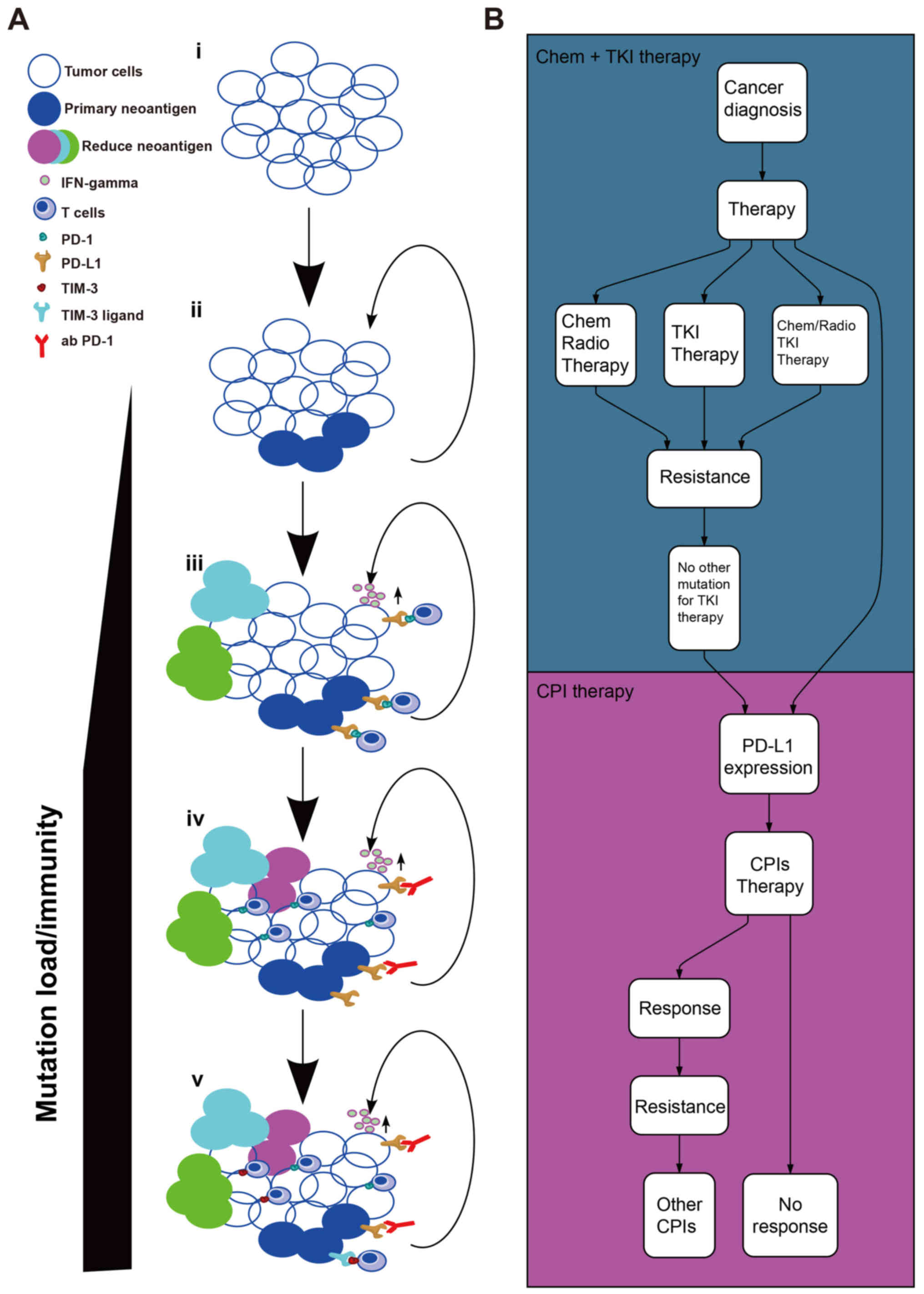
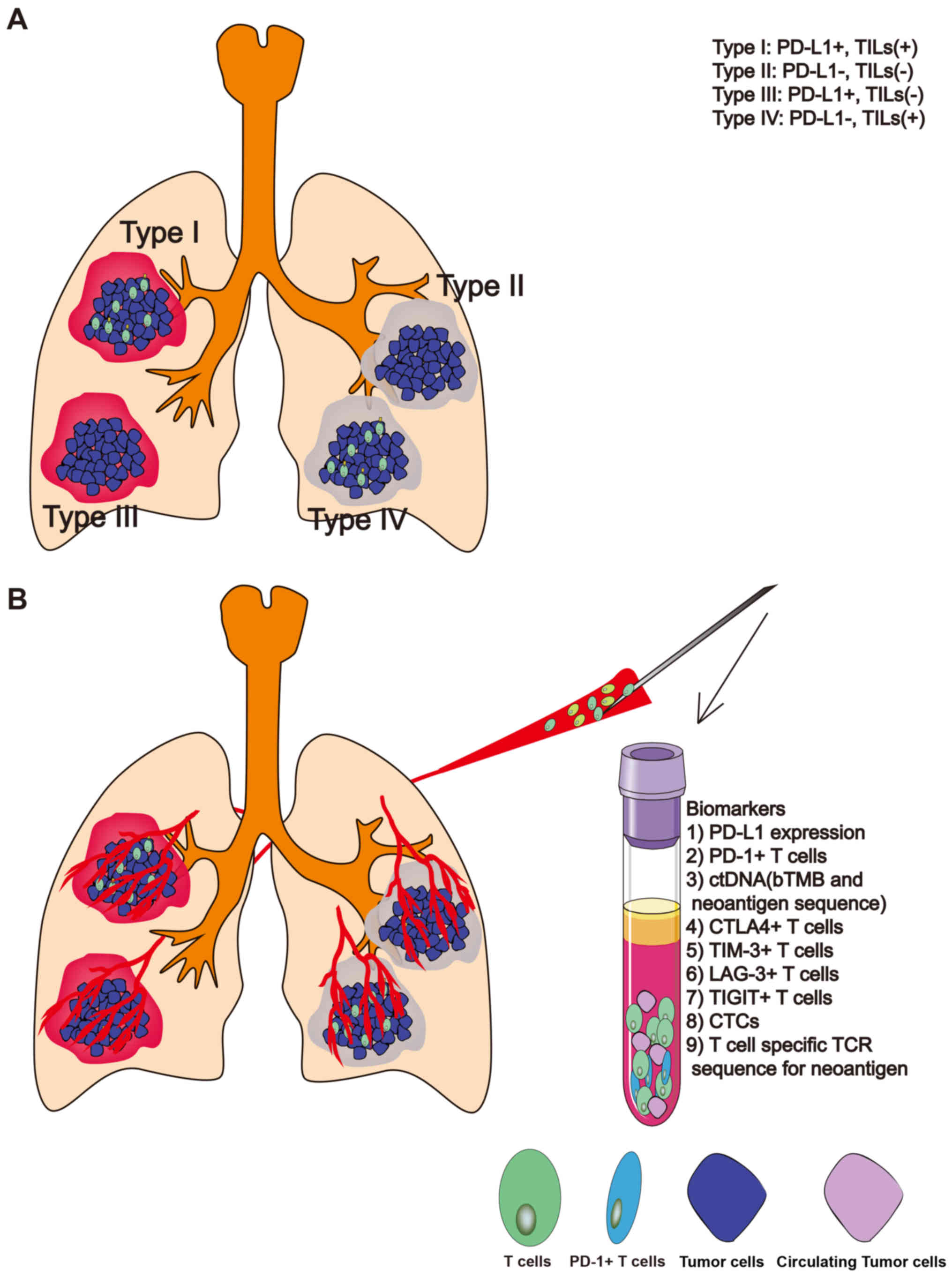
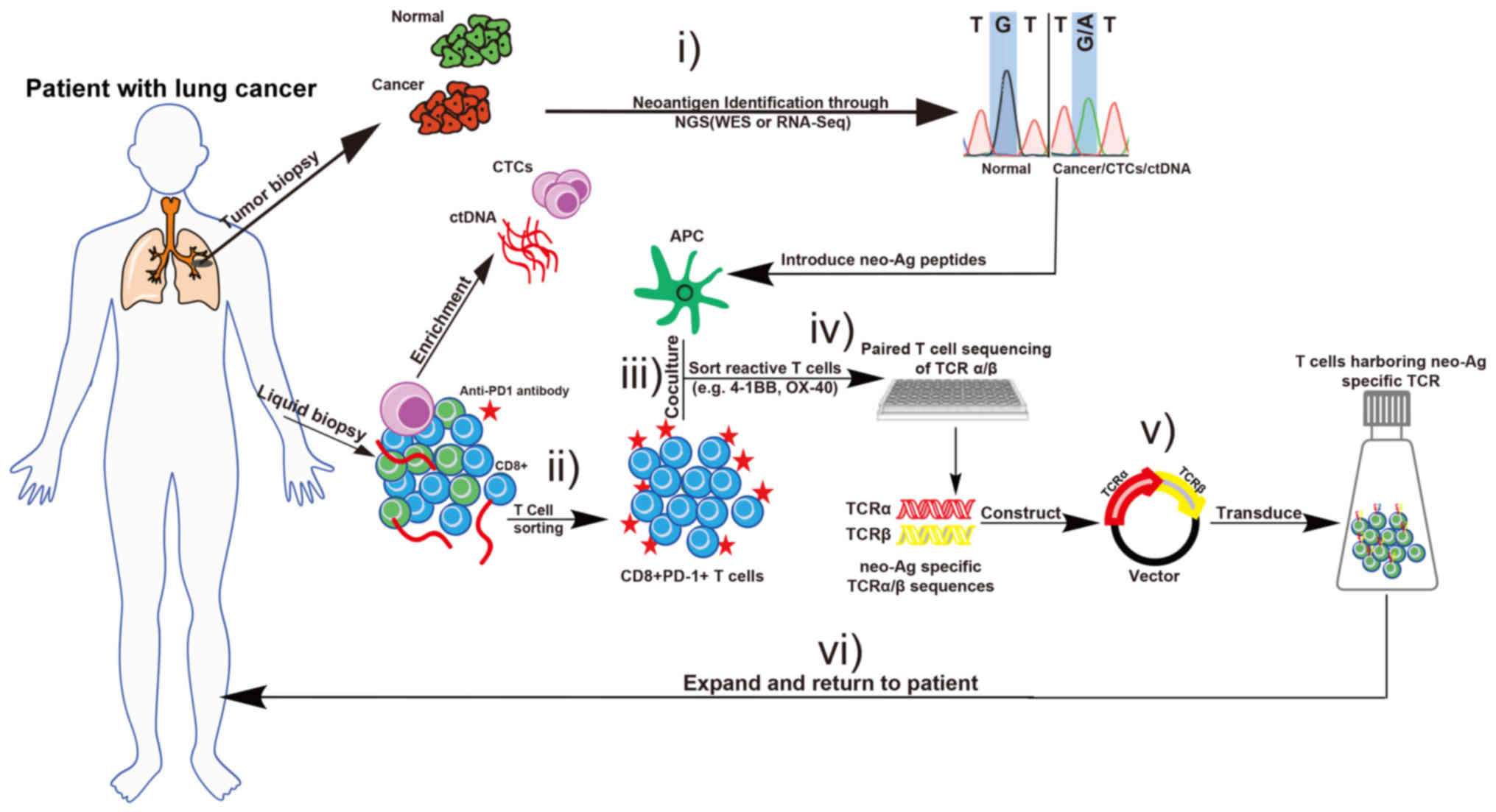
|
1
|
Cameron F, Whiteside G and Perry C:
Ipilimumab: First global approval. Drugs. 71:1093–1104. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou
J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, et al:
Bevacizumab plus ipilimumab in patients with metastatic melanoma.
Cancer Immunol Res. 2:632–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L and Han X: Anti-PD-1/PD-L1 therapy
of human cancer: Past, present, and future. J Clin Invest.
125:3384–3391. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brahmer J, Reckamp KL, Baas P, Crino L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizvi NA, Mazieres J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herbst RS, Baas P, Kim DW, Felip E,
Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borghaei H and Brahmer J: Nivolumab in
nonsquamous non-small-cell lung cancer. N Engl J Med. 374:493–494.
2016.PubMed/NCBI
|
|
15
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spigel DR, Schrock AB, Fabrizio D,
Frampton GM, Sun J and He J: Total mutation burden (TMB) in lung
cancer (LC) and relationship with response to PD-1/PD-L1 targeted
therapies. Am Soc Clin Oncol. 2016. View Article : Google Scholar
|
|
18
|
Anagnostou V, Smith KN, Forde PM, Niknafs
N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N,
et al: Evolution of neoantigen landscape during immune checkpoint
blockade in non-small cell lung cancer. Cancer Discov. 7:264–276.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vitale SG, Lagana AS, Capriglione S,
Angioli R, La Rosa VL, Lopez S, Valenti G, Sapia F, Sarpietro G,
Butticè S, et al: Target therapies for uterine carcinosarcomas:
Current evidence and future perspectives. Int J Mol Sci.
18:E11002017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai H, Mao L, Wang HS, Zhao J, Yang L, An
TT, Wang X, Duan CJ, Wu NM, Guo ZQ, et al: Epidermal growth factor
receptor mutations in plasma DNA samples predict tumor response in
Chinese patients with stages IIIB to IV non-small-cell lung cancer.
J Clin Oncol. 27:2653–2659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai H, Wang Z, Chen K, Zhao J, Lee JJ,
Wang S, Zhou Q, Zhuo M, Mao L, An T, et al: Influence of
chemotherapy on EGFR mutation status among patients with
non-small-cell lung cancer. J Clin Oncol. 30:3077–3083. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aparicio S and Caldas C: The implications
of clonal genome evolution for cancer medicine. N Engl J Med.
368:842–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aguiar PN Jr, De Mello RA, Barreto CMN,
Perry LA, Penny-Dimri J, Tadokoro H and Lopes GL Jr: Immune
checkpoint inhibitors for advanced non-small cell lung cancer:
Emerging sequencing for new treatment targets. ESMO Open.
2:e0002002017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alexander W: European society for medical
oncology 2016 congress. P T. 41:796–800. 2016.PubMed/NCBI
|
|
25
|
Cyriac G and Gandhi L: Emerging biomarkers
for immune checkpoint inhibition in lung cancer. Semin Cancer Biol.
52:269–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hegde PS, Karanikas V and Evers S: The
where, the when, and the how of immune monitoring for cancer
immunotherapies in the era of checkpoint inhibition. Clin Cancer
Res. 22:1865–1874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bobisse S, Foukas PG, Coukos G and Harari
A: Neoantigen-based cancer immunotherapy. Ann Transl Med.
4:2622016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miller A, Asmann Y, Cattaneo L, Braggio E,
Keats J, Auclair D, Lonial S; MMRF CoMMpass Network, ; Russell SJ
and Stewart AK: High somatic mutation and neoantigen burden are
correlated with decreased progression-free survival in multiple
myeloma. Blood Cancer J. 7:e6122017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Turajlic S, Litchfield K, Xu H, Rosenthal
R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M,
et al: Insertion-and-deletion-derived tumour-specific neoantigens
and the immunogenic phenotype: A pan-cancer analysis. Lancet Oncol.
18:1009–1021. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tran E, Robbins PF and Rosenberg SA:
‘Final common pathway’ of human cancer immunotherapy: Targeting
random somatic mutations. Nat Immunol. 18:255–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tran E, Turcotte S, Gros A, Robbins PF, Lu
YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS,
et al: Cancer immunotherapy based on mutation-specific CD4+ T cells
in a patient with epithelial cancer. Science. 344:641–645. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McGranahan N, Furness AJ, Rosenthal R,
Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak
NJ, Hiley CT, et al: Clonal neoantigens elicit T cell
immunoreactivity and sensitivity to immune checkpoint blockade.
Science. 351:1463–1469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jenkins RW, Barbie DA and Flaherty KT:
Mechanisms of resistance to immune checkpoint inhibitors. Br J
Cancer. 118:9–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McGranahan N and Swanton C: Clonal
heterogeneity and tumor evolution: Past, present, and the future.
Cell. 168:613–628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reck M, Bondarenko I, Luft A, Serwatowski
P, Barlesi F, Chacko R, Sebastian M, Lu H, Cuillerot JM and Lynch
TJ: Ipilimumab in combination with paclitaxel and carboplatin as
first-line therapy in extensive-disease-small-cell lung cancer:
Results from a randomized, double-blind, multicenter phase 2 trial.
Ann Oncol. 24:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Forde PM, Chaft JE and Pardoll DM:
Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med.
379:e142018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samstein RM and Riaz N: The DNA damage
response in immunotherapy and radiation. Adv Radiat Oncol.
3:527–533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakamura Y: Immunopharmacogenomics.
Springer; Japan: pp. 4832015
|
|
41
|
Schumacher TN and Scheper W: A liquid
biopsy for cancer immunotherapy. Nat Med. 22:340–341. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heitzer E, Ulz P and Geigl JB: Circulating
tumor DNA as a liquid biopsy for cancer. Clin Chem. 61:112–123.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng F, Su L and Qian C: Circulating
tumor DNA: A promising biomarker in the liquid biopsy of cancer.
Oncotarget. 7:48832–48841. 2016.PubMed/NCBI
|
|
44
|
Akyuz N, Brandt A, Stein A, Schliffke S,
Mahrle T, Quidde J, Goekkurt E, Loges S, Haalck T, Ford CT, et al:
T-cell diversification reflects antigen selection in the blood of
patients on immune checkpoint inhibition and may be exploited as
liquid biopsy biomarker. Int J Cancer. 140:2535–2544. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gedvilaitė V, Schveigert D and Cicėnas S:
Cell-free DNA in non-small cell lung cancer. Acta Med Litu.
24:138–144. 2017.PubMed/NCBI
|
|
46
|
Mingari MC and Moretta L: Surface markers
of human T lymphocytes. Ric Clin Lab. 12:439–448. 1982.PubMed/NCBI
|
|
47
|
Gajewski TF: The next hurdle in cancer
immunotherapy: Overcoming the Non-T-Cell-inflamed tumor
microenvironment. Semin Oncol. 42:663–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Inoue H, Park JH, Kiyotani K, Zewde M,
Miyashita A, Jinnin M, Kiniwa Y, Okuyama R, Tanaka R, Fujisawa Y,
et al: Intratumoral expression levels of PD-L1, GZMA, and HLA-A
along with oligoclonal T cell expansion associate with response to
nivolumab in metastatic melanoma. Oncoimmunology. 5:e12045072016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duan J, Wang Y and Jiao S: Checkpoint
blockade-based immunotherapy in the context of tumor
microenvironment: Opportunities and challenges. Cancer Med.
7:4517–4529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kandoth C, McLellan MD, Vandin F, Ye K,
Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al:
Mutational landscape and significance across 12 major cancer types.
Nature. 502:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kinoshita Y, Ishiguro T, Sano Y, Azuma Y,
Tsunenari T, Ono N, Kayukawa Y, Ueda O, Wada NA, Hino H, et al:
Anti-GPC3 TRAB, a first-in-class T cell-redirecting bispecific
antibody targeting glypican-3 with potent in vitro and in vivo
antitumor efficacy against solid tumors. Cancer Research.
76:14822016. View Article : Google Scholar
|
|
54
|
Teng MW, Ngiow SF, Ribas A and Smyth MJ:
Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer
Res. 75:2139–2145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Smyth MJ, Ngiow SF, Ribas A and Teng MW:
Combination cancer immunotherapies tailored to the tumour
microenvironment. Nat Rev Clin Oncol. 13:143–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kawai O, Ishii G, Kubota K, Murata Y,
Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, et al:
Predominant infiltration of macrophages and CD8(+) T Cells in
cancer nests is a significant predictor of survival in stage IV
nonsmall cell lung cancer. Cancer. 113:1387–1395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mu CY, Huang JA, Chen Y, Chen C and Zhang
XG: High expression of PD-L1 in lung cancer may contribute to poor
prognosis and tumor cells immune escape through suppressing tumor
infiltrating dendritic cells maturation. Med Oncol. 28:682–688.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
He Y, Yu H, Rozeboom L, Rivard CJ, Ellison
K, Dziadziuszko R, Suda K, Ren S, Wu C, Hou L, et al: LAG-3 protein
expression in non-small cell lung cancer and its relationship with
PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol.
12:814–823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Y, Cai P, Liang T, Wang L and Hu L:
TIM-3 is a potential prognostic marker for patients with solid
tumors: A systematic review and meta-analysis. Oncotarget.
8:31705–31713. 2017.PubMed/NCBI
|
|
61
|
Liu XG, Hou M and Liu Y: TIGIT, a novel
therapeutic target for tumor immunotherapy. Immunol Invest.
46:172–182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Anderson AC, Joller N and Kuchroo VK:
Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized
functions in immune regulation. Immunity. 44:989–1004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Koyama S, Akbay EA, Li YY, Herter-Sprie
GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ,
Asahina H, et al: Adaptive resistance to therapeutic PD-1 blockade
is associated with upregulation of alternative immune checkpoints.
Nat Commun. 7:105012016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gros A, Parkhurst MR, Tran E, Pasetto A,
Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts
IM, et al: Prospective identification of neoantigen-specific
lymphocytes in the peripheral blood of melanoma patients. Nat Med.
22:433–438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nicolazzo C, Raimondi C, Mancini M,
Caponnetto S, Gradilone A, Gandini O, Mastromartino M, Del Bene G,
Prete A, Longo F, et al: Monitoring PD-L1 positive circulating
tumor cells in non-small cell lung cancer patients treated with the
PD-1 inhibitor Nivolumab. Sci Rep. 6:317262016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gandara DR, Paul SM, Kowanetz M,
Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G,
Malboeuf C, et al: Blood-based tumor mutational burden as a
predictor of clinical benefit in non-small-cell lung cancer
patients treated with atezolizumab. Nat Med. 24:1441–1448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gandara DR, Kowanetz M, Mok TSK, Rittmeyer
A, Fehrenbacher L, Fabrizio D, Otto G, Malboeuf C, Lieber D, Paul
SM, et al: 1295OBlood-based biomarkers for cancer immunotherapy:
Tumor mutational burden in blood (bTMB) is associated with improved
atezolizumab (atezo) efficacy in 2L+ NSCLC (POPLAR and OAK). Ann
Oncol. 28 (Suppl 5):2017. View Article : Google Scholar
|
|
69
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Durgeau A, Virk Y, Corgnac S and
Mami-Chouaib F: Recent advances in targeting CD8 T-Cell immunity
for more effective cancer immunotherapy. Front Immunol. 9:142018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pasetto A, Gros A, Robbins PF, Deniger DC,
Prickett TD, Matus-Nicodemos R, Douek DC, Howie B, Robins H,
Parkhurst MR, et al: Tumor-and neoantigen-reactive T-cell receptors
can be identified based on their frequency in fresh tumor. Cancer
Immunol Res. 4:734–743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bassani-Sternberg M and Coukos G: Mass
spectrometry-based antigen discovery for cancer immunotherapy. Curr
Opin Immunol. 41:9–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Caron E, Kowalewski DJ, Chiek Koh C, Sturm
T, Schuster H and Aebersold R: Analysis of major histocompatibility
complex (MHC) immunopeptidomes using mass spectrometry. Mol Cell
Proteomics. 14:3105–3117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Khodadoust MS, Olsson N, Wagar LE, Haabeth
OA, Chen B, Swaminathan K, Rawson K, Liu CL, Steiner D, Lund P, et
al: Antigen presentation profiling reveals recognition of lymphoma
immunoglobulin neoantigens. Nature. 543:723–727. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cai LL, Ye HM, Zheng LM, Ruan RS and Tzeng
CM: Circulating tumor cells (CTCs) as a liquid biopsy material and
drug target. Curr Drug Targets. 15:965–972. 2014.PubMed/NCBI
|
|
77
|
Rizvi NA, Hellmann MD, Brahmer JR,
Juergens RA, Borghaei H, Gettinger S, Chow LQ, Gerber DE, Laurie
SA, Goldman JW, et al: Nivolumab in combination with platinum-based
doublet chemotherapy for first-line treatment of advanced
non-small-cell lung cancer. J Clin Oncol. 34:2969–2979. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu SY and Wu YL: Ongoing clinical trials
of PD-1 and PD-L1 inhibitors for lung cancer in China. J Hematol
Oncol. 10:1362017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ribas A, Puzanov I, Dummer R, Schadendorf
D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD,
et al: Pembrolizumab versus investigator-choice chemotherapy for
ipilimumab-refractory melanoma (KEYNOTE-002): A randomised,
controlled, phase 2 trial. Lancet Oncol. 16:908–918. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen Q, Li T and Yue W: Drug response to
PD-1/PD-L1 blockade: Based on biomarkers. Onco Targets Ther.
11:4673–4683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Koeppel F, Blanchard S, Jovelet C, Genin
B, Marcaillou C, Martin E, Rouleau E, Solary E, Soria JC, André F
and Lacroix L: Whole exome sequencing for determination of tumor
mutation load in liquid biopsy from advanced cancer patients. PLoS
One. 12:e01881742017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ilie M, Khambata-Ford S, Copie-Bergman C,
Huang L, Juco J, Hofman V and Hofman P: Use of the 22C3 anti-PD-L1
antibody to determine PD-L1 expression in multiple automated
immunohistochemistry platforms. PLoS One. 12:e01830232017.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Marchetti A, Barberis M, Franco R, De Luca
G, Pace MV, Staibano S, Volante M, Buttitta F, Guerini-Rocco E,
Righi L, et al: Multicenter comparison of 22c3 pharmDx (Agilent)
and SP263 (Ventana) assays to test PD-L1 expression for NSCLC
patients to be treated with immune checkpoint inhibitors. J
Thoracic Oncol. 12:1654–1663. 2017. View Article : Google Scholar
|
|
86
|
Kowanetz M, Koeppen H, Zou W, Mariathasan
S, Hellmann M, Kockx M, Chappey C, Kadel E, Smith D, Miley N, et
al: Abstract A017: PD-L1 as a predictive biomarker for atezolizumab
(MPDL3280A; anti-PDL1) in non-small cell lung cancer (NSCLC). AACR.
2016.
|
|
87
|
Hu X and Hay JW: First-line pembrolizumab
in PD-L1 positive non-small-cell lung cancer: A cost-effectiveness
analysis from the UK health care perspective. Lung Cancer.
123:166–171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dang TO, Ogunniyi A, Barbee MS and Drilon
A: Pembrolizumab for the treatment of PD-L1 positive advanced or
metastatic non-small cell lung cancer. Expert Rev Anticancer Ther.
16:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ibrahim RA, Berman DM, Depril V, Humphrey
RW, Chen T and Messina M: Ipilimumab safety profile: Summary of
findings from completed trials in advanced melanoma. J Clin Oncol.
29 (15-Suppl):85832011. View Article : Google Scholar
|
|
90
|
Teply BA and Lipson EJ: Identification and
management of toxicities from immune checkpoint-blocking drugs.
Oncology (Williston Park). 3 (28 Suppl):30–38. 2014.
|
|
91
|
Efremova M, Finotello F, Rieder D and
Trajanoski Z: Neoantigens generated by individual mutations and
their role in cancer immunity and immunotherapy. Front Immunol.
8:16792017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wilson EA and Anderson KS: Lost in the
crowd: Identifying targetable MHC class I neoepitopes for cancer
immunotherapy. Expert Rev Proteomics. 15:1065–1077. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bassani-Sternberg M, Bräunlein E, Klar R,
Engleitner T, Sinitcyn P, Audehm S, Straub M, Weber J,
Slotta-Huspenina J, Specht K, et al: Direct identification of
clinically relevant neoepitopes presented on native human melanoma
tissue by mass spectrometry. Nat Commun. 7:134042016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Klebanoff CA, Rosenberg SA and Restifo NP:
Prospects for gene-engineered T cell immunotherapy for solid
cancers. Nat Med. 22:26–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Politi K and Herbst RS: Lung cancer in the
era of precision medicine. Clin Cancer Res. 21:2213–2220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ramalingam S, Hui R, Gandhi L, Carcereny
E, Felip E, Ahn MJ, Eder JP, Balmanoukian AS, Leighl N, Aggarwal C,
et al: P2. 39: Long-Term OS for patients with advanced NSCLC
enrolled in the KEYNOTE-001 study of pembrolizumab. J Thoracic
Oncol. 11:S241–S242. 2016. View Article : Google Scholar
|
|
97
|
Gettinger S, Rizvi NA, Chow LQ, Borghaei
H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman
JW, et al: Nivolumab monotherapy for first-line treatment of
advanced non-small-cell lung cancer. J Clin Oncol. 34:2980–2987.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Forde PM, Smith KN, Chaft JE, Hellmann M,
Merghoub T, Wolchok JD, Yang SC, Battafarano RJ, Gabrielson E,
Georgiades CS, et al: NSCLC, early stageNeoadjuvant anti-PD1,
nivolumab, in early stage resectable non-small-cell lung cancer.
Ann Oncol. 27 (Suppl 6):LBA41–PR. 2016. View Article : Google Scholar
|
|
99
|
Antonia S, Rizvi N, Brahmer JR, Ou SHL,
Khleif SN, Hwu WJ, Gutierrez M, Schoffski P, Hamid O, Weiss J, et
al: Abstract A047: Safety and clinical activity of durvalumab
(MEDI4736), an anti-programmed cell death ligand-1 (PD-L1)
antibody, in patients with non-small cell lung cancer (NSCLC).
AACR. 2016.
|