Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of
the most prevalent types of head and neck malignancies, and it has
an estimated incidence of 5.1–10 cases per 100,000 worldwide
(1). The majority of patients (~60%)
present with stage III or stage IV disease at diagnosis (2). LSCC has been one of the few cancer
types with a decreased survival outcome over the past 40 years in
the United States of America (2,3). Due to
the large population, China has reported ~13.0% of laryngeal cancer
(LC) cases and 14.7% of LC-specific mortalities within the global
LC population (4).
The treatment modalities for LSCC include surgery,
chemotherapy, radiotherapy or the combination of these therapies
(1,5). Adjuvant chemoradiotherapy (CRT) is
recommended for resected locally advanced head and neck cancer with
positive surgical margins or extracapsular extension, according to
2 randomized clinical trials: The Intergroup Radiation Therapy
Oncology Group (RTOG) 95–01 and European Organization for Research
and Treatment of Cancer 22931 (6–8).
Advanced LSCC has failed to demonstrate a satisfactory prognosis
despite the progress in the diagnosis and treatment of LSCC, and
the optimal treatment modality continues to be debated (9–11).
Previous study on the treatment of LSCC has focused
on the comparison between primary radiotherapy and CRT (12), and there have been few studies
performed to compare surgery and surgery + adjuvant CRT for
patients with advanced LSCC. The aim of the present study was to
determine the clinical effect of adjuvant CRT for patients with
advanced LSCC who underwent initial surgeries in a single
institution over a 10-year post-treatment follow-up.
Materials and methods
Patient selection
The medical records of patients with previously
untreated LSCC were retrospectively reviewed. The patients were
recruited between January 2005 and December 2010 from the
Department of Otorhinolaryngology-Head and Neck Surgery, Eye, Ear,
Nose and Throat Hospital of the Fudan University (Shanghai, China).
The total number of patients with complete medical records during
the time interval was 235. The diagnosis and treatment of all
patients with LSCC were based on collaboration within a
multidisciplinary medical team. Data regarding patient
demographics, tumor characteristics and treatment modalities were
obtained by reviewing medical records. Approval from the
Institutional Review Board of the Eye, Ear, Nose and Throat
Hospital of Fudan University (Shanghai, China) was obtained for the
present study. All patients gave their full consent to participate
in the present study, and a written consent form was obtained from
each patient.
Treatment and follow-up
All the patients were initially treated with total
laryngectomy and neck dissection. The type of neck dissection
depended on the particular clinical situation. Adjuvant CRT was
primarily performed in cases of pN2+ stage disease (according to
the AJCC TNM staging system, 7th edition) or in cases of positive
surgical margins and/or extracapsular spread in the pathological
examination (13). Physical
examination and computerized tomography were performed for regular
follow-up if required. For the first 2 years, follow-up was
performed every 1–3 months. Subsequently, it was performed every
4–6 months for the third year. Following that, the follow-ups
continued annually until the end of the study period. No patients
were lost to follow-up.
Statistical analysis
Descriptive statistics were compiled to characterize
the patients receiving surgery vs. surgery + adjuvant CRT. The
differences between these two groups were evaluated using a
χ2 test or Fisher's exact test for categorical
variables. Time-to-event was measured from the date of initial
surgical resection. Outcome measures were laryngeal cancer-specific
survival (CSS), overall survival (OS) and disease-free survival
(DFS). Patients were censored at the time of their last follow-up.
Survival times were calculated from the day of surgery to the date
of occurrence of an event or from the date of the last follow-up.
Survival outcomes were calculated using the Kaplan-Meier method.
The differences between survival curves were analyzed using a
log-rank test. Univariate and multivariate analyses of survival
outcomes were accomplished using Cox proportional hazards modeling.
Variables with P<0.05 in the univariate analysis were entered
into the multivariate analysis to determine independent indicators
of treatment outcome. Propensity-score matching (PSM) was then
performed to decrease the effects of potential confounding factors:
One-to-one propensity matching without replacement was completed
using the nearest-neighbor matching algorithm (14,15).
Analyses were performed using the SPSS v24.0 statistical software
package (IBM Corporation, Armonk, NY, USA) and Stata 14.0
(StataCorp LP, College Station, TX, USA). All statistics were
double-sided, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A total of 232 patients with LSCC [230 males (99.2%)
and 2 females (0.8%)] were included in the present study (Table I). The median age was 61.0 years
(range, 36–82 years) and 59.1% (137 patients) were ≥60 years of
age. At diagnosis, 83 patients (35.8%) were diagnosed with stage
III disease, while 149 patients (64.2%) were diagnosed with stage
IV disease. N classification included N0 (n=45; 19.4%), N1 (n=61;
26.3%), N2 (n=108; 46.6%) and N3 (n=18; 7.8%). In total, 167
patients received surgery, and 65 patients received surgery +
adjuvant CRT. An increased number of patients with N+ disease
received adjuvant CRT compared with those patients with N0 disease
(31.0 vs. 18.4%). Of all the 232 patients, 25 patients exhibited
positive surgical margins and/or extracapsular spread, and they
were all in the surgical + adjuvant CRT group.
 | Table I.Baseline patient characteristics in
the 232 unmatched patients with resected laryngeal cancer who had
undergone surgery alone or surgery with adjuvant CRT. |
Table I.
Baseline patient characteristics in
the 232 unmatched patients with resected laryngeal cancer who had
undergone surgery alone or surgery with adjuvant CRT.
|
| Treatment groups, no.
of patients (%) |
|
|---|
|
|
|
|
|---|
| Variable | Total | Surgery | Surgery + CRT | P-value |
|---|
| Age, years |
|
|
| 0.681 |
|
<60 | 95 (40.9) | 67 (40.1) | 28 (43.1) |
|
|
≥60 | 137 (59.1) | 100 (59.9) | 37 (56.9) |
|
| Sex |
|
|
| 1.000 |
|
Male | 230 (99.1) | 165 (98.8) | 65 (100.0) |
|
|
Female | 2 (0.9) | 2 (1.2) | 0 (0.0) |
|
| Smoking
history |
|
|
| 0.385 |
|
Yes | 188 (81.0) | 133 (79.6) | 55 (84.6) |
|
| No | 44 (19.0) | 34 (20.4) | 10 (15.4) |
|
| Alcohol
consumption |
|
|
| 0.508 |
|
Yes | 124 (53.4) | 87 (52.1) | 37 (56.9) |
|
| No | 108 (46.6) | 80 (47.9) | 28 (43.1) |
|
| Primary tumor
site |
|
|
| 0.473 |
|
Supraglottis | 161 (69.4) | 112 (67.1) | 49 (75.4) |
|
|
Glottis | 70 (30.2) | 54 (32.3) | 16 (24.6) |
|
|
Subglottis | 1 (0.4) | 1 (0.6) | 0 (0.0) |
|
| T
classification |
|
|
| 0.771 |
|
T1-T2 | 42 (18.1) | 31 (18.6) | 11 (16.9) |
|
|
T3-T4 | 190 (81.9) | 136 (81.4) | 54 (83.1) |
|
| N
classification |
|
|
| 0.038 |
| N0 | 45 (19.4) | 38 (22.8) | 7 (10.8) |
|
|
N+ | 187 (80.6) | 129 (77.2) | 58 (89.2) |
|
| Clinical Stage |
|
|
| 0.005 |
|
III | 83 (35.8) | 69 (41.3) | 14 (21.5) |
|
| IV | 149 (64.2) | 98 (58.7) | 51 (78.5) |
|
Survival analysis
The median follow-up for all patients was 46.8
months (range, 4.9–140.8 months). Of the 232 patients, 114 patients
(49.1%) succumbed to LSCC and 6 patients (2.6%) succumbed as a
result of other causes. Cumulatively, the 3/5-year OS, DFS and CSS
rates were 69.4/55.2, 58.6/47.4 and 69.4/56.5%, respectively.
Prognostic factors for a poorer OS identified in the univariate
analysis were age (<60 vs. ≥60 years; P=0.045), N classification
(N0 vs. N+; P=0.006) and clinical stage (III vs. IV; P=0.004). N
classification and stage were also statistically significant
factors in DFS and CSS according to univariate analysis (P<0.05;
Table II). No differences regarding
primary tumor localization, T classification, smoking and alcohol
consumption history were demonstrated for these 3 endpoints.
Multivariate analysis revealed that age (<60 vs. ≥60 years;
P=0.035) and stage (III vs. IV; P=0.022) were statistically
significant factors in OS, whereas N classification (N0 vs. N+) was
the independent factor in DFS (P=0.015) and CSS (P=0.043) (Table III).
 | Table II.Univariate analysis of survival in
232 unmatched patients with laryngeal cancer. |
Table II.
Univariate analysis of survival in
232 unmatched patients with laryngeal cancer.
|
| Overall
survival | Disease-free
survival | Cancer-specific
survival |
|---|
|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<60 vs.
≥60) | 1.47
(1.01–2.14) | 0.045 | 1.06
(0.75–1.50) | 0.740 | 1.42
(0.97–2.08) | 0.073 |
| Smoking history
(yes vs. no) | 0.87
(0.54–1.40) | 0.568 | 0.79
(0.50–1.23) | 0.294 | 0.82
(0.50–1.34) | 0.423 |
| Alcohol consumption
(yes vs. no) | 1.04
(0.73–1.50) | 0.835 | 1.10
(0.78–1.54) | 0.593 | 0.99
(0.69–1.43) | 0.953 |
| Primary site
(supraglottis vs. glottis) | 1.02
(0.69–1.51) | 0.925 | 0.96
(0.67–1.40) | 0.841 | 1.08
(0.73–1.61) | 0.705 |
| T classification
(T1-2 vs. T3-4) | 1.09
(0.68–1.76) | 0.722 | 1.23
(0.77–1.96) | 0.383 | 1.05
(0.64–1.72) | 0.845 |
| N classification
(N0 vs. N+) | 2.10
(1.24–3.57) | 0.006 | 2.05
(1.26–3.34) | 0.004 | 2.11
(1.22–3.64) | 0.007 |
| Clinical Stage (III
vs. IV) | 1.80
(1.21–2.67) | 0.004 | 1.48
(1.03–2.13) | 0.036 | 1.74
(1.17–2.61) | 0.007 |
| Treatment (Surgery
vs. Surgery + CRT) | 1.40
(0.96–2.05) | 0.083 | 1.06
(0.73–1.53) | 0.776 | 1.33
(0.90–1.97) | 0.152 |
 | Table III.Multivariate analysis of survival in
232 unmatched patients with laryngeal cancer. |
Table III.
Multivariate analysis of survival in
232 unmatched patients with laryngeal cancer.
|
| Overall
survival | Disease-free
survival | Cancer-specific
survival |
|---|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<60 vs.
≥60) | 1.50
(1.03–2.19) | 0.035 | – | – | – | – |
| N classification
(N0 vs. N+) | 1.70
(0.98–2.96) | 0.059 | 1.88
(1.13–3.13) | 0.015 | 1.80
(1.02–3.17) | 0.043 |
| Stage (III vs.
IV) | 1.62
(1.07–2.46) | 0.022 | 1.25
(0.85–1.83) | 0.261 | 1.50
(0.99–2.29) | 0.058 |
Comparison of treatment modalities
prior to and following PSM
As the N classification and clinical stage were the
significant indicators from the survival analysis and they were not
well balanced for the surgery and surgery + CRT groups, PSM was
additionally performed to obtain a matched cohort and to
investigate if there were any survival differences between the two
groups (16–18). The patient characteristics following
matching with well-balanced N classification and stage are
summarized in Table IV. Prior to
PSM, there was no statistical significance between the two
treatment groups by log-rank tests, but it appeared that the
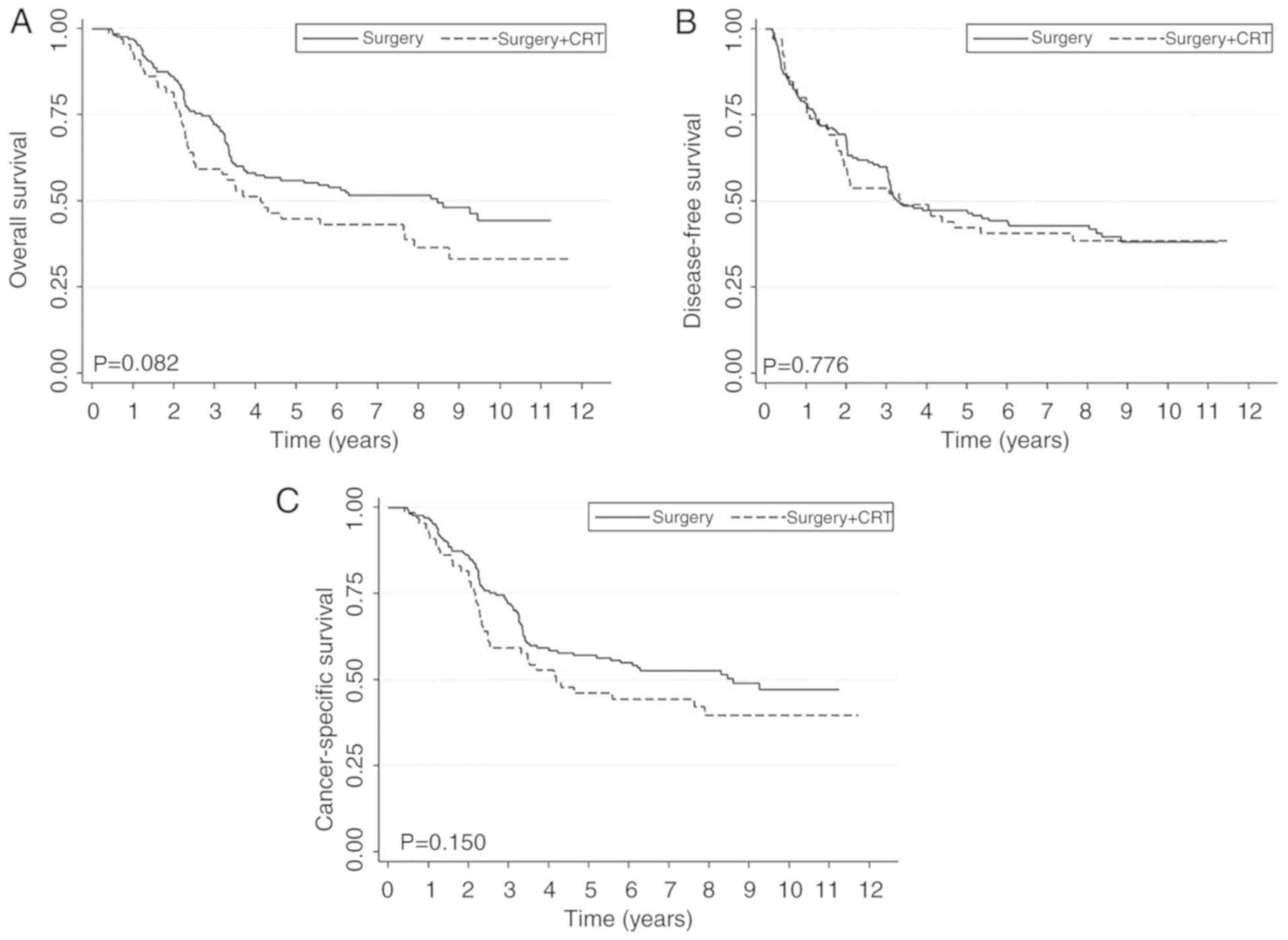
surgery-only group may have exhibited improved survival outcomes
compared with the surgery + CRT group in OS and CSS (Fig. 1). Following PSM, it was revealed that
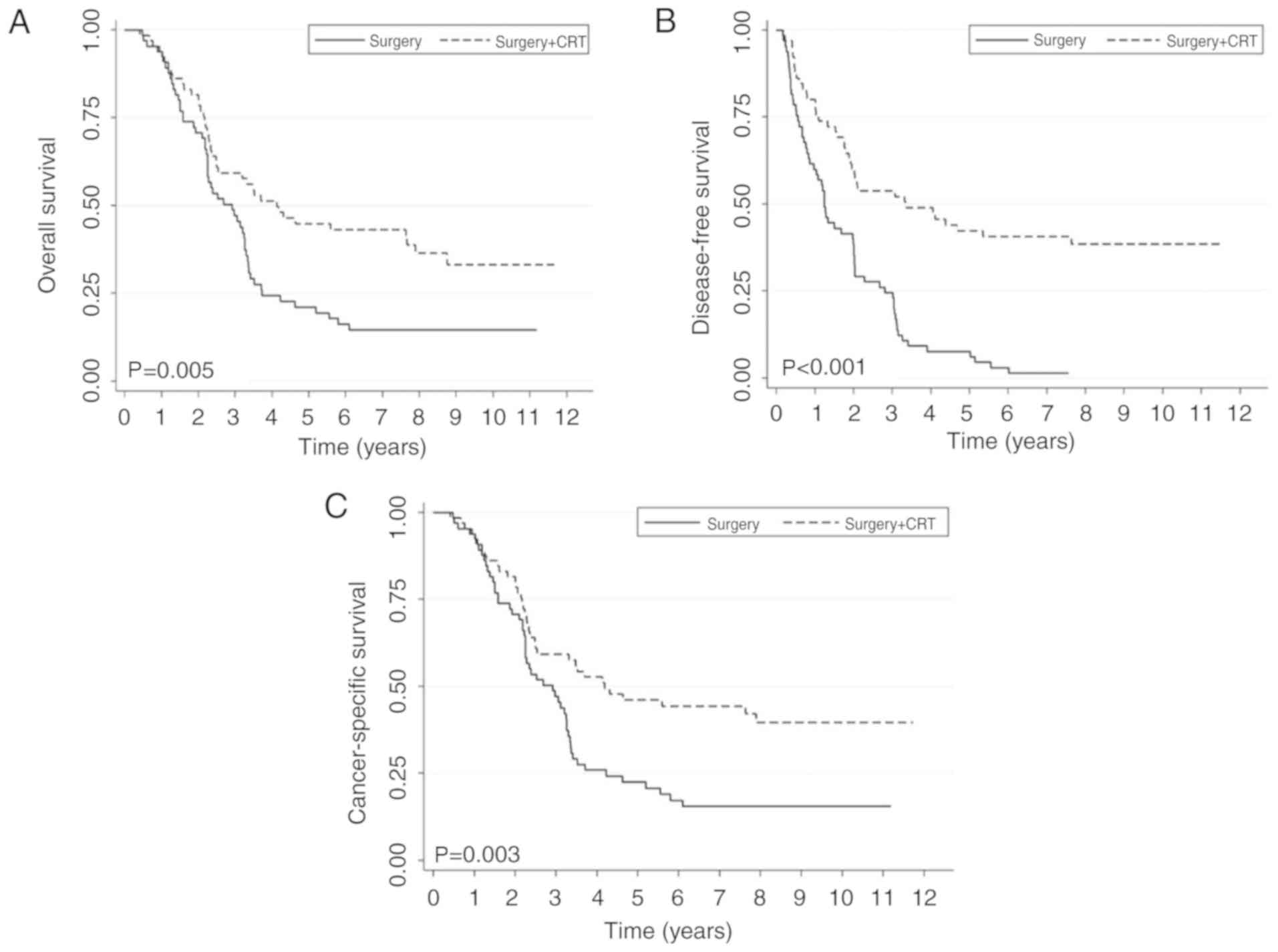
patients who underwent surgery + CRT exhibited an improved survival
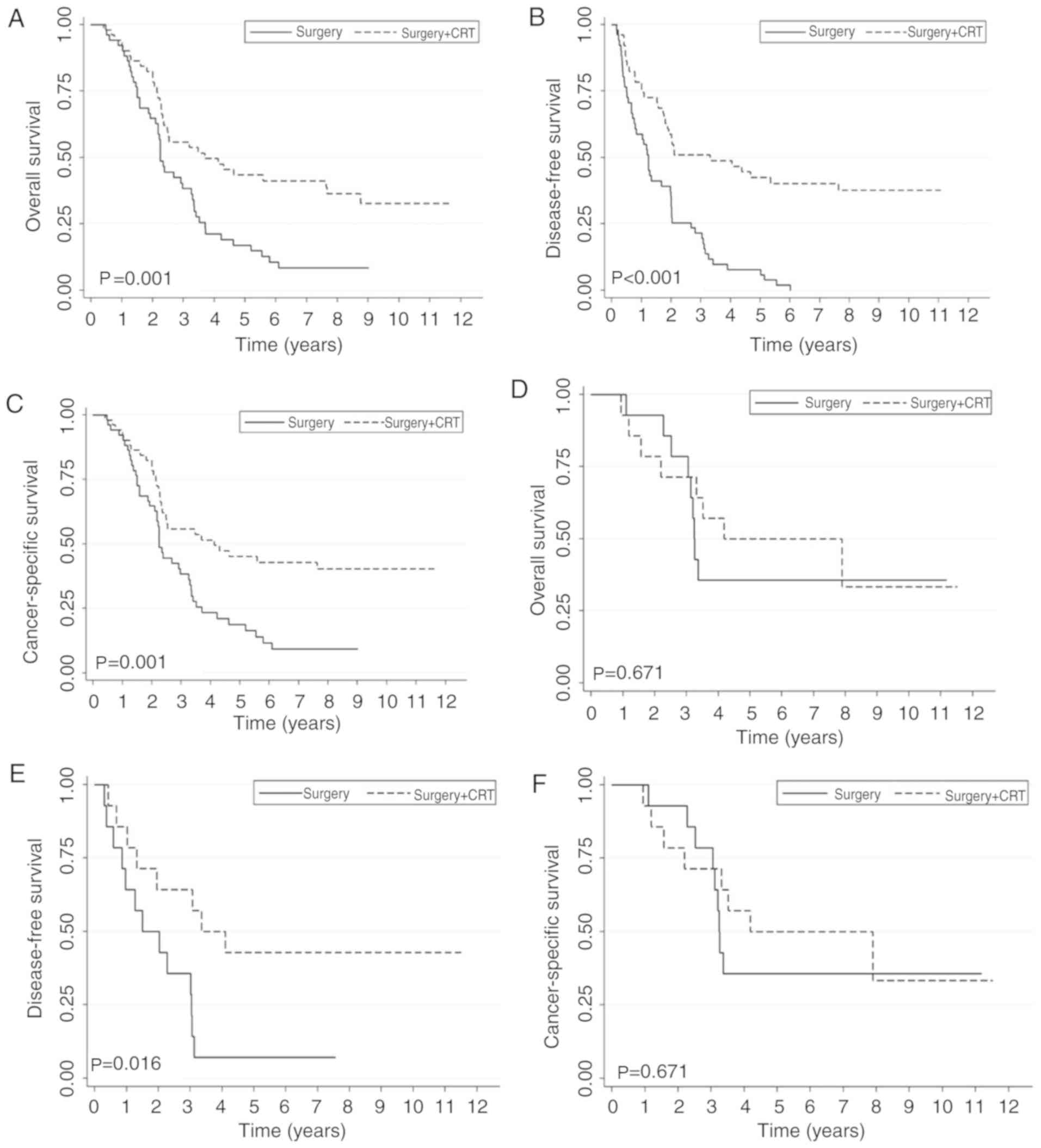
outcome compared with patients who only underwent surgery (Fig. 2). Following stratification by stage,
patients at stage IV continued to exhibit an improved survival
benefit in the surgery + CRT group compared with the surgery-only
group for all 3 endpoints. No significant difference in OS or CSS
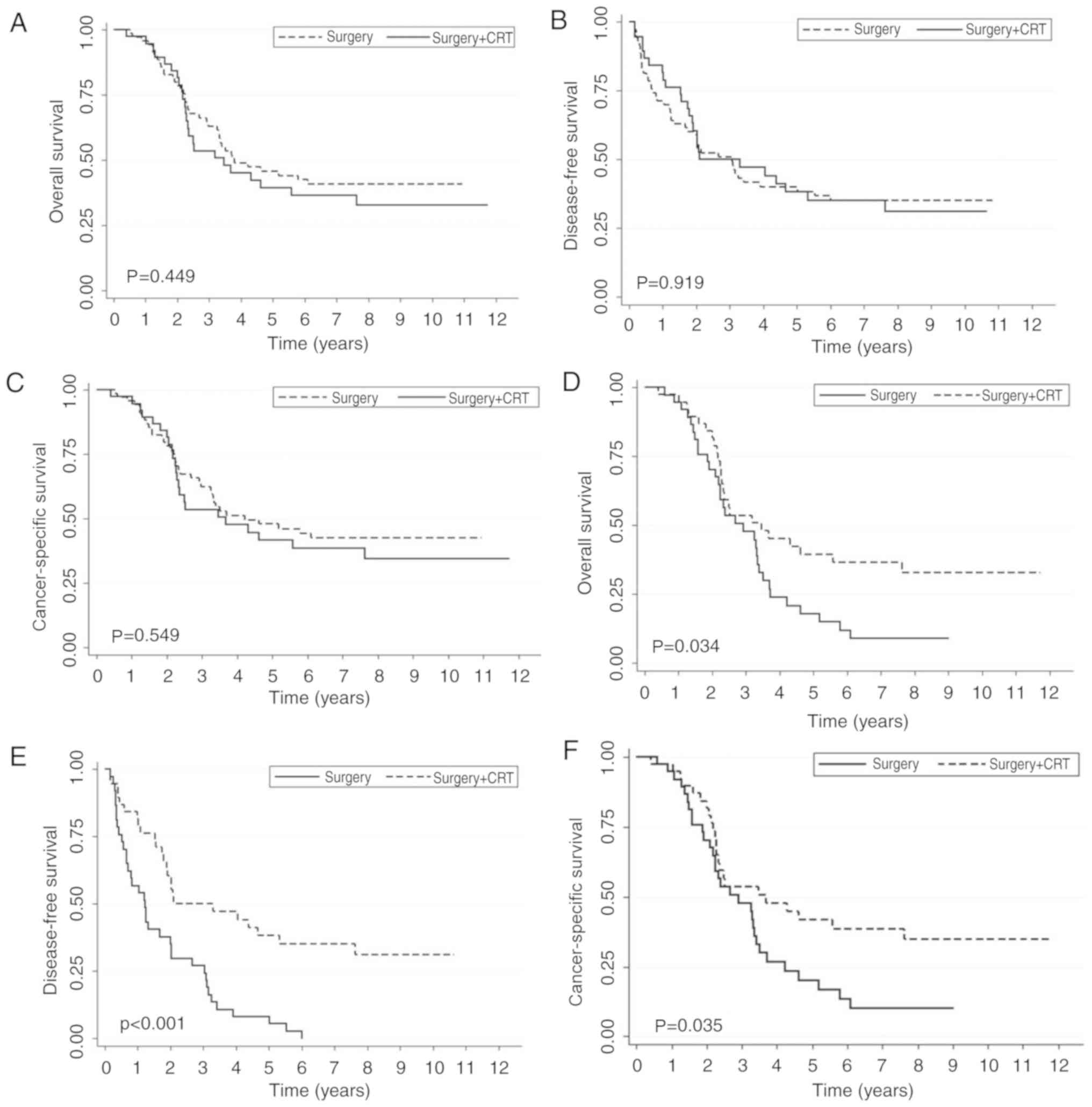
was observed in patients with stage III disease (Fig. 3). For patients with pN2 disease, no
statistical significance was observed between the two groups.
Following matching the T stage, patients with pN2 stage LC
exhibited an improved survival benefit in the surgery + CRT group
compared with the surgery-only group (Fig. 4).
 | Table IV.Baseline patient characteristics in
the 130 propensity score-matched patients with resected laryngeal
cancer who had undergone surgery alone or surgery with adjuvant
CRT. |
Table IV.
Baseline patient characteristics in
the 130 propensity score-matched patients with resected laryngeal
cancer who had undergone surgery alone or surgery with adjuvant
CRT.
|
| Treatment groups,
no. of patients (%) |
|
|
|---|
|
|
|
|
|
|---|
| Variable | Total | Surgery | Surgery + CRT | Standardized
differences | P-value |
|---|
| Age, y |
|
|
|
| 0.681 |
|
<60 | 56 (43.1) | 28 (43.1) | 28 (43.1) | 0.000 |
|
|
≥60 | 74 (56.9) | 37 (56.9) | 37 (56.9) |
|
|
| Sex |
|
|
|
| 1.000 |
|
Male | 129 (99.2) | 64 (98.5) | 65 (100.0) | 0.175 |
|
|
Female | 1 (0.8) | 1 (1.5) | 0 (0.00) |
|
|
| Smoking
history |
|
|
|
| 0.385 |
|
Yes | 111 (85.4) | 56 (86.2) | 55 (84.6) | −0.043 |
|
| No | 19 (14.6) | 9 (13.8) | 10 (15.4) |
|
|
| Alcohol
consumption |
|
|
|
| 0.508 |
|
Yes | 71 (54.6) | 34 (52.3) | 37 (56.9) | 0.092 |
|
| No | 59 (45.4) | 31 (47.7) | 28 (43.1) |
|
|
| Primary site |
|
|
|
| 0.473 |
|
Supraglottis | 92 (70.8) | 43 (66.2) | 49 (75.4) | 0.202 |
|
|
Glottis | 38 (29.2) | 22 (33.8) | 16 (24.6) |
|
|
| T
classification |
|
|
|
| 0.771 |
|
T1-T2 | 21 (16.2) | 10 (15.4) | 11 (16.9) | 0.041 |
|
|
T3-T4 | 109 (83.8) | 55 (84.6) | 54 (83.1) |
|
|
| N
classification |
|
|
|
| 0.038 |
| N0 | 14 (10.8) | 7 (10.8) | 7 (10.8) | 0.000 |
|
| N+ | 116 (89.2) | 58 (89.2) | 58 (89.2) |
|
|
| Clinical Stage |
|
|
|
| 0.005 |
|
III | 28 (21.5) | 14 (21.5) | 14 (21.5) | 0.000 |
|
| IV | 102 (78.5) | 51 (78.5) | 51 (78.5) |
|
|
Discussion
Previously, the primary approach to therapy for
advanced LSCC was surgical treatment (19,20). At
present, multiple treatment modalities, including radiotherapy and
chemoradiotherapy, have been introduced to treat advanced LSCC.
These were based on published clinical trials that revealed that
increased rates of larynx preservation may be obtained with
chemoradiotherapy for advanced LC (21,22).
However, different treatment modalities or combinations have varied
in different developing and developed countries (23).
Several clinical trials have been conducted to
investigate the optimal treatment for LC (21,24,25). The
Veterans Administration randomized clinical trial (RCT) comprising
two treatment arms revealed that the usage of primary CRT offered
patients who underwent surgery an equal survival chance (17). Concomitantly, the CRT group had a
two-thirds likelihood of preservation of the larynx for stage
III/IV laryngeal cancer (21). An
additional RCT, the RTOG 91–11 trial, comprising three treatment
arms (induction chemotherapy + radiation (RT) vs. concurrent CRT
vs. RT) indicated superior locoregional control and larynx
preservation rate in the concurrent CRT treatment arm; however, no
difference was observed in OS (22).
The updated 10-year follow-up for the RTOG 91-11 study revealed
that there was a trend toward a poorer survival in the concurrent
CRT treatment arm, which may be attributed to the increased
incidence of toxicity (24).
There has been a decreased survival rate in
laryngeal cancer in previous decades with the increased application
of different treatment approaches (26). Hoffman et al (26) suggested that RT alone or CRT was
correlated with an increased mortality rate compared with surgery
in T3 glottic cancer. An RCT conducted in France including patients
with T3 primary laryngeal tumors demonstrated significantly
improved survival in the group undergoing immediate surgery
compared with patients receiving induction chemotherapy followed by
RT (25). Previous studies revealed
that the 5-year OS and DFS rates in patients with advanced LC were
60.8–71.6 and 41.0–57.8%, respectively, with regard to different
treatment regimens (23,27). The present study demonstrated that
the 5-year OS and DFS rates were 55.2 and 47.4%, respectively,
which supported previously published data.
Studies have indicated that the lymph node status is
one of the most significant prognostic factors in patients with LC
(28,29). Smoking and being of an elderly age
are also risks factors in patients with LC, but the effects of age
in the prognosis of LC remain controversial (30–32). The
multivariate analysis in the present study demonstrated that the
patients' clinical stage and nodal status were independent
indicators in survival outcome. Age was statistically significant
in univariate analysis, but it was not an independent factor.
Smoking was not a risk factor in the present study and requires
verification with an increased number of study participants.
Furthermore, there is a concern for the long-term
toxicity and for the methods of selecting patients utilizing
surgical or non-surgical treatments as the primary treatment
strategies (24,33). Treatment varies in different
geographical locations as there is currently no optimal treatment
in clinical practice (34). Certain
studies have supported the hypothesis that postoperative RT does
not provide additional survival effects in patients with LC, while
other studies have suggested that patients receiving surgery with
postoperative RT have improved survival (32,35,36).
Chen and Halpern (37) demonstrated
almost equivalent efficacy for CRT and total laryngectomy for stage
III disease, but significant differences in survival for all
patients with advanced LC in a hospital-based study. Analysis of
the National Cancer Data Base revealed that patients with stage III
and IV disease treated with surgery as the primary treatment
strategy had improved 5-year relative survival compared with those
treated with irradiation (with or without chemotherapy) among
158,426 LC cases (26). A
cross-section study using Surveillance, Epidemiology and End
Results-Medicare data demonstrated that an improved survival
benefit was observed following surgery with adjuvant RT of the
whole LC cohort (36). However,
there remains a paucity in the current literature of data to
provide detailed descriptions of the comparison of the treatment
modalities for advanced LSCC. In the present study, 232 patients
with advanced LSCC were compared with 2 treatment modalities:
Surgery vs. surgery + CRT. The results revealed that patients who
underwent surgery + adjuvant CRT had improved survival outcomes
compared with patients who were treated with surgery alone in the
overall cohort. Subsequent stratification demonstrated that the
clinical effect was limited in patients with pN2 disease and
patients with stage IV disease. The inadequate patient number meant
that whether patients with stage III LC would receive a survival
benefit with adjuvant CRT could not be discerned. Additional
studies will be required in the coming years to elucidate the
causes of this apparent decrease in larynx cancer survival, and/or
improve the selection of patients for surgical and non-surgical
treatments.
Several limitations of the present study should be
noted: In the subgroup analysis, with regard to the potential
benefit from the adjuvant CRT in patients with or without positive
surgical margins, the benefit of adjuvant CRT in the subgroup of
patients with positive surgical margins was not determined, as all
the patients with positive surgical margins were in the surgical +
adjuvant CRT group. For the patients with pN2+ disease, no
statistical significance was observed among the unmatched 232
patients. Following matching, an additional survival benefit was
observed in the patients with LC receiving adjuvant CRT. In
addition, as LSCC is a predominantly male disease, and because
there were a limited number of females in the present study due to
the relatively small sample size, the potential effects of sex were
not assessed.
In conclusion, patients with stage IV LSCC may
benefit from adjuvant CRT following initial total laryngectomy. It
is worth noting that a significant limitation of the present study
was its retrospective design. As it includes the data from only 1
institution, the results require validation by a prospective
multicenter study.
Acknowledgements
The authors would like to thank Dr Jian Zhou and Dr
Chiyao Hsueh (Eye, Ear, Nose and Throat Hospital of Fudan
University, Shanghai, China) for their collection and follow-up of
clinical cases. They would also like to thank Dr Patricia A.
McEvoy-Jamil (The University of Texas School of Public Health) for
her assistance and editing of the English language.
Funding
The present study was supported in part by Shen Kang
Hospital Development of Shanghai Municipality (grant no.
SHDC12015114), Science and Technology Commission of Shanghai
Municipality (grant no. 16411950101), Shanghai Natural Science
Foundation of China (grant no. 17ZR1404700) and Shanghai Pudong
District Health Bureau of Health (IPPF) Technology Projects (grant
no. PW2016D-11).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ and WD conceived the study and completed the
manuscript. WD and XL analyzed and interpreted the patient data.
HG, CL and YW reviewed, collected and analyzed data. LT and LZ
designed the study acquired the data. LZ supervised the whole
study, revised the manuscript and gave final approval of the
version to be published. All the authors contributed to write the
manuscript.
Ethics approval and consent to
participate
All patients gave their full consent to participate
in the present study, and a written consent form was obtained from
each patient. The research protocol was approved by the
Institutional Review Board of Eye, Ear, Nose and Throat Hospital of
Fudan University.
Patient consent for publication
A written consent form was obtained from each
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eskiizmir G, Tanyeri Toker G, Celik O,
Gunhan K, Tan A and Ellidokuz H: Predictive and prognostic factors
for patients with locoregionally advanced laryngeal carcinoma
treated with surgical multimodality protocol. Eur Arch
Otorhinolaryngol. 274:1701–1711. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steuer CE, El-Deiry M, Parks JR, Higgins
KA and Saba NF: An update on larynx cancer. CA Cancer J Clin.
67:31–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du L, Li H, Zhu C, Zheng R, Zhang S and
Chen W: Incidence and mortality of laryngeal cancer in China, 2011.
Chin J Cancer Res. 27:52–58. 2015.PubMed/NCBI
|
|
5
|
Skora T, Nowak-Sadzikowska J,
Mucha-Malecka A, Szyszka-Charewicz B, Jakubowicz J and Glinski B:
Postoperative irradiation in patients with pT3-4N0 laryngeal
cancer: Results and prognostic factors. Eur Arch Otorhinolaryngol.
272:673–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trifiletti DM, Smith A, Mitra N, Grover S,
Lukens JN, Cohen RB, Read P, Mendenhall WM, Lin A and
Swisher-McClure S: Beyond positive margins and extracapsular
extension: Evaluating the utilization and clinical impact of
postoperative chemoradiotherapy in resected locally advanced head
and neck cancer. J Clin Oncol. 35:1550–1560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cooper JS, Pajak TF, Forastiere AA, Jacobs
J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et
al: Postoperative concurrent radiotherapy and chemotherapy for
high-risk squamous-cell carcinoma of the head and neck. N Engl J
Med. 350:1937–1944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bernier J, Cooper JS, Pajak TF, van
Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem
J, Ang KK and Lefèbvre JL: Defining risk levels in locally advanced
head and neck cancers: A comparative analysis of concurrent
postoperative radiation plus chemotherapy trials of the EORTC
(#22931) and RTOG (# 9501). Head Neck. 27:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davis GE, Schwartz SR, Veenstra DL and
Yueh B: Cost comparison of surgery vs. organ preservation for
laryngeal cancer. Arch Otolaryngol Head Neck Surg. 131:21–26. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah JP, Karnell LH, Hoffman HT, Ariyan S,
Brown GS, Fee WE, Glass AG, Goepfert H, Ossoff RH and Fremgen A:
Patterns of care for cancer of the larynx in the United States.
Arch Otolaryngol Head Neck Surg. 123:475–483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karatzanis AD, Psychogios G, Waldfahrer F,
Kapsreiter M, Zenk J, Velegrakis GA and Iro H: Management of
locally advanced laryngeal cancer. J Otolaryngol Head Neck Surg.
43:42014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Felice F, Blanchard P, Levy A, Nguyen
F, Gorphe P, Janot F, Temam S and Tao Y: Treatment of squamous cell
carcinoma of the posterior pharyngeal wall: Radiotherapy versus
surgery. Head Neck. 1 Suppl 38:E1722–E1729. 2016. View Article : Google Scholar
|
|
13
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: American joint committee on cancer. AJCC
cancer staging manual (7th). (New York, NY). Springer. 2010.
|
|
14
|
Austin PC: Optimal caliper widths for
propensity-score matching when estimating differences in means and
differences in proportions in observational studies. Pharm Stat.
10:150–161. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Austin PC: An introduction to propensity
score methods for reducing the effects of confounding in
observational studies. Multivariate Behav Res. 46:399–424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vlachtsis K, Nikolaou A, Markou K,
Fountzilas G and Daniilidis I: Clinical and molecular prognostic
factors in operable laryngeal cancer. Eur Arch Otorhinolaryngol.
262:890–898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Byrd SA, Xu MJ, Cass LM, Wehrmann DJ,
Naunheim M, Christopher K, Dombrowski JJ, Walker RJ, Wirth L, Clark
J, et al: Oncologic and functional outcomes of pretreatment
tracheotomy in advanced laryngeal squamous cell carcinoma: A
multi-institutional analysis. Oral Oncol. 78:171–176. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu X, Zhou Q and Zhang X: Efficacy
comparison between total laryngectomy and nonsurgical
organ-preservation modalities in treatment of advanced stage
laryngeal cancer: A meta-analysis. Medicine (Baltimore).
95:e31422016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheahan P: Management of advanced
laryngeal cancer. Rambam Maimonides Med J. 5:e00152014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Neill CB, O'Neill JP, Atoria CL, Baxi
SS, Henman MC, Ganly I and Elkin EB: Treatment complications and
survival in advanced laryngeal cancer: A population-based analysis.
Laryngoscope. 124:2707–2713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Department of Veterans Affairs Laryngeal
Cancer Study Group, ; Wolf GT, Fisher SG, Hong WK, Hillman R,
Spaulding M, Laramore GE, Endicott JW, McClatchey K and Henderson
WG: Induction chemotherapy plus radiation compared with surgery
plus radiation in patients with advanced laryngeal cancer. N Engl J
Med. 324:1685–1690. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo XN, Chen LS, Zhang SY, Lu ZM and Huang
Y: Effectiveness of chemotherapy and radiotherapy for laryngeal
preservation in advanced laryngeal cancer: A meta-analysis and
systematic review. Radiol Med. 120:1153–1169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Forastiere AA, Zhang Q, Weber RS, Maor MH,
Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, et
al: Long-term results of RTOG 91-11: A comparison of three
nonsurgical treatment strategies to preserve the larynx in patients
with locally advanced larynx cancer. J Clin Oncol. 31:845–852.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Richard JM, Sancho-Garnier H, Pessey JJ,
Luboinski B, Lefebvre JL, Dehesdin D, Stromboni-Luboinski M and
Hill C: Randomized trial of induction chemotherapy in larynx
carcinoma. Oral Oncol. 34:224–228. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoffman HT, Porter K, Karnell LH, Cooper
JS, Weber RS, Langer CJ, Ang KK, Gay G, Stewart A and Robinson RA:
Laryngeal cancer in the United States: Changes in demographics,
patterns of care, and survival. Laryngoscope. 116 Suppl 111:1–13.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gourin CG, Conger BT, Sheils WC, Bilodeau
PA, Coleman TA and Porubsky ES: The effect of treatment on survival
in patients with advanced laryngeal carcinoma. Laryngoscope.
119:1312–1317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gonzalez-Marquez R, Rodrigo JP and Suarez
Nieto C: Prognostic significance of postoperative wound infections
after total laryngectomy. Head Neck. 34:1023–1027. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jose J, Coatesworth AP, Johnston C and
MacLennan K: Cervical node metastases in squamous cell carcinoma of
the upper aerodigestive tract: The significance of extracapsular
spread and soft tissue deposits. Head Neck. 25:451–456. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lubin JH, Gaudet MM, Olshan AF, Kelsey K,
Boffetta P, Brennan P, Castellsague X, Chen C, Curado MP, Dal Maso
L, et al: Body mass index, cigarette smoking, and alcohol
consumption and cancers of the oral cavity, pharynx, and larynx:
Modeling odds ratios in pooled case-control data. Am J Epidemiol.
171:1250–1261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramroth H, Schoeps A, Rudolph E, Dyckhoff
G, Plinkert P, Lippert B, Feist K, Delank KW, Scheuermann K, Baier
G, et al: Factors predicting survival after diagnosis of laryngeal
cancer. Oral Oncol. 47:1154–1158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang SY, Lu ZM, Luo XN, Chen LS, Ge PJ,
Song XH, Chen SH and Wu YL: Retrospective analysis of prognostic
factors in 205 patients with laryngeal squamous cell carcinoma who
underwent surgical treatment. PLoS One. 8:e601572013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Megwalu UC and Sikora AG: Survival
outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck
Surg. 140:855–860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Corry J, Peters L, Kleid S and Rischin D:
Larynx preservation for patients with locally advanced laryngeal
cancer. J Clin Oncol. 31:840–844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Papadas TA, Alexopoulos EC, Mallis A,
Jelastopulu E, Mastronikolis NS and Goumas P: Survival after
laryngectomy: A review of 133 patients with laryngeal carcinoma.
Eur Arch Otorhinolaryngol. 267:1095–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gourin CG, Dy SM, Herbert RJ, Blackford
AL, Quon H, Forastiere AA, Eisele DW and Frick KD: Treatment,
survival, and costs of laryngeal cancer care in the elderly.
Laryngoscope. 124:1827–1835. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen AY and Halpern M: Factors predictive
of survival in advanced laryngeal cancer. Arch Otolaryngol Head
Neck Surg. 133:1270–1276. 2007. View Article : Google Scholar : PubMed/NCBI
|