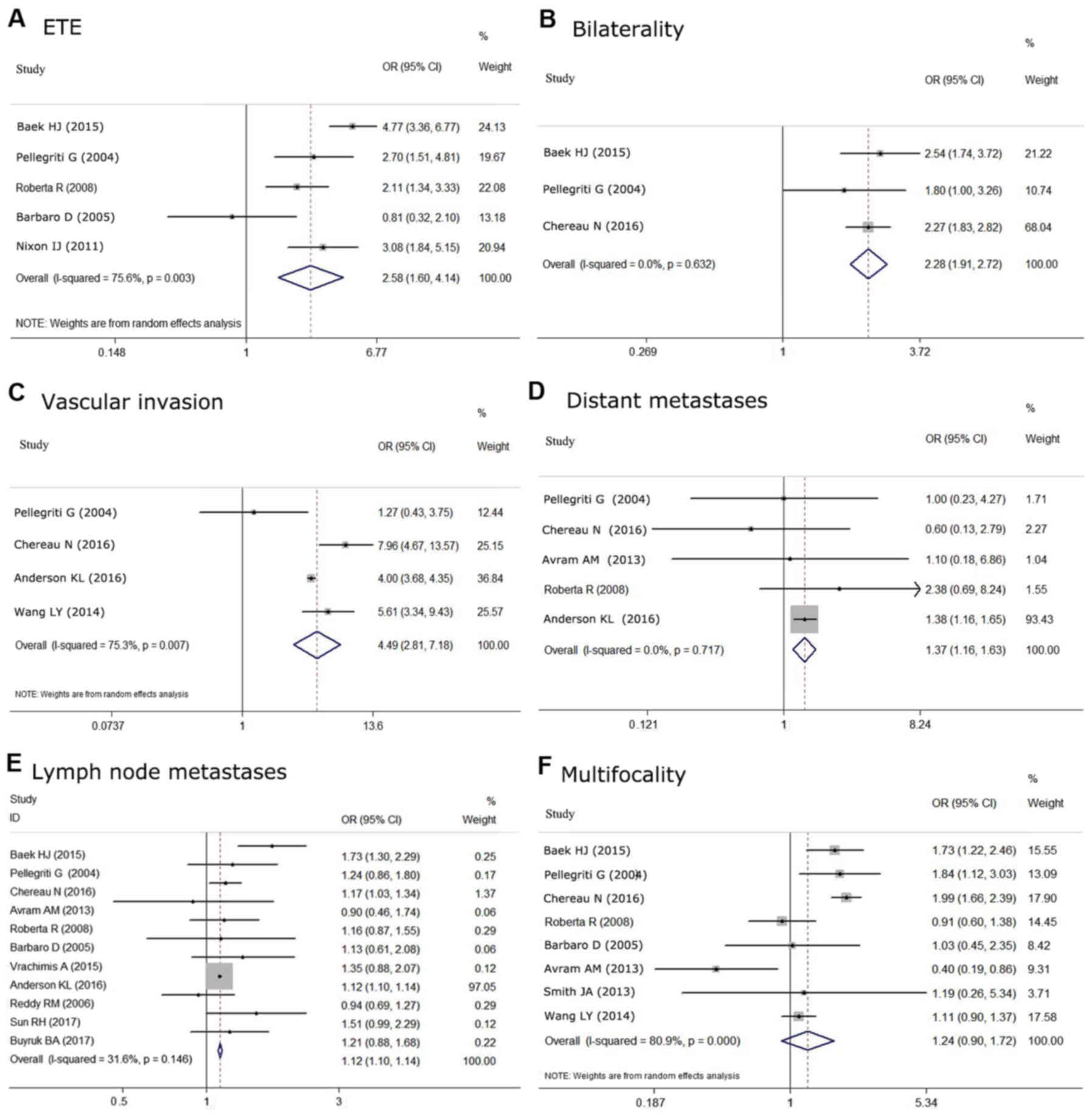
studies, respectively. Based on the meta-analysis, S2 was
found to be associated with aggressive histological features more
often compared with S1, including ETE (OR=2.575, 95%
CI=1.603–4.135; P<0.05) (Fig.
6A), bilaterality (OR=2.278, 95% CI=1.905–2.723;
P<0.05) (Fig. 6B),
vascular invasion (OR=4.494, 95% CI=2.812–7.183, P<0.05)
(Fig. 6C), lymph node metastases
(OR=1.12, 95% CI=1.10–1.14, P<0.05) (Fig. 6E) and distant metastases (OR=1.373,
95% CI=1.155–1.631; P<0.05) (Fig. 6D). Although it has been reported that
tumors >1 cm are more frequently associated with multifocality
than tumors ≤1 cm, no significant association was found in our
meta-analysis (OR=1.242, 95% CI 0.899–1.716; P=0.188)
(Fig. 6F). There was no statistical
heterogeneity among these studies when evaluating the association
with bilaterality (P=0.632, I2=0.0%),
lymph node metastases (P=0.146, I2=31.6%), and
distant metastases (P=0.717, I2=0.0%).
However, significant statistical heterogeneity was detected among
the studies predicting correlations between tumor size and
multifocality (P<0.05, I2=80.9%),
extrathyroidal extension (P<0.05, I2=75.6%)
and vascular invasion (P<0.05, I2=75.3%).
Furthermore, Begg's test was negative for all analyses.
Discussion
Primary tumor diameter has been described as a
determinant for outcome in DTC (9).
The subdivision of sDTC is based on previous studies that indicated
tumors >1 cm had worse prognoses (8). However, it remains controversial
whether the subgroups (S1 vs. S2) influence the prognosis of
sDTC and what the appropriate therapeutic strategies are for
patients in each subgroup. Thus, we determined whether tumor size
>1 cm would impact recurrence and survival in sDTC. The results
of our study offer some guidelines for physicians dealing with sDTC
patients.
The current meta-analysis focused on the effects of
tumor size (S2 vs. S1) on postoperative recurrence and
survival. The results indicated that patients with S2 had a
higher risk of postoperative recurrence compared with S1
during follow-up. However, there was no statistical difference in
survival between patients within the groups, and significant
statistical heterogeneity was detected during analysis. Based on
meta-regression analysis, publication year potentially caused the
statistical heterogeneity (P<0.05). In the subgroup
meta-analysis, the subgroup including nine studies published after
2009 showed that S2 was associated with worse prognoses
compared with S1, but the subgroup of two studies published
before 2009 showed the opposite; there were no statistical
heterogeneities in either subgroup. Of note, the 7th AJCC's TNM
staging guide was published in 2009, which subdivided T1 to T1a and
T1b (9). This subdivision may
heighten differences between the two subgroups. Further prospective
studies are necessary to confirm the influence of tumor size on
survival in sDTC.
To remove the effects of aggressive histological
features such as ETE, we analyzed the influence of T stage (T1a
vs. T1b) independently on postoperative prognosis. The
results also showed that T1b increased the risk of recurrence and
death compared with T1a. Since different pathological patterns of
DTC indicate different prognoses, we analyzed the two groups (S1
vs. S2) in PTC independently. The meta-analysis revealed that
S2 increased the risk of recurrence for sPTC patients
compared with S1, but there was no statistical difference
between the two groups on survival. Given that the prognostic
influence of tumor size is partly due to its association with
aggressive histological features, our study explored the
correlation between tumor size and other clinicopathological
factors. The results confirmed that S2 sDTC was more often
associated with ETE, bilaterality, vascular invasion, lymph node
metastases and distant metastases than S1.
The optimal treatment for the patients with sDTC is
controversial. Bilimoria et al (8) reported a retrospective study enrolling
52,173 papillary thyroid carcinoma patients (including 28,016 sPTC
patients) that concluded lobectomy as initial treatment was
inadequate and had a worse prognosis regarding recurrence and death
events compared with total thyroidectomy for the patients with
S2 disease (for recurrence: HR=1.24, 95% CI=1.01–1.65,
P=0.04; for survival: HR=1.49, 95% CI=1.02–2.17,
P=0.04), but for patients with S1 disease, the two
surgical approaches made no difference in prognosis. However, the
2015 ATA guidelines also recommended that thyroid lobectomy alone
may be sufficient initial treatment for low-risk DTC ≤4 cm, unless
there are clear indications to remove the contralateral lobe
(11) Momesso et al (26) retrospectively analyzed clinical data
from 176 DTC patients and argued that the surgical approach (total
thyroidectomy or subtotal thyroidectomy) did not influence
postoperative survival or recurrence for patients in either group;
they also found no influence from radioiodine therapy. Ito et
al (17) investigated the
prognosis of 2,638 patients with T1N0M0 papillary thyroid carcinoma
(PTC) who underwent initial surgery without radioiodine therapy.
Those authors concluded that total thyroidectomy is not mandatory
for T1N0M0 PTC patients unless other diseases coexisted requiring
total thyroidectomy if a 1% risk of recurrence to the remnant
thyroid is acceptable, and radioiodine ablation therapy is also not
necessary. Based on a retrospective study of 1522 T1N0M0 DTC
patients, Wang et al (13)
found no difference in disease-specific survival among 1,522
patients with T1 tumors, and there was no difference in the risk of
recurrence between total thyroidectomy and less than total
thyroidectomy both for the patients with T1a and T1b (T1a: P=0.105;
T1b: P=0.868).
The major limitation of this meta-analysis was that
only 21 studies were suitable based on the inclusion and exclusion
criteria. Although 358 articles were identified, most of the
studies did not offer sufficient statistics about recurrence or
survival subdivided among patients with S1 and S2
disease. There were few studies with the specific aim of
investigating the effect of tumor size on patient prognosis. Thus,
large-scale prospective studies are required to confirm the
influence of tumor size on prognosis in sDTC. Moreover, since there
were few data regarding the association between pathological type
and subgroup (S1 and S2), we could not adequately
perform analyses on these factors. The limitation of insufficient
availability of studies included resulted in some findings being
heavily weighed by these few studies. Finally, publication bias is
a major concern in all forms of pooled analyses, while our analysis
revealed that this was not a complicating variable for any of the
included studies.
Our meta-analysis suggested that patients with
S2 have an increased risk of postoperative recurrence and
mortality compared with S1 patients, and comparisons between
T1a and T1b came to the same conclusions. Furthermore, the
meta-analysis indicated that S2 sDTC was more commonly
associated with ETE, bilaterality, vascular invasion, lymph node
metastases and distant metastases compared with S1. In
summary, our analysis suggests that it is necessary to subdivide
sDTC into S1 and S2 subgroups due to their different
effects on prognosis, especially recurrence. Future prospective
studies are required to confirm the influence of tumor size on
prognosis in sDTC.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from the
National Natural Science Foundation of China (no. 81702649 to NQ,
nos. 81572622 and 81272934 to QHJ.
Availability of data and materials
The analyzed datasets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TTZ, CFL, NQ, RLS and QHJ were involved in the
conception and design of the study. SSW, DZH and GHS were involved
in the acquisition of the clinical data and reviewing. TTZ, NQ, YXZ
and YW analyzed and interpreted the data. TTZ, NQ, RLS and QHJ
wrote the manuscript. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Ethics
Committee/Institutional Review Board of Fudan University Shanghai
Cancer Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hedinger C, Williams E and Sobin L:
Histological typing of thyroid tumour. (2nd). World Health
Organization. (New York, NY). 15–17. 1993.
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim H, Devesa SS, Sosa JA, Check D and
Kitahara CM: Trends in thyroid cancer incidence and mortality in
the United States, 1974–2013. JAMA. 317:1338–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nilubol N and Kebebew E: Should small
papillary thyroid cancer be observed? A population-based study.
Cancer. 121:1017–1024. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vrachimis A, Wenning C, Gerß J, Dralle H,
Vaez Tabassi M, Schober O and Riemann B; MSDS study Group, : Not
all DTC patients with N positive disease deserve the attribution
‘high risk’. Contribution of the MSDS trial. J Surg Oncol.
112:9–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anderson KL Jr, Youngwirth LM, Scheri RP,
Stang MT, Roman SA and Sosa JA: T1a versus T1b differentiated
thyroid cancers: Do we need to make the distinction? Thyroid.
26:1046–1052. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bilimoria KY, Bentrem DJ, Ko CY, Stewart
AK, Winchester DP, Talamonti MS and Sturgeon C: Extent of surgery
affects survival for papillary thyroid cancer. Ann Surg.
246:375–381; discussion 381–374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edge SB and Compton CC: The american joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. (Eighth). Springer.
(New York, NY). 873–887. 2017.PubMed/NCBI
|
|
11
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The american thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chereau N, Tresallet C, Noullet S,
Godiris-Petit G, Tissier F, Leenhardt L and Menegaux F: Does the T1
subdivision correlate with the risk of recurrence of papillary
thyroid cancer? Langenbecks Arch Surg. 401:223–230. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang LY, Nixon IJ, Palmer FL, Thomas D,
Tuttle RM, Shaha AR, Patel SG, Shah JP and Ganly I: Comparable
outcomes for patients with pT1a and pT1b differentiated thyroid
cancer: Is there a need for change in the AJCC classification
system? Surgery. 156:1484–1490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rossi R, Roti E, Trasforini G, Pansini G,
Cavazzini L, Zatelli MC, Pearce EN, Braverman LE and Uberti EC:
Differentiated thyroid cancers 11–20 mm in diameter have clinical
and histopathologic characteristics suggesting higher
aggressiveness than those ≤10 mm. Thyroid. 18:309–315. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moher D, Shamseer L, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P and Stewart LA; PRISMA-PGroup,
: Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015 statement. Syst Rev. 4:12015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito Y, Masuoka H, Fukushima M, Inoue H,
Kihara M, Tomoda C, Higashiyama T, Takamura Y, Kobayashi K, Miya A
and Miyauchi A: Excellent prognosis of patients with solitary
T1N0M0 papillary thyroid carcinoma who underwent thyroidectomy and
elective lymph node dissection without radioiodine therapy. World J
Surg. 34:1285–1290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pellegriti G, Scollo C, Lumera G,
Regalbuto C, Vigneri R and Belfiore A: Clinical behavior and
outcome of papillary thyroid cancers smaller than 1.5 cm in
diameter: Study of 299 cases. J Clin Endocrinol Metab.
89:3713–3720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barbaro D, Simi U, Meucci G, Lapi P,
Orsini P and Pasquini C: Thyroid papillary cancers: Microcarcinoma
and carcinoma, incidental cancers and non-incidental cancers-are
they different diseases? Clin Endocrinol (Oxf). 63:577–581. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reddy RM, Grigsby PW, Moley JF and Hall
BL: Lymph node metastases in differentiated thyroid cancer under 2
cm. Surgery. 140:1050–1054; discussion 1054–1055. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krämer JA, Schmid KW, Dralle H, Dietlein
M, Schicha H, Lerch H, Gerss J, Frankewitsch T, Schober O and
Riemann B; MSDS study Group, : Primary tumour size is a prognostic
parameter in patients suffering from differentiated thyroid
carcinoma with extrathyroidal growth: Results of the MSDS trial.
Eur J Endocrinol. 163:637–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nixon IJ, Ganly I, Patel S, Palmer FL,
Whitcher MM, Tuttle RM, Shaha AR and Shah JP: The impact of
microscopic extrathyroid extension on outcome in patients with
clinical T1 and T2 well-differentiated thyroid cancer. Surgery.
150:1242–1249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ibrahimpasic T, Nixon IJ, Palmer FL,
Whitcher MM, Tuttle RM, Shaha A, Patel SG, Shah JP and Ganly I:
Undetectable thyroglobulin after total thyroidectomy in patients
with low- and intermediate-risk papillary thyroid cancer-is there a
need for radioactive iodine therapy? Surgery. 152:1096–1105. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Avram AM, Fig LM, Frey KA, Gross MD and
Wong KK: Preablation 131-I scans with SPECT/CT in postoperative
thyroid cancer patients: What is the impact on staging? J Clin
Endocrinol Metab. 98:1163–1171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smith JA, Sharma N, Nankivell P and
Watkinson JC: The presentation, natural history and outcome of T1
a/b thyroid cancer with regard to new grading systems. Eur Arch
Otorhinolaryngol. 272:439–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Momesso DP, Vaisman F, Caminha LS, Pessoa
CH, Corbo R and Vaisman M: Surgical approach and radioactive iodine
therapy for small well-differentiated thyroid cancer. J Endocrinol
Invest. 37:57–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han JM, Kim WG, Kim TY, Jeon MJ, Ryu JS,
Song DE, Hong SJ, Shong YK and Kim WB: Effects of low-dose and
high-dose postoperative radioiodine therapy on the clinical outcome
in patients with small differentiated thyroid cancer having
microscopic extrathyroidal extension. Thyroid. 24:820–825. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baek HJ, Kim DW and Ryu JH: Association
between TNM staging system and histopathological features in
patients with papillary thyroid carcinoma. Endocrine. 48:589–594.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun R, Zhang H, Liu K, Fan J, Li G, Song X
and Li C: Clinicopathologic predictive factors of cervical lymph
node metastasis in differentiated thyroid cancer. Acta
Otorrinolaringol Esp. 69:149–155. 2018.(In English, Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aydin Buyruk B, Kebapci N, Yorulmaz G,
Buyruk A and Kebapci M: An evaluation of clinicopathological
factors effective in the development of central and lateral lymph
node metastasis in papillary thyroid cancer. J Natl Med Assoc.
110:384–390. 2018. View Article : Google Scholar : PubMed/NCBI
|