Introduction
Glioblastomas (GBM), one of the most aggressive and
lethal types of brain tumor, accounts for more than half of all
gliomas (1). The GBM prognosis is
still very poor, although the standard of care has improved
survival (2,3). The dismal outcome makes GBM an urgent
subject of cancer research. Thus, detecting suitable biomarkers is
urgently needed for GBM diagnosis as well as prognosis.
Well-defined risks for GBM are radiation exposure
and certain genetic syndromes (4).
For example, mutation of PTEN, EGFR, TP53, PIK3CA, IDH1, NF1,
RB1 and PDGFRA have been identified in GBM genome,
whereas the functional consequences of many of these alterations
are still unknown (5–7). With the development of high-througput
technology, it has been well accepted that at least 90% of genome
sequences is transcribed into noncoding RNAs (ncRNAs), but <2%
of the genome encode proteins (8,9). It is
increasingly recognized that the roles of ncRNAs constitute an
important component in the most biological processes, in part due
to the findings that most of the transcripts do not encode for
proteins (10,11). According to the size of ncRNA, they
are classfied into small ncRNAs as well as long ncRNAs (lncRNAs).
Growing evidence has demonstrated that lncRNAs participate in many
kinds of biological pathways, for example, neural lineage
commitment, and immune response (12–14).
Furthermore, lncRNAs have been demonstrated to be important
regulators of disease processes (15,16).
Significantly, lncRNAs have been revealed to regulate tumor growth
and metastasis, including GBM. For example, Pastori et al
indicated that lncRNA HOTAIR plays important roles in glioblastoma
through promoting cell proliferation (17). Another study has indicated that the
oncogenic lncRNA MALAT1 is related to survival and metastasis in
GBM (18). However, the overall
study of lncRNAs in brain tumors has lagged behind (19,20).
Therefore, the extraction of lncRNA signatures is challenging.
Significantly, competing endogenous RNAs (ceRNAs)
have been reported to be crucial in illuminating how lncRNAs
regulate the expression of coding genes (21,22). For
instance, a previous study has reported that the ceRNA interaction
network for GBM reveals oncogenic pathways (23). Moreover, Zhang et al (24) have proposed the biological functions
of lncRNA associated-ceRNAs in GBM. Collectively, the dysregulated
ceRNA network might offer new hope for revealing the pathogenesis
underlying GBM.
Herein, in order to better understand the pathogenic
processes of GBM, we sought to identify the candidate lncRNA
signatures through establishing a functional lncRNA-mediated ceRNA
network (LMCN) for GBM.
Materials and methods
The specific steps of our study were divided into
the following procedures: Firstly, mRNA as well as lncRNA
expression data of GBM were obtained from the GEO database
(http://www.ncbi.nlm.nih.gov/geo/) based
on the platform of exon-array data on the Affymetrix Human 1.0 ST
Array. Simultaneously, miRNA-target interactions were downloaded
from starBase v2.0 (25). Then, a
hypergeometric test was applied to identify the competing
lncRNA-mRNA interactions, following by co-expression analysis to
extract the co-expressed lncRNA-mRNA pairs to further establish the
LMCN. Functional analyses for the mRNAs in the LMCN to reveal the
biological roles of lncRNAs.
Collecting miRNA-target interactions
and affymetrix microarray
The mRNA as well as lncRNA data of GBM (accession
number GSE9385) (26) were received
from the GEO database, which is on the basis of the (GPL5188)
platform of GeneChip Human Exon 1.0 ST Array. The GSE9385 included
26 GBMs, 22 oligodendrogliomas (ODs) as well as 6 control samples.
In our study, with the goal of better understanding the molecular
mechanisms of GBM, we only selected 26 GBMs and 6 control samples
for subsequent analysis. After the probes were mapped to the gene
symbols, we obtained a total of 35,367 genes for the following
study.
Identification of miRNA-target
interactions
starBase v2.0 provides high-quality and
experimentally validated miRNA-target interactions. In the present
study, the experimentally confirmed lncRNA-miRNA as well as
miRNA-mRNA interactions were obtained from starBase 2.0. Then, we
aligned the obtained 35,367 genes to the lncRNA-miRNA as well as
miRNA-mRNA interactions obtained from starBase 2.0, and we derived
the new expression profiles of 11,423 genes (including 212 lncRNAs
and 11,211 mRNAs). Moreover, we obtained the interactions covering
any genes of 212 lncRNAs and 11,211 mRNAs from the lncRNA-miRNA and
miRNA-mRNA interactions, respectively. Ultimately, 2551
lncRNA-miRNA interactions and 352,647 miRNA-mRNA interactions were
obtained.
Identifying potential ceRNA
interactions
Herein, to get the competing lncRNA-mRNA
interactions, the hypergeometric test was used. In an attempt to
measure the enrichment significance of the common miRNAs, we
computed the P-values. Subsequently, false discovery rate (FDR) was
utilized to correct the original P-values based on Benjamini &
Hochberg method (27). The
interactions with FDR <0.01 was considered as the significant
and competing interactions.
Co-expression analysis
With an attempt of measuring the co-expression
probability of lncRNA-mRNA interactions, we calculated the PCC
relying on the expression of the potential lncRNA-mRNAs pair. In
this study, the PCC absolute value of an edge was determined as the
weight values, and only edges having weight value >1.15 were
reserved to establish the LMCN based on Pearson correlation
coefficient. Cytoscape (28) was
employed to illustrate the highly competitive LMCN.
Topological analysis for LMCN
As documented, topological centralities (degree,
closeness as well as betweenness) are broadly utilized to reveal
the properties of network (29).
Among these centralities, degree is the simplest index. Herein, we
analyzed the degree distribution of all nodes in LMCN and we
determined the nodes having degrees greater than 50 as hubs.
Mining synergistic, competing lncRNA
modules from the LMCN
Although LMCN can supply an overall view of all
lncRNA-mRNA interactions, the sub-networks or clusters reveal a
more detailed picture of how lncRNAs synergized with the competing
mRNAs. Thus, in this study, the jActiveModule plug-in of Cytoscape
was employed to further screened out the synergistic competing
modules from the LMCN as previously described (30).
Analysis of functional annotation of
synergistic, competing lncRNA modules
DAVID is widely used to analyze genes obtained from
the genomic experiments. In the present study, we utilized the
DAVID to extract significant pathway terms enriched in genes in the
module based on the ‘guilt by association’ strategy, thereby
revealing the underlying biological process of lncRNAs. We used
Fisher's exact test to detect the significant pathways using the
criteria of FDR <0.001.
Results
Constructing a highly competitive
LMCN
To establish a highly competitive LMCN, the
hypergeometric test was first utilized to detect the candidate
lncRNA-mRNA interactions via measuring the significance of the
common miRNAs among each lncRNA-mRNA interaction. Eventually, using
the criteria of FDR <0.01, we identified 212 lncRNAs, 11,199
mRNAs, as well as 218,696 ceRNA interactions. Based on a weight
value >1.15, a highly competive LMCN was built, which included
199 lncRNAs, 2052 mRNAs, and 2715 ceRNA interactions. This LMCN is
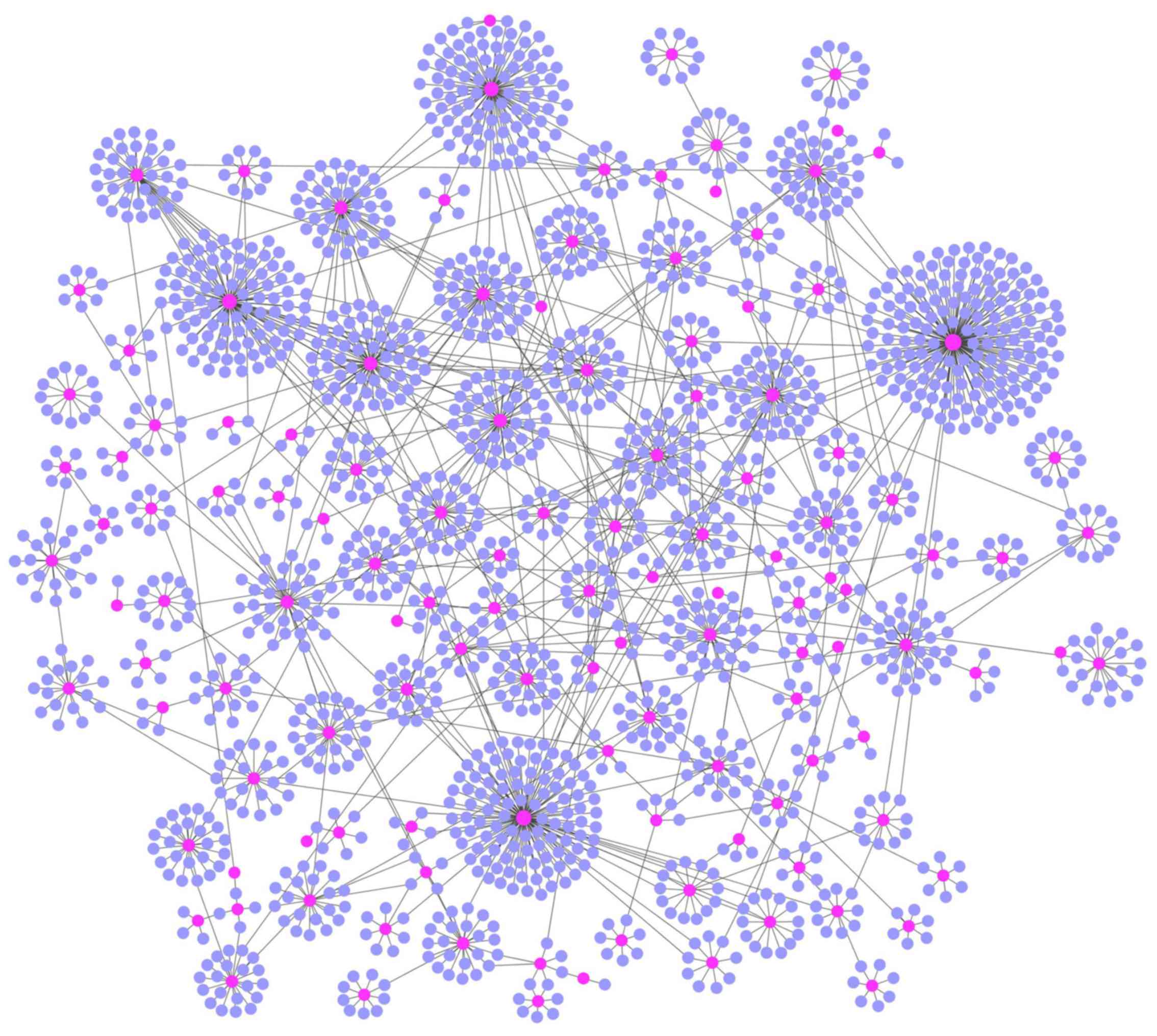
shown in Fig. 1. We observed that
the lncRNAs were distributed in the central area of the LMCN, while
the mRNAs were distributed in the outside layer (Fig. 1).
Degree distribution of the LMCN
Then, we investigated the degree properties of LMCN
to reveal the organization of the LMCN in GBM patients. The degree
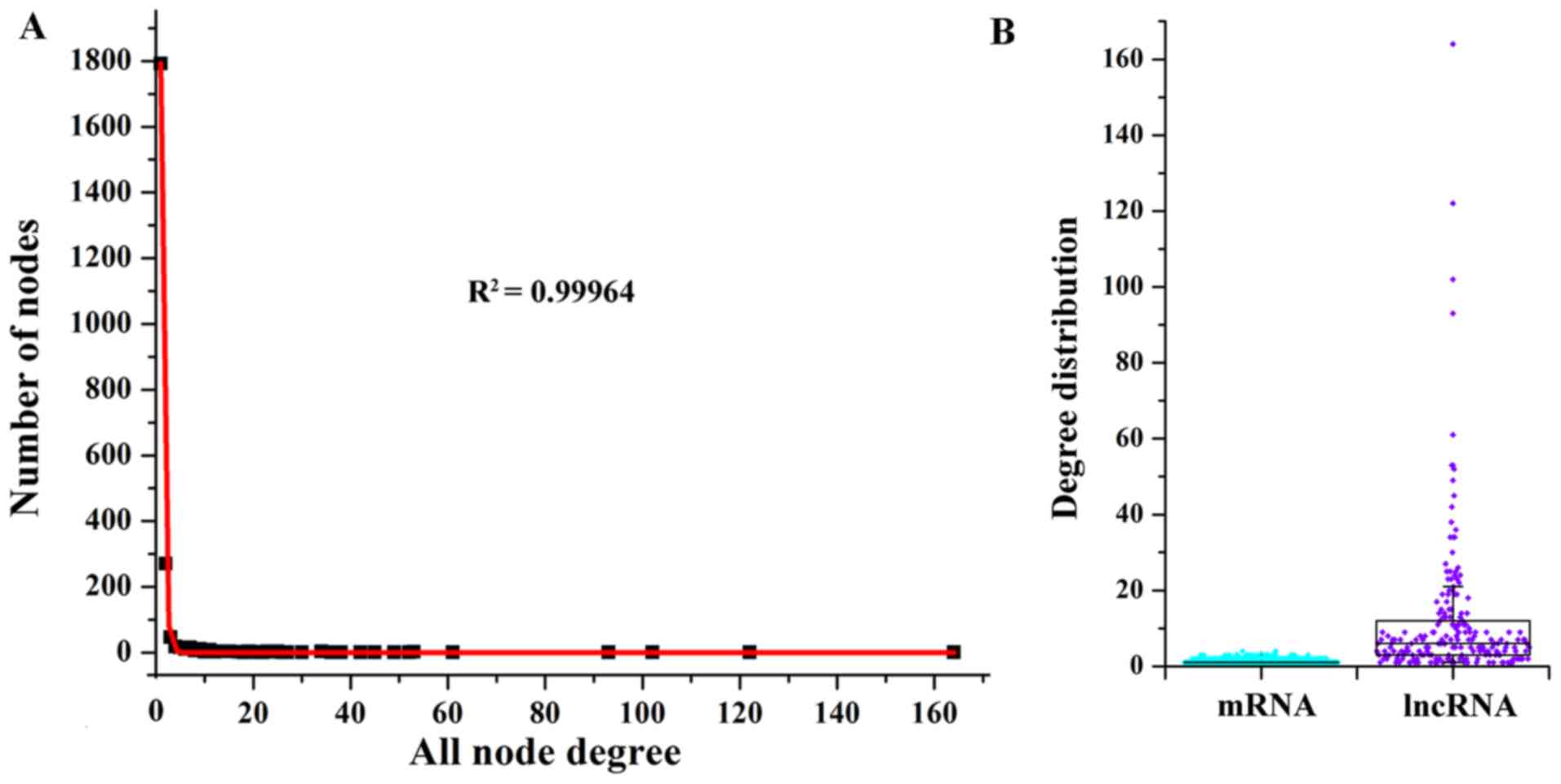
distribution of the LMCN (R2=0.99964) demonstrated power
law distributions (Fig. 2A). In
general, the node with a higher degree demonstrated a hub, which
regulated more ceRNA interactions. As displayed in Fig. 2B, the degree distribution of the
mRNAs was smaller than that of the lncRNAs, which suggested that
though lncRNAs did not encode for proteins, these lncRNAs showed
more degree distribution than mRNAs within the LMCN. On the basis
of the traits of the lncRNAs of the LMCN, some hub nodes were
considered as risk lncRNAs for GBM. With the goal of identifying
the hub nodes of the LMCN, the degrees of all nodes in the entire
network were ordered in a descending rank on the basis of their
degree distribution. Using the degrees >50 as the cut-off
threshold, there were 8 hub genes, including EPB41L4A-AS1
(degree=164), ZRANB2-AS2 (degree=122), XIST (degree=102), HOTAIR
(degree=93), TRAF3IP2-AS1 (degree=61), TPT1-AS1 (degree=53), PVT1
(degree=53), and DLG1-AS1 (degree=52).
Identifying synergistic, competing
lncRNA modules from the LMCN
To explore the modularity features of the LMCN, the
jActiveModule plug-in of Cytoscape was employed. One synergistic,
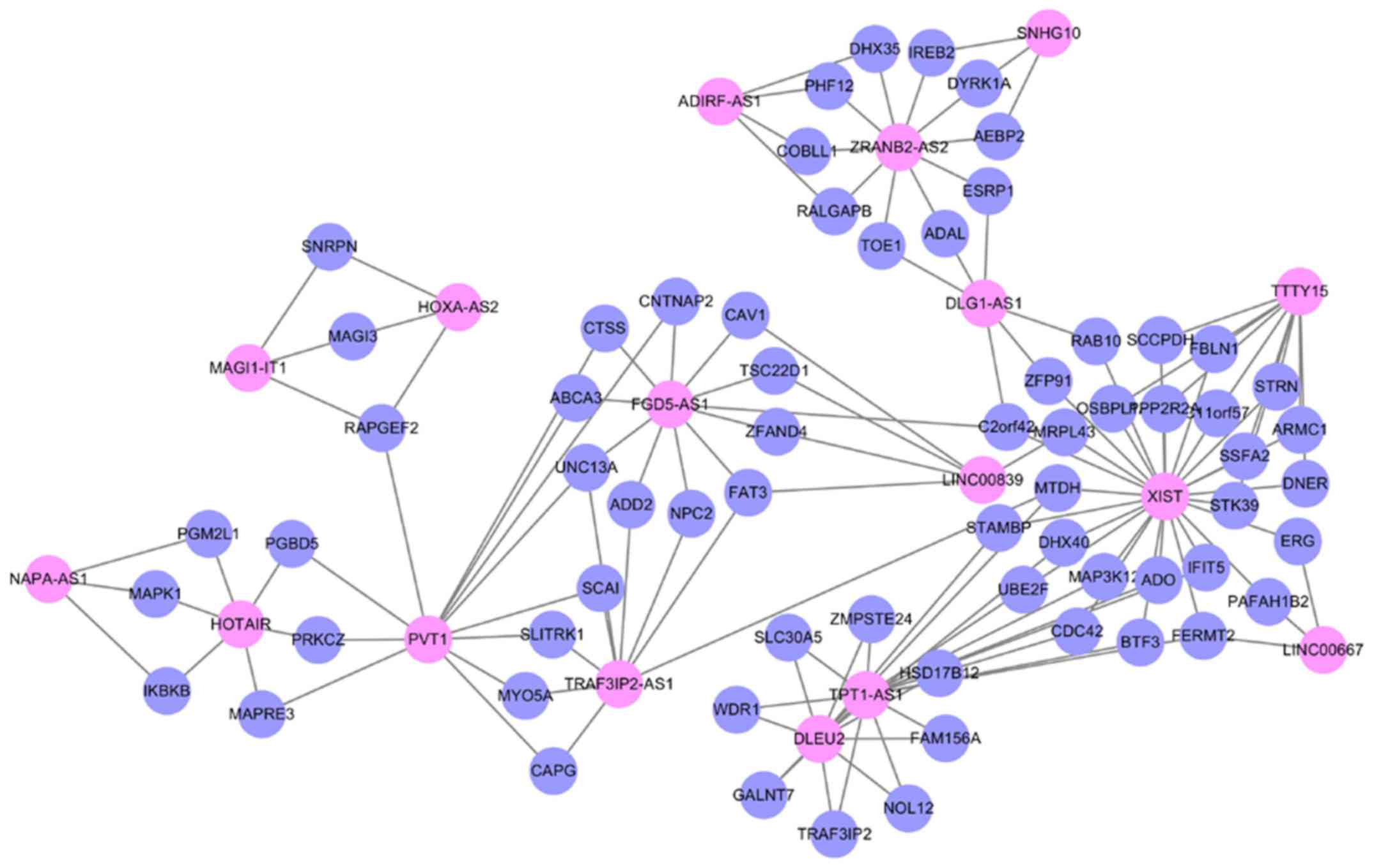
competitive module was identified, which contained 83 nodes, as
listed in Fig. 3. Similar to the
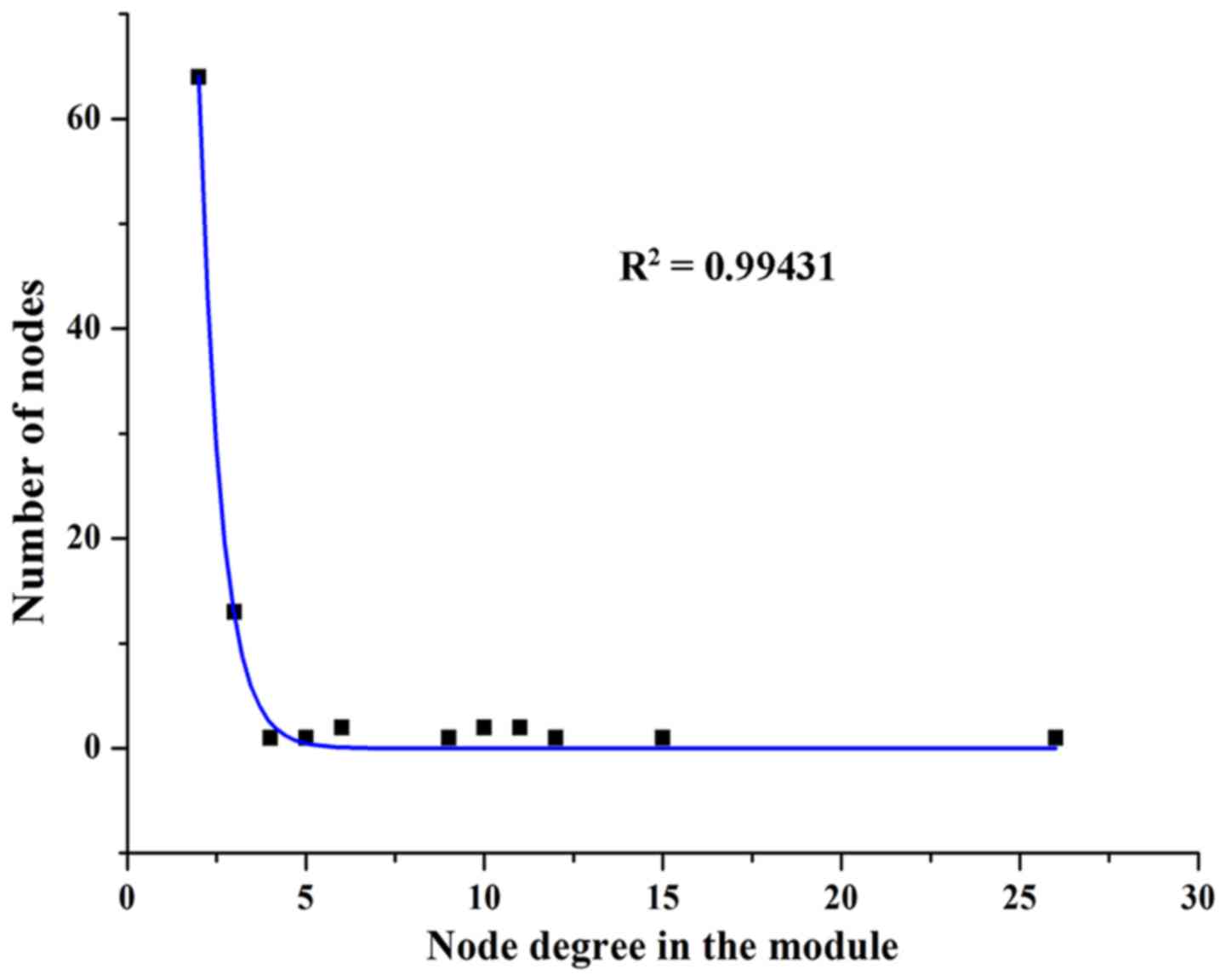
LMCN, synergistic, competitive module possessed a scale-free
characteristics with power law degree distribution (Fig. 4, R2=0.99431). Based on the
network organization, we found that lncRNA XIST competed with 26
mRNAs and 6 lncRNAs (LINC00667, TTTY15, TPT1-AS1, DLG1-AS1, DLEU2
and LINC00839) in the module (Fig.
3), suggesting its significant roles in GBM. Of note, three
lncRNAs XIST, TPT1-AS1 and PVT1 in the LMCN were also the hubs in
the synergistic, competing lncRNA module.
Analysis of functional annotation
To further investigate the biological significance
of lncRNAs in GBM, pathway enrichment analyses of mRNAs in the
synergistic, competing lncRNA module were conducted. According to
the FDR <0.001, 5 pathways enriched by the mRNAs of the module
were identified, including cytokine-cytokine receptor interaction,
neuroactive ligand-receptor interaction, and mTOR signaling
pathway. Specific information is shown in Table I.
 | Table I.The significant pathways relying on
FDR<0.001. |
Table I.
The significant pathways relying on
FDR<0.001.
| Pathway terms | FDR values |
|---|
| Neuroactive
ligand-receptor interaction |
4.28×10−12 |
| Cytokine-cytokine
receptor interaction |
4.38×10−05 |
| mTOR signaling
pathway |
1.99×10−04 |
| Oocyte meiosis |
2.40×10−04 |
| Spliceosome |
3.63×10−04 |
Discussion
The ceRNAs have been demonstrated to regulate the
expression based on the competing mechanisms, which play important
roles in diverse tumor pathological as well as physiological
processes. The ‘guilt by association’ method is typically, utilized
to explore lncRNA roles (31). Thus,
we decided to use a more comprehensive method to establish a
functional LMCN, module extraction, and functional analysis. The
results suggested that the GBM-related LMCN can be applied to
accelerate biosignature identification and therapeutic development
for GBM.
In the present study, hub lncRNAs XIST, and PVT1 in
the LMCN were also the hubs in the synergistic, competing lncRNA
module. The lncRNA XIST is the main regulator of X inactivation in
mammals (32). Previous studies have
indicated that XIST exerts critical functions in cell
proliferation, cell differentiation, and genome maintenance
(33). Significantly, a former study
has implicated that XIST is overexpressed in glioma tissues and
glioblastoma stem cells, and knockdown of XIST plays
tumor-suppressive roles via decreasing cell proliferation, and
migration as well as inducing apoptosis (34). PVT1 is an oncogene as well as a Myc
protein target known to be upregulated in transformed cells
(35). As reported, lncRNA PVT1 is
related to cell proliferation, lymph node invasion, cell apoptosis,
and metastasis (36,37). The aberrant expression of PVT1 has
been identified in cancers, including gastric cancer, prostate
cancer, and breast cancer (38).
However, no knowledge on the relationship between PVT1 and GBM has
been reported. Demonstrated here, we infer that regulating the
expression of XIST and PVT1 might be of therapeutic benefit in GBM
patients.
Neuroactive ligand-receptor interaction was the most
significant pathway in this study. Neuroactive ligand-receptor
interactions involve in signaling molecules and interactions which
exert critical functions in many cellular processes, for example,
apoptosis and cell proliferation. Moreover, apoptosis and cell
proliferation are key processes for cancer onset and progression
(39,40). Cytokine-cytokine receptor interaction
is another significant pathway. Cytokines are crucial regulators
involved in cell growth, and angiogenesis (41). As reported, cytokines can induce
inflammatory responses which are induced through binding to the
corresponding receptors of cytokines (42). Significantly, cell growth,
angiogenesis, and inflammation contribute to cancer development and
progression (43,44). Importantly, cytokine-cytokine
receptor interaction has been indicated to play key roles in cancer
(45). Remarkably, neuroactive
ligand-receptor interactions and cytokine-cytokine receptor
interactions have been indicated to be highly associated with GBM
(41).
Therefore, we suggested that altered expression of
neuroactive ligand-receptor interactions and cytokine-cytokine
receptor interactions caused GBM occurrence.
In the present study, computational method of LMCN
network was used to identify the potential lncRNAs and significant
pathways. This approach potentially helps us move closer to
understanding the biological phenomena of GBM. However, some
limitations still remained. To begin with, samples were limited. In
addition, study was performed using bioinformatics method, but was
not validated using experiments. Moreover, the data used in our
study were retrieved from the public database, not produced by us.
Therefore, it is urgent to develop chip data on GBM and to do
animal experiments to confirm our obtained findings.
In short, we identified several hub lncRNAs (such as
XIST and PVT1) and significant pathways (for instance neuroactive
ligand-receptor interactions and cytokine-cytokine receptor
interactions) for GBM based on the establishment of LMCN. These
findings might offer potential biomarkers to early diagnose, and
predict GBM prognosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XYZ conceived the study, analyzed the data and
drafted the manuscript; LL conceived the study and revised the
manuscript. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liang Y, Diehn M, Watson N, Bollen AW,
Aldape KD, Nicholas MK, Lamborn KR, Berger MS, Botstein D, Brown
PO, et al: Gene expression profiling reveals molecularly and
clinically distinct subtypes of glioblastoma multiforme. Proc Natl
Acad Sci USA. 102:5814–5819. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan J, Xiao G, Peng G, Liu D, Wang Z,
Liao Y, Liu Q, Wu M and Yuan X: MiRNA-125a-5p inhibits glioblastoma
cell proliferation and promotes cell differentiation by targeting
TAZ. Biochem Biophys Res Commun. 457:171–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al TCGA Research Network, : The somatic genomic
landscape of glioblastoma. Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parsons DW, Jones S, Zhang X, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et
al: An integrated genomic analysis of human glioblastoma
multiforme. Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frattini V, Trifonov V, Chan JM, Castano
A, Lia M, Abate F, Keir ST, Ji AX, Zoppoli P, Niola F, et al: The
integrated landscape of driver genomic alterations in glioblastoma.
Nat Genet. 45:1141–1149. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: Novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katayama S, Tomaru Y, Kasukawa T, Waki K,
Nakanishi M, Nakamura M, Nishida H, Yap CC, Suzuki M, Kawai J, et
al FANTOM Consortium, : Antisense transcription in the mammalian
transcriptome. Science. 309:1564–1566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Birney E, Stamatoyannopoulos JA, Dutta A,
Guigó R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis
ET, Thurman RE, et al Children's Hospital Oakland Research
Institute, : Identification and analysis of functional elements in
1% of the human genome by the ENCODE pilot project. Nature.
447:799–816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gomez JA, Wapinski OL, Yang YW, Bureau JF,
Gopinath S, Monack DM, Chang HY, Brahic M and Kirkegaard K: The
NeST long ncRNA controls microbial susceptibility and epigenetic
activation of the interferon-γ locus. Cell. 152:743–754. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramos AD, Andersen RE, Liu SJ, Nowakowski
TJ, Hong SJ, Gertz C, Salinas RD, Zarabi H, Kriegstein AR and Lim
DA: The long noncoding RNA Pnky regulates neuronal differentiation
of embryonic and postnatal neural stem cells. Cell Stem Cell.
16:439–447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aprea J, Prenninger S, Dori M, Ghosh T,
Monasor LS, Wessendorf E, Zocher S, Massalini S, Alexopoulou D,
Lesche M, et al: Transcriptome sequencing during mouse brain
development identifies long non-coding RNAs functionally involved
in neurogenic commitment. EMBO J. 32:3145–3160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao X, Tang Z, Zhang H, Atianjoh FE, Zhao
JY, Liang L, Wang W, Guan X, Kao SC, Tiwari V, et al: A long
noncoding RNA contributes to neuropathic pain by silencing Kcna2 in
primary afferent neurons. Nat Neurosci. 16:1024–1031. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kerin T, Ramanathan A, Rivas K, Grepo N,
Coetzee GA and Campbell DB: A noncoding RNA antisense to moesin at
5p14.1 in autism. Sci Transl Med. 4:128ra402012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pastori C, Daniel M, Penas C, Volmar CH,
Johnstone AL, Brothers SP, Graham RM, Allen B, Sarkaria JN, Komotar
RJ, et al: BET bromodomain proteins are required for glioblastoma
cell proliferation. Epigenetics. 9:611–620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Wang Y, Li J, Zhang Y, Yin H and
Han B: CRNDE, a long-noncoding RNA, promotes glioma cell growth and
invasion through mTOR signaling. Cancer Lett. 367:122–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Kiang KM, Zhang GP and Leung GK:
Long non-coding RNAs dysregulation and function in glioblastoma
stem cells. Noncoding RNA. 1:69–86. 2015.PubMed/NCBI
|
|
21
|
Ulitsky I and Bartel DP: lincRNAs:
Genomics, evolution, and mechanisms. Cell. 154:26–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lü M, Tang B, Zeng S, Hu C, Xie R, Wu Y,
Wang S, He F and Yang S: Long noncoding RNA BC032469, a novel
competing endogenous RNA, upregulates hTERT expression by sponging
miR-1207-5p and promotes proliferation in gastric cancer. Oncogene.
35:3524–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer
A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J,
et al: An extensive microRNA-mediated network of RNA-RNA
interactions regulates established oncogenic pathways in
glioblastoma. Cell. 147:370–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang K, Qi L, Kang X, Wang Y and Wang S:
Identification and functional characterization of lncRNAs acting as
ceRNA involved in the malignant progression of glioblastoma
multiforme. Oncol Rep. 36:2911–2925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
French PJ, Peeters J, Horsman S, Duijm E,
Siccama I, van den Bent MJ, Luider TM, Kros JM, van der Spek P and
Sillevis Smitt PA: Identification of differentially regulated
splice variants and novel exons in glial brain tumors using exon
expression arrays. Cancer Res. 67:5635–5642. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benjamini Y, Drai D, Elmer G, Kafkafi N
and Golani I: Controlling the false discovery rate in behavior
genetics research. Behav Brain Res. 125:279–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Osareh F, Khademi R, Rostami MK and
Shirazi MS: Co-authorship Network Structure Analysis of Iranian
Researchers' scientific outputs from 1991 to 2013 based on the
Social Science Citation Index (SSCI). Collnet J Scientometrics Inf
Manage. 8:263–271. 2014. View Article : Google Scholar
|
|
30
|
Kohl M, Wiese S and Warscheid B:
Cytoscape: software for visualization and analysis of biological
networks. Methods Mol Biol. 696:291–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo Q, Cheng Y, Liang T, He Y, Ren C, Sun
L and Zhang G: Comprehensive analysis of lncRNA-mRNA co-expression
patterns identifies immune-associated lncRNA biomarkers in ovarian
cancer malignant progression. Sci Rep. 5:17683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brown CJ, Ballabio A, Rupert JL,
Lafreniere RG, Grompe M, Tonlorenzi R and Willard HF: A gene from
the region of the human X inactivation centre is expressed
exclusively from the inactive X chromosome. Nature. 349:38–44.
1991. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weakley SM, Wang H, Yao Q and Chen C:
Expression and function of a large non-coding RNA gene XIST in
human cancer. World J Surg. 35:1751–1756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J,
Chen L, Xi Z, Teng H, Wang Z, et al: Knockdown of long non-coding
RNA XIST exerts tumor-suppressive functions in human glioblastoma
stem cells by up-regulating miR-152. Cancer Lett. 359:75–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carramusa L, Contino F, Ferro A, Minafra
L, Perconti G, Giallongo A and Feo S: The PVT-1 oncogene is a Myc
protein target that is overexpressed in transformed cells. J Cell
Physiol. 213:511–518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS
and Feng XJ: Increased expression of the lncRNA PVT1 promotes
tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol.
7:6929–6935. 2014.PubMed/NCBI
|
|
37
|
Takahashi Y, Sawada G, Kurashige J, Uchi
R, Matsumura T, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, et
al: Amplification of PVT-1 is involved in poor prognosis via
apoptosis inhibition in colorectal cancers. Br J Cancer.
110:164–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meyer KB, Maia AT, O'Reilly M, Ghoussaini
M, Prathalingam R, Porter-Gill P, Ambs S, Prokunina-Olsson L,
Carroll J and Ponder BA: A functional variant at a prostate cancer
predisposition locus at 8q24 is associated with PVT1 expression.
PLoS Genet. 7:e10021652011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lorenz HM, Herrmann M, Winkler T, Gaipl U
and Kalden JR: Role of apoptosis in autoimmunity. Apoptosis.
5:443–449. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Urrego D, Tomczak AP, Zahed F, Stühmer W
and Pardo LA: Potassium channels in cell cycle and cell
proliferation. Philos Trans R Soc Lond B Biol Sci.
369:201300942014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu H and Li L: Biological pathway
selection through nonlinear dimension reduction. Biostatistics.
12:429–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liao D and Johnson RS: Hypoxia: A key
regulator of angiogenesis in cancer. Cancer Metastasis Rev.
26:281–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weidle UH, Klostermann S, Eggle D and
Krüger A: Interleukin 6/interleukin 6 receptor interaction and its
role as a therapeutic target for treatment of cachexia and cancer.
Cancer Genomics Proteomics. 7:287–302. 2010.PubMed/NCBI
|