Introduction
Ovarian cancer is the fifth leading cause of
cancer-associated mortality among women with >20,000 new cases
and 14, 000 mortalities estimated in the USA in 2018 (1). Ovarian cancer has an asymptomatic onset
and the majority of cases are detected in the late stages, when
tumour metastasis has occurred (2).
Despite the best possible treatment, the mortality rate remains
high, and the 5-year overall survival rate is <40%. Early
diagnosis leads to a better prognosis, with a 95% survival rate if
the disease is confined to the ovary, 79% if there is adjacent
tissue infiltration, and 28% if the disease is advanced (3). Industrialized nations have been
reported to have higher incidence of ovarian cancer due to
environmental factors. It has also been observed that the risk of
developing epithelial ovarian cancer (EOC) is ~1.5-fold higher in
women who have never breastfed, compared to those who have
breastfed for >18 months. This observation is linked to the
reduction in ovarian cancer incidence observed with the use of oral
contraceptives (4,5). Overall, ~90% cases of ovarian cancer
arise from the epithelium, 3% from the germ cells and 2% from the
sex-cord stromal cells. EOC is histologically subdivided into
serous (70%), endometrioid (10%), mucinous (6%) and clear cell
(6%), differing in their genetic status and therapeutic response
(6).
Despite conventional debulking surgery and the
development of adjuvant chemotherapy using platinum/taxane-based
drugs, no significant improvement has been noted in ovarian cancer
prognosis. This is largely due to the emergence of multidrug
resistance, predisposing the patient to relapse (7). Novel agents and molecules are currently
being investigated, and, notably, mesenchymal stem cells (MSCs) and
their soluble factors are reported to exhibit beneficial anticancer
effects (8,9). MSCs migrate to damaged tissues, sites
of inflammation and tumour sites, and contribute either directly or
indirectly towards restoring homeostasis (10). These cells generally have low
immunogenicity and escape immune surveillance, enabling them to
reach the intended site of action (11). Within the tumour environment, MSCs
are reported to dynamically interact with tumour-associated cells
and release soluble factors (cytokines, chemokines and growth
factors) with autocrine and paracrine functions (12). However, the interaction between MSCs
and tumour cells depends on a number of factors, and the overall
outcome determines whether the tumour subsides or progresses.
Cytokines are low-molecular-weight glycoproteins
produced by immune and non-immune cells. Functionally, these
proteins are pleotropic, and the expression of receptors to various
cytokines on the cell surface facilitates cell-cytokine
interactions. Dysregulation of cytokines may result in disease or a
pathological state, and the measurement of their levels helps
monitor disease severity or therapeutic intervention (13). Research on cytokines has evolved
rapidly in the last decade, contributing to molecular and
immunological insights in health and disease. Cancer is a state of
chronic inflammation, and signalling involving tumour progression,
inhibition, metastasis or immunomodulation is partially regulated
by cytokines and/or chemokines (14). A previous study has suggested that
EOC is immunogenic, as indicated by the presence of tumour-reactive
T cells in the tumour microenvironment, which is associated with
higher production of IFN-γ induced monkine and secondary lymphoid
tissue chemokine contibuting to improvement in the overall survival
rate (15).
Considering the beneficial effects of MSCs against
cancer, and the role of cytokines in tumour inhibition and
therapeutics, the in vitro effect of human Wharton's Jelly
stem cell conditioned medium (hWJSC-CM) and cell lysate (hWJSC-CL)
against an ovarian cancer cell line (OVCAR3) was evaluated. The two
hWJSC extracts inhibited OVCAR3 cell proliferation, partly mediated
by their soluble factors, leading to the decreased expression of
oncogenic cytokines, chemokines and growth factors.
Materials and methods
Ethical approval
The present study was performed in accordance with
the recommendations of the Bioethics Committee of the King
Abdulaziz University, Jeddah, Saudi Arabia. All subjects provided
written informed consent, in accordance with the Declaration of
Helsinki. The protocol for the derivation and use of hWJSCs, and
the commercial human ovarian cancer cell line (OVCAR3) was approved
by the Bioethics Committee of the King Abdulaziz University
(approval no. 33-15/KAU).
Derivation of hWJSCs
Human umbilical cord specimens (n=5) were collected
from patients undergoing full-term delivery at the Department of
Obstetrics and Gynaecology, King Abdulaziz University Hospital. The
hWJSCs were derived as previously described (16,17).
Briefly, the umbilical cord was cut into ~2-cm pieces, opened
lengthways, and the blood vessels were removed. The cut pieces were
treated with an enzyme cocktail containing 2 mg/ml collagenase
type-I, 2 mg/ml collagenase type-IV and 100 IU hyaluronidase for 30
min (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The matrix
contents were gently scraped and the medium containing the cells
was centrifuged at 500 × g for 5 min. The cell pellet was washed
twice with PBS and centrifuged at 500 × g for 5 min again. The
resultant pellet was resuspended in hWJSC culture medium comprised
of high-glucose Dulbecco's Modified Eagle's Medium (DMEM;
Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal bovine serum
(FBS; Sigma-Aldrich; Merck KGaA), 2 mM Glutamax (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 1% non-essential amino acids
(Thermo Fisher Scientific, Inc.), 16 ng/ml basic fibroblast growth
factor (bFGF; Sigma-Aldrich; Merck KGaA) and 1% antibiotics (50
IU/ml penicillin and 50 µg/ml streptomycin, Sigma-Aldrich; Merck
KGaA), and incubated under standard culture conditions of 37°C in a
5% CO2 incubator. The cultures were left undisturbed
until cell growth was evident, except for gentle changes of the
growth medium every 72 h.
CD marker analysis
Cultures of hWJSCs were analyzed for expression of
MSC related cluster of differentiation (CD) markers as reported
earlier (18). Briefly, monolayer
cultures of hWJSCs were dissociated using 0.25% Trypsin-EDTA (Life
Technologies, Carlsbad, CA, USA) for 3 min. Trypsin activity was
inhibited by addition of culture medium containing 10% FBS
(Sigma-Aldrich; Merck KGaA). The cell suspension was centrifuged at
300 g × 5 min and the cell pellet was then resuspended in phosphate
buffered saline without calcium and magnesium (PBS-) containing 3%
FBS to obtain single cell suspension. Separate aliquots
(2×105 cells) were used for MSC isotype cocktail
(Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), MSC phenotyping
cocktail (Miltenyi Biotec GmbH) or in combination with other
primary monoclonal antibodies (CD44, CD29; BD Biosciences, Franklin
Lakes, NJ, USA) to avoid interference with same fluorochromes. The
MSC isotype cocktail comprised of fluorochrome conjugated
monoclonal antibodies, namely mouse IgG1-FITC, mouse IgG1-PE, mouse
IgG1-APC, mouse IgG1-PerCp and mouse IG2a-PerCp. The MSC
phenotyping cocktail comprised of both positive (CD73-APC,
CD90-FITC, CD105-PE) and negative (CD34/CD45/CD14/CD20-PerCp)
fluorochrome conjugated monoclonal antibodies. The cells were
incubated with respective antibodies at 1:10 dilution for 15 min at
4°C; then washed with 1 ml of 3% FBS and centrifuged at 300 g × 5
min. The supernatant was discarded, and the cells were resuspended
in 500 µl of 3% FBS before analysis using a FACS Aria III
instrument (BD Biosciences), which is equipped with a 488 nm (blue)
laser and a 561 nm (yellow-green) laser for uncoupled excitation
and detection of FITC and PE fluorochromes.
Preparation of hWJSC-CM
Early passages of hWJSCs (P2-P4) were grown under
standard culture conditions and the medium was changed every 48 h.
When the cells were 70% confluent, the culture medium was replaced
with fresh medium and the cells were cultured for up to 72 h. The
hWJSC-CM was then harvested, sterilized using 0.2 µm syringe
filters and stored in aliquots at 4°C until further use (17).
Preparation of hWJSC-CL
The hWJSCs were grown as described above, and at 80%
confluence the cells were trypsinized, pelleted, washed twice in
PBS, and centrifuged at 500 g for 5 min. The resultant cell pellet
was lysed in cell lysis buffer (Sigma-Aldrich; Merck KGaA) with a
protease inhibitor cocktail. The cells were gently pipetted up and
down to lyse the membranes and release the cellular contents. The
cell lysate (in 2 ml Eppendorf tubes) were then placed on ice and
continuously agitated in a rocker platform for 15 min. The cell
suspension was then centrifuged at 25,000 g for 15 min and the
clear supernatant was collected and stored in aliquots at 4°C until
further use. The total protein content was quantified using a
NanoDrop™ 2000 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.) (17).
Culture of OVCAR3 cells
The commercial human ovarian cancer OVCAR3 cell line
was purchased from the European Collection of Authenticated Cell
Cultures (Salisbury, UK). The OVCAR3 cells were rapidly thawed in a
37°C water bath following the standard cell-thawing procedure and
cultured at optimal conditions of 37°C in a 5% CO2
incubator. The cells were cultured in low-glucose Dulbecco's
Modified Eagles Medium DMEM supplemented with 10% FBS, 2 mM
Glutamax and 1% penicillin-streptomycin.
Cell morphology
OVCAR3 cells were seeded at a density of
2×104 cells/well in a 24-well plate and tested with
hWJSC-CM (25, 50 and 75%), hWJSC-CL (5, 10 and 15 µg/ml) and
paclitaxel (2.5, 5 and 10 nM). The control and experimental groups
were then cultured at 37°C in a 5% CO2 incubator for
24–72 h and any changes in cell morphology were observed using an
inverted-phase contrast microscope (Nikon Corporation, Tokyo,
Japan).
Cell proliferation
OVCAR3 cells were seeded at 2×104
cells/well in a 24-well plate and allowed to attach overnight. The
culture medium was then replaced with medium containing various
concentrations of hWJSC-CM (25, 50 and 75%), hWJSC-CL (5, 10 and 15
µg/ml) or paclitaxel (2.5, 5 and 10 nM) for 24, 48 and 72 h. The
control cells were cultured in normal culture medium without any
pharmacological agents. The cell proliferation/inhibition under
various experimental conditions was analysed using the MTT assay
according to the manufacturer's instructions. Briefly, the culture
medium was removed and 200 µl fresh medium containing 10 µl MTT
reagent (Sigma-Aldrich; Merck KGaA) was added, followed by
incubation under standard culture conditions for up to 4 h. The
medium was removed and 200 µl solubilisation reagent was added. The
absorbance at 570 nm, with a reference wavelength of 630 nm, was
measured using a SpectraMax i3 Multimode Reader (Molecular Devices,
LLC, Sunnyvale, CA, USA). The differences between the treated and
control groups were analyzed with one-way analysis of variance with
Bonferroni's multiple comparisons post hoc analyis using SPSS
version 22.0 (IBM Corp., Armonk, NY, USA). The results are
presented as the mean ± standard error of the mean from 3
replicates for individual assays and P<0.05 was considered to
indicate a statistically significant difference.
Multiplex cytokine analysis
Multiplex cytokine analysis was performed using the
Human Cytokine Magnetic 30-Plex Panel (LHC6003M; Thermo Fisher
Scientific, Inc.). The assay was performed on the cell culture
supernatant of OVCAR3 cells collected at 48 h following treatment
with 50% hWJSC-CM, 10 µg/ml hWJSC-CL and 5 nM paclitaxel. The assay
was performed in a 96-well plate format, according to the
manufacturer's instructions. Briefly, the antibody-coated
polystyrene magnetic beads in solution (with different spectral
intensities) were vortexed for 30 sec and sonicated for 30 sec for
5 min (at 50Hz; 37°C). Then 25 µl of the antibody-coated
polystyrene magnetic beads was added to the 96 well flat bottom
plate and washed twice with 1X wash buffer. The standards (1:3
serial dilution) and samples (undiluted cell culture supernatants)
were prepared, added to the beads and incubated at room temperature
(RT) on an orbital shaker at 500 RPM for 2 h to enable the capture
of analytes. The plate was then incubated at RT with biotinylated
detection antibodies (100 µl/well) for 1 h, followed by
streptavidin-R-phycoerythrin antibodies (100 µl/well) for 30 min
(both antibodies are supplied with the kit-LHC6003M as 10X
concentration and were diluted to 1X using respective diluents).
Between incubations with different antibodies, the plate was washed
twice with wash buffer. All washing steps were performed using a
hand-held magnetic bottom in order to facilitate the retention of
the magnetic antibody beads. Finally, the plate was washed three
times, the beads resuspended in wash buffer and analysed using the
Luminex MAGPIX® instrument (Luminex Corporation, Austin,
TX, USA). The data obtained were analysed using the Luminex
xPONENT® multiplex assay analysis software
(v.4.2.1324.0, Luminex Corporation).
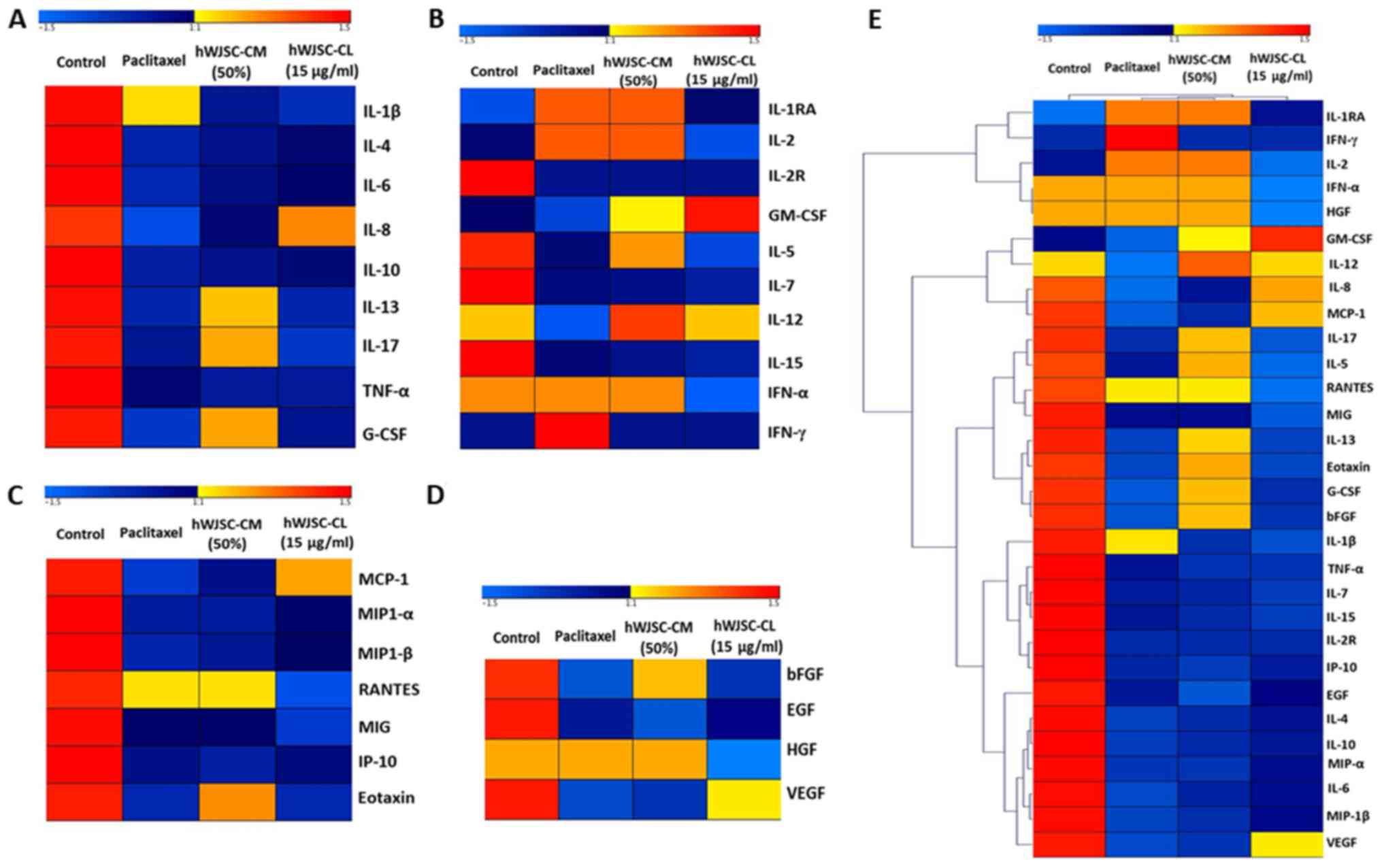
Heatmaps and hierarchical cluster
analysis
The differential expression of cytokines, chemokines
and growth factors, calculated based on the log2-fold
change between the control and the experimental groups, was used as
an input for Genesis Software (Graz University of Technology, Graz,
Austria) (19) to generate the
heatmaps. Furthermore, the hierarchical clustering function with
the complete linkage method in Genesis was used to generate the
hierarchical clusters of pro- and antitumour cytokines, chemokines
and growth factors.
Results
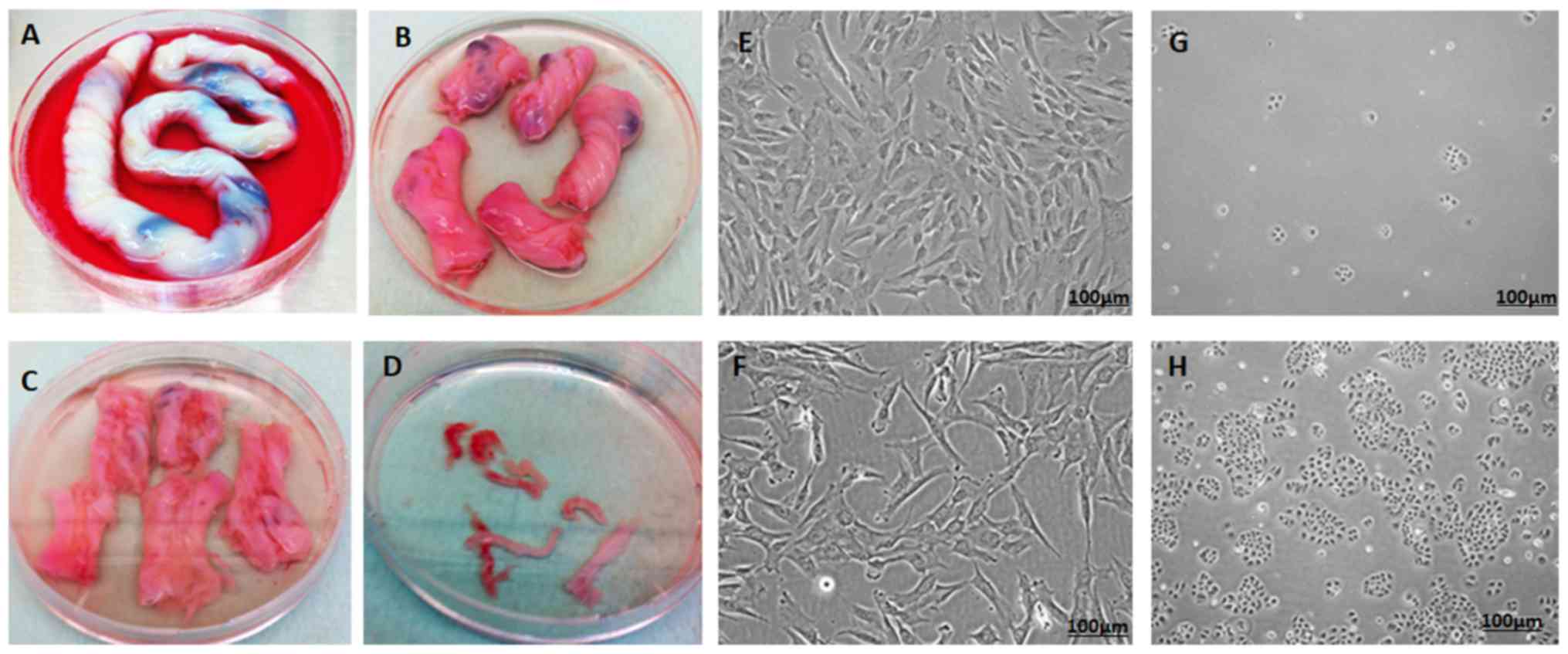
Derivation and culture of hWJSCs
The hWJSCs were successfully derived from all human
umbilical cords and their primary cultures were established. The
hWJSCs appeared as epitheliod cells that resembled short
fibroblasts in the initial passages, and transformed to long
fibroblasts in subsequent passages (Fig.
1A-F).
Culture of OVCAR3 cells
Following thawing, the OVCAR3 cells exhibited
minimal attachment and slow growth. However, with subsequent
passages they displayed improved proliferation rates and
demonstrated their characteristic epithelial morphology in culture
(Fig. 1G and H).
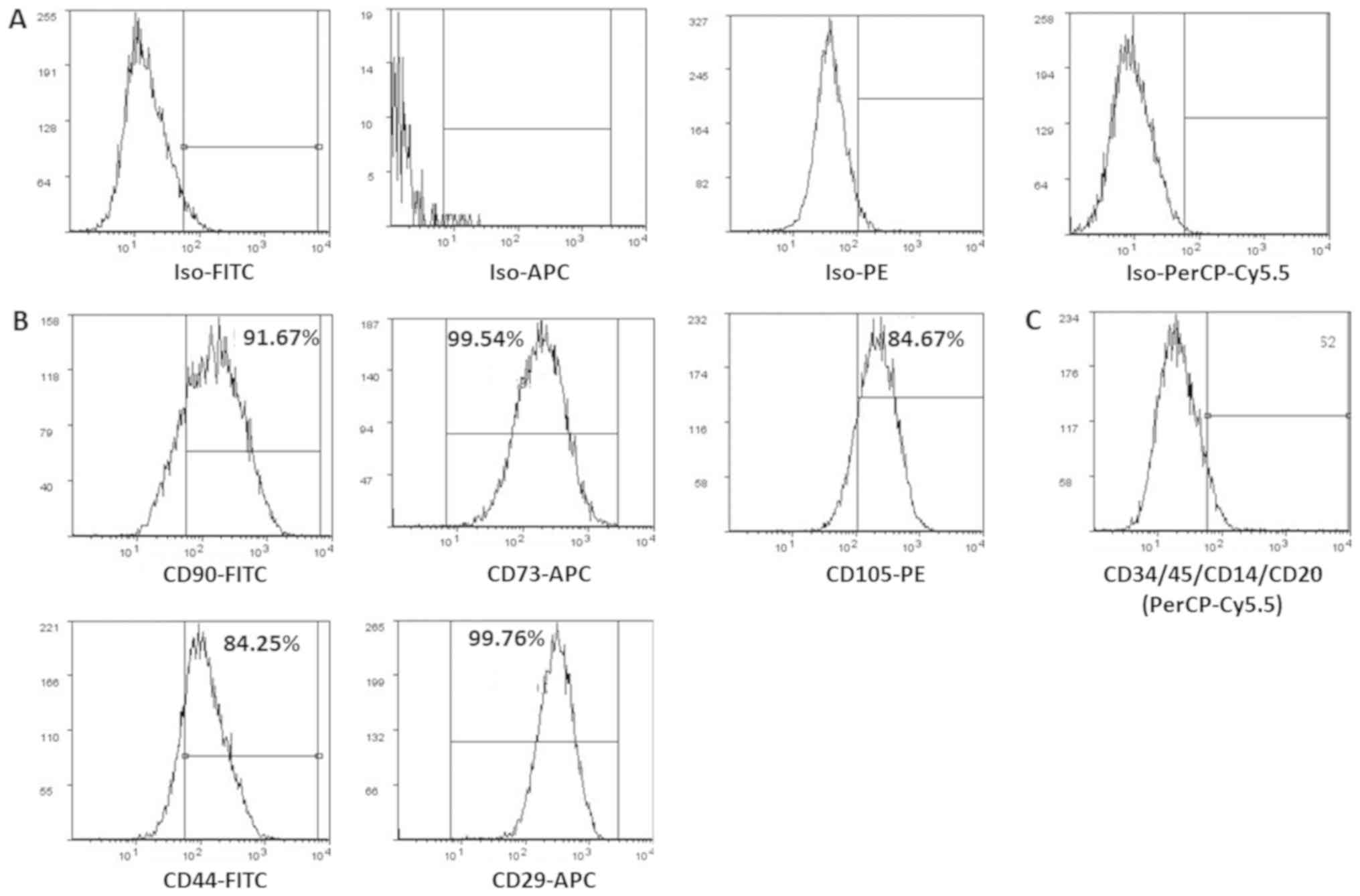
Surface marker characterization of
hWJSCs
The derived cells analyzed for CD markers expression
demonstrated high percentages of positive MSC related CD markers,
namely CD73 (99.54%), CD90 (91.67%), CD105 (84.67%), CD44 (84.25%)
and CD29 (99.76%) compared with respective isotype matched controls
(Fig. 2A and B). These cells were
negative for CD34 and CD45, the haematopoietic stem cell related CD
markers (Fig. 2C).
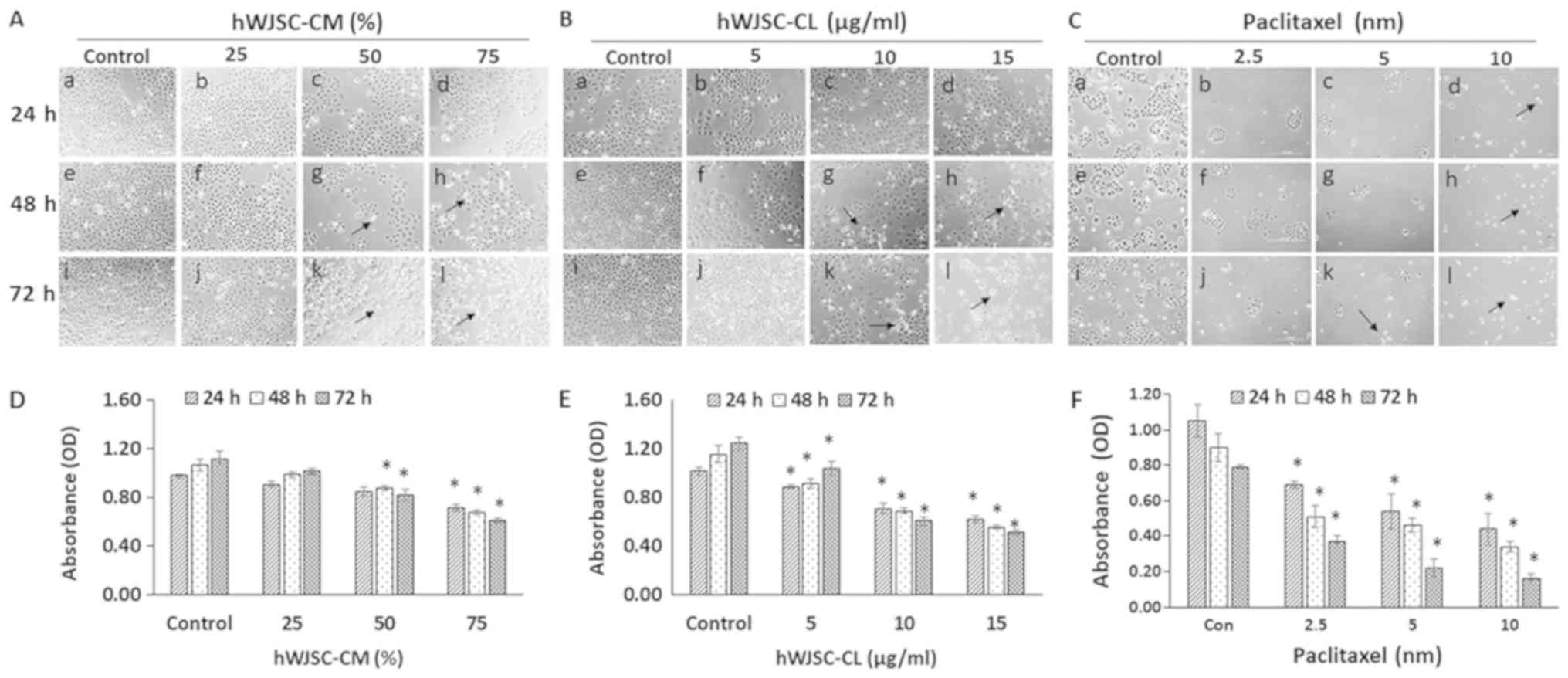
Morphology of OVCAR3 cells (phase
contrast microscopy)
The OVCAR3 cells exhibited various morphological
changes leading to cell death following exposure to hWJSC-CM (25,
50 and 75%); hWJSC-CL (5, 10 and 15 µg/ml) and paclitaxel (2.5, 5
and 10 nM) for 24, 48 and 72 h (Fig.
3A-C). An overall decrease in live cells was observed,
demonstrating morphological changes, including cell shrinkage,
membrane damage and cell death. These cellular changes were more
pronounced in the cells treated with paclitaxel, followed by those
treated with hWJSC-CL and hWJSC-CM. The morphological changes and
cell death were time- and concentration-dependent.
OVCAR3 cell proliferation (MTT
assay)
A concentration-dependent decrease in the
proliferation of the OVCAR3 cells was revealed following exposure
to hWJSC-CM (25, 50 and 75%); hWJSC-CL (5, 10 and 15 µg/ml) and
paclitaxel (2.5, 5 and 10 nM) for 24, 48 and 72 h. The mean
decrease in OVCAR3 cells observed following treatment with 25, 50
and 75% hWJSC-CM was 7.14, 13.27 and 26.53%, respectively at 24 h;
7.48, 17.76 and 36.45%, respectively at 48 h; and 8.93, 26.79 and
45.54%, respectively at 72 h (Fig.
3D). The mean decrease in OVCAR3 cells observed with the 50%
hWJSC-CM at 48 and 72 h, and the 75% hWJSC-CM atall three time
points was statistically significant (P<0.05) compared with the
control.
The mean decrease in OVCAR3 cells following
treatment with 5, 10 and 15 µg/ml hWJSC-CL was 12.75, 30.39 and
39.22%, respectively at 24 h; 20.69, 40.52 and 51.72%, respectively
at 48 h; and 16.80, 51.20 and 58.57%, respectively at 72 h
(Fig. 3E). The mean decrease
observed with all three concentrations of hWJSC-CL at all three
time points was statistically significant (P<0.05) compared with
the control.
The mean decrease in OVCAR3 cells observed with 2.5,
5 and 10 nM paclitaxel was 34.29, 48.57 and 58.10%, respectively at
24 h; 43.33, 48.89 and 62.22%, respectively at 48 h; and 53.16,
72.15 and 79.75%, respectively at 72 h (Fig. 3F). The mean decrease following
treatment with all three concentrations of paclitaxel at all three
time points was statistically significant (P<0.05) compared with
the control.
Cytokine secretion profile in OVCAR3
cells treated with hWJSC extracts
The analysis of the cell culture supernatant of
OVCAR3 cells following treatment with hWJSC-CM (50%), hWJSC-CL (10
µg/ml) and paclitaxel (5 nM) for 48 h, demonstrated a decrease in
the secreted cytokines, chemokines and growth factors compared with
the untreated control.
The levels of cytokines that are reported to be
involved in tumour growth, invasion and migration, namely
interleukin (IL)-1β, IL-4, IL-6, IL-8, IL-10, IL-13, IL-17,
interferon (IFN)-γ, tumour necrosis factor α (TNF-α) and
granulocyte colony-stimulating factor (G-CSF), were decreased
following treatment with hWJSC extracts and paclitaxel (Fig. 4). The mean decrease following
treatment with 5 nM paclitaxel, 50% hWJSC-CM and 10 µg/ml hWJSC-CL
was as follows: IL-1β by 14.73, 22.33 and 26.17%; IL-4 by 55.56,
50.48 and 45.40%; IL-6 by 92.55, 87.38 and 83.54%; IL-8 by 95.61,
81.08 and 46.97%; IL-10 by 73.78, 69.90 and 66.16%; IL-13 by 42.74,
24.51 and 42.74%; IL-17 by 15.27, 7.60 and 19.17%; TNF-α by 35.59,
46.23 and 46.23%; and G-CSF by 19.73, 7.55 and 15.39% respectively.
Of these, the decrease in IL-1β with hWJSC-CL alone; IL-4, IL-6,
IL-8, IL-10, IL-13, IL-17 and TNF-α with paclitaxel, hWJSC-CM and
hWJSC-CL; and G-CSF with paclitaxel alone, were statistically
significant (P<0.05) compared with the control.
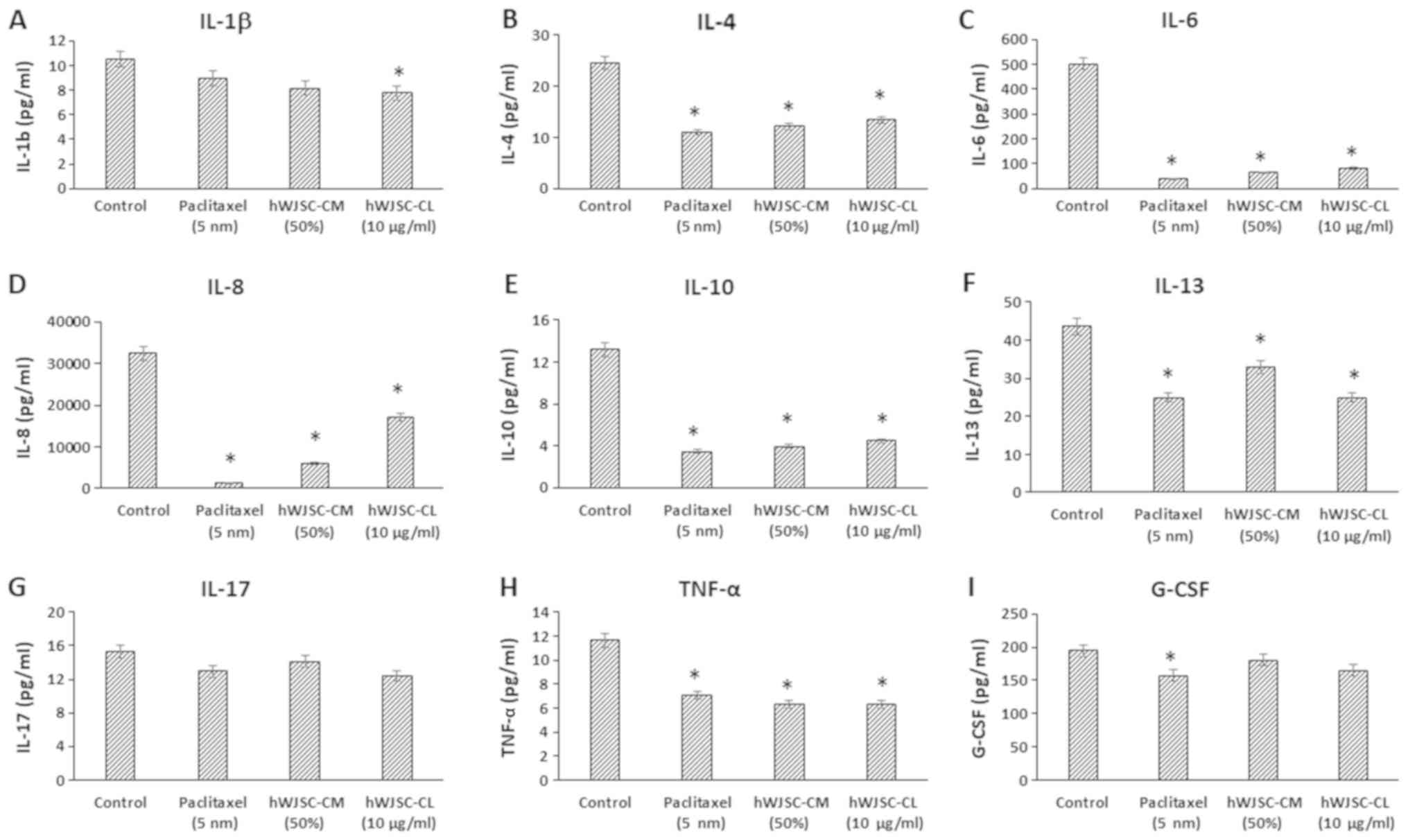 | Figure 4.Expression levels of cytokines in the
cell culture supernatant of OVCAR3 cells following treatment with
hWJSC-CM (50%), hWJSC-CL (10 µg/ml) and paclitaxel (5 nm) for 48 h,
and analyzed using multiplex cytokine assay. The following
cytokines namely, (A) IL-1β, (B) IL-4, (C) IL-6, (D) IL-8, (E)
IL-10, (F) IL-13, (G) IL-17, (H) TNF-α and (I) G-CSF that are
reported to increase tumour growth and progression were decreased.
The values are expressed as mean ± SEM of three different
experiments. *P<0.05 vs. the control. hWJSC-CM, human Wharton's
jelly stem cells conditioned medium; hWJSC-CL, human Wharton's
jelly stem cell lysate. |
The levels of cytokines that are reported to exhibit
antitumour effects, namely IL-1 receptor antagonist (IL-1RA), IL-2,
IL-2 receptor (IL-2R), IL-5, IL-7, IL-12, IL-15, IFN-α, IFN-γ and
granulocyte-macrophage colony-stimulating factor (GM-CSF), either
increased or decreased following treatment with hWJSC extracts and
paclitaxel (Fig. 5). Of these, only
the changes in IL-1RA with paclitaxel (32.47% increase), hWJSC-CM
(32.47% increase) and hWJSC-CL (16.47% increase); GM-CSF with
hWJSC-CL (35.08% increase); and IL-5 with hWJSC-CL (38.66%
decrease) were statistically significant (P<0.05) compared with
the control.
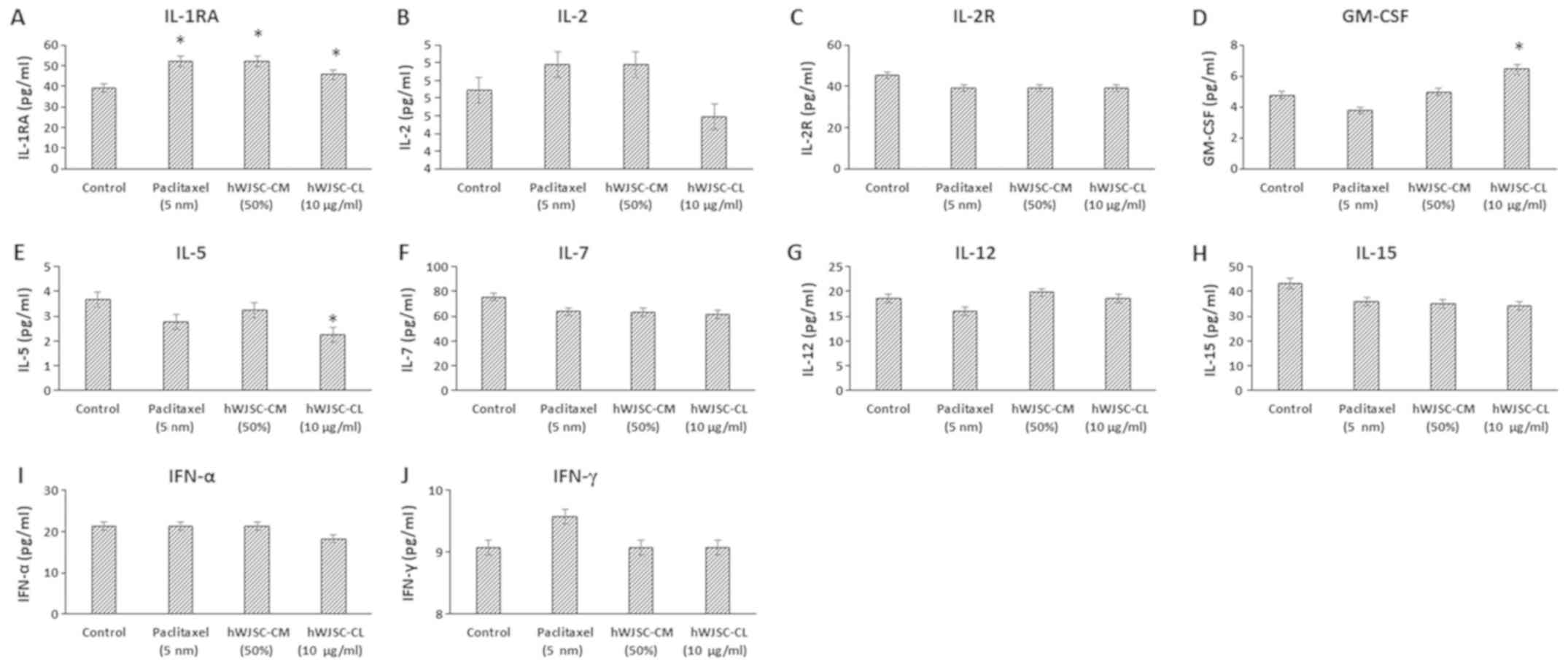 | Figure 5.Expression levels of cytokines in the
cell culture supernatant of OVCAR3 cells following treatment with
hWJSC-CM (50%), hWJSC-CL (10 µg/ml) and paclitaxel (5 nm) for 48 h,
and analyzed using multiplex cytokine assay. The following
cytokines namely, (A) IL-1RA, (B) IL-2, (C) IL2R, (D) GM-CSF, (E)
IL-5, (F) IL-7, (G) IL-12, (H) IL-15, (I) IFN-α and (J) IFN-γ were
either increased or mildly decreased. The values are expressed as
mean ± SEM of three different experiments. *P<0.05 vs. the
control. hWJSC-CM, human Wharton's jelly stem cells conditioned
medium; hWJSC-CL, human Wharton's jelly stem cell lysate. |
The levels of chemokines that are primarily
associated with tumour progression, namely monocyte chemoattractant
protein 1 (MCP-1); macrophage inflammatory protein (MIP)-1α;
MIP-1β; Regulated Upon Activation, Normally T-Expressed, and
Secreted (RANTES); monokine induced by INF-γ (MIG); INF-γ induced
protein (IP-10) and Eotaxin, decreased following treatment with
hWJSC extracts and paclitaxel (Fig.
6). The mean decrease was as follows: MCP-1 by 55.36, 43.87 and
24.20%; MIP-1α by 50.65, 50.65 and 42.13%; MIP-1β by 46.29, 42.27
and 34.31%; RANTES by 3.47, 3.47 and 8.39%; MIG by 27.45, 27.43 and
40.78%; IP-10 by 15.90, 17.73 and 14.90%; and Eotoxin by 6.17, 2.04
and 6.18%, following treatment with 5 nM paclitaxel, 50% hWJSC-CM
and 10 µg/ml hWJSC-CL, respectively. Of these, only the decrease in
MCP-1, MIP-1α and MIP-1β with paclitaxel, hWJSC-CM and hWJSC-CL;
and RANTES and MIG with hWJSC-CL was statistically significant
(P<0.05) compared with the control.
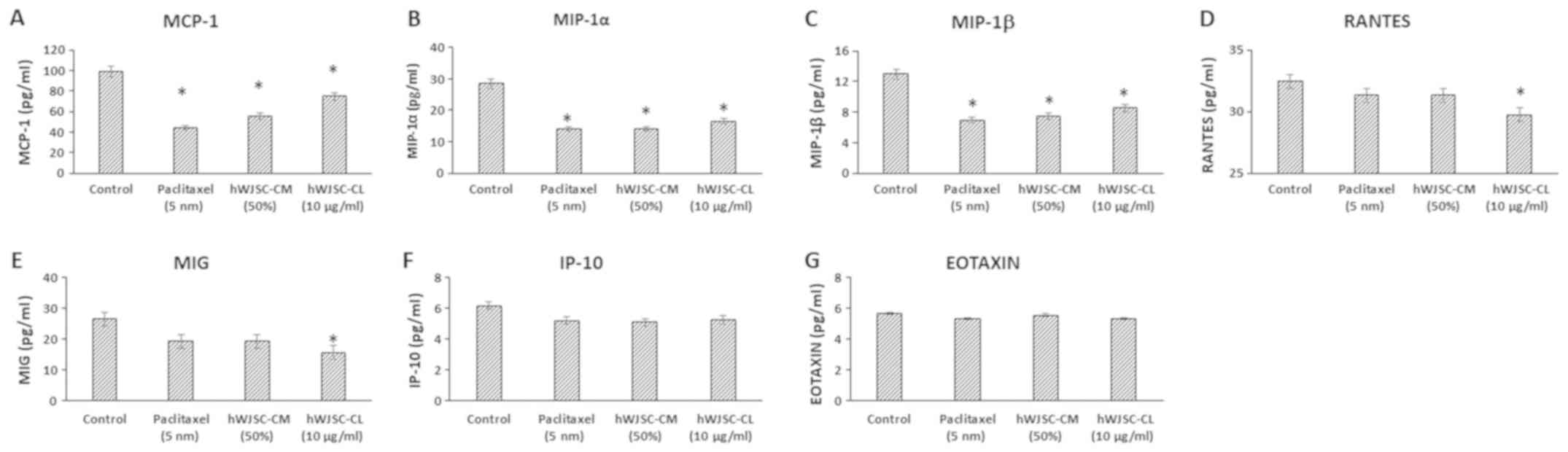 | Figure 6.Expression levels of chemokines in
the cell culture supernatant of OVCAR3 cells following treatment
with hWJSC-CM (50%), hWJSC-CL (10 µg/ml) and paclitaxel (5 nm) for
48 h, and analyzed using multiplex cytokine assay. The following
chemokines namely, (A) MCP-1, (B) MIP-1α, (C) MIP-1β, (D) RANTES,
(E) MIG, (F) IP-10 and (G) Eotaxin, that are reported to increase
tumour growth and progression were decreased. The values are
expressed as mean ± SEM of three different experiments. *P<0.05
vs. the control. hWJSC-CM, human Wharton's jelly stem cells
conditioned medium; hWJSC-CL, human Wharton's jelly stem cell
lysate. |
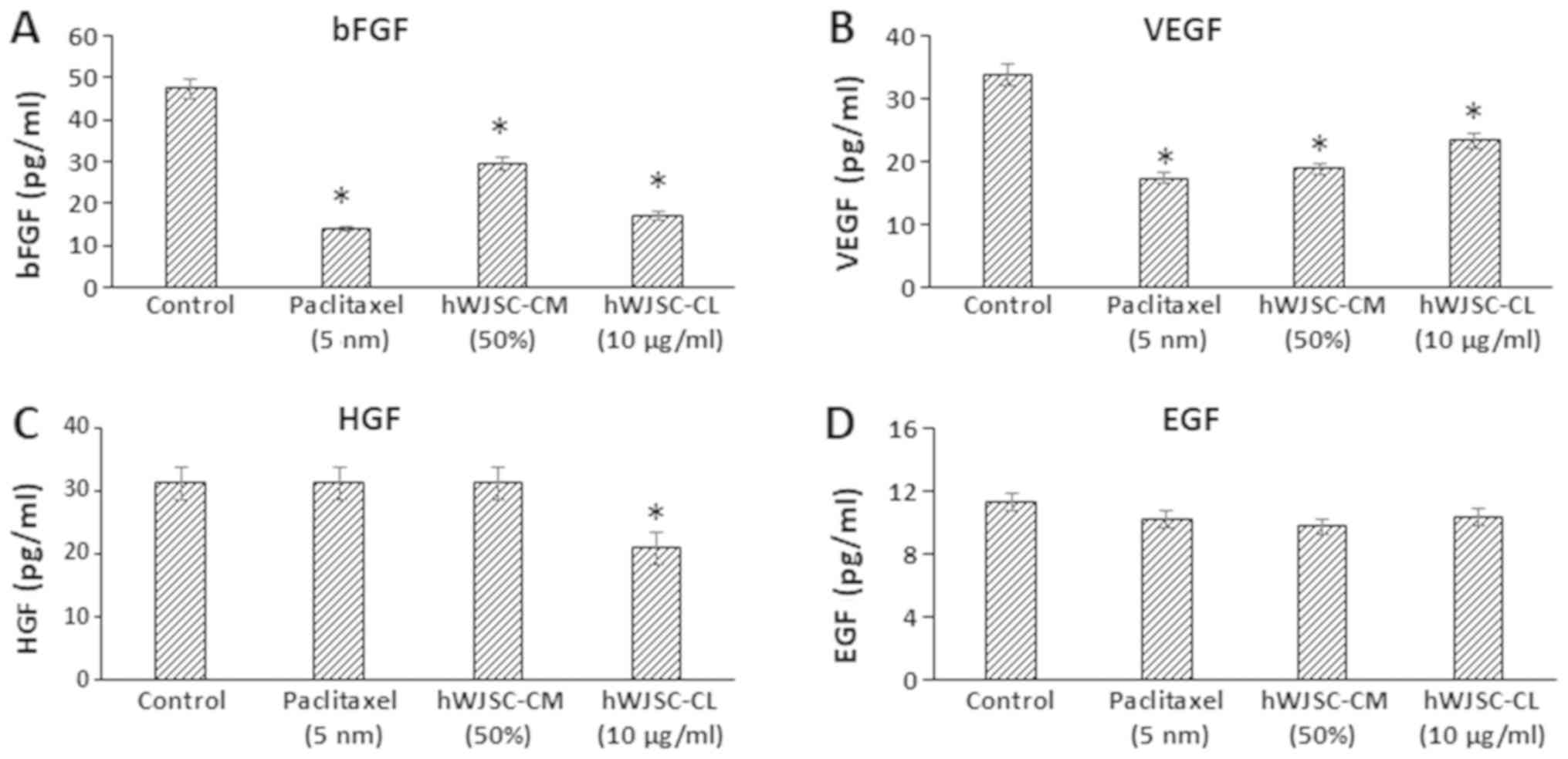
The majority of the growth factors known to support
oncogenic activity, namely bFGF, vascular endothelial growth factor
(VEGF), hepatocyte growth factor (HGF) and epidermal growth factor
(EGF), decreased following treatment with hWJSC extracts and
paclitaxel (Fig. 7). The mean
decrease was as follows: bFGF by 70.65, 37.41 and 63.98%; VEGF by
48.92, 44.49 and 31.20%; and EGF by 9.59, 13.68 and 8.34% following
treatment with 5 nM paclitaxel, 50% hWJSC-CM and 10 µg/ml hWJSC-CL,
respectively. HGF only exhibited a decrease by 33.11% with
hWJSC-CL. Of these, only the changes observed with bFGF, VEGF and
HGF were statistically significant (P<0.05) compared with the
control.
Heatmaps and hierarchical cluster
analysis
The mean expression values of cytokines in OVCAR3
cells treated with 50% hWJSC-CM, 10 µg/ml hWJSC-CL and 5 nM
paclitaxel displayed variable fold changes as depicted by heatmaps,
where the blue and red colours indicate the lower and higher
expression limits, respectively (Fig.
8A-D). Certain oncogenic cytokines revealed high fold changes
with paclitaxel (IL-1β alone); hWJSC-CM (G-CSF > IL-17 >
IL-13); and hWJSC-CL (IL-8 alone), whereas other cytokines revealed
moderate to low fold changes (Fig.
8A). Certain antitumour cytokines exhibited high fold changes
with paclitaxel (IFN-γ>IL-1RA> IL-2>IFN-α); hWJSC-CM
(IL-12>IL-1RA>IL-2>IFN-α >IL-5>GM-CSF); and hWJSC-CL
(GM-CSF>IL-12), whereas other cytokines revealed moderate to low
fold changes (Fig. 8B). Certain
chemokines exhibited high fold changes with paclitaxel (RANTES
alone); hWJSC-CM (RANTES >Eotaxin); and hWJSC-CL (MCP-1 alone),
whereas other chemokines revealed moderate to low fold changes
(Fig. 8C). Certain growth factors
exhibited higher fold changes with paclitaxel (HGF alone); hWJSC-CM
(HGF>bFGF); and hWJSC-CL (VEGF alone), whereas other growth
factors revealed moderate to low fold changes (Fig. 8D). Hierarchical clustering was
determined based on the level of fold changes exhibited by the
cytokines, chemokines and growth factors, and the distances within
and between each cluster (Fig. 8E).
A total of 29 clusters consisted of three major clusters linked to
7 small sub-clusters, of which each was linked to ≥2 closely
associated cytokines, chemokines or growth factors (Fig. 7E).
Discussion
Tumour cells develop abilities to escape the immune
surveillance of the body and manage to proliferate, invade local or
adjacent tissues, and migrate to distant sites. MSCs have gained a
lot of attraction as an anticancer agent, with certain protocols
undergoing clinical trials (9). MSCs
can be modified to express antitumour cytokines, chemokines, growth
factor antagonists and apoptosis-inducing agents. Given their
tumour tropism, modified or naïve MSCs have been used against a
number of tumours, including melanoma, colon cancer, hepatic
cancer, lung cancer, breast cancer, prostate cancer and ovarian
cancer (12,20). Compared with other currently
available MSCs, the hWJSCs isolated from within the human umbilical
cord present several advantages, including high proliferative
potential, long telomeres and multipotency (16). The present study demonstrated the
inhibition of the growth and proliferation of OVCAR3 cells in
vitro in freshly prepared hWJSC-CM and hWJSC-CL, which may
partly be mediated by the soluble factors/cytokines in the hWJSC
extracts.
Overall, cancer is an inflammatory condition, and
cell growth, differentiation, migration and signaling are regulated
by numerous molecules, including cytokines, chemokines and growth
factors. The cytokine expression pattern may vary according to the
tumour type, and they can serve as biomarkers for diagnosis and
prognosis (14). The present study
revealed a decrease in cytokines that are reported to have
cancer-promoting properties, namely IL-1β, IL-4, IL-6, IL-8, IL-10,
IL-13, IL-17, TNF-α and G-CSF. A prospective randomized
placebo-controlled multicenter trial that evaluated the association
between proinflammatory cytokines and cancer incidence identified
IL-1β, IL-6 and TNF-α to be associated with increased risk of
cancer (21). Cathespin, a cysteine
protease, promotes tumour growth and proliferation, and IL-4
induces cathespin activity in tumour-associated macrophages
(22). High levels of IL-6 and IL-8
were dectected in patients with EOC and were associated with poor
prognosis and short disease-free survival time (23). Increased levels of IL-10 have been
reported in patients with ovarian cancer, and IL-10, being
immunosuppresive, contributes to the disease progression (24). IL-13 enhanced the invasion of cancer
cells that were IL-13 receptor subunit α-2 (IL-13Rα2)+,
but not IL-13Rα2−, in a murine model of human pancreatic
cancer (25). Increased levels of
TNF-α in the tumour environment were associated with ovarian cancer
progression in humans and mice in a 1L-17-dependent manner
(26). Furthermore, the treatment of
breast cancer MDA-MB231 and MDA-MB435 cell lines with IL-17
resulted in enhanced Matrigel invasion (27). The G-CSF receptor was highly
expressed in serous epithelial ovarian tumour and the stimulation
of G-CSFR+ OVCA429 and TOV21 G cells with G-CSF enhanced
cell migration, mediated by the tyrosine-protein kinase JAK2/signal
transducer and activator of transcription 3 signaling pathway
(28). There appears to be efficient
signaling and interaction between the oncogenic cytokines, and
their inhibiton, or a decrease in their expression, as accomplished
by hWJSC extracts, may be beneficial.
In the present study, the cytokines associated with
antitumour effects, namely IL-1RA, IL-2, IL2R, GM-CSF, IL-5, IL-7,
IL-12, IL-15 IFN-α and IFN-γ, either increased or slightly
decreased following treatment with hWJSC-CM and hWJSC-CL. Whereas
IL-1 cytokines promote tumour growth and progression, IL-1RA, a
member of the IL-1 family, blocks the IL-1 receptor competitively
and supports tumour inhibiton (29).
It has previously been reported that IL-1RA also inhibits the
expression of VEGF in colorectal carcinoma (30). Adoptive T cell therapy appears to be
a promising strategy in the inhibition of EOC. Tumour infiltrating
lymphocytes (TILs) were expanded in freshly resected ovarian
tumours using a combination of IL-2, anti-cluster of
differentiation (CD)3 and anti-CD28 magnetic beads, and these TILs
demonstrated tumour-inhibitory effects (31). In a rodent model of orthoptic liver
tumour, a combination of IL-2 and GM-CSF administered
intratumourally or intravenously was more effective than IL-2
monotherapy against the tumour, and these effects were mediated by
CD8+ T cells, natural killer T cells and macrophages
(32). The efficacy of IL-2 depends
on its potential to expand regulatory T cells, and this is mediated
by its receptor, IL-2R, which directs IL-2 back to the cell surface
(33). IL-5 was demonstrated to be
predominantly expressed in benign ovarian neoplasms (14) and the antitumour effects of GM-CSF
were revealed to be mediated by cytokine receptors shared by IL3
and IL-5 (34). The levels of IL-7,
a cytokine essential for the adaptive immune response, were
significantly decreased in the ascites compared with the plasma of
patients with advanced ovarian cancer (35). Furthermore, the administration of
cytotoxic T lymphocytes cultured with IL-7 and IL-15 led to
regression of melanoma and mammary tumours in a murine model,
compared with those cultured with IL-12 alone (36). IFN-α and IFN-γ have been used
intraperitonealy (cytokine therapy) against ovarian cancer with
promising results; autologus monocytes infusion (cellular therapy)
has also been reported to have antitumour effects, therefore the
combination of cytokine and cellular therapy may help overcome
resistant tumours (37).
The chemokines MCP-1, MIP-1α, MIP-1β, RANTES, MIG,
IP-10 and Eotaxin, which are associated with tumour growth and
proliferation, decreased following treatment with hWJSC extracts in
the present study. MCP-1, MIP-1β and RANTES are highly expressed in
ovarian cancers having the presence of T cells intraepithelially or
in the tumour microenvironment, whereas IP-10 is expressed in
tumours even in the absence of tumour-infiltrating T cells
(38). MCP-1 and MIP-1α are
expressed in a number of tumour tissues and are associated with the
regulation of cancer progression (39). Increased overall survival was
associated with a high expression of MIG and IP-10 in high grade
serous ovarian cancer, and the tumour-suppresive effects were
mediated by the recruitment of tumour-infiltrating lymphocytes
(40).
The growth factors bFGF, EGF, HGF and VEGF are
implicated in tumour cell proliferation, growth and
differentiation. Combined treatment of VEGF and HGF, or VEGF, HGF
and EGF was revealed to increase telomerase activity in ovarian
cancer cell lines (41),
demonstrating the role of these growth factors in tumour survival
and progression. In the present study, the levels of the majority
of the cytokines, chemokines and growth factors that have oncogenic
effects decreased following treatment with hWJSC extracts.
In conclusion, the tumour microenvironment serves an
important role in tumor progression or inhibibtion via
cytokine regulation, and MSCs and/or their secretory products can
contribute to tumour inhibition by modifying molecular signalling
pathways. The higher fold changes observed in the heatmap with
anti- and pro-oncogenic chemokines and growth factors, and the
hierarchical clustering between them, indicate that hWJSC extracts
inhibit OVCAR3 cells in vitro in a coordinated manner,
mediated by cytokines. MSCs and their secretome are known to arrest
various tumours by epithelial mesenchymal transition inhibition,
immune regulation, extracellular matrix remodelling and through
paracrine effects (42). Given the
inhibitory effects of tumour-promoting cytokines, the hWJSC
extracts may be useful in the inhibition of solid tumours. Unlike
other existing stem cell types the hWJSCs has several advantages as
they, (i) can be harvested in abundance without infliciting any
pain as they are derived from umbilical cords obtained following
delivery; (ii) are highly proliferative with wide differentiation
potenital; (iii) have no/less ethical constraints compared to human
embryonic stem cells; (iv) are hypo-immunogeneic and
non-tumorigenic (43). However,
evaluation on a single cancer line is a limitiation to the present
study and additional studies on different types of cancer cell
lines will be required to understand the real potential of hWJSCs
in cancer inhibition.
Acknowledgements
The authors acknowledge with thanks the King
Abdulaziz City for Science and Technology (KACST), Riyadh, Kingdom
of Saudi Arabia for the financial support (grant no. AT-34-330). We
also acknowledge the Science and Technology unit, King Abdulaziz
University for the technical support; the Department of Obstetrics
and Gynaecology, King Abdulaziz University Hospital for providing
the clinical material and the Centre of Excellence in Genomic
Medicine Research (CEGMR) for providing the logistics.
Funding
The present study was funded by the King Abdulaziz
City for Science and Technology (KACST), Riyadh, Kingdom of Saudi
Arabia (grant no. AT-34-330).
Availability of data and materials
All data generated and analysed in the present study
are included in this article.
Authors' contributions
GK was involved in conceptualization, intellectual
contribution, statistical evaluation and manuscript writing. KHWS
and NA are the clinicians and were involved in providing clinical
materials/information and intellectual support. RK, FA, MR, MIN,
PNP and MAQ were involved in assisting the experimental work, data
analysis and manuscript editing.
Ethics approval and consent to
participate
The present study was performed in accordance with
the recommendations of the Bioethics Committee of the King
Abdulaziz University, Jeddah, Saudi Arabia. All subjects provided
written informed consent, in accordance with the Declaration of
Helsinki. The protocol for the derivation and use of hWJSCs, and
the commercial human ovarian cancer cell line (OVCAR3) was approved
by the Bioethics Committee of the King Abdulaziz University
(approval no. 33-15/KAU).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang JY, Yoshihara K, Tanaka K, Hatae M,
Masuzaki H, Itamochi H; Cancer Genome Atlas (TCGA) Research
Network, ; Takano M, Ushijima K, Tanyi JL, et al: Predicting time
to ovarian carcinoma recurrence using protein markers. J Clin
Invest. 123:3740–3750. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosenberg L, Palmer JR, Zauber AG,
Warshauer ME, Lewis JL Jr, Strom BL, Harlap S and Shapiro S: A
case-control study of oral contraceptive use and invasive
epithelial ovarian cancer. Am J Epidemiol. 139:654–661. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barbieri F, Bajetto A and Florio T: Role
of chemokine network in the development and progression of ovarian
cancer: A potential novel pharmacological target. J Oncol.
2010:4269562010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren L, Xiao L, Hu J, Li Z and Wang Z: MDR1
and MDR3 genes and drug resistance to cisplatin of ovarian cancer
cells. J Huazhong Univ Sci Technolog Med Sci. 27:721–724. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu J, Qu Z, Fei ZW, Wu JH and Jiang CP:
Role of stem cell-derived exosomes in cancer. Oncol Lett.
13:2855–2866. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mohr A and Zwacka R: The future of
mesenchymal stem cell-based therapeutic approaches for cancer-From
cells to ghosts. Cancer Lett. 414:239–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
D'souza N, Burns JS, Grisendi G, Candini
O, Veronesi E, Piccinno S, Horwitz EM, Paolucci P, Conte P and
Dominici M: MSC and tumors: Homing, differentiation, and secretion
influence therapeutic potential. Adv Biochem Eng Biotechnol.
130:209–266. 2012.
|
|
11
|
Sohni A and Verfaillie CM: Mesenchymal
stem cells migration homing and tracking. Stem Cells Int.
2013:1307632013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rhee KJ, Lee JI and Eom YW: Mesenchymal
stem cell-mediated effects of tumor support or suppression. Int J
Mol Sci. 16:30015–30033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rea IM, Gibson DS, McGilligan V, McNerlan
SE, Alexander HD and Ross OA: Age and age-related diseases: Role of
inflammation triggers and cytokines. Front Immunol. 9:5862018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jammal MP, Martins-Filho A, Silveira TP,
Murta EF and Nomelini RS: Cytokines and prognostic factors in
epithelial ovarian cancer. Clin Med Insights Oncol. 10:71–76. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fong CY, Subramanian A, Biswas A,
Gauthaman K, Srikanth P, Hande MP and Bongso A: Derivation
efficiency, cell proliferation, freeze-thaw survival, stem-cell
properties and differentiation of human Wharton's jelly stem cells.
Reprod Biomed Online. 21:391–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gauthaman K, Yee FC, Cheyyatraivendran S,
Biswas A, Choolani M and Bongso A: Human umbilical cord Wharton's
jelly stem cell (hWJSC) extracts inhibit cancer cell growth in
vitro. J Cell Biochem. 113:2027–2039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gauthaman K, Abbas M, Gari M, Alsehli H,
Kadam R, Alkaff M, Chaudhary A, Al-Qahtani M, Abuzenadah A,
Kafienah W and Mobasheri A: Pellet culture protects bone marrow
derived mesenchymal stem cells from the effects of heat shock.
Front Physiol. 7:1802016.PubMed/NCBI
|
|
19
|
Sturn A, Quackenbush J and Trajanoski Z:
Genesis: Cluster analysis of microarray data. Bioinformatics.
18:207–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HY and Hong IS: Double-edged sword of
mesenchymal stem cells: Cancer-promoting versus therapeutic
potential. Cancer Sci. 108:1939–1946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trompet S, de Craen AJ, Mooijaart S, Stott
DJ, Ford I, Sattar N, Jukema W and Westendorp RG: High innate
production capacity of proinflammatory cytokines increases risk for
death from cancer: Results of the PROSPER study. Clin Cancer Res.
15:7744–7748. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gocheva V, Chen X, Peters C, Reinheckel T
and Joyce JA: Deletion of cathepsin H perturbs angiogenic
switching, vascularization and growth of tumors in a mouse model of
pancreatic islet cell cancer. Biol Chem. 391:937–945. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dobrzycka B, Mackowiak-Matejczyk B,
Terlikowska KM, Kulesza-Bronczyk B, Kinalski M and Terlikowski SJ:
Serum levels of IL-6, IL-8 and CRP as prognostic factors in
epithelial ovarian cancer. Eur Cytokine Netw. 24:106–113.
2013.PubMed/NCBI
|
|
24
|
Mustea A, Könsgen D, Braicu EI, Pirvulescu
C, Sun P, Sofroni D, Lichtenegger W and Sehouli J: Expression of
IL-10 in patients with ovarian carcinoma. Anticancer Res.
26:1715–1718. 2006.PubMed/NCBI
|
|
25
|
Fujisawa T, Joshi B, Nakajima A and Puri
RK: A novel role of interleukin-13 receptor alpha2 in pancreatic
cancer invasion and metastasis. Cancer Res. 69:8678–8685. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Charles KA, Kulbe H, Soper R,
Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P,
Thompson RG, Kollias G, Smyth JF, et al: The tumor-promoting
actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in
mice and humans. J Clin Invest. 119:3011–3023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H,
Yoshida M, Naito AT, Nishi J, Ueno H, Umezawa A, et al: IGFBP-4 is
an inhibitor of canonical Wnt signalling required for
cardiogenesis. Nature. 454:345–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar J, Fraser FW, Riley C, Ahmed N,
McCulloch DR and Ward AC: Granulocyte colony-stimulating factor
receptor signalling via Janus kinase 2/signal transducer and
activator of transcription 3 in ovarian cancer. Br J Cancer.
110:133–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lewis AM, Varghese S, Xu H and Alexander
HR: Interleukin-1 and cancer progression: The emerging role of
interleukin-1 receptor antagonist as a novel therapeutic agent in
cancer treatment. J Transl Med. 4:482006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Konishi N, Miki C, Yoshida T, Tanaka K,
Toiyama Y and Kusunoki M: Interleukin-1 receptor antagonist
inhibits the expression of vascular endothelial growth factor in
colorectal carcinoma. Oncology. 68:138–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Owens GL, Price MJ, Cheadle EJ, Hawkins
RE, Gilham DE and Edmondson RJ: Ex vivo expanded
tumour-infiltrating lymphocytes from ovarian cancer patients
release anti-tumour cytokines in response to autologous primary
ovarian cancer cells. Cancer Immunol Immunother. 67:1519–1531.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang CJ, Chen YH, Huang KW, Cheng HW,
Chan SF, Tai KF and Hwang LH: Combined GM-CSF and IL-12 gene
therapy synergistically suppresses the growth of orthotopic liver
tumors. Hepatology. 45:746–754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su EW, Moore CJ, Suriano S, Johnson CB,
Songalia N, Patterson A, Neitzke DJ, Andrijauskaite K,
Garrett-Mayer E, Mehrotra S, et al: IL-2Rα mediates temporal
regulation of IL-2 signaling and enhances immunotherapy. Sci Transl
Med. 7:311ra1702015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Broughton SE, Dhagat U, Hercus TR, Nero
TL, Grimbaldeston MA, Bonder CS, Lopez AF and Parker MW: The
GM-CSF/IL-3/IL-5 cytokine receptor family: From ligand recognition
to initiation of signaling. Immunol Rev. 250:277–302. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giuntoli RL II, Webb TJ, Zoso A, Rogers O,
Diaz-Montes TP, Bristow RE and Oelke M: Ovarian cancer-associated
ascites demonstrates altered immune environment: Implications for
antitumor immunity. Anticancer Res. 29:2875–2884. 2009.PubMed/NCBI
|
|
36
|
Gao J, Zhao L, Wan YY and Zhu B: Mechanism
of action of IL-7 and its potential applications and limitations in
cancer immunotherapy. Int J Mol Sci. 16:10267–10280. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Green DS, Nunes AT, Annunziata CM and Zoon
KC: Monocyte and interferon based therapy for the treatment of
ovarian cancer. Cytokine Growth Factor Rev. 29:109–115. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zsiros E, Duttagupta P, Dangaj D, Li H,
Frank R, Garrabrant T, Hagemann IS, Levine BL, June CH, Zhang L, et
al: The ovarian cancer chemokine landscape is conducive to homing
of vaccine-primed and CD3/CD28-costimulated T cells prepared for
adoptive therapy. Clin Cancer Res. 21:2840–2850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding L, Li B, Zhao Y, Fu YF, Hu EL, Hu QG,
Ni YH and Hou YY: Serum CCL2 and CCL3 as potential biomarkers for
the diagnosis of oral squamous cell carcinoma. Tumor Biol.
35:10539–10546. 2014. View Article : Google Scholar
|
|
40
|
Bronger H, Singer J, Windmüller C, Reuning
U, Zech D, Delbridge C, Dorn J, Kiechle M, Schmalfeldt B, Schmitt M
and Avril S: CXCL9 and CXCL10 predict survival and are regulated by
cyclooxygenase inhibition in advanced serous ovarian cancer. Br J
Cancer. 115:553–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bermudez Y, Aquino MM, Saunders BO,
Coppola D, Nicosia SV and Kruk PA: Cytokines modulate telomerase
activity in human ovarian cancer cell lines. AACR. 64:2242004.
|
|
42
|
Zhang C, Yang SJ, Wen Q, Zhong JF, Chen
XL, Stucky A, Press MF and Zhang X: Human-derived normal
mesenchymal stem/stromal cells in anticancer therapies. J Cancer.
8:85–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gauthaman K, Fong CY, Subramanian A,
Biswas A and Bongso A: ROCK inhibitor Y-27632 increases
thaw-survival rates and preserves stemness and differentiation
potential of human Wharton's jelly stem cells after
cryopreservation. Stem Cell Rev. 6:665–676. 2010. View Article : Google Scholar : PubMed/NCBI
|