Introduction
In 2012 ovarian cancer (OCa) is the second most
prevalent type of gynecological cancer globally (1,2). Due to
OCa being asymptomatic during early stages, the majority of
patients are diagnosed in advanced stages, resulting in a notably
poor survival rate (3,4). Ovarian tumors are classified into
benign, cystadenoma, borderline and malignant lesions (5). A total of ~70-80% of OCa cases are
epithelial in origin, and the most common histological type is
serous carcinoma (6). The most
frequent subtypes are endometrial, clear cells, mucinous and
undifferentiated carcinomas (5,7).
Currently, only two biomarkers [cancer antigen (CA)125 and CA119]
are used for the clinical diagnosis of OCa (8); however, these markers are not increased
in all patients with OCa, and they can also be elevated in other
cancer types (8,9). Therefore, their decreased sensitivity
and specificity limit the merit of these biomarkers as screening
tools and increase the requirement for novel diagnostic and
prognostic markers.
Focal adhesion kinase (FAK), a non-receptor tyrosine
kinase that serves a key role in the integration of signals from
activated membrane receptors, the majority of which are within the
integrin family (10), has been
proposed as a potential marker of OCa (11–13). FAK
is expressed in a wide range of human tissue and cell types, and it
has been associated with the control of survival, proliferation and
motility via integrin-dependent adhesion and signaling pathways
(10,14). FAK consists of the following three
domains: An amino-terminal band 4.1, ezrin, radixin, moesin (FERM)
domain; a central kinase domain; and a C-terminal focal adhesion
targeting (FAT) domain (15). The
FERM domain is a non-catalytic motif that binds a number of growth
factor receptors, including epidermal growth factor receptor,
platelet derived growth factor receptor and vascular endothelial
growth factor receptor 2. FAK possesses a nuclear localization
sequence and it is able to interact with nuclear proteins,
including p53 (16). The kinase
domain phosphorylates downstream substrates to convey cellular
signals from the aforementioned receptors. The FAT domain contains
two proline-rich sequences and is required for localization at the
focal adhesions (17).
There are multiple isoforms of FAK, with multiple
FAK transcripts resulting from alternative splicing and/or
promoters. Schaller et al (18) identified a truncated isoform of FAK
lacking the kinase domain. This truncated isoform is known as
FAK-related non-kinase (FRNK) and functions as a dominant negative
regulator of FAK (18). The standard
transcript of FAK, lacking exons 13, 14, 16 and 31, is termed FAK°.
Additional FAK isoforms include FAK+, which contains a
three-amino acid insertion in the FAT sequence of exon 31.
FAK6 contains six additional residues inserted following
residue 392 (exon 14). FAK7 contains seven additional
residues in exon 16. FAK+6,7 contains all insertions
from the former three isoforms. Finally, FAK+6,7,28
(exons 13, 14 and 16), which contains 28 additional residues in the
vicinity of box 6 (19,20). FAK variants are differentially
expressed in various tissues at different stages of maturation and
appear to differ in their phosphorylation ability (21).
Alternative splicing also alters the
autophosphorylation rate of FAK, with FAK+ and FAK°
having a low autophosphorylation capacity, while FAK+6,7
and FAK+6,7,28 display increased autophosphorylation
(19). A number of studies have
associated FAK with oncological diseases. Additionally, Despeaux
et al (22) determined that
FAK6, FAK6,7 and FAK6,28 are
expressed by myeloid leukemia cells and are associated with
increased mortality rate of patients.
Increased expression of FAK has been detected in
ovarian carcinomas and is associated with a poor prognosis
(13,23). Increased levels of the active form of
FAK have also been associated with the aggressiveness of the tumor
(24). However, to the best of our
knowledge, a comprehensive study of the expression of FAK
throughout the development of different histological types of
ovarian tumor has not been conducted to date; therefore, the aim of
the present study was to investigate the FAK expression level in
serous and mucinous cystadenoma, borderline tumor and carcinoma
samples, along with healthy ovary samples. Additionally, the
expression of FAK°, FAK28 and FAK28,6
isoforms was determined in human OCa-derived cell lines.
Materials and methods
Tissue samples
A total of 161 archival, paraffin-embedded ovarian
tissue samples were obtained in 2015 from the Pathological Oncology
Service of the Century XXI National Medical Center (CMN-SXXI), the
General Hospital of Mexico and the Hospital of Gynaecology and
Obstetrics No. 4 IMSS (Mexico City, Mexico), under approval of the
Committee of Ethics of each hospital. The samples were obtained
from patients treated in the aforementioned hospitals between
January 2010 and December 2013, the samples were from
postmenopausal patients prior to treatment and with definitive
diagnosis of ovarian cancer. Slides (5-µm) were obtained, the
slides were deparaffinized in an oven at 60°C for 20 min, then
incubated in xylene at room temperature for 15 min, and a graded
series of ethanol (100, 70 and 30%) for 5 min and rinsed in
H2O. All slides were incubated with hematoxylin for 1
min at room temperature, subsequently slides were then washed with
PBS solution and finally were incubated with eosin for 30 sec and
evaluated with an optical microscope at ×20 magnification by an
experienced pathologist. Upon histopathological examination, the
samples were classified as follows: 50 serous carcinoma samples; 25
serous borderline tumor samples; 25 serous cystadenoma samples; 6
mucinous carcinoma samples; 14 mucinous borderline tumor samples;
25 mucinous cystadenoma samples; and 16 healthy ovary samples.
Additionally, archival, paraffin-embedded 25 tumor samples (5
cervical cancer, 10 breast cancer, 5 colon cancer and 5 prostate
cancer) positive for the expression of FAK, were obtained from the
Pathological Oncology Service of the CMN-SXXI in 2015, were from
patients (men and women) with a definitive diagnosis of cancer,
prior to treatment and without an age range and were included as
positive controls. These patients were treated at the CMN-SXXI
between 2010 and 2013.
Immunohistochemistry for the analysis
of FAK expression
Areas containing tumor tissue were identified in
H&E-stained slides from each paraffin-embedded sample. These
samples were assembled into a multi-tissue block, according to the
methodology reported by Hidalgo et al (25). Slices (5 µm) were cut from the
multi-tissue block, placed on glass slides, deparaffinized using
xylene in an oven at 60°C for 20 min, and then rehydrated in graded
concentrations of ethanol (100, 70 and 30%) and rinsed in
H2O. The slides were incubated in citrate buffer
(Biocare Decloaker DIVA; Biocare Medical, LLC, Paheco, CA, USA) at
90°C for 10 min for antigen retrieval, and were then washed with
PBS solution. The Mouse/Rabbit Immunodetector HRB/DAB Detection kit
(Bio, Sb, 0003LH Santa Barbara, CA, USA) was used for the
visualization of the antibody, endogenous peroxidase was inhibited
by incubation with Peroxide Immunodetector Blocker (Bio, Sb, 0003LH
Santa Barbara) at room temperature for 15 min. Following washing
with PBS, the slides were incubated with anti-FAK kinase domain
(dilution 1:200, cat. no. GTX50666; GeneTex, Inc., Irvine, CA, USA)
and anti-FAT domain (dilution 1:200, cat. no. GTX50489; GeneTex
Inc.) primary antibodies for 24 h at 4°C. Following washing with
PBS, tissues were incubated with Biotin Immunodetector (Bio, Sb,
0003LH) at room temperature for 20 min, followed by incubation with
Label Immunodetector (Bio, Sb, 0003LH Santa Barbara, CA, USA). To
detect the reaction, slides were incubated with an Immunodetector
DAB Chromogen kit (Bio, Sb, 0003LH Santa Barbara, CA, USA), and
then counterstained with haematoxylin and mounted with resin,
according to the manufacturer's protocol. Each sample was studied
in assays conducted in triplicate.
Semi-quantitative analysis of the reaction was
performed under an optical microscope at 20× magnification,
according to the system described by Allred et al (26), which considers two criteria: The
number of positive cells; and the intensity of the reaction. Visual
analysis was conducted by three independent observers. A sample was
considered negative when <5% cells exhibited immunostaining.
Samples with low reaction intensity and 6–25% positive cells were
considered low positive (+), samples with moderate intensity of
reaction and 26–75% positive cells were considered intermediate
positive (++), and samples with high-intensity reaction and >76%
cells exhibiting immunoreaction were considered highly positive
(+++). For densitometric analysis, three microphotographs were
captured of each sample with an Olympus BX40 optical microscope at
20× magnification. The samples were analyzed using Image-Pro Plus
ver. 5.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Cell lines
Cell lines SKOV3 and NIH-OVCAR3 were donated by Dr.
Fabián Arechavaleta-Velasco and Dr. Laura Díaz-Cueto (Hospital of
Gynecology and Obstetrics No. 4 IMSS, Mexico City, Mexico).
TOV-112D and HeLa cell lines were purchased from American Type
Culture Collection (Manassas, VA, USA). The human ovary cancer cell
line SKOV3 was derived from the ascites fluid of a 64-year-old
Caucasian female with an invasive ovarian adenocarcinoma (27). The SKOV3 cell line exhibits
epithelial and adherent morphology. The NIH-OVCAR3 was derived from
malignant ascites fluid from a patient with progressive ovarian
adenocarcinoma and grown as a cobblestone-like monolayer with
multilayered foci (28). The
TOV-112D cell line was derived from a primary malignant ovarian
adenocarcinoma grade 3, stage III (29). The human cervical cancer-derived HeLa
cell line was included as a positive control for the expression of
FAK (30). The SKOV3, NIH-OVCAR3,
TOV-112D and HeLa cell lines were cultured in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) and 0.1 mg/ml
streptomycin.
Western blot analysis
SKOV3, HIH-OVCAR3 and TOV-112D cells were
resuspended in lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1
mM EDTA; 1% NP40; and 0.25% sodium deoxycholate) containing
Complete Protease Inhibitors (Roche Applied Science, Mannheim,
Germany). Protein concentration was determined using a DC Protein
Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
according to manufacturer's protocol. A total of 30 µg protein was
resolved by 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were incubated with the anti-FAK kinase domain (cat. no. GTX50666
GeneTex Inc., Irvine, CA, USA) diluted at 1:1,000 or anti-GAPDH
(cat. no. PA1-987 Zymed; Thermo Fisher Scientific, Inc.) diluted at
1:20,000 as a control, at 4°C overnight. Membranes were then washed
and incubated with the appropriate goat anti-rabbit IgG secondary
antibody (horseradish peroxidase-conjugated) diluted at 1:5,000, at
room temperature for 40 min (cat. no. A27036 Zymed; Thermo Fisher
Scientific, Inc.). Proteins were detected by chemiluminescence
using the Amersham ECL plus Western Blotting Detection System (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA).
RNA extraction and reverse
transcription-semi-quantitative polymerase chain reaction (RT-qPCR)
of alternative transcripts
SKOV3, NIH-OVCAR3 and TOV-112D cells were
trypsinized, centrifuged and incubated with 1 ml TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) to obtain the
RNA, according to manufacturer's protocols. Following
centrifugation at 12,000 × g for 10 min at 4°C, the aqueous phase
was recovered, and 500 µl isopropanol was added. Subsequently, an
additional centrifugation at 12,000 × g for 10 min at 4°C was
performed, and then the pellet was obtained, washed in 70% ethanol
and homogenized. Following centrifugation at 7,500 × g for 5 min at
4°C, the pellets were dissolved in 50 µl H2O.
Subsequently, oligoDT cDNA was synthesized with a Promega Reverse
Transcription system GoScript™ (cat. no. A5000; Promega
Corporation, Madison, WI, USA). A total of 1 µg RNA was incubated
at 70°C for 5 min in the presence of 0.5 mg oligoDT, followed by
incubation at 4°C for 5 min. Finally, Master mix (Recombinant
RNasin® Ribonuclease Inhibitor and GoScript™ Reverse
Transcriptase) was added, according to the manufacturer's
protocols, and incubated at 25°C for 5 min and then at 42°C for 30
min, cDNA was stored at −80°C until further use. For amplification
of FAK isoforms, the PCR primers reported by Corsi et al
(21) were employed (primer M-R2,
M-F2, M-F3 and M-F4). These primers were designed to amplify the
region between exons 12 and 17. The oligonucleotides used for the
amplification of the region comprising exons 12 to 17 were: M-R2,
Forward, 5′-AGCGAAAAGCAAGGCATGCGG-3′, and M-F2 reverse,
5′-CTGACGCATTGTTAAGGCTTC-3′ for the isoforma FAK28,6.
For amplification of the remaining isoforms, the R2 reverse
oligonucleotide was used in combination with different forward
primers as follows: M-F3 reverse, 5′-TCTCTGTGTCAGAAACAGATGATT-3′
for the isoform FAK° without exons 13 and 14; and M-F4 reverse
5′-CTCCTTCTACGGAAACAGATGATT-3′ for the isoform FAK8
lacking exon 14. For PCR reactions, 1.25 U GoTaq®Flexi
DNA Polymerase (cat. no. M8295 Promega Corporation, Madison, WI,
USA) were employed under the following conditions: 95°C for 2 min,
then 30 cycles at 95°C for 30 sec, 57°C for 30 sec and 68°C for 30
sec, followed by a final cycle of 68°C for 2 min. Amplification of
the ne cycle at 957. For PCR reactions 1.25 U GoTaqs follows:
5′-TCGGGTCAGAAGGATTCCTATG-3′, and reverse
5′-GGTCTCAAACATGATCTGGG-3′ oligonucleotides under the conditions
aforementioned for the FAK isoforms. PCR products were visualized
on 1% agarose gels stained with ethidium bromide. Band intensities
were visualized using the Stratagene Eagle Eye II Gel Imaging
System and software EagleSight v3.22 (Stratagene, La Jolla, CA,
USA).
Statistical analysis
Data are reported as the means ± standard deviations
of three independent experiments. Data from densitometric
evaluation were analyzed using one-way analysis of variance test
followed by Tukey analysis and Duncan test to compare the level of
expression between the experimental groups. In order to compare
data from the semi-quantitative analysis, samples showing low,
moderate and high expression from each experimental group were
grouped together and considered as the overall positive expression
group. Then differences among the experimental groups were
evaluated using the Kruskal-Wallis test and Bonferroni correction
for pairwise comparisons, Allred et al (26) and Pizon et al (31). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of the kinase and FAT
domains of FAK in OCa
Expression of FAK kinase and FAT domains was
analyzed in healthy ovary, cystadenoma, borderline tumor and
ovarian carcinoma tissue samples, which were assembled into a
multi-tissue block by immunohistochemistry. The expression of FAK
kinase domain was observed in 14 out of 16 samples of healthy
ovaries, but immunostaining was low or moderate, with none of the
samples exhibiting a high positive expression (Table I). A similar pattern of expression
was detected in serous cystadenoma samples, with 56% of samples
exhibiting low or moderate expression and again, with no samples
exhibiting a high positive expression. In contrast, all borderline
serous tumor samples were positive for expression of the kinase
domain, but only one exhibited high positivity. Similarly, all
serous carcinoma samples were positive for the expression of the
FAK kinase domain, but 74% of these exhibited high positive
expression; additionally, no samples exhibited low positive
staining (Table I). The proportion
of samples exhibiting expression of the kinase domain in serous
cystadenoma, borderline tumor and carcinoma samples was
significantly increased, compared with healthy ovary samples
(P<0.05; Table I) and a
significant difference between borderline tumors and carcinomas was
observed (P<0.05). The expression of the FAK kinase domain was
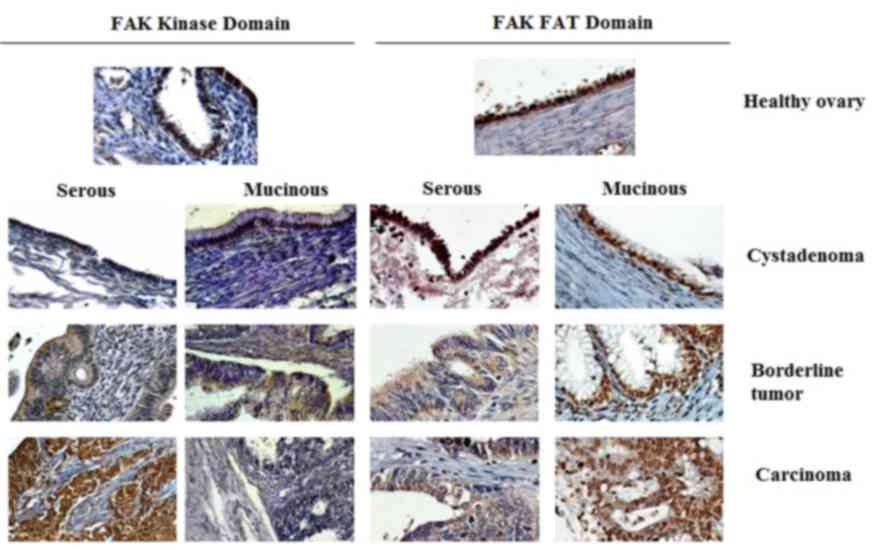
exclusively cytoplasmic in all positive serous samples (Fig. 1). In contrast, none of the mucinous
tumor groups exhibited a significantly increased proportion of
positive expression of the kinase domain, compared with the healthy
ovary samples (Table I). However, it
is notable that the staining detected in positive samples was also
cytoplasmic (Fig. 1). In agreement
with the semi-quantitative analysis, densitometric evaluation
demonstrated a significantly increased expression of FAK kinase in
borderline tumor and carcinoma samples, compared with healthy ovary
samples (P<0.05; Fig. 2). In
contrast, no significant difference was detected in mucinous
lesions (Fig. 2A).
 | Table I.Expression of focal adhesion kinase
kinase domain in healthy ovary, serous and mucinous samples. |
Table I.
Expression of focal adhesion kinase
kinase domain in healthy ovary, serous and mucinous samples.
| Histological
type | No. of cases | Negative expression
(−) | Low expression
(+) | Moderate expression
(++) | High expression
(+++) | P-value |
|---|
| Healthy ovary
samples | 16 | 2 | 6 | 8 | 0 |
|
| Serous tumor
types |
|
|
|
|
|
|
|
Cystadenoma | 25 | 11 | 10 | 4 | 0 |
<0.05a |
|
Borderline | 25 | 0 | 5 | 19 | 1 |
<0.05a,b |
|
Carcinoma | 50 | 0 | 0 | 13 | 37 |
<0.05a–c |
| Mucinous tumor
types |
|
|
|
|
|
|
|
Cystadenoma | 25 | 7 | 8 | 10 | 0 | NSa |
|
Borderline | 14 | 2 | 4 | 6 | 2 | NSa,b |
|
Carcinoma | 6 | 1 | 3 | 1 | 1 | NSa–c |
However, the expression of the FAT domain was
observed in 85% of healthy ovary samples (Fig. 1). Additionally, a high positive
expression was detected in 11/16 healthy ovary samples (Table II). There were 88% of serous
cystadenoma samples that exhibited positive expression of the FAT
domain, and the difference between this group and the healthy ovary
samples was statistically significant (P<0.05; Table II). All serous borderline tumor
samples were positive for expression of the FAT domain, exhibiting
low-to-high levels of expression (Table
II). Similarly, all serous borderline tumor samples analyzed
expressed the FAT domain, with 68% of samples exhibiting
moderate-to-high expression (Table
II). The proportion of carcinoma samples expressing the FAT
domain was significantly increased compared with the serous
borderline samples (P<0.01; Table
II). For the mucinous tumor samples, it was observed that 68%
of cystadenoma samples expressed low-to-moderate levels of the FAT
domain, while all borderline and carcinoma samples demonstrated
positive staining, with levels of positivity ranging from low to
high (Table II). Unlike the kinase
domain, the FAT domain exhibited a nuclear and cytoplasmic
localization (Fig. 1). Densitometric
analysis demonstrated that the expression of FAT was significantly
increased in healthy ovary samples, compared with serous and
mucinous samples (P<0.05; Fig.
2B).
 | Table II.Expression of focal adhesion kinase
focal adhesion targeting domain in healthy ovary, serous and
mucinous samples. |
Table II.
Expression of focal adhesion kinase
focal adhesion targeting domain in healthy ovary, serous and
mucinous samples.
| Histological
type | No. of cases | Negative expression
(−) | Low expression
(+) | Moderate expression
(++) | High expression
(+++) | P-value |
|---|
| Healthy ovary
samples | 16 | 1 | 2 | 2 | 11 |
|
| Serous tumor
types |
|
|
|
|
|
|
|
Cystadenoma | 25 | 3 | 7 | 9 | 6 |
<0.05a |
|
Borderline | 25 | 0 | 8 | 14 | 3 |
<0.05a |
|
|
|
|
|
|
| NSb |
|
Carcinoma | 50 | 0 | 10 | 18 | 22 | NSa |
|
|
|
|
|
|
| 0.02b |
|
|
|
|
|
|
| 0.01c |
| Mucinous tumor
types |
|
|
|
|
|
|
|
Cystadenoma | 25 | 8 | 11 | 6 | 0 |
<0.05a |
|
Borderline | 14 | 0 | 3 | 8 | 3 | NSa |
|
|
|
|
|
|
|
<0.05b |
|
Carcinoma | 6 | 0 | 1 | 3 | 2 | NSa |
|
|
|
|
|
|
|
<0.05b |
|
|
|
|
|
|
| NSc |
Expression of FAK kinase and
alternative transcripts in OCa cell lines
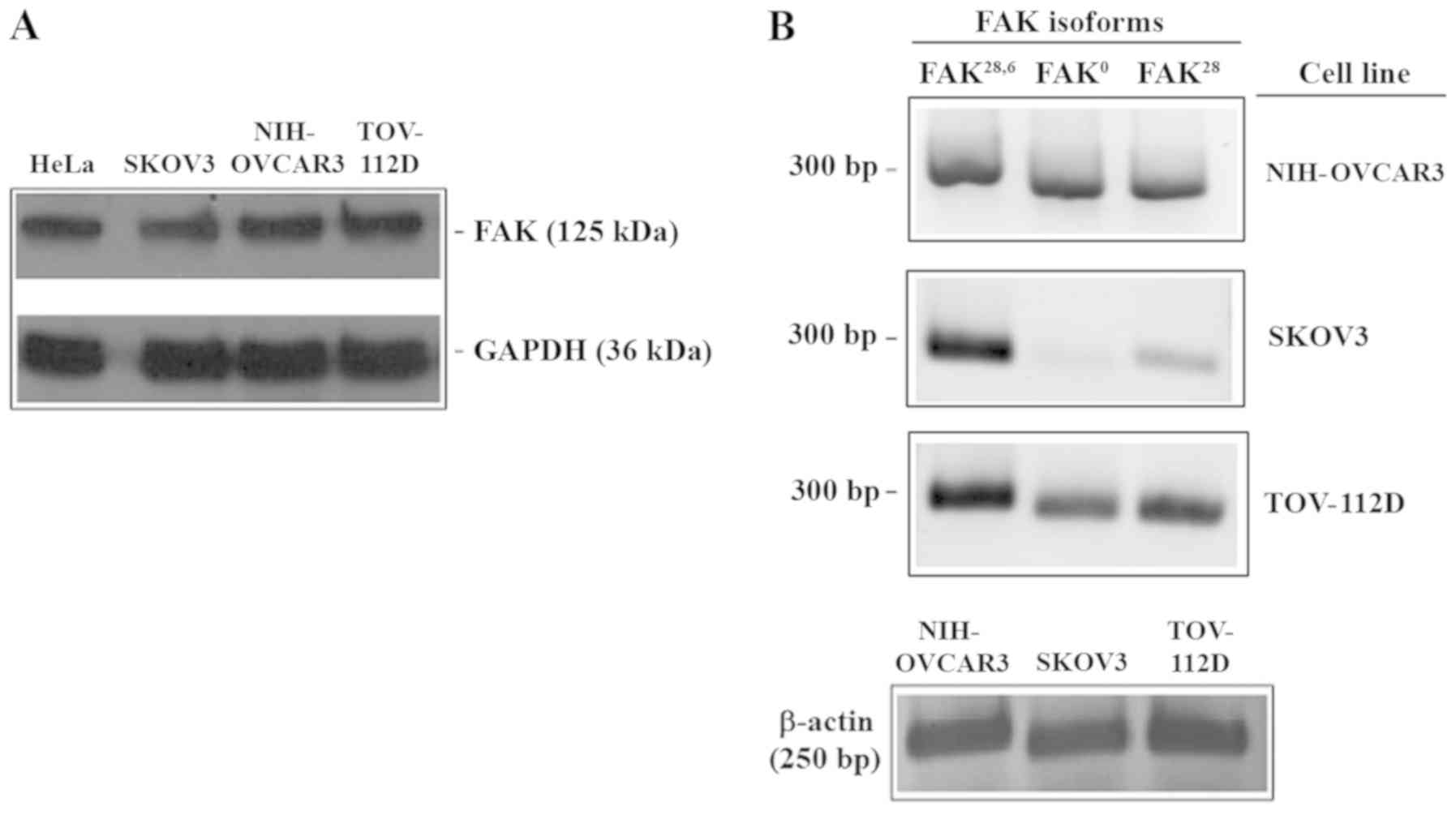
The present results demonstrated the expression of
FAK in OCa biopsies. In order to evaluate whether FAK is expressed
in OCa-derived cell lines, the expression of FAK kinase domain in
SKOV3, NIH-OVCAR3 and TOV-112D cells was evaluated with western
blotting, with HeLa cells included as a FAK expression control.
Incubation with the anti-FAK kinase domain antibody demonstrated
the presence of a band of a molecular weight similar to that
observed in the positive control. The present results indicated
that all three cell lines tested expressed FAK (Fig. 3A). Additionally, to address whether
OCa cells expressing different FAK isoforms, the three cell lines
were analyzed with RT-qPCR. As depicted in Fig. 3B, three different transcripts were
detected. A 300-bp fragment was observed in all cell lines, this
transcript corresponds to FAK28,6 isoform. Amplification
of the FAK° isoform was also detected in all cell lines. Finally,
the FAK28 fragment was detected in all cell lines.
However, SKOV3 cells demonstrated a very weak expression of FAK°
compared with that observed in NIH-OVCAR3 and TOV-112D cells
(Fig. 3B).
Discussion
FAK has been considered an important kinase for the
development of tumor types due to it participating in the processes
of angiogenesis, proliferation and cell migration (14). Although numerous studies have
examined the functions of FAK (15),
there are, to the best of our knowledge, limited data evaluating
the expression of FAK isoforms in OCa (13).
In the present study, the expression of FAK in
serous and mucinous ovarian tumor samples was evaluated with two
antibodies (anti-FAK kinase domain and anti-FAK FAT domain). It was
observed that the expression of FAK, as demonstrated by positive
immunostaining of the kinase domain, was increased in more advanced
tumor samples. This observation is consistent with data reported by
other research groups, in which FAK increases in advanced stages of
serous tumor cases (10,11,22–24). In
contrast, when the expression of FAT domain was analyzed, an
increased level of expression was observed in healthy ovary samples
compared with carcinoma samples. Sood et al (13) determined that the endogenous
inhibitor FRNK negatively regulates the phosphorylation of FAK. In
the present study, it was observed that kinase domain is increased,
but FAT domain is reduced, in carcinoma samples; it has previously
been demonstrated that the FAK COOH-terminal region, containing the
FAT domain, reduces the tyrosine phosphorylation of FAK, inducing
apoptosis and loss of adhesion of cancer cells (32). Thus, our observation of a reduction
of FAT domain expression in ovarian cancer may be in line with the
hypothesis that FAT is negative regulator of FAK activity.
FAK is a cytoplasmic protein, and it is activated
and localized in focal adhesions (33). Accordingly, a cytoplasmic expression
of FAK kinase domain was observed in serous and mucinous tumor
samples; however, the FAT domain was located in the cytoplasm of
serous tumor samples and also in the nucleus of mucinous tumor
samples. Previous studies demonstrated that nuclear FAK has the
ability to modify gene expression (16), providing kinase-independent survival
signals to cells; additionally, it is associated with poor
prognosis in colorectal cancer (34). The result of the present study
indicated that the FRNK isoform is localized in the nucleus of OCa
cells.
In addition, the present study determined the
expression of alternative FAK transcripts in OCa, which may
originate different isoforms of the protein. A limitation of our
study was that the evaluation of the FAK isoforms was not carried
out simultaneously with that of an internal control. Although this
is not standard practice, the expression of β-actin was
demonstrated in samples from the same RNA used for the study of the
FAK isoforms, but in an independent reaction. This might cause
variability among gels, however, all samples showed the expression
of the internal control gene (β-actin), and in addition they
demonstrated different levels of expression for the three isoforms
analyzed. It was observed that all cell lines tested exhibited a
high expression of FAK28,6. In an extensive molecular
analysis, Corsi et al (21)
determined that the expression of FAK 28 and 6 boxes is strongly
conserved among vertebrates, indicating an important function for
the FAK28,6 isoform. Notably, it has been proposed that
the inclusion of box 6 is associated with increased
autophosphorylation of FAK (35),
indicating that in OCa, this may produce hyperactivation of
multiple downstream signaling pathways. This protein has been
proposed as a prognostic marker and as a potential therapeutic
target in numerous types of tumor; however, novel evidence of FAK
isoforms opens up new questions and perspectives in the treatment
of OCa.
Acknowledgements
The authors would like to thank to Dr. Fabián Jesús
Arechavaleta-Velasco and Dr. Laura Díaz-Cueto from the Hospital of
Gynecology and Obstetrics No. 4 IMSS, Mexico City, Mexico for their
academic support. Additionally, the authors would like to thank
Cecilia Aguilar-Zacarías from the department of Molecular Biology
and Biotechnology, Institute of Biomedical Research, National
University of Mexico, Mexico City, for their technical
assistance.
Funding
The present study was supported by a grant from IMSS
(grant no. R-2011-785-066). The present study was performed in
partial fulfillment of the requirements of the Programa de
Doctorado en Ciencias Biológicas of Manuel Nolasco-Quiroga at the
Universidad Nacional Autónoma de México, with doctoral fellowships
from CONACyT (Reg: 211158), COMECyT (Grant no. 15BEPD0036-II), and
IMSS (Grant no. 087-2013). The authors would like to thank Posgrado
en Ciencias Biológicas, UNAM, Mexico, for academic support.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MNQ performed the experiments, analyzed and
interpreted data, and wrote the manuscript. MRD, JM, RGA, MJLI,
PPS, IAC, GVG performed RT-qPCR analysis of cell lines. MNQ, MRD,
MJLI, AIC, PPS constructed tissue microarrays, determined FAK on
microarrays and evaluated cellular stains. LRZ designed the
experiments using cell lines, analyzed and interpreted data. DAA
made substantial contributions to the conception of the study. FSG
designed the experiments on tissue samples and wrote the
manuscript.
Ethics approval and consent to
participate
Ethical approval was awarded collectively by
Committee of Ethics of the Century XXI National Medical Center, the
General Hospital of Mexico, and the Hospital of Gynecology and
Obstetrics No. 4 IMSS (approval no. R-2011-785-066) (Mexico City,
Mexico).
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coward JI, Middleton K and Murphy F: New
perspectives on targeted therapy in ovarian cancer. Int J Womens
Health. 7:189–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iyoke CA and Ugwu GO: Burden of
gynaecological cancers in developing countries. World J Obstet
Gynecol. 2:1–7. 2013. View Article : Google Scholar
|
|
3
|
Rauh-Hain JA, Krivak TC, Del Carmen MG and
Olawaiye AB: Ovarian cancer screening and early detection in the
general population. Rev Obstet Gynecol. 4:15–21. 2011.PubMed/NCBI
|
|
4
|
Goff B: Symptoms associated with ovarian
cancer. Clin Obstet Gynecol. 55:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaku T, Ogawa S, Kawano Y, Ohishi Y,
Kobayashi H, Hirakawa T and Nakano H: Histological classification
of ovarian cancer. Med Electron Microsc. 36:9–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leong HS, Galletta L, Etemadmoghadam D,
George J; Australian Ovarian Cancer Study, ; Köbel M, Ramus SJ and
Bowtell D: Efficient molecular subtype classification of high-grade
serous ovarian cancer. J Pathol. 236:272–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Köbel M, Kalloger SE, Boyd N, McKinney S,
Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, et al:
Ovarian carcinoma subtypes are different diseases: Implications for
biomarker studies. PLoS Med. 5:e2322008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Canney PA, Moore M, Wilkinson PM and James
RD: Ovarian cancer antigen CA125: A prospective clinical assessment
of its role as a tumour marker. Br J Cancer. 50:765–769. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pepin K, Carmen MD, Brown A and Dizon DS:
CA 125 and epithelial ovarian cancer: Role in screening, diagnosis,
and surveillance. Am J Hematol Oncol. 10:22–29. 2014.
|
|
10
|
Mitra SK and Schlaepfer DD:
Integrin-regulated FAK-Src signaling in normal and cancer cells.
Curr Opin Cell Biol. 18:516–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Judson PL, He X, Cance WG and Van Le L:
Overexpression of focal adhesion kinase, a protein tyrosine kinase,
in ovarian carcinoma. Cancer. 86:1551–1556. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li M, Hong LI, Liao M and Guo G:
Expression and clinical significance of focal adhesion kinase and
adrenomedullin in epithelial ovarian cancer. Oncol Lett.
10:1003–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sood AK, Coffin JE, Schneider GB, Fletcher
MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD and Hendrix
MJ: Biological significance of focal adhesion kinase in ovarian
cancer: Role in migration and invasion. Am J Pathol. 165:1087–1095.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao X and Guan JL: Focal adhesion kinase
and its signaling pathways in cell migration and angiogenesis. Adv
Drug Deliv Rev. 63:610–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hall JE, Fu W and Schaller MD: Focal
adhesion kinase: Exploring Fak structure to gain insight into
function. Int Rev Cell Mol Biol. 288:185–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lim ST: Nuclear FAK: A new mode of gene
regulation from cellular adhesions. Mol Cells. 36:1–6. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dixon RD, Chen Y, Ding F, Khare SD,
Prutzman KC, Schaller MD, Campbell SL and Dokholyan NV: New
insights into FAK signaling and localization based on detection of
a FAT domain folding intermediate. Structure. 12:2161–2171. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schaller MD, Borgman CA and Parsons JT:
Autonomous expression of a noncatalytic domain of the focal
adhesion-associated protein tyrosine kinase pp125FAK. Mol Cell
Biol. 13:785–791. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toutant M, Costa A, Studler J, Kadaré G,
Carnaud M and Girault JA: Alternative splicing controls the
mechanisms of FAK autophosphorylation. Mol Cell Biol. 22:7731–7743.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Contestabile A, Bonanomi D, Burgaya F,
Girault JA and Valtorta F: Localization of focal adhesion kinase
isoforms in cells of the central nervous system. Int J Dev
Neurosci. 21:83–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Corsi JM, Rouer E, Girault JA and Enslen
H: Organization and post-transcriptional processing of focal
adhesion kinase gene. BMC Genomics. 7:1982006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Despeaux M, Chicanne G, Rouer E, De
Toni-Costes F, Bertrand J, Mansat-De Mas V, Vergnolle N, Eaves C,
Payrastre B, Girault JA and Racaud-Sultan C: Focal adhesion kinase
splice variants maintain primitive acute myeloid leukemia cells
through altered Wnt signaling. Stem Cells. 30:1597–1610. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grisaru-Granovsky S, Salah Z, Maoz M,
Pruss D, Beller U and Bar-Shavit R: Differential expression of
protease activated receptor 1 (Par1) and pY397FAK in benign and
malignant human ovarian tissue samples. Int J Cancer. 113:372–378.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lark AL, Livasy CA, Dressler L, Moore DT,
Millikan RC, Geradts J, Iacocca M, Cowan D, Little D, Craven RJ and
Cance W: High focal adhesion kinase expression in invasive breast
carcinomas is associated with an aggressive phenotype. Mod Pathol.
18:1289–1294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hidalgo A, Piña P, Guerrero G, Lazos M and
Salcedo M: A simple method for the construction of small format
tissue arrays. J Clin Pathol. 56:144–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
27
|
Fogh J: Human tumor cells in vitro. Plenum
Press; New York: pp. 115–141. 1975
|
|
28
|
Hamilton TC, Young RC, Mckoy WM,
Grotzinger KR, Green JA, Chu EW, Whang-Peng J, Rogan AM, Green WR
and Ozols RF: Characterization of a human ovarian carcinoma cell
line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer
Res. 43:5379–5389. 1983.PubMed/NCBI
|
|
29
|
Provencher DM, Lounis H, Champoux L,
Tétrault M, Manderson EN, Wang JC, Eydoux P, Savoie R, Tonin PN and
Mes-Masson AM: Characterization of four novel epithelial ovarian
cancer cell lines. In Vitro Cell Dev Biol Anim. 36:357–361. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McCormack SJ, Brazinski SE, Moore JL Jr,
Werness BA and Goldstein DJ: Activation of the focal adhesion
kinase signal transduction pathway in cervical carcinoma cell lines
and human genital epithelial cells immortalized with human
papillomavirus type 18. Oncogene. 15:265–274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pizon M, Schott DS, Pachmann U and
Pachmann K: B7-H3 on circulating epithelial tumor cells correlates
with the proliferation marker, Ki-67, and may be associated with
the aggressiveness of tumors in breast cancer patients. Int J
Oncol. 53:2289–2299. 2018.PubMed/NCBI
|
|
32
|
Xu LH, Yang X, Craven RJ and Cance WG: The
COOH-terminal domain of the focal adhesion kinase induces loss of
adhesion and cell death in human tumor cells. Cell Growth Differ.
9:999–1005. 1998.PubMed/NCBI
|
|
33
|
Parsons JT: Focal adhesion kinase: The
first ten years. J Cell Sci. 116:1409–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Albasri A, Fadhil W, Scholefield JH,
Durrant LG and Ilyas M: Nuclear expression of phosphorylated focal
adhesion kinase is associated with poor prognosis in human
colorectal cancer. Anticancer Res. 34:3969–3974. 2014.PubMed/NCBI
|
|
35
|
Toutant M, Studler JM, Burgaya F, Costa A,
Ezan P, Gelman M and Girault JA: Autophosphorylation of Tyr397 and
its phosphorylation by Src-family kinases are altered in
focal-adhesion-kinase neuronal isoforms. Biochem J. 348:119–128.
2000. View Article : Google Scholar : PubMed/NCBI
|