Introduction
Acute lymphoblastic leukemia (ALL) has an extremely
rapid progression and is more common in children aged 3–7 years
(1). According to statistics, ALL
accounts for ~80% of leukemia, and this number is rising year by
year (2). Data show that, in 2016,
the number of new ALL patients in the world exceeded 2.1 million,
and the cumulative number of patients reached >300 million
(3,4). Because of the rapid progression of ALL,
there is a high probability of death caused by the absence of
effective treatment within 5–10 days after its onset (5). As there are no obvious special symptoms
in the early stages of ALL, many patients often miss the best
treatment period because of limited medical general knowledge or
because they are misdiagnosed, which is also one of the key
problems that lead to poor long-term prognosis in ALL (6). It has been reported that the 5-year
survival rate of the prognosis of ALL patients corresponds to only
20–40% (7). Because of its high
incidence and high risk, ALL has been included in the key research
of disease, but its pathogenesis has not yet obtained breakthrough
research results. In clinic, methotrexate (MTX) is the most common
drug in the treatment of leukemia, lymphadenoma, osteosarcoma and
other autoimmune diseases (8).
MTX, an oncology drug of antifolate metabolism, that
has a similar chemical structure to folic acid and can inhibit DNA
synthesis by inhibiting the activity of dihydrofolate reductase in
cells, has been proven to have a high application value in ALL
(9–11). With the deepening of research, an
increasing number of scholars worldwide consider that the efficacy
of high-dose MTX in the treatment of ALL is more significant
(12–14). However, most of the current studies
are limited to the wide application of MTX, and there is little
research on the exact effect of MTX in the treatment of ALL
patients with different subtypes and different disease courses.
Since 2014, the People's Hospital of Pingyi County (Linyi, China)
has begun to promote the use of MTX in clinical practice as the
first choice for the treatment of ALL, and has accumulated a large
number of sample cases. Therefore, by comparing efficacy
differences of high-dose MTX in ALL patients with different
subtypes and disease courses, the efficacy of high-dose MTX in the
treatment of ALL was studied in depth to provide reference and
guidance for clinical practice.
Patients and methods
Patients
A retrospective analysis of 207 children with ALL,
treated with high-dose MTX in The People's Hospital of Pingyi
County from March 2014 to June 2017, was carried out, including 124
males and 83 females, with an age range of 3–11 years and an
average age of 6.13±3.27 years. Inclusion criteria: children
conforming to the clinical manifestations of ALL (15) and diagnosed with ALL in the People's
Hospital of Pingyi County; children with complete data; children
not receiving related medical treatment in other hospitals.
Exclusion criteria: children unwilling to receive relevant medical
treatment; children allergic to drugs; children with other serious
cardiovascular diseases or tumors; children with severe liver and
renal insufficiency; children with communication or cognitive
impairment. This study was approved by the Ethics Committee of the
People's Hospital of Pingyi County. Guardians of the patients
signed informed consent and agreed that the tissue of the patient
could be used in this study, and cooperated with the medical staff
to complete the diagnosis and treatment.
Main reagents
MTX was purchased from Beijing Baiaolaibo Technology
Co., Ltd., Beijing, China (cat. no. QN0838-IKA). Dexamethasone was
purchased from Hubei Yuancheng Saichuang Technology Co., Ltd.,
Wuhan, China (cat. no. 50-02-2). Cytosine arabinoside was purchased
from Shanghai Baoman Biotechnology Co., Ltd., Shanghai, China (cat.
no. N0072). Calcium formyltetrahydrofolate (CF) was purchased from
Shanghai Yijing industrial Co., Ltd., Shanghai, China (cat. no.
151533-22-1).
Methods
All patients received chemotherapy according to the
2013 Leukemia Diagnosis and Treatment Guidelines (16). Intravenous infusion of 1/6 of the
total amount of MTX (≤500 mg each time) was performed within 30
min, and the remaining 5/6 was administered within 24 h. A triple
sheath injection was performed 1 h after the infusion of the first
1/6 MTX (including MTX 12.5 mg, dexamethasone 5 mg, cytosine
arabinoside 35 mg). CF rescue (15 mg/m2) was performed
after 12 h of complete infusion of MTX, and in the case of delayed
excretion, the number and dose of CF rescue were increased.
Sufficient hydration (3 l/m2) and urine alkalization (pH
>8) were conducted on the same day and 3 days after
administration. The changes of the vital signs in children were
monitored during chemotherapy. Venous blood (2 ml) was drawn in the
anticoagulant tube on an empty stomach at 12 h (T1), 48 h (T2), 72
h (T3) after infusion, and separated by centrifuging at 2,500 × g
for 15 min at 4°C to collect serum. The concentration of MTX in the
serum of children and the adverse reactions were determined by
Siemens Viva-E automatic drug concentration monitoring system
(Siemens AG, Munich, Germany), including bone marrow suppression,
gastrointestinal reaction and liver function injury.
Statistical analysis
SPSS 24.0 software (Beijing Strong Vinda Information
Technology Co., Ltd., Beijing, China) was used for statistical
analysis. The clinical enumeration data of children with different
subtype of ALL and different course of disease were expressed as
percentage [n (%)] and analyzed using χ2 test. The
measurement data were expressed as mean ± standard deviation and
t-test was used for their comparison. F analysis was used to
compare the differences between multiple groups. LSD test was used
as a post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of clinical data of ALL
children with different subtypes
Of the 207 children, there were 128 cases of
B-lineage (B-lineage group) and 79 cases of T-lineage (T-lineage
group). There was no significant difference between the two groups
of patients regarding the clinical data of age, body weight, living
environment, whether he/she was the only child, whether there was a
family history of hereditary disease, and whether it was the first
onset (P>0.05), proving that the two groups of patients were
comparable (Table I).
 | Table I.Clinical data of children with
different subtypes [n (%)]. |
Table I.
Clinical data of children with
different subtypes [n (%)].
| Factor | B-lineage group
(n=128) | T-lineage group
(n=79) | χ2 or t
value | P-value |
|---|
| Age (years) | 5.94±3.11 | 6.23±2.97 | 0.663 | 0.508 |
| Body weight (kg) | 27.62±8.94 | 28.92±9.14 | 1.008 | 0.315 |
| Living
environment |
|
| 0.451 | 0.502 |
|
Urban | 75 (58.59) | 50 (63.29) |
|
|
|
Rural | 53 (41.41) | 29 (36.71) |
|
|
| Only child |
|
| 0.044 | 0.834 |
| Yes | 71 (55.47) | 45 (56.96) |
|
|
| No | 57 (44.53) | 34 (43.04) |
|
|
| Family history of
hereditary diseases |
|
| 0.076 | 0.783 |
| Yes | 35 (27.34) | 23 (29.11) |
|
|
| No | 93 (72.66) | 56 (70.89) |
|
|
| First onset |
|
| 0.131 | 0.718 |
| Yes | 96 (75.00) | 61 (77.22) |
|
|
| No | 32 (25.00) | 18 (22.79) |
|
|
Comparison of plasma concentration and
CF rescue times in ALL children with different subtypes
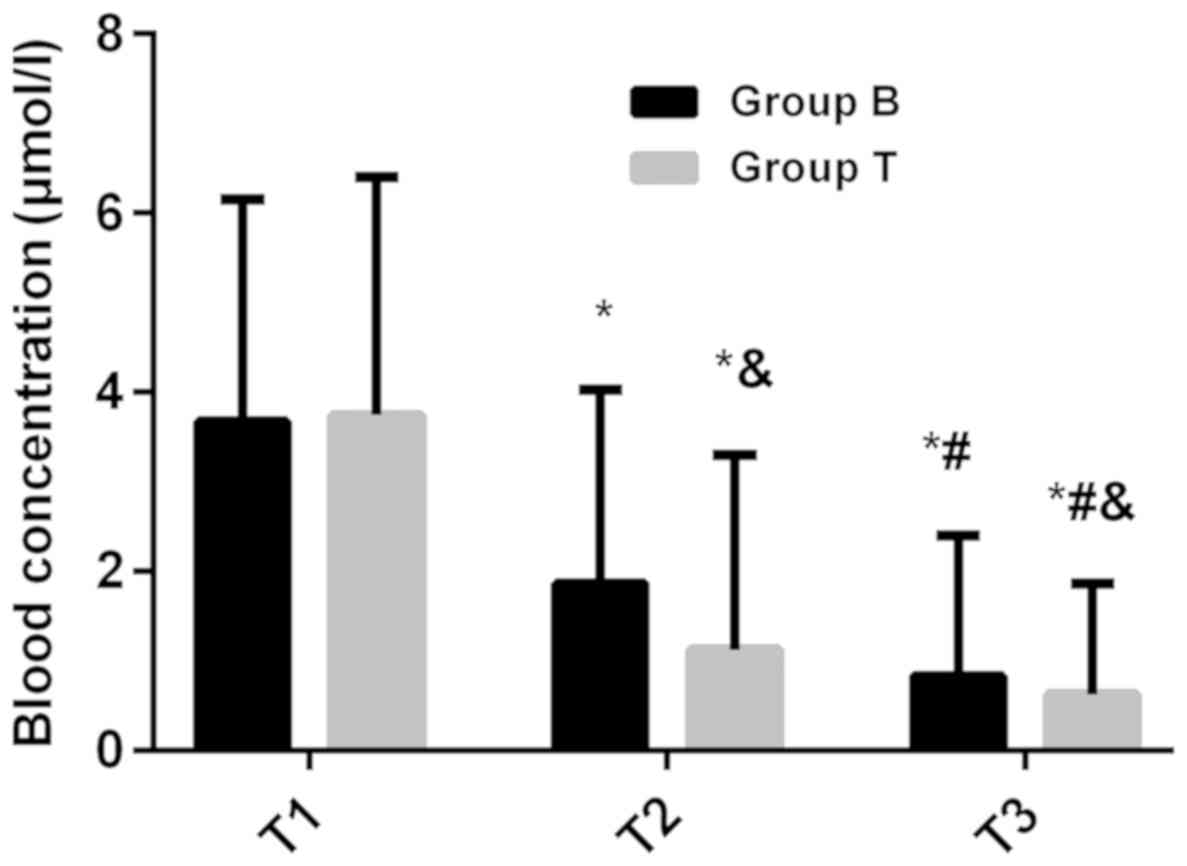
There was no significant difference in plasma
concentration between the two groups at T1 (P>0.05). The plasma
concentration in B-lineage group (1.86±2.17 µmol/l) was
significantly higher than that in T-lineage group (1.12±2.18
µmol/l) at T2 (P<0.05). The plasma concentration in B-lineage
group (0.83±1.57 µmol/l) was also significantly higher than that in
T-lineage group (0.62±1.24 µmol/l) at T3 (P<0.05). The plasma
concentration at T2 was significantly lower than that at T1, and at
T3 was lower than that at T2 in both groups (P<0.05). There was
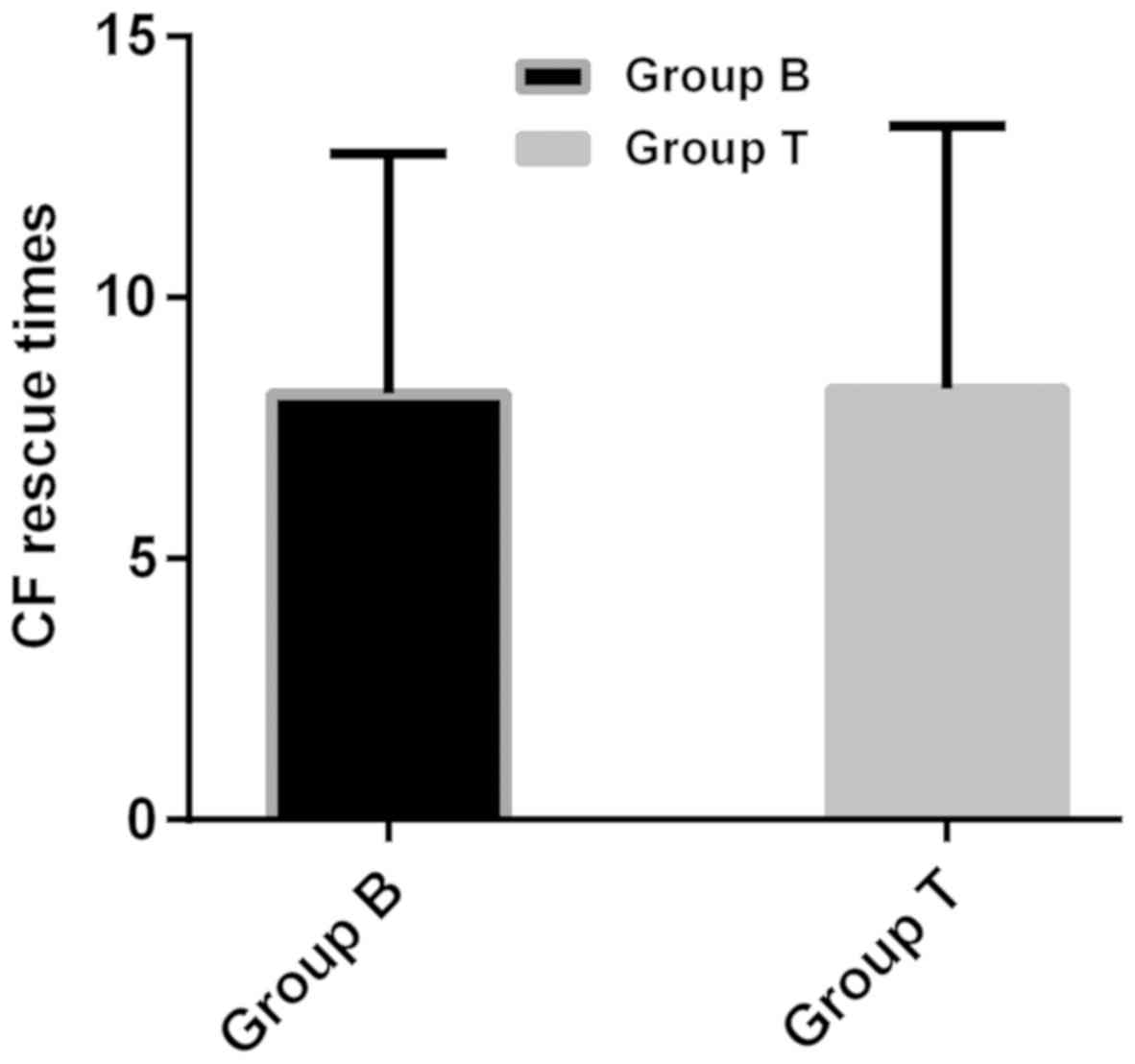
no significant difference in CF rescue times between B-lineage
group (8.17±4.58 times) and T-lineage group (8.26±5.03 times)
(P>0.05) (Figs. 1 and 2).
Comparison of adverse reactions in ALL
children with different subtypes
The incidence of adverse reactions in children with
ALL in B-lineage group was significantly higher than that in the
T-lineage group, and the difference was statistically significant
(P<0.05) (Table II).
 | Table II.Adverse reactions in children with
different subtypes [n (%)]. |
Table II.
Adverse reactions in children with
different subtypes [n (%)].
| Adverse reaction | B-lineage group
(n=128) | T-lineage group
(n=79) | χ2
value | P-value |
|---|
| Bone marrow
suppression | 11 (8.59) | 3 (3.80) |
1.782 | 0.182 |
| Gastrointestinal
reaction | 37
(28.91) | 16 (20.25) |
1.920 | 0.166 |
| Skin allergic
reaction | 12 (9.38) | 6 (7.60) |
0.195 | 0.659 |
| Liver function
injury | 8
(6.25) | 2 (2.53) |
1.469 | 0.226 |
| Respiratory tract
reaction | 16 (12.5) | 7 (8.86) |
0.655 | 0.418 |
| Adverse reaction rate
(%) | 84
(65.63) | 34 (43.04) | 10.170 | 0.001 |
Comparison of clinical data of
children with different disease courses
According to the disease course, the children were
divided into three groups. High-risk group: disease course >15
days (n=67); moderate-risk group: disease course >8 and <15
days (n=58); low-risk group: disease course <8 days (n=82).
There was no significant difference between the three groups in the
clinical data of age, body weight, living environment, whether
he/she was the only child, whether there was a family history of
hereditary disease, and whether it was the first onset (P>0.05),
confirming that the three groups of patients were comparable
(Table III).
 | Table III.Clinical data of children with
different disease courses [n (%)]. |
Table III.
Clinical data of children with
different disease courses [n (%)].
| Factor | High-risk group
(n=67) | Moderate-risk group
(n=58) | Low-risk group
(n=82) | F/χ2
value | P-value |
|---|
| Age (years) | 5.32±2.86 | 6.17±2.35 | 5.81±3.02 | 1.467 | 0.233 |
| Body weight
(kg) | 26.69±7.37 | 27.36±7.84 | 28.23±8.58 | 0.694 | 0.501 |
| Living
environment |
|
|
| 0.574 | 0.615 |
|
Urban | 37 (55.22) | 35 (60.34) | 53 (64.63) |
|
|
|
Rural | 30 (44.78) | 23 (39.66) | 29 (35.37) |
|
|
| Only child |
|
|
| 1.068 | 0.447 |
|
Yes | 35 (52.24) | 39 (67.24) | 42 (51.22) |
|
|
| No | 32 (47.76) | 19 (32.76) | 40 (48.78) |
|
|
| Family history of
hereditary diseases |
|
|
| 0.150 | 0.867 |
|
Yes | 23 (34.33) | 14 (24.14) | 21 (25.61) |
|
|
| No | 44 (65.67) | 44 (75.86) | 61 (74.39) |
|
|
| First onset |
|
|
| 0.094 | 0.913 |
|
Yes | 46 (68.66) | 40 (68.97) | 71 (86.59) |
|
|
| No | 21 (31.34) | 18 (31.03) | 11 (13.41) |
|
|
Comparison of plasma concentration and
CF rescue times in children with different disease courses
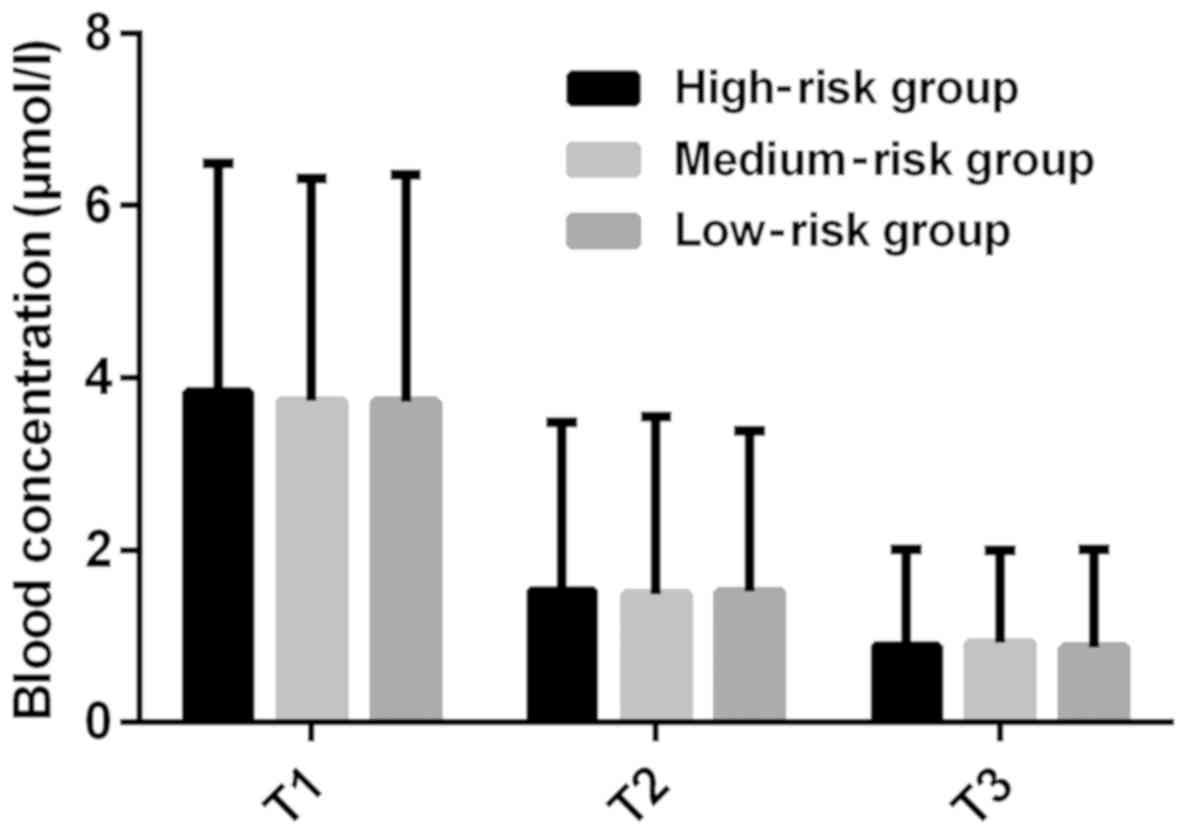
There was no significant difference in plasma
concentration at T1, T2 and T3 between the three groups
(P>0.05). The plasma concentration at T2 was lower than that at
T1, and at T3 was lower than that at T2 in the three groups, and
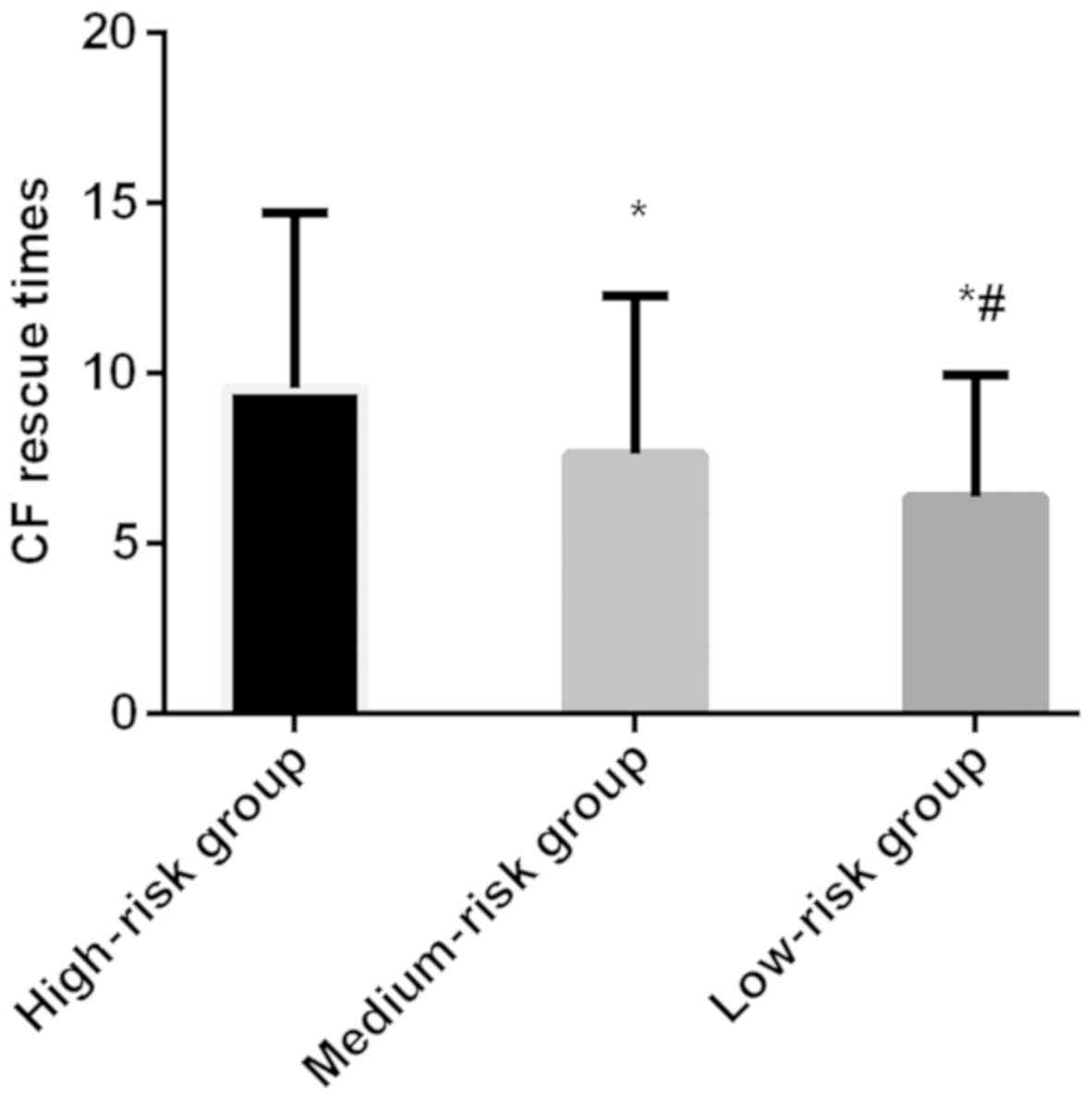
the differences were statistically significant (P<0.05). The CF
rescue times in high-risk group (9.57±5.16 times) were more than
that in moderate-risk group (7.63±4.66 times) and low-risk group
(6.37±3.58 times), and the differences were statistically
significant (P<0.05) (Figs. 3 and
4).
Comparison of adverse reactions in
children with different disease courses
The incidence of a gastrointestinal reaction, skin
allergic reaction and respiratory tract reaction, as well as the
adverse reaction rate in the high- and moderate-risk groups were
significantly higher than that in the low-risk group (P<0.05).
Also, the adverse reactions rate in the high-risk group was
significantly higher than that in the low-risk group (P<0.05)
(Table IV).
 | Table IV.Adverse reactions in children with
different disease courses [n (%)]. |
Table IV.
Adverse reactions in children with
different disease courses [n (%)].
| Adverse
reaction | High-risk group
(n=67) | Moderate-risk group
(n=58) | Low-risk group
(n=82) | F value | P-value |
|---|
| Bone marrow
suppression | 9 (13.43) | 4 (6.90) | 1 (1.22) | 0.039 | 0.962 |
| Gastrointestinal
reaction | 24 (35.82) | 18
(31.03)a | 11
(13.41)a | 0.099 | 0.908 |
| Skin allergic
reaction | 9 (13.43) | 7
(12.07)a | 2
(2.44)a | 0.042 | 0.959 |
| Liver function
injury | 5 (7.46) | 3 (5.17) | 2 (2.44) | 0.037 | 0.965 |
| Respiratory tract
reaction | 13 (19.40) | 6 (10.35) | 4 (4.88) | 0.048 | 0.954 |
| Adverse reaction
rate (%) | 60 (89.55) | 38
(65.52)a | 20
(24.39)a,b | 0.090 | 0.916 |
Discussion
As an antifolate metabolite, MTX combined with
dihydrofolate reductase can inhibit the conversion of dihydrofolic
acid to tetrahydrofolic acid, block the synthesis of pyrimidine and
purine, inhibit the synthesis of DNA, thus achieving an antitumor
effect (17). MTX is usually not
able to penetrate the blood-brain barrier and is difficult to be
detected in cerebrospinal fluid during tumor therapy (18). To achieve the goal of treating
leukocyte, the concentration of MTX in cerebrospinal fluid needs to
be >107 µmol/l, at which time the inhibitory effect
on the synthesis of DNA in leukemic cells could be completed
(19). Therefore, the dosage of MTX
needs to be increased. When MTX is highly concentrated and
continuously injected into the patient's body, it can enter the
cells through the reductive folate carrier on the cell membrane,
thus affecting leukemic cells (20).
MTX can affect leukemic cells, but also produce toxicity to normal
cells of patients, so it is necessary to carry out CF rescue in the
treatment of MTX (21), which can
reduce the toxicity of MTX and increase the effectiveness of the
treatment by providing tetrahydrofolate coenzyme to the normal
cells of the patient (22).
In the present study, the analysis on the efficacy
differences of high-dose MTX in ALL patients with different
subtypes and disease courses showed that the plasma concentration
of B-lineage ALL children with different subtypes is significantly
higher than that of T-lineage ALL children after 48 h of MTX
infusion, and the incidence of adverse reactions of B-lineage ALL
children is significantly higher than that of T-lineage ALL
children, which indicates that MTX is less toxic and more suitable
for T-lineage ALL children. The reason is presumed to be that
methylene tetrahydrofolate reductase and γ-glutamyl transferase are
very important metabolites during the treatment of MTX, and the
higher their expression, the better metabolic circulation
conditions of MTX in children (23).
B-lineage ALL originates from B-lineage lymphocytes that are mainly
distributed in human plasma, and belong to the first line of
defense (24). The onset of B
lymphoid causes the patient's plasma antibody ability to be in an
extremely poor condition, and then the toxicity caused by the
injection of MTX begins to invade the patient's blood, but the
blood tissues cannot resist the toxicity of MTX and could only be
counteracted by the injection of CF. Therefore, the toxic effect of
MTX in vivo is greater than its metabolic effect, which
results in higher plasma concentration in patients with B-lineage
ALL. T-lineage ALL originates from T-lineage lymphocytes that are
mainly distributed in the cell membrane and play a role in immune
metabolism through surface antigen and surface receptor (25). Therefore, the immune metabolic
function of B cells in patients can form the first filter device
after the MTX injection, and the toxicity of MTX can be eliminated
completely after CF injection, which indicates that MTX can
directly undergo metabolic reactions for effective treatment after
entering the cells of the patient. The results of Conter et
al (26) in the study of
high-dose MTX in ALL patients are consistent with this experiment.
Among patients with different disease courses, we found that there
is no significant difference in plasma concentration among the
three groups at different time-points. Also, the adverse reaction
rate in the high-risk group was found to be significantly higher
than that in moderate- and low-risk groups, and in the
moderate-risk group was significantly higher than that in the
low-risk group. Moreover, the CF rescue times in high-risk group
were found to be more than that in the other two groups, which
suggested that high-dose MTX is more toxic to children with more
severe disease. The cause may be that the disease course in the
high-risk group is significantly higher than that in the other two
groups, and the internal environment of children is severely
damaged by leukemic cells, and is totally unable to resist the
incidental toxicity of MTX injected into the body, and only
continuous CF rescue can neutralize the efficacy of MTX. Therefore,
more attention should be paid to children with serious ALL in
clinic. The vital signs of children should be paid close attention
in order to prevent the toxic effect of MTX treatment from being
greater than its efficacy and having a negative effect on the
children.
The purpose of this study was to analyze the
efficacy differences of high-dose MTX in ALL children with
different subtypes and disease courses. However, because of the
limited experimental conditions, there are still some shortcomings,
such as the small base of the study subjects for statistical
analysis. Also there may be differences in the results among
different ethnic and age groups. Thus, further studies are still
needed.
In conclusion, compared with T-lineage ALL children,
high-dose MTX caused more toxic injury to B-lineage ALL children.
During clinical application of MTX in the treatment of ALL, close
attention should be paid to the changes of vital signs of patients,
and timely CF rescue should be performed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FG and QM were responsible for the treatment of the
patients. CL and YZ analyzed the patient data and revised the
manuscript. FG drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the People's Hospital of Pingyi County (Linyi, China). Signed
informed consents were obtained from the guardians of the
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang W, Zimmerman G, Huang X, Yu S, Myers
J, Wang Y, Moreton S, Nthale J, Awadallah A, Beck R, et al:
Aberrant Notch signaling in the bone marrow microenvironment of
acute lymphoid leukemia suppresses osteoblast-mediated support of
hematopoietic niche function. Cancer Res. 76:1641–1652. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nicolato A, Nouér SA, Garnica M, Portugal
R, Maiolino A and Nucci M: Invasive fungal diseases in patients
with acute lymphoid leukemia. Leuk Lymphoma. 57:2084–2089. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Jawahri A, Li S, Ballen KK, Cutler C,
Dey BR, Driscoll J, Hunnewell C, Ho VT, McAfee SL, Poliquin C, et
al: Phase II trial of reduced-intensity busulfan/clofarabine
conditioning with allogeneic hematopoietic stem cell
transplantation for patients with acute myeloid leukemia,
myelodysplastic syndromes, and acute lymphoid leukemia. Biol Blood
Marrow Transplant. 22:80–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duque-Afonso J, Lin CH, Han K, Wei MC,
Feng J, Kurzer JH, Schneidawind C, Wong SH, Bassik MC and Cleary
ML: E2A-PBX1 remodels oncogenic signaling networks in B-cell
precursor acute lymphoid leukemia. Cancer Res. 76:6937–6949. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fitzgerald JC, Weiss SL, Maude SL, Barrett
DM, Lacey SF, Melenhorst JJ, Shaw P, Berg RA, June CH, Porter DL,
et al: Cytokine release syndrome after chimeric antigen receptor T
cell therapy for acute lymphoblastic leukemia. Crit Care Med.
45:e124–e131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jacoby E, Nguyen SM, Fountaine TJ, Welp K,
Gryder B, Qin H, Yang Y, Chien CD, Seif AE, Lei H, et al: CD19 CAR
immune pressure induces B-precursor acute lymphoblastic leukaemia
lineage switch exposing inherent leukaemic plasticity. Nat Commun.
7:123202016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frey NV and Porter DL: Cytokine release
syndrome with novel therapeutics for acute lymphoblastic leukemia.
Hematology (Am Soc Hematol Educ Program). 2016:567–572. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferreri AJ, Cwynarski K, Pulczynski E,
Ponzoni M, Deckert M, Politi LS, Torri V, Fox CP, Rosée PL, Schorb
E, et al International Extranodal Lymphoma Study Group (IELSG), :
Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and
rituximab (MATRix regimen) in patients with primary CNS lymphoma:
Results of the first randomisation of the International Extranodal
Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol.
3:e217–e227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Larsen EC, Devidas M, Chen S, Salzer WL,
Raetz EA, Loh ML, Mattano LA Jr, Cole C, Eicher A, Haugan M, et al:
Dexamethasone and high-dose methotrexate improve outcome for
children and young adults with high-risk B-acute lymphoblastic
leukemia: A report from Children's Oncology Group Study AALL0232. J
Clin Oncol. 34:2380–2388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmiegelow K, Attarbaschi A, Barzilai S,
Escherich G, Frandsen TL, Halsey C, Hough R, Jeha S, Kato M, Liang
DC, et al Ponte di Legno toxicity working group, : Consensus
definitions of 14 severe acute toxic effects for childhood
lymphoblastic leukaemia treatment: A Delphi consensus. Lancet
Oncol. 17:e231–e239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krull KR, Cheung YT, Liu W, Fellah S,
Reddick WE, Brinkman TM, Kimberg C, Ogg R, Srivastava D, Pui CH, et
al: Chemotherapy pharmacodynamics and neuroimaging and
neurocognitive outcomes in long-term survivors of childhood acute
lymphoblastic leukemia. J Clin Oncol. 34:2644–2653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asselin BL, Devidas M, Chen L, Franco VI,
Pullen J, Borowitz MJ, Hutchison RE, Ravindranath Y, Armenian SH,
Camitta BM, et al: Cardioprotection and safety of dexrazoxane in
patients treated for newly diagnosed T-cell acute lymphoblastic
leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: A
report of the Children's Oncology Group Randomized Trial Pediatric
Oncology Group 9404. J Clin Oncol. 34:854–862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iparraguirre L, Gutierrez-Camino A, Umerez
M, Martin-Guerrero I, Astigarraga I, Navajas A, Sastre A, Garcia de
Andoin N and Garcia-Orad A: MiR-pharmacogenetics of methotrexate in
childhood B-cell acute lymphoblastic leukemia. Pharmacogenet
Genomics. 26:517–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wojtuszkiewicz A, Raz S, Stark M, Assaraf
YG, Jansen G, Peters GJ, Sonneveld E, Kaspers GJ and Cloos J:
Folylpolyglutamate synthetase splicing alterations in acute
lymphoblastic leukemia are provoked by methotrexate and other
chemotherapeutics and mediate chemoresistance. Int J Cancer.
138:1645–1656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jabbour E and Kantarjian H: Chronic
myeloid leukemia: 2014 update on diagnosis, monitoring, and
management. Am J Hematol. 89:547–556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burnett AK, Russell NH, Hills RK, Hunter
AE, Kjeldsen L, Yin J, Gibson BE, Wheatley K and Milligan D:
Optimization of chemotherapy for younger patients with acute
myeloid leukemia: Results of the medical research council AML15
trial. J Clin Oncol. 31:3360–3368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hough R, Rowntree C, Goulden N, Mitchell
C, Moorman A, Wade R and Vora A: Efficacy and toxicity of a
paediatric protocol in teenagers and young adults with Philadelphia
chromosome negative acute lymphoblastic leukaemia: Results from
UKALL 2003. Br J Haematol. 172:439–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoelzer D, Bassan R, Dombret H, Fielding
A, Ribera JM and Buske C; ESMO Guidelines Committee, : Acute
lymphoblastic leukaemia in adult patients: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
Suppl 5:v69–v82. 2016.PubMed/NCBI
|
|
19
|
Liang DC, Yang CP, Liu HC, Jaing TH, Chen
SH, Hung IJ, Yeh TC, Lin TH, Lai CL, Lai CY, et al: NUDT15 gene
polymorphism related to mercaptopurine intolerance in Taiwan
Chinese children with acute lymphoblastic leukemia.
Pharmacogenomics J. 16:536–539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Al-Ghobashy MA, Hassan SA, Abdelaziz DH,
Elhosseiny NM, Sabry NA, Attia AS and El-Sayed MH: Development and
validation of LC-MS/MS assay for the simultaneous determination of
methotrexate, 6-mercaptopurine and its active metabolite
6-thioguanine in plasma of children with acute lymphoblastic
leukemia: Correlation with genetic polymorphism. J Chromatogr B
Analyt Technol Biomed Life Sci. 1038:88–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Howard SC, McCormick J, Pui CH, Buddington
RK and Harvey RD: Preventing and managing toxicities of high-dose
Methotrexate. Oncologist. 21:1471–1482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carbonnel F, Colombel JF, Filippi J,
Katsanos KH, Peyrin-Biroulet L, Allez M, Nachury M, Novacek G,
Danese S, Abitbol V, et al: European Crohn's and Colitis
Organisation; Groupe d'Étude Thérapeutique des Affections
Inflammatoires Digestives: Methotrexate is not superior to placebo
for inducing steroid-free remission, but induces steroid-free
clinical remission in a larger proportion of patients with
ulcerative colitis. Gastroenterology. 150:380–388.e4. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamanaka H, Tanaka Y, Takeuchi T, Sugiyama
N, Yuasa H, Toyoizumi S, Morishima Y, Hirose T and Zwillich S:
Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with
background methotrexate, in Japanese patients with rheumatoid
arthritis: An open-label, long-term extension study. Arthritis Res
Ther. 18:342016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gitlin AD, von Boehmer L, Gazumyan A,
Shulman Z, Oliveira TY and Nussenzweig MC: Independent roles of
switching and hypermutation in the development and persistence of B
lymphocyte memory. Immunity. 44:769–781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woessmann W, Seidemann K, Mann G,
Zimmermann M, Burkhardt B, Oschlies I, Ludwig WD, Klingebiel T,
Graf N, Gruhn B, et al BFM Group, : The impact of the methotrexate
administration schedule and dose in the treatment of children and
adolescents with B-cell neoplasms: A report of the BFM Group Study
NHL-BFM95. Blood. 105:948–958. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Conter V, Valsecchi MG, Buldini B,
Parasole R, Locatelli F, Colombini A, Rizzari C, Putti MC, Barisone
E, Lo Nigro L, et al: Early T-cell precursor acute lymphoblastic
leukaemia in children treated in AIEOP centres with AIEOP-BFM
protocols: A retrospective analysis. Lancet Haematol. 3:e80–e86.
2016. View Article : Google Scholar : PubMed/NCBI
|