Introduction
New cancer cases are rising worldwide because of the
growing aging population, and the increasing prevalence of risk
factors including smoking, drinking, and obesity. Approximately
14.1 million new cancer cases and 8.2 million deaths occurred
worldwide in 2012 (1). In Japan, the
most common cause of death was malignant neoplasm (2). A substantial portion of cancer cases
and deaths has declined by effective prevention methods, such as
tobacco and alcohol control, vaccination, and the use of early
detection tests. Inherited genetic mutations play a major role in
determining the risk for cancers, and may provide useful
information to determine the candidates for early detection tests
(3).
Cytoskeletal components regulate cell migration,
polarity, and morphology. A neuroepithelial stem cell marker,
nestin (NES), is a cytoskeletal protein belonging to the group of
class VI intermediate filament (IF) proteins (4,5). NES
protein has head, coil, and tail structures. The tail structure of
NES is known to interact with other IF proteins, including
vimentin, desmin, α-internexin, and synemin, to form heterodimers
(6). NES contributes to the
disassembly of vimentin during mitosis (7) and to the inactivation of the
proapoptotic cyclin-dependent kinase 5 (CDK5) (8). Mouse Cdk5 and Cdc2 induce
phosphorylation at both threonine 316 (Thr316) and threonine 1495
(Thr1495) of NES protein (8,9), and phosphorylation of NES modulates
mitosis-associated cytoplasmic reorganization during cell mitosis
(10).
We have reported that expression of NES in various
tumors such as pancreatic cancer (11,12),
glioblastoma (13), lung cancer
(14), malignant melanoma (15), and uterine cancer (16), regulates cell proliferation,
migration, invasion and metastasis. NES regulates stemness in
glioblastoma cells through the alteration of cyclin D1 and heat
shock cognate 71 kDa protein (13).
Phosphorylation of NES at Thr315 and/or Thr1299 regulates cell
proliferation (9), and inhibition of
both phosphorylation sites suppresses invasion and metastasis of
human pancreatic cancer (17).
Data from previous studies (11,12,18–21)
indicate that inhibition of either NES expression or
phosphorylation may be a therapeutic target for several cancers
(22). NES is not merely a
cytoskeletal protein that serves as a progenitor cell marker, but
also is a key regulator of cancer progression processes such as
migration, invasion, and metastasis (5,23);
therefore, we hypothesized that NES might play important roles in
pathogenesis of various cancers. Multiple reports have shown that
single nucleotide polymorphisms (SNPs) affect cancer
predispositions. However, there have been no reports of a
relationship between NES gene variations and cancer. Autopsy is a
precious source to analyze various malignant tumors as well as of
precursor lesions. In the present study, we comparatively analyzed
serially autopsied patients with various malignant neoplasms, based
on their clinical information and SNPs.
Patients and methods
Study population
Consecutive autopsy cases (N=2,206) were collected
at the Tokyo Metropolitan Geriatric Hospital (Tokyo, Japan) between
1995 and 2012 (24). Participants
with family relationships (n=26) were excluded from this study.
There were 1,225 men and 981 women with a median age of 80.7 years
(range, 33–104 years) and a median body mass index (BMI) of 17.4
kg/m2 (range, 8.1–37.9). The patients were enrolled in
the Internet Database of Japanese Single Nucleotide Polymorphisms
for Geriatric Research (JG-SNP) (25). We collected information about smoking
and drinking from the medical records. The most frequent causes of
death were malignancies, infections, and cardiovascular diseases.
Approximately 60% of patients had malignant tumors (26). Cancer-bearing subjects include those
with any type of cancer, including pathologically verified surgical
resected cancer as a past history and occult cancer found on
autopsy. We reviewed all the pancreatic specimens from autopsies to
determine the presence or absence of pancreatic cancers and
pancreatic intraepithelial neoplasia (PanIN). PanIN was defined as
microscopic, papillary or flat, non-invasive, epithelial lesions
with diameters of 5 mm or less (27). PanIN lesions were classified as
PanIN-1A, −1B, −2, or −3 according to previously described criteria
(28,29). The present study was approved by the
Tokyo Metropolitan Geriatric Hospital Ethics Committee (approval
no. 15-02). This study was conducted in accordance with the
principles embodied in the Declaration of Helsinki, 2013. Written
informed consent was obtained prior to the autopsy from the family
members of all participants involved in this study.
Genotyping and genotype calling
Genomic DNA was extracted from the renal cortex
using a standard procedure as previously reported (24). All samples were analyzed with
Illumina Infinium HumanExome BeadChip Version 1.1 (Illumina, San
Diego, CA) by iScan (26). Genotype
calling was performed using the Genotyping Module (version 1.9) of
the GenomeStudio data analysis software package. Initial genotype
clustering was performed using the default Illumina cluster file
(HumanExome 12v1-1_A.egt) and the manifest file
(HumanExome-12v1-1_A.bmp), using the GenTrain2 clustering
algorithm. Validation of the polymorphisms was performed by direct
sequencing, using the BigDyeTerminator v3.1 Cycle Sequencing kit on
a 3130 Genetic Analyzer (both Applied Biosystems, Foster City, CA,
USA) (26). The pathological
assessment (YM and TA) and genotyping (MM and MNM) were performed
in different institutions in a double-blind fashion to minimize
bias. We could not provide the raw data of the present study
because we are analyzing our data for use in future studies.
Statistical analysis
We performed Fisher's exact test to determine the
association between the phenotypes and SNPs using SPSS version 22
(IBM Corp., Armonk, NY, USA). Power was analyzed using PASS 15.0.5.
(NCSS, LLC., Kaysville, UT, USA). P<0.05 was considered to
indicate a statistically significant difference. We also analyzed
the odds ratio (OR) and 95% confidence interval (CI).
Results
The five SNPs we analyzed in the present study are
shown in Table I. They are in exons
and located in the tail structure of the NES protein, and four SNPs
except for NES p.P1275L are rare variants. All SNPs are miss sense
mutations. Two SNPs are possibly damaging. We analyzed the
association between SNPs of NES and various cancers in major
organs. NES p.A1199P did associate with pancreatic cancer (OR, 4.4;
95% CI, 1.9–10.0, P=0.001 by Fisher's exact test, Table II). Large cell lung carcinoma also
showed association to NES p.A1199P (OR, 9.2; 95% CI, 0.9–90.9,
P=0.02 by Fisher's exact test, Table
II), but few patients harbored this change. The urinary tract
malignancies showed an association with NES p.A1199P (P=0.053).
Malignant neoplasms in other organs such as lung, colon, stomach,
brain, and blood cancers did not associate with NES p.A1199P
(Fisher's exact test, Tables II and
III).
 | Table I.Single nucleotide polymorphisms of
nestin. |
Table I.
Single nucleotide polymorphisms of
nestin.
| Number | Alleles | In-exon | Mutation(s) | rs number | Prediction | Minor allele
frequency |
|---|
| exm109872 | [A/G] | EXON |
Missense_P1275L | rs3748570 | Benign | 0.2413 |
| exm109911 | [T/C] | EXON |
Missense_S1016N | rs2365718 | Possibly
damaging | 0.0043 |
| exm109937 | [T/G] | EXON | Missense_L791I | rs77202633 | Benign | 0.0553 |
| exm1719129 | [G/C] | EXON |
Missense_A1199P | rs78303930 | Possibly
damaging | 0.0170 |
| exm1719137 | [A/G] | EXON | Missense_V876A | rs143673331 | Benign | 0.0028 |
 | Table II.Malignant tumors in major organs and
NES p.A1199P. |
Table II.
Malignant tumors in major organs and
NES p.A1199P.
| Organ | Type | Tumor + (%) | Tumor - (%) | OR | 95% CI | P-value |
|---|
| Lung | CC | 253 (11.5) | 1,871 (85.0) |
|
|
|
|
| GC+GG | 7 (0.3) | 71 (3.2) | 0.729 | 0.332–1.603 | 0.430 |
| Large cell
carcinoma/lung | CC | 3 (0.1) | 2,121 (96.3) |
|
|
|
|
| GC+GG | 1 (0) | 77 (3.5) | 9.174 | 0.944–90.0909 | 0.020a |
| Stomach | CC | 239 (10.8) | 1,887 (85.6) |
|
|
|
|
| GC+GG | 7 (0.3) | 72 (3.3) | 0.767 | 0.349–1.686 | 0.509 |
| Colorectum | CC | 209 (9.5) | 1,917 (86.9) |
|
|
|
|
| GC+GG | 4 (0.2) | 75 (3.4) | 0.489 | 0.177–1.351 | 0.159 |
| Pancreas | CC | 47 (2.1) | 2,078 (94.3) |
|
|
|
|
| GC+GG | 7 (0.3) | 71 (3.2) | 4.367 | 1.905–10 | 0.001b |
| Liver | CC | 68 (3.1) | 2,058 (93.3) |
|
|
|
|
| GC+GG | 0 (0) | 79 (3.6) | N.D. | N.D. | 0.106 |
| Biliary tract | CC | 61 (2.8) | 2,065 (93.7) |
|
|
|
|
| GC+GG | 2 (0.1) | 77 (3.5) | 0.880 | 0.211–4.219 | 0.860 |
| Kidney | CC | 32 (1.5) | 2,094 (95.0) |
|
|
|
|
| GC+GG | 1
(0) | 78 (3.5) | 0.839 | 0.113–6.211 | 0.863 |
| Urinary tract | CC | 41 (1.9) | 2,085 (94.6) |
|
|
|
|
| GC+GG | 4 (0.2) | 75 (3.4) | 2.710 | 0.947–7.752 | 0.053 |
| Prostate | CC | 197 (16.0) | 987 (80.4) |
|
|
|
|
| GC+GG | 7 (0.6) | 37 (3.0) | 0.948 | 0.416–2.155 | 0.898 |
| Blood | CC | 195 (8.9) | 1,930 (87.6) |
|
|
|
|
| GC+GG | 4 (0.2) | 74 (3.4) | 0.535 | 0.194–1.479 | 0.221 |
| Brain | CC | 2 (0.1) | 2,122 (96.4) |
|
|
|
|
| GC+GG | 0 (0) | 78 (3.5) | N.D. | N.D. | 0.786 |
 | Table III.Other malignant tumors and NES
p.A1199P. |
Table III.
Other malignant tumors and NES
p.A1199P.
| Organ | Type | Tumor + (%) | Tumor - (%) | OR | 95% CI | P-value |
|---|
|
Adenocarcinoma/lung | CC | 115 (5.2) | 2,009 (91.2) |
|
|
|
|
| GC+GG | 2 (0.1) | 76 (3.5) | 0.460 | 0.112–1.894 | 0.270 |
| Squamous cell
carcinoma/lung | CC | 77 (3.5) | 2,047 (93.0) |
|
|
|
|
| GC+GG | 3 (0.1) | 75 (3.4) | 1.064 | 0.328–4.785 | 0.918 |
| Adenosquamous
carcinoma lung | CC | 8 (0.4) | 2,116 (96.1) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.587 |
| Small cell
carcinoma lung | CC | 55 (2.5) | 2,069 (94.0) |
|
|
|
|
| GC+GG | 2 (0.1) | 76 (3.5) | 0.990 | 0.237–4.132 | 0.989 |
| Unclassified cancer
lung | CC | 11 (0.5) | 2,113 (96.0) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.524 |
| Mesothelioma | CC | 1 (0.0) | 2,124 (96.4) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.848 |
| Esophageal
cancer | CC | 32 (1.5) | 2,091 (95.0) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.275 |
| Colon cancer | CC | 163 (7.4) | 1,963 (89.0) |
|
|
|
|
| GC+GG | 3 (0.1) | 76 (3.4) | 0.475 | 0.148–1.524 | 0.201 |
| Rectal cancer | CC | 52 (2.4) | 2,074 (94.1) |
|
|
|
|
| GC+GG | 1 (0.0) | 78 (3.5) | 0.511 | 0.070–3.745 | 0.501 |
| Small intestine
cancer | CC | 11 (0.5) | 2,115 (95.9) |
|
|
|
|
| GC+GG | 0 (0.0) | 79 (3.6) | N.D. | N.D. | 0.522 |
| Lymphocytic
leukemia | CC | 18 (0.8) | 2,107 (95.6) |
|
|
|
|
| GC+GG | 1 (0.0) | 77 (3.5) | 1.520 | 0.200–11.494 | 0.683 |
| Malignant
lymphoma | CC | 119 (5.4) | 2,005 (91.1) |
|
|
|
|
| GC+GG | 1 (0.0) | 77 (3.5) | N.D. | N.D. | 0.099 |
| Myelodysplastic
syndrome | CC | 39 (1.8) | 2,086 (94.7) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.227 |
| Myelogenous
leukemia | CC | 104 (4.7) | 2,021 (91.7) |
|
|
|
|
| GC+GG | 2 (0.1) | 76 (3.4) | 0.512 | 0.124–2.110 | 0.345 |
| Myeloma | CC | 38 (1.7) | 2,087 (94.7) |
|
|
|
|
| GC+GG | 1 (0.0) | 77 (3.5) | 0.713 | 0.097–5.263 | 0.739 |
| Breast cancer | CC | 74 (3.4) | 2,050 (93.1) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.094 |
| Uterine cancer | CC | 20 (2.1) | 919 (94.4) |
|
|
|
|
| GC+GG | 0 (0.0) | 35 (3.6) | N.D. | N.D. | 0.383 |
| Ovarian cancer | CC | 5 (0.5) | 939 (95.9) |
|
|
|
|
| GC+GG | 0 (0.0) | 35 (3.6) | N.D. | N.D. | 0.666 |
| Thyroid cancer | CC | 52 (2.4) | 2,072 (94.1) |
|
|
|
|
| GC+GG | 2 (0.1) | 76 (3.5) | 1.048 | 0.250–4.386 | 0.948 |
| Sarcoma | CC | 7 (0.3) | 2,117 (96.1) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.612 |
| Melanoma | CC | 1 (0.0) | 2,123 (96.4) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.848 |
| Skin cancer | CC | 9 (0.4) | 2,112 (96.0) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.564 |
| Head and neck
cancer | CC | 25 (1.1) | 2,099 (95.3) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.335 |
| Other tumor | CC | 9 (0.4) | 2,115 (99.2) |
|
|
|
|
| GC+GG | 0 (0.0) | 9 (0.4) | N.D. | N.D. | 0.565 |
| Unclassified
tumor | CC | 4 (0.2) | 2,120 (96.3) |
|
|
|
|
| GC+GG | 0 (0.0) | 78 (3.5) | N.D. | N.D. | 0.701 |
Other SNPs except for NES p.A1199P did not associate
with pancreatic cancer (Table IV);
therefore, we performed further analysis about NES p.A1199P.
Alleles of NES p.A1199P were CC (n=2,127), GC (n=78) and GG (n=1);
GC and GG alleles showed that amino acid number 1199 was changed
from alanine to proline. It did not associate with sex, drinking,
smoking, or BMI (Table V).
 | Table IV.SNPs of nestin and pancreatic
cancer. |
Table IV.
SNPs of nestin and pancreatic
cancer.
| SNP | Reference no. | OR | 95% CI | P-value |
|---|
| P1275L | rs3748570 | 0.630 | 0.366–1.085 | 0.116 |
| S1016N | rs2365718 | 1.047 | 0.058–18.790 | 1.000 |
| L791I | rs77202633 | 0.738 | 0.289–1.885 | 0.670 |
| A1199P | rs78303930 | 4.367 | 1.905–10 | 0.001a |
| V876A | rs143673331 | 1.951 | 0.100–38.174 | 1.000 |
 | Table V.Patients and NES p.A1199P. |
Table V.
Patients and NES p.A1199P.
| Comparison | CC | GC+GG | OR | 95% CI | P-value |
|---|
| Sex (%) |
|
|
|
|
|
|
Male | 1,182 (53.6) | 43 (1.9) |
|
|
|
|
Female | 945 (42.8) | 36 (1.6) | 0.955 | 0.608–1.499 | 0.841 |
| Drinking habit
(%) |
|
|
|
|
|
|
Drinker | 674 (34.0) | 27 (1.4) |
|
|
|
|
Non-drinker | 1,240 (62.6) | 41 (2.1) | 1.212 | 0.739–1.988 | 0.447 |
| Smoking habit
(%) |
|
|
|
|
|
|
Smoker | 1,023 (50.4) | 38 (1.9) |
|
|
|
|
Non-smoker | 936 (46.1) | 33 (1.6) | 1.054 | 0.655–1.695 | 0.829 |
| BMI (%) |
|
|
|
|
|
| BMI
≥25 | 948 (43.4) | 36 (1.6) |
|
|
|
| BMI
<25 | 1,159 (53.0) | 43 (2.0) | 1.024 | 0.652–1.608 | 0.919 |
We examined the association of NES p.A1199P with
precancerous lesions, PanINs (Table
VI). PanIN-1A, −1B, −2 and −3 did not associate with NES
p.A1199P. In addition, there were no significance between NES
p.A1199P and low grade PanIN (PpanIN-1 and −2), or PanIN and
cancer. Presence of PanIN-3 and pancreatic cancer was associated
with NES.p.A1199P (P=0.007, Table
VI). All pancreatic cancers were invasive ductal
adenocarcinomas. Pancreatic cancer cases with GC+GG of NES p.A1199P
showed a tendency to be well differentiated as compared to CC
(P=0.085, data not shown). Sex, age, and tumor stage had no
association with NES p.A1199P.
 | Table VI.Pancreatic intraepithelial neoplasia
and NES p.A1199P. |
Table VI.
Pancreatic intraepithelial neoplasia
and NES p.A1199P.
| Variable | Type | + (%) | − (%) | OR | 95% CI | P-value |
|---|
| PanIN-1A | CC | 1,137 (51.6) | 990 (44.9) |
|
|
|
|
| GC+GG | 42 (1.9) | 36 (1.6) | 1.016 | 0.646–1.597 | 0.646 |
| PanIN-1B | CC | 911 (41.3) | 1,216 (55.1) |
|
|
|
|
| GC+GG | 31 (1.4) | 47 (2.1) | 0.880 | 0.555–1.397 | 0.595 |
| PanIN-2 | CC | 255 (11.6) | 1,870 (84.9) |
|
|
|
|
| GC+GG | 7 (0.3) | 71 (3.2) | 0.723 | 0.329–1.590 | 0.673 |
| PanIN-3 | CC | 29 (1.3) | 2,096 (95.1) |
|
|
|
|
| GC+GG | 2 (0.1) | 76 (3.4) | 1.901 | 0.446–8.130 | 0.672 |
| Low grade
PanIN | CC | 1,174 (53.2) | 953 (43.2) |
|
|
|
|
| GC+GG | 43 (2.0) | 35 (1.6) | 0.997 | 0.633–1.570 | 0.666 |
| PanIN and
cancer | CC | 1,189 (53.9) | 938 (42.5) |
|
|
|
|
| GC+GG | 44 (2.0) | 34 (1.5) | 1.020 | 0.647–1.610 | 0.672 |
| PanIN-3 and
cancer | CC | 72 (3.3) | 2,054 (93.2) |
|
|
|
|
| GC+GG | 8 (0.4) | 70 (3.2) | 3.215 | 1.493–6.944 | 0.007a |
Discussion
In the present study, we investigated the
relationship between SNPs of NES and malignant neoplasm
predispositions in autopsied Japanese patients. Our data suggests
that NES p.A1199P associates with the occurrence of pancreatic
cancer, though other malignant neoplasms did not show any
association to SNPs of NES. Furthermore, NES p.A1199P did not
associate with occurrence of PanIN, suggesting that only a small
portion of PanINs are precancerous lesions (24). The high incidence rate of PanINs and
our previous study (24) both
support this conclusion.
Morbidity and mortality of pancreatic cancers have
been increasing worldwide (30,31). In
Japan, pancreatic cancer is the fifth and fourth leading cause of
cancer-related death in men and women, respectively (32). Risk factors for pancreatic cancer are
tobacco use (33), heavy alcohol
consumption, diabetes, obesity, pancreatitis, low 25-(OH) vitamin D
levels, and aging (34,35). The vast majority of pancreatic
cancers are thought to arise from PanINs; high-grade PanINs
(carcinomas in situ) are considered as precursors of
pancreatic cancer (29,36–38).
Approximately 5–10% of patients with pancreatic cancer have family
histories of pancreatic cancer (39,40).
Recent studies for pancreatic ductal adenocarcinoma (PDAC) using
Caucasian populations have identified associations with chromosome
bands of ABO, KLF5, NR5A2, CLPTM1L-TERT (41,42);
LINC-PINT, BRCAR1, PDX1, ZNRF3, PVT1 (43); LINC00673, SUGCT and TP63 (44). A recent study also showed that three
SNPs in NR5A2, MYC and CLPTM1L-TERT represent independent risk
factors of pancreatic cancer; NR5A2 expression in the pancreatic
cancers was markedly decreased (45). In Japanese populations, SNPs of NR5A2
have shown a significant association with PDAC (46,47).
Previously, we have reported that six SNPs (rs7016880, rs10096633,
rs10503669, rs12678919, rs17482753, and rs328) that associated with
blood lipid levels were associated with the risk for pancreatic
cancer in the same cohort (24).
In the present study, we focused on SNPs of NES in
autopsied patients, because NES plays important roles in many
processes in various organ neoplasms as well as tissue
regeneration. A previous report has shown that SNPs of NES
(rs11582300 and rs3748570) were associated with early-onset
coronary heart diseases in Irish people (48). The present study is the first report
to clarify the relationship between SNPs of NES and various
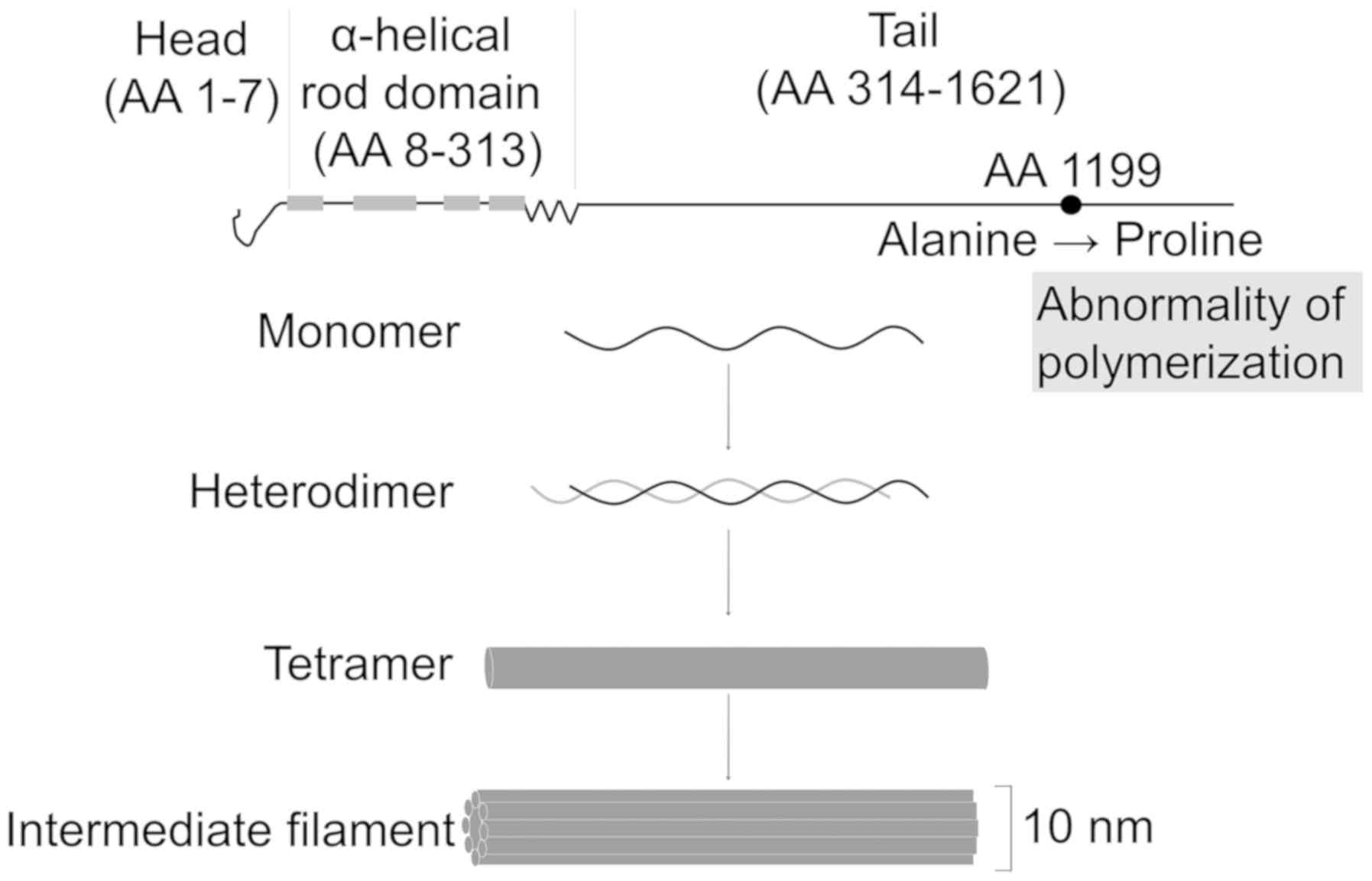
malignancies. Amino acid 1199 of NES is conserved in various
mammals including primates and pigs, and is located in the tail
lesion of NES (Fig. 1). The tail
lesion polymerizes with other IF proteins, and regulates cell
morphology, migration, and mitosis. In the present study, we did
not find any association between clinicopathological
characteristics of pancreatic cancer patients and NES p.A1199P.
Pancreatic exocrine progenitor cells of mice express NES protein
(49), and pancreatic cancer might
originate from pancreatic exocrine progenitor cells. These data
suggest that NES p.A1199P might influence carcinogenesis steps in
the pancreas. We need biological studies and a larger cohort study
to clarify molecular mechanisms of NES p.A1199P.
The present study has several limitations. The
average age of our patients is much higher than that observed in
most patients with pancreatic cancer as previously reported
(24). Japan is experiencing a
‘super-aging’ society. PDAC is projected to surpass breast,
prostate, and colorectal cancers to become the second leading cause
of cancer-related deaths by 2030 in the U.S (50). In this context, it is definitely
important to identify the characteristics of age-related pancreatic
carcinogenesis. Furthermore, the power of statistical analysis in
the present study was 48.6% between presence of pancreatic cancer
and NES p.A1199P. We need further analysis using large scale
different cohort.
In conclusion, we found that missense variations of
NES appear to affect pancreatic carcinogenesis in Japanese patients
by an undetermined mechanism.
Acknowledgements
Not applicable.
Funding
The present study was supported, in part, by a grant
from the Smoking Research Foundation and a grant-in-aid from the
Japan Society for the Promotion of Science (C; grant no. 16KT0125).
The present study was also supported by grants-in-aid from the
Ministry of Education, Culture, Sports, Science, and Technology of
Japan, GMEXT/JSPS KAKENHI Grants (grant nos. A-16H01872,
A-25242062, A-22240072, B-21390459, C-26670481, C-21590411 and
CER-24650414), Grants-in-Aid for Research on Intractable Diseases
(Mitochondrial Disorders) from the Ministry of Health, Labor, and
Welfare of Japan (grant nos. 23-016, 23–116 and 24-005), the
Practical Research Project for Rare/Intractable Diseases from the
Japan Agency for Medical Research and Development, AMED (grant nos.
15ek0109088h0001 and 15ek0109088s0401), the Takeda Science
Foundation, and the Joint Usage/Research Program of the Medical
Research Institute, Tokyo Medical and Dental University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM, MT and TA conceived and directed the project,
analyzed the data and wrote the manuscript. MS, SM, MM, MNM and TI
analyzed data and supervised. All authors have read and approved
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Tokyo
Metropolitan Geriatric Hospital Ethics Committee (approval no.
15-02). This study was conducted in accordance with the principles
embodied in the Declaration of Helsinki, 2013. Written informed
consent was obtained prior to the autopsy from the family members
of all participants involved in this study.
Patient consent for publication
Written informed consent was obtained prior to the
autopsy from the family members of all participants involved in
this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirata M, Nagai A, Kamatani Y, Ninomiya T,
Tamakoshi A, Yamagata Z, Kubo M, Muto K, Kiyohara Y, Mushiroda T,
et al: Overview of BioBank Japan follow-up data in 32 diseases. J
Epidemiol. 27 (3 Suppl):S22–S28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Srinivasan S, Clements JA and Batra J:
Single nucleotide polymorphisms in clinics: Fantasy or reality for
cancer? Crit Rev Clin Lab Sci. 53:29–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lendahl U, Zimmerman LB and McKay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishiwata T, Matsuda Y and Naito Z: Nestin
in gastrointestinal and other cancers: Effects on cells and tumor
angiogenesis. World J Gastroenterol. 17:409–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sjöberg G, Jiang WQ, Ringertz NR, Lendahl
U and Sejersen T: Colocalization of nestin and vimentin/desmin in
skeletal muscle cells demonstrated by three-dimensional
fluorescence digital imaging microscopy. Exp Cell Res. 214:447–458.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chou YH, Khuon S, Herrmann H and Goldman
RD: Nestin promotes the phosphorylation-dependent disassembly of
vimentin intermediate filaments during mitosis. Mol Biol Cell.
14:1468–1478. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sahlgren CM, Mikhailov A, Vaittinen S,
Pallari HM, Kalimo H, Pant HC and Eriksson JE: Cdk5 regulates the
organization of Nestin and its association with p35. Mol Cell Biol.
23:5090–5106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuda Y, Suzuki G, Kusano T, Kawamoto Y,
Yoshimura H, Fuse A, Yokota H, Naito Z and Ishiwata T:
Phosphorylation of Thr(1495) of nestin in a mouse model of cerebral
ischemia and reperfusion damage. Pathol Int. 63:448–456. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sahlgren CM, Mikhailov A, Hellman J, Chou
YH, Lendahl U, Goldman RD and Eriksson JE: Mitotic reorganization
of the intermediate filament protein nestin involves
phosphorylation by cdc2 kinase. J Biol Chem. 276:16456–16463. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuda Y, Yoshimura H, Ueda J, Naito Z,
Korc M and Ishiwata T: Nestin delineates pancreatic cancer stem
cells in metastatic foci of NOD/Shi-scid IL2Rγ(null) (NOG) mice. Am
J Pathol. 184:674–685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuda Y, Naito Z, Kawahara K, Nakazawa
N, Korc M and Ishiwata T: Nestin is a novel target for suppressing
pancreatic cancer cell migration, invasion and metastasis. Cancer
Biol Ther. 11:512–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuda Y, Ishiwata T, Yoshimura H, Hagio
M and Arai T: Inhibition of nestin suppresses stem cell phenotype
of glioblastomas through the alteration of post-translational
modification of heat shock protein HSPA8/HSC71. Cancer Lett.
357:602–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Narita K, Matsuda Y, Seike M, Naito Z,
Gemma A and Ishiwata T: Nestin regulates proliferation, migration,
invasion and stemness of lung adenocarcinoma. Int J Oncol.
44:1118–1130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akiyama M, Matsuda Y, Ishiwata T, Naito Z
and Kawana S: Inhibition of the stem cell marker nestin reduces
tumor growth and invasion of malignant melanoma. J Invest Dermatol.
133:1384–1387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato A, Ishiwata T, Matsuda Y, Yamamoto T,
Asakura H, Takeshita T and Naito Z: Expression and role of nestin
in human cervical intraepithelial neoplasia and cervical cancer.
Int J Oncol. 41:441–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuda Y, Ishiwata T, Yoshimura H,
Yamahatsu K, Minamoto T and Arai T: Nestin phosphorylation at
threonines 315 and 1299 correlates with proliferation and
metastasis of human pancreatic cancer. Cancer Sci. 108:354–361.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawamoto M, Ishiwata T, Cho K, Uchida E,
Korc M, Naito Z and Tajiri T: Nestin expression correlates with
nerve and retroperitoneal tissue invasion in pancreatic cancer. Hum
Pathol. 40:189–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuda Y, Kure S and Ishiwata T: Nestin
and other putative cancer stem cell markers in pancreatic cancer.
Med Mol Morphol. 45:59–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamahatsu K, Matsuda Y, Ishiwata T, Uchida
E and Naito Z: Nestin as a novel therapeutic target for pancreatic
cancer via tumor angiogenesis. Int J Oncol. 40:1345–1357.
2012.PubMed/NCBI
|
|
21
|
Liu C, Chen B, Zhu J, Zhang R, Yao F, Jin
F, Xu H and Lu P: Clinical implications for nestin protein
expression in breast cancer. Cancer Sci. 101:815–819. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuda Y, Ishiwata T, Yoshimura H,
Yamashita S, Ushijima T and Arai T: Systemic administration of
small interfering RNA targeting human nestin inhibits pancreatic
cancer cell proliferation and metastasis. Pancreas. 45:93–100.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neradil J and Veselska R: Nestin as a
marker of cancer stem cells. Cancer Sci. 106:803–811. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuda Y, Tanaka M, Sawabe M, Mori S,
Muramatsu M, Mieno MN, Furukawa T and Arai T: Relationship between
pancreatic intraepithelial neoplasias, pancreatic ductal
adenocarcinomas, and single nucleotide polymorphisms in autopsied
elderly patients. Genes Chromosomes Cancer. 57:12–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sawabe M, Arai T, Kasahara I, Esaki Y,
Nakahara K, Hosoi T, Orimo H, Takubo K, Murayama S, Tanaka N, et
al: Developments of geriatric autopsy database and Internet-based
database of Japanese single nucleotide polymorphisms for geriatric
research (JG-SNP). Mech Ageing Dev. 125:547–552. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada M, Sato N, Ikeda S, Arai T, Sawabe
M, Mori S, Yamada Y, Muramatsu M and Tanaka M: Association of the
chromodomain helicase DNA-binding protein 4 (CHD4) missense
variation p.D140E with cancer: Potential interaction with smoking.
Genes Chromosomes Cancer. 54:122–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Longnecker DS, Adsay NV, Fernandez-del
Castillo C, Hruban RH, Kasugai T, Klimstra DS, Klöppel G, Lüttges
J, Memoli VA, Tosteson TD, et al: Histopathological diagnosis of
pancreatic intraepithelial neoplasia and intraductal
papillary-mucinous neoplasms: Interobserver agreement. Pancreas.
31:344–349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hruban RH, Boffetta P, Hiraoka N,
Iacobuzio-Donahue C, Kato Y, Kern SE, Kloppel G, Marita A,
Offerhaus GJA and Pitman MB: Tumours of the pancreas. Bosman F.T,
Carneiro F, Hruban R.H and Theise N.D: WHO classification of
tumours of the digestive system. IARC; Lyon: 2010
|
|
29
|
Hruban RH, Adsay NV, Albores-Saavedra J,
Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G,
Longnecker DS, et al: Pancreatic intraepithelial neoplasia: A new
nomenclature and classification system for pancreatic duct lesions.
Am J Surg Pathol. 25:579–586. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Warshaw AL and Fernández-del Castillo C:
Pancreatic carcinoma. N Engl J Med. 326:455–465. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bidoli E, Fratino L, Bruzzone S,
Pappagallo M, De Paoli P, Tirelli U and Serraino D: Time trends of
cancer mortality among elderly in Italy, 1970–2008: An
observational study. BMC Cancer. 12:4432012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Japan TEBotCSi: Cancer statics in Japan
2017. Found Pro Cancer Res. 2018.
|
|
33
|
MacLeod SL and Chowdhury P: The genetics
of nicotine dependence: Relationship to pancreatic cancer. World J
Gastroenterol. 12:7433–7439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pandol S, Gukovskaya A, Edderkaoui M,
Dawson D, Eibl G and Lugea A: Epidemiology, risk factors, and the
promotion of pancreatic cancer: Role of the stellate cell. J
Gastroenterol Hepatol. 27 Suppl 2:S127–S134. 2012. View Article : Google Scholar
|
|
35
|
Matsuda Y, Ishiwata T, Yachida S, Suzuki
A, Hamashima Y, Hamayasu H, Yoshimura H, Honma N, Aida J, Takubo K
and Arai T: Clinicopathological features of 15 occult and 178
clinical pancreatic ductal adenocarcinomas in 8339 autopsied
elderly patients. Pancreas. 45:234–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kanda M, Matthaei H, Wu J, Hong SM, Yu J,
Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B and Goggins
M: Presence of somatic mutations in most early-stage pancreatic
intraepithelial neoplasia. Gastroenterology. 142:730–733.e9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mukada T and Yamada S: Dysplasia and
carcinoma in situ of the exocrine pancreas. Tohoku J Exp
Med. 137:115–124. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Basturk O, Hong SM, Wood LD, Adsay NV,
Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M,
Hruban RH, et al: A revised classification system and
recommendations from the baltimore consensus meeting for neoplastic
precursor lesions in the pancreas. Am J Surg Pathol. 39:1730–1741.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hruban RH, Canto MI, Goggins M, Schulick R
and Klein AP: Update on familial pancreatic cancer. Adv Surg.
44:293–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klein AP: Genetic susceptibility to
pancreatic cancer. Mol Carcinog. 51:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amundadottir L, Kraft P,
Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA,
Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, et al:
Genome-wide association study identifies variants in the ABO locus
associated with susceptibility to pancreatic cancer. Nat Genet.
41:986–990. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Petersen GM, Amundadottir L, Fuchs CS,
Kraft P, Stolzenberg-Solomon RZ, Jacobs KB, Arslan AA,
Bueno-de-Mesquita HB, Gallinger S, Gross M, et al: A genome-wide
association study identifies pancreatic cancer susceptibility loci
on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 42:224–228.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wolpin BM, Rizzato C, Kraft P, Kooperberg
C, Petersen GM, Wang Z, Arslan AA, Beane-Freeman L, Bracci PM,
Buring J, et al: Genome-wide association study identifies multiple
susceptibility loci for pancreatic cancer. Nat Genet. 46:994–1000.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Childs EJ, Mocci E, Campa D, Bracci PM,
Gallinger S, Goggins M, Li D, Neale RE, Olson SH, Scelo G, et al:
Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with
susceptibility to pancreatic cancer. Nat Genet. 47:911–916. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang M, Wang Z, Obazee O, Jia J, Childs
EJ, Hoskins J, Figlioli G, Mocci E, Collins I, Chung CC, et al:
Three new pancreatic cancer susceptibility signals identified on
chromosomes 1q32.1, 5p15.33 and 8q24.21. Oncotarget. 7:66328–66343.
2016.PubMed/NCBI
|
|
46
|
Low SK, Kuchiba A, Zembutsu H, Saito A,
Takahashi A, Kubo M, Daigo Y, Kamatani N, Chiku S, Totsuka H, et
al: Genome-wide association study of pancreatic cancer in Japanese
population. PLoS One. 5:e118242010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ueno M, Ohkawa S, Morimoto M, Ishii H,
Matsuyama M, Kuruma S, Egawa N, Nakao H, Mori M, Matsuo K, et al:
Genome-wide association study-identified SNPs (rs3790844,
rs3790843) in the NR5A2 gene and risk of pancreatic cancer in
Japanese. Sci Rep. 5:170182015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Meng W, Patterson CC, Belton C, Hughes A
and McKeown PP: Variants in the nestin gene and coronary heart
disease. Circ J. 72:1538–1539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rovira M, Scott SG, Liss AS, Jensen J,
Thayer SP and Leach SD: Isolation and characterization of
centroacinar/terminal ductal progenitor cells in adult mouse
pancreas. Proc Natl Acad Sci USA. 107:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|