Introduction
Differentiated thyroid cancer (DTC) has a relatively
good prognosis, but distant metastasis can lead to death due to
progressive disease (PD) (1). The
primary treatment strategy for patients with DTC and distant
metastasis is total thyroidectomy and regional lymph node
dissection, followed by I-131 radioactive iodine (RAI) therapy.
Patients that develop distant metastasis after hemi-thyroidectomy
typically undergo total thyroidectomy followed by RAI therapy.
Other standard treatments include surgery, RAI therapy (2), and thyroid hormone suppression therapy
(1). External irradiation can be
performed to control pain due to bone metastases or local
recurrence lesions. Long-term survival with remnant tumors is
possible if local disease control is good; the 10-year survival
rate for patients with distant metastatic thyroid cancer ranges
from 26–60% (3–5). However, activities of daily living may
decline due to bone or pulmonary metastasis.
According to the American Thyroid Association
(6) and the Union for International
Cancer Control guidelines (7),
tyrosine kinase inhibitor (TKI) treatment is recommended for
progressive RAI-refractory DTC. The TKIs sorafenib (8) and lenvatinib (9) were previously described for treating
RAI-refractory DTC following disease progression. Both lenvatinib
and sorafenib have been reported to target RAI refractory
metastatic lesions after total thyroidectomy. For lenvatinib, there
was at least one measurable lesion without iodine uptake on any
iodine-131 scan and at least one measurable lesion that had
progressed (10). For sorafenib,
there was at least one target lesion without iodine uptake
(11). To the best of our knowledge,
no study has investigated the frequency of disease progression in
distant metastasis from DTC nor has anyone reported on patient
prognosis in those with PD that were treated with sorafenib and/or
lenvatinib or not treated with TKI. Sorafenib has been approved for
the treatment of RAI-refractory and progressive DTC in Japan since
June 2014, with lenvatinib being cleared the following year
(12). In this study, we
retrospectively investigated whether the use of TKIs improved the
long-term treatment outcomes of distant metastasis from DTC. The
aims of this study were to clarify the factors associated with
prognosis among patients with DTC with distant metastasis and
evaluate TKI drugs in clinical practice.
Materials and methods
Patients
The current study included 147 patients (59 men, 88
women; median age, 71; range, 33–91 years) diagnosed with distant
metastasis from DTC who were treated in our hospitals between April
2015 and August 2018. Informed consent was obtained from all
individual participants included in the study. Cancer staging was
determined according to the cancer eighth edition cancer staging
manual by the American joint committee (13). DTC patients already diagnosed with
distant metastasis in stage IV-B (age >55) and in stage II (age
<55), follow-up cases, and those diagnosed with other stage
initially and recognized distant metastasis later were included.
Patients not continuing treatment at our hospitals were excluded.
Pathological diagnoses included papillary carcinoma in 122 patients
and follicular carcinoma in 25. Patients with medullary carcinoma
were excluded due to varying treatment strategy and tumor markers.
Overall, 77 patients (52.4%) exhibiting stable disease (SD) were
followed up in our outpatient clinics (SD group). These patients
underwent total thyroidectomy as the initial surgery, or completion
surgery was performed when distant metastases were recognized, with
all patients undergoing at least one round of RAI therapy.
Denosumab therapy and external irradiation were performed to
relieve pain caused by bone metastasis and to prevent osteoporosis
(14), and 120 mg denosumab was
administered subcutaneously at least once every 2 months.
Decompression and volume reduction surgery in addition to external
irradiation were performed to prevent paralysis in cases of
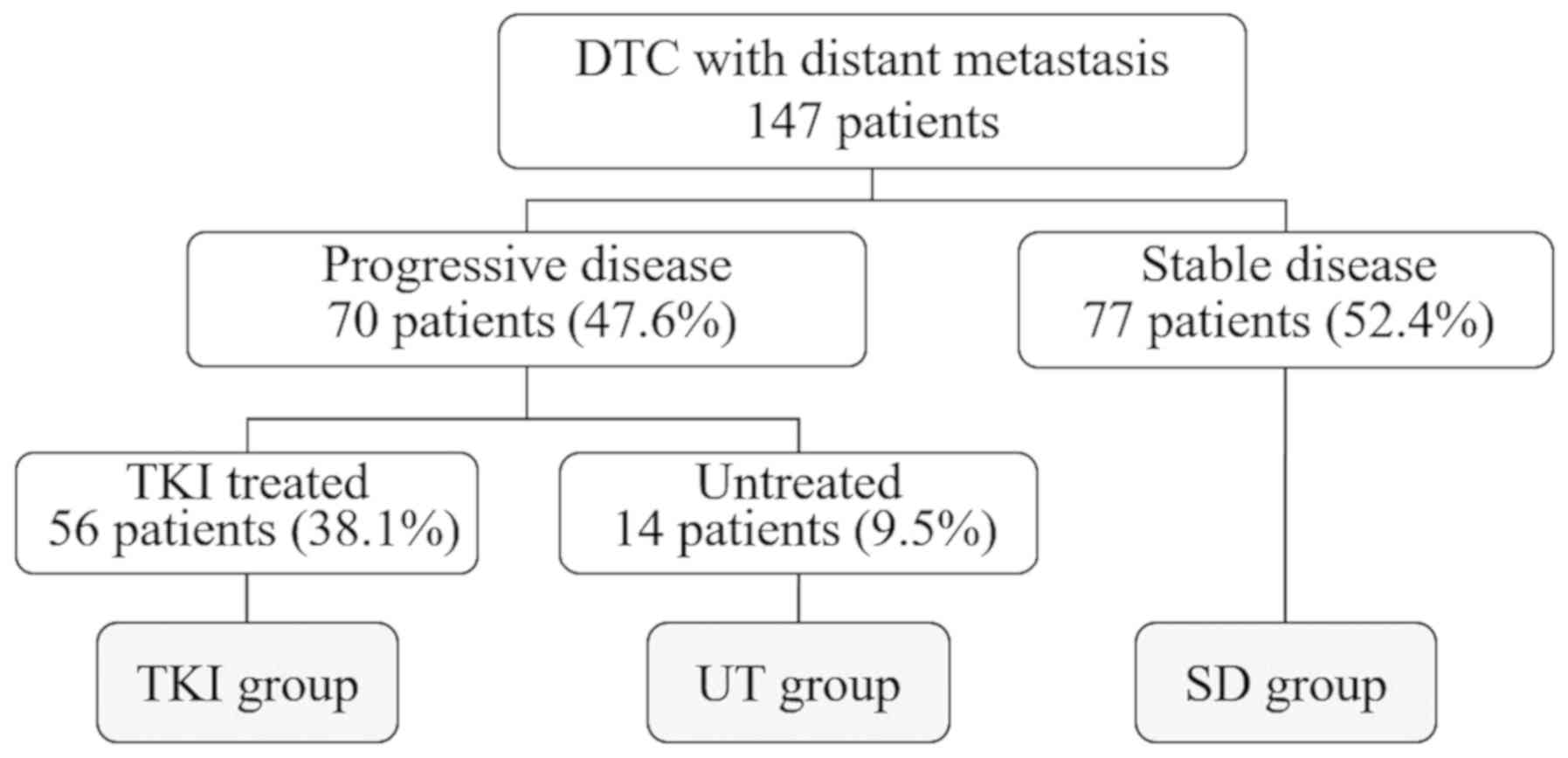
vertebral bone metastasis. Of the 147 total cases, disease
progression was observed in 70 (47.6%), 56 of which were treated
with TKI (TKI group). Disease progression was diagnosed as an
increase of ≥20% based on the response evaluation criteria in solid
tumors (RECIST) (version 1.1) criteria (15) or the appearance of a new lesion on
imaging within 1 year. Of the 14 patients (9.5%) who were not
treated with TKIs (untreated [UT] group), three refused treatment
and nine were excluded because they exhibited no treatment
indications for TKI or other treatments due to a wide range of
tracheal invasion, common carotid infiltration, and high risk of
bleeding. Patient subgroups were listed in Fig. 1. Numbers and parameters of the
patient groups are listed in Table
I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| A, Factor |
|---|
|
|---|
|
| Subgroup |
|
|---|
|
|
|
|
|---|
| Group | SD | TKI | UT | P-value |
|---|
| n | 77 | 56 | 14 |
|
| Age | 71.00 (33.00,
91.00) | 70.00 (41.00,
84.00) | 70.00 (47.00,
84.00) | 0.983 |
| Sex (%) |
|
Female | 45 (58.4) | 35 (62.5) | 8
(57.1) | 0.874 |
|
Male | 32 (41.6) | 21 (37.5) | 6
(42.9) |
|
| Pathology (%) |
|
FTC | 12 (15.6) | 11 (19.6) | 2
(14.3) | 0.795 |
|
PTC | 65 (84.4) | 45 (80.4) | 12 (85.7) |
|
| PS (%) |
| 0 | 66 (85.7) | 25 (44.6) | 4 (28.6) |
<0.001a |
| 1 | 11 (14.3) | 25 (44.6) | 5 (35.7) |
|
| 2 | 0
(0.0) | 6
(10.7) | 5 (35.7) |
|
|
| B,
Characteristics |
|
|
|
Subgroup |
|
|
| Group | SD | TKI | UT | P-value |
|
| Pulmonary
metastasis (%) | 58 (75.3) | 47 (83.9) | 11 (78.6) | 0.486 |
| Bone metastasis
(%) | 12 (15.6) | 22 (40.0) | 5
(35.7) | 0.005a |
| LN recurrence
(%) | 12 (15.8) | 31 (55.4) | 7
(70.0) |
<0.001a |
| Local recurrence
(%) | 12 (16.0) | 15 (27.3) | 8
(72.7) |
<0.001a |
| Died (%) | 1 (1.3) | 16 (29.1) | 10 (71.4) |
<0.001a |
| Diameter | 7.00 (3.00,
76.00) | 25.00 (9.00,
99.00) | 30.00 (3.00,
60.00) |
<0.001a |
| Follow up | 8.78 (0.13,
31.20) | 9.93 (2.12,
30.53) | 5.38 (0.52,
14.19) | 0.032a |
| Tg-DT | 2.23 (0.14,
9.69) | 1.53 (0.31,
7.29) | 1.20 (0.38,
2.51) | 0.182 |
| TgAb (%) | 9 (11.7) | 17 (32.1) | 4 (28.6) | 0.014a |
TKI treatment
Sorafenib and lenvatinib were administered to 22 and
49 patients, respectively. Among patients who were administered
both drugs, the initial treatment was sorafenib in 12 patients and
lenvatinib in three. Because only sorafenib could be used until
June 2015, we decided to use it as the first-choice TKI. After June
2015, lenvatinib, which has greater efficacy, was selected. The
maximum size of the evaluated lesion, pathological diagnosis,
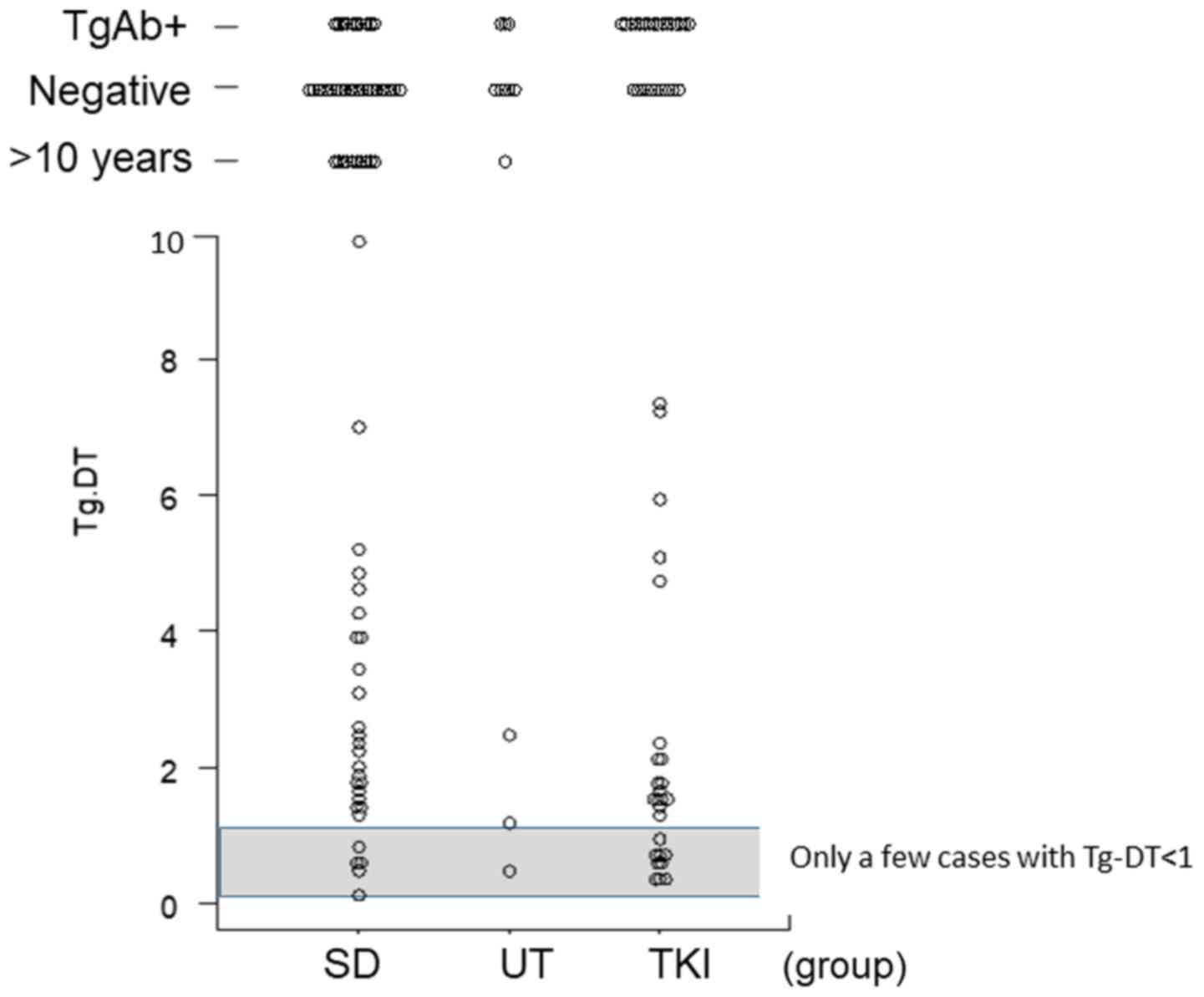
thyroglobulin (Tg) level, and Tg doubling time (Tg-DT) (16) were compared between the SD, UT, and
TKI groups. In the course of treatment, transient Tg oscillations
represent a frequent phenomenon that may not necessarily reflect
morphologic tumor progression (17).
A Tg level of <1 ng/ml during SD indicated a lack of disease
progression and rendered it impossible to calculate Tg-DT. That is,
when calculated with a trace amount Tg of ≤1 ng/ml, the calculated
Tg-DT becomes a strange numerical value, and it cannot be
evaluated. Meanwhile, decreases in Tg levels, which are calculated
as negative Tg-DT values, denoted successful treatment using RAI
therapy and/or external irradiation. Tg antibody (TgAb) negativity
was determined as previously described (16). Tg levels before and after RAI therapy
were excluded from the calculation due to the effect of
discontinuing thyroid hormone therapy. We also excluded patients
failing to comply with daily thyroid hormone use and those with
elevated thyroid-stimulating hormone levels. Patients for whom
Tg-DT could be calculated in the SD and TKI groups are described in
the scatter plot (Fig. 2). In
addition, we determined the treatment outcomes for each target
lesion, as the timing of TKI treatment depends on the metastatic
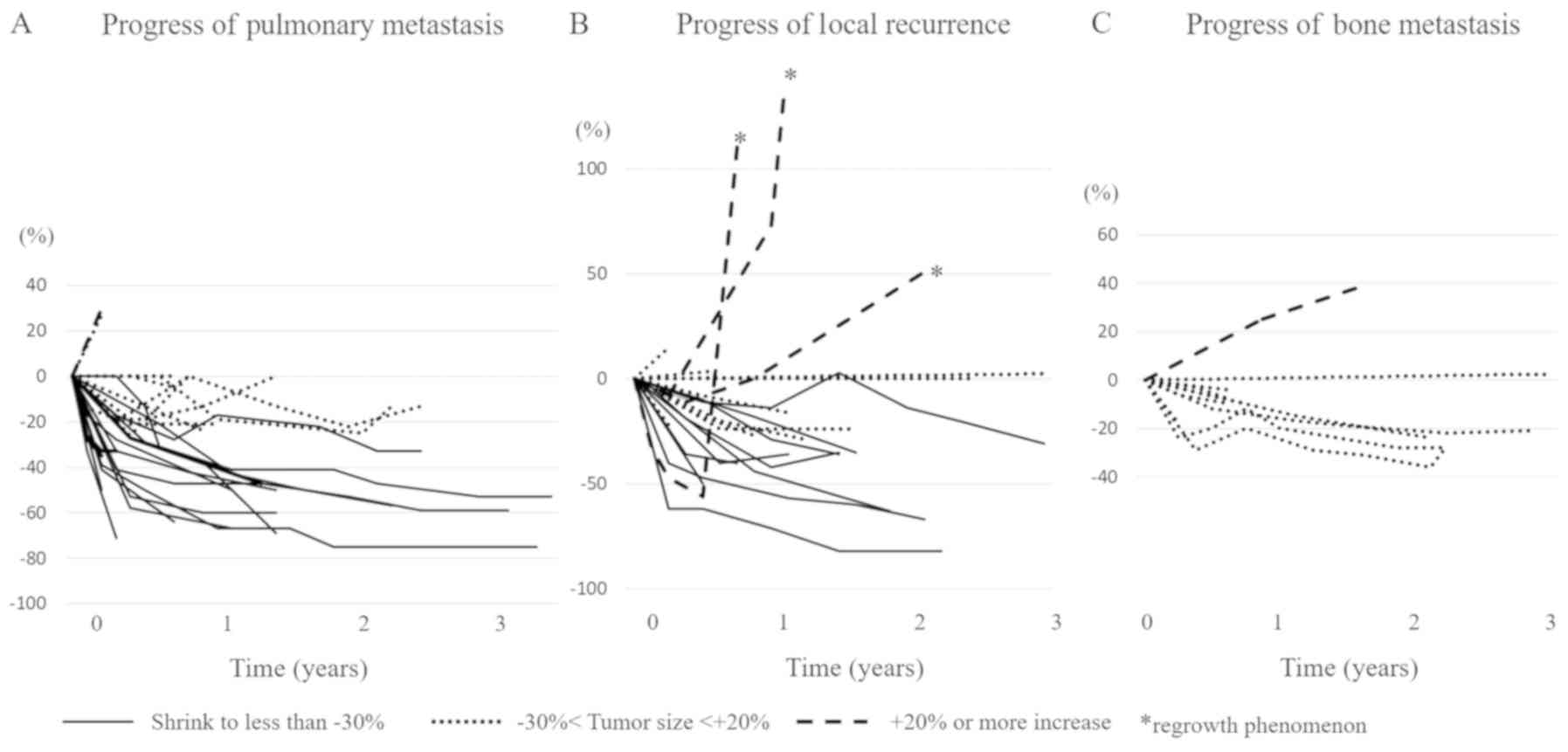
lesion. Changes in tumor diameter before and after TKI treatment
are illustrated for each target lesion (Fig. 3A-C). The radiologic response to TKI
therapy was classified according to RECIST version 1.1 criteria as
complete remission (CR), partial response (PR), SD, or PD. Safety
was assessed based on the Common Terminology Criteria for Adverse
Events version 3.0. The disease control rate (DCR) was defined as
the percentage of patients with CR, PR, and SD. Patients treated
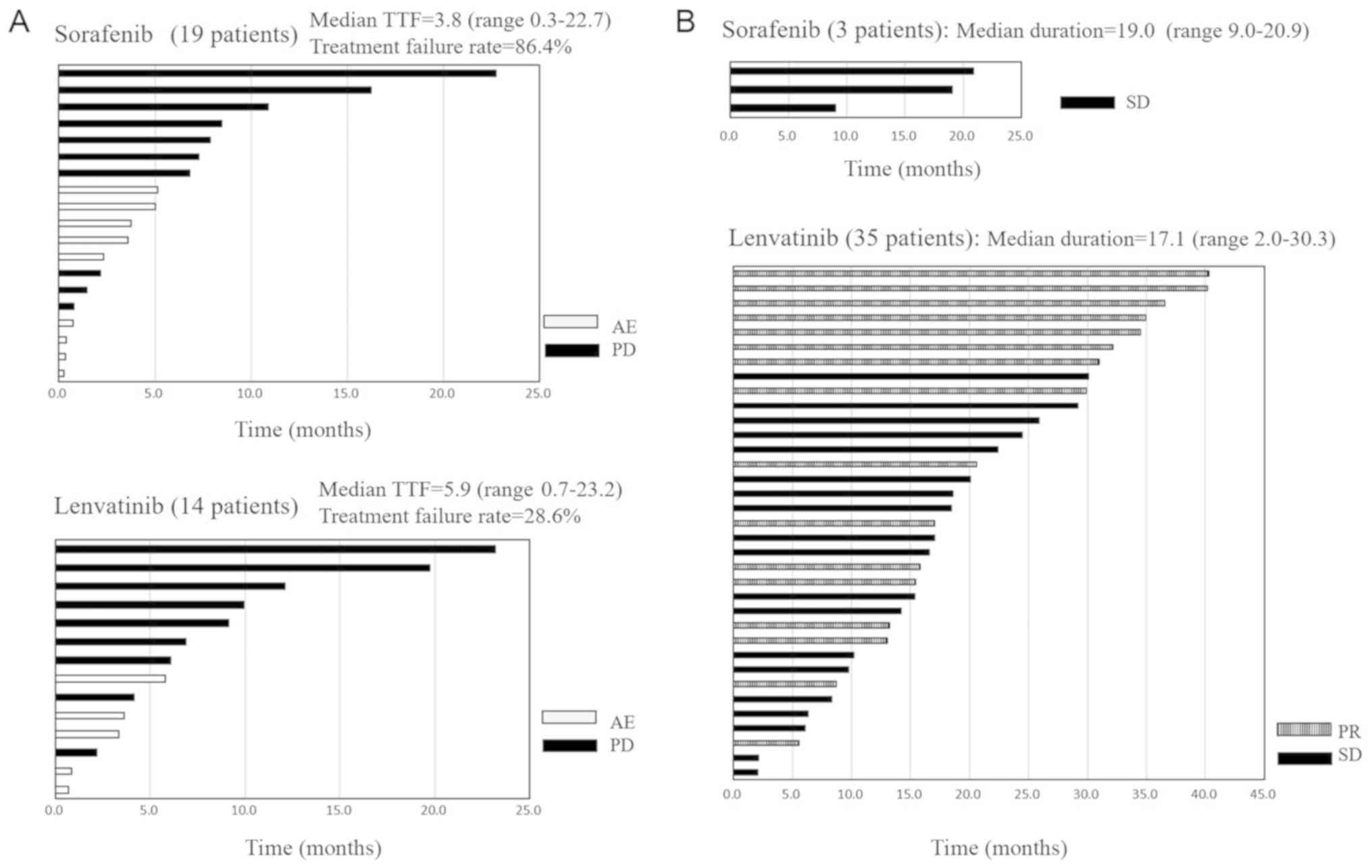
with sorafenib and those treated with lenvatinib were separately
examined to determine the respective treatment efficacy; the
administration period for cases in which treatment was discontinued
is graphically shown as the time to treatment failure (TTF)
together with the reason for discontinuation. The treatment period
is also graphically shown for each ongoing treatment case, and the
treatment effect is described (Fig. 4A
and B).
Evaluation
Next, we investigated whether TKI treatment improved
the treatment outcomes of distant metastasis from DTC by comparing
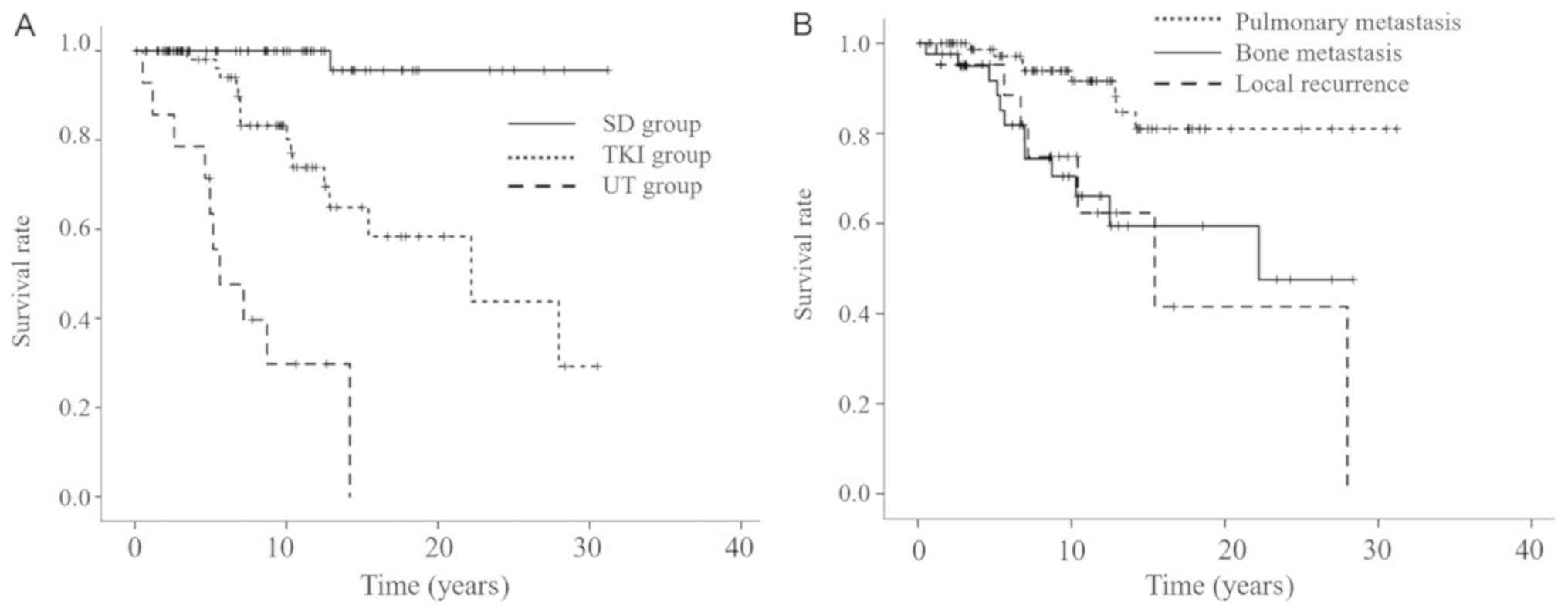
the overall survival (OS) between the UT and TKI groups (Fig. 5A). We compared the OS by target
lesions, pulmonary metastasis, bone metastasis, and local
recurrence (Fig. 5B). OS was defined
as the time between the date of initial diagnosis or the initial
surgery in the UT group, the date of the initial surgery in the TKI
group, and the date of death or final evaluation.
Statistical analysis
The OS was calculated using the Kaplan-Meier method
on the SPSS software. Kaplan-Meier estimator on the SPSS software
(version 24; IBM Corp., Armonk, NY, USA) was used to calculate the
OS, and log-rank and Bonferroni tests were applied. P<0.05 was
considered to indicate a statistically significant difference. The
OS was not calculated in the SD group because all of those patients
survived, except one who died from other disease. The OS of the TKI
and UT group were validated by log-rank test only. Comparison of
the median values among three groups was done using the
Kruskal-Wallis test and the statistically significant difference
was set at P<0.05. These statistical analyses were performed
using EZR (Saitama Medical Center, Jichi Medical University,
Saitama, Japan), a graphical user interface for R (The R Foundation
for Statistical Computing, Vienna, Austria); more precisely, it is
a modified version of R commander designed to add statistical
functions frequently used in biostatistics (18).
Results
Patients
Patient characteristics and parameters are shown in
Table I. The median maximum diameter
of the metastatic lesion in the SD group was 7 mm, which was
statistically smaller (P<0.001) than in the TKI group (25 mm)
and in the UT group (30 mm). Performance status was better in the
SD group, which also had a lower rate of bone metastasis and
recurrence of regional node metastasis. Meanwhile, other
parameters, such as age, sex, histology etc. were similar between
the groups. Treatment outcomes are shown in Table II. In the TKI group, 16 (28.6%), 26
(46.4%), and nine patients (16.1%) exhibited PR, SD, and PD,
respectively, whereas five patients (8.9%) were not evaluable, thus
the DCR was 75.0% (42 of 56). Sixteen patients (29.1%) in the TKI
group died, with causes of death being adverse events (AEs) in
three (two grade 5 bleeding cases and one gastrointestinal
perforation case), disease progression following treatment
discontinuation in eight, anaplastic transformation in two, and
aspiration pneumonitis and other complications in three. Meanwhile,
one patient in the SD group died from other disease, whereas 10 of
14 (71.4%) in the UT group died. The median observation periods
were 8.78 (range, 0.13–31.20) years for the SD group, 9.93 (range,
2.12–30.53) years for the TKI group, and 5.38 (range, 0.52–14.19)
years for the UT group.
 | Table II.Antitumor efficacy of TKIs. |
Table II.
Antitumor efficacy of TKIs.
|
| Metastatic
sites |
|---|
|
|
|
|---|
|
Characteristics | Total | Pulmonary | Bone | Others |
|---|
| N | 56 | 23 | 9 | 24 |
| Tumor size prior to
TKI (mm) | 24.0 (9.99) | 17.0 (12.44) | 50.0
(20.99)a | 27.5 (9.55) |
| Complete
response | 0 | 0 | 0 | 0 |
| Partial
response | 16 | 9 | 0 | 7 |
| Stable disease | 26 | 8 | 7 | 11 |
| Progressive
disease | 9 | 4 | 1 | 4 |
| Not evaluable | 5 | 2 | 1 | 2 |
| Response rate
(%) | 28.5 | 39.1 | 0.0 | 29.2 |
| Disease control
rate (%) | 75.0 | 73.9 | 77.8 | 75.0 |
TgAb and Tg-DT
TgAb positivity and Tg level were not informative in
nine of 77 patients (11.7%) in the SD group, 17 of 56 (32.1%) in
the TKI group, and three of 14 (21.4%) in the UT group, and these
rates were statistically significant (P=0.014). The TgAb-positive
rate increased with disease progression. The median Tg-DTs were
2.23 (range, 0.14–9.69), 1.53 (range, 0.31–7.29), and 1.20 (range,
0.38–2.51) in the SD, TKI, and UT groups, respectively, and these
rates were not statistically significant (P=0.18). If Tg-DT is
measured for a value of 10 years or more, or it becomes a negative
value, it can be concluded that there is no disease progression.
The number of patients with such values was 36 of 77 (41.6%) in the
SD group, 9 of 56 (16.1%) in the TKI group, and 9 of 14 (64.3%) in
the UT group. The total number of informative cases was 32 of 77
(41.6%), 30 of 56 (53.6%), and 2 of 14 (14.3%) in the SD, TKI, and
UT groups, respectively. These Tg-DT distributions were graphically
demonstrated in Fig. 2.
Treatment outcomes
In total, nine patients (39.1%) with pulmonary
metastasis and seven (29.2%) with local and lymph node recurrence
exhibited PRs (Table II). Two
patients with pulmonary metastasis did not respond to TKI and died,
whereas tumors shrunk in the remaining 21 cases. (Fig. 3A) In those with lymph node
recurrence, three cases had quick regrowth following
discontinuation of lenvatinib (Fig.
3B). Moreover, although tumor shrinkage was noted in patients
with bone metastasis, no patient exhibited PR, and the rest
exhibited SDs (Table II and
Fig. 3C). In terms of target
lesions, TKI treatment was most effective for pulmonary metastasis.
(Table II and Fig. 3A-C).
The median TTF for sorafenib was 3.8 (range,
0.3–22.7) months, and the treatment failure rate was 86.4% (19 of
22 patients). Conversely, the median TTF for lenvatinib was 5.9
(range, 0.7–23.2) months, and the treatment failure rate was 14 of
49 (28.6%). The reasons for discontinuation of sorafenib were as
follows: 10 cases were PD, 9 cases were AE (4 cases of allergic
dermatitis, 2 cases of gastrointestinal bleeding, and 1 case each
of liver dysfunction, anorexia, and whole body malaise). For
lenvatinib, nine cases were PD and five cases were AE (two cases of
grade 5 bleeding and one case each of gastrointestinal perforation,
allergic dermatitis, and anorexia). The median treatment duration
for sorafenib was 5.1 (range, 0.3–22.7) months and that for
lenvatinib was 14.1 (range, 0.7–40.3) months (Fig. 4A and B). Drug evaluation was
performed with TTF and on-going TKI, i.e., a drug with a longer TTF
period and lower failure rate was better, and clearly a drug with a
larger number of on-going TKI was better. The TTF period was longer
for lenvatinib, and treatment failure rate was higher for
sorafenib. On-going treatment included 35 cases of lenvatinib and 3
cases of sorafenib. Lenvatinib is widely-used in clinical
practice.
The median OS was 5.61 years (95% confidence
interval, 2.18–9.04) in the UT group whereas that was 22.2 years
(95% confidence interval, 8.85–35.57) in the TKI group (Fig. 5A). The OS of TKI-treated patients was
better than that of UT patients, with statistically significant
difference between the groups (log-rank test, P<0.001). The
median OS was 15.42 years (95% confidence interval, 6.17–24.67) for
bone metastasis, 22.21 years (95% confidence interval, 10.41-NA)
for local recurrence, and was not attained for pulmonary metastasis
(Fig. 5B). The prognosis by target
lesion was satisfactory for pulmonary metastasis, and there is
statistically significant difference between the groups (log-rank
test, P<0.001).
Discussion
Efficacy of TKI therapy by target
lesion
The lesion evaluation criterion of RECIST is a tumor
diameter of at least 10 mm on CT (19), thus only tumors of this size should
be assessed to determine the initial treatment effect. In other
words, in order to diagnose a PR (30% decrease) at a tumor diameter
of 10 mm, the diameter before treatment must be at least 14.3 mm.
Therefore, in patients with pulmonary metastasis, TKI therapy is
only considered in those with a maximum tumor diameter of at least
15 mm or alternatively 10 mm in patients with symptomatic lesions;
hence, we established these standards so that TKI introduction
timing is not too late to stop disease progression. In the TKI
group, two patients with pulmonary metastasis (10–15 mm) were
symptomatic; the remainder of metastases were >15 mm. We did not
experience a symptomatic patient with pulmonary metastasis <10
mm. As shown in Figs. 3A and
5B, the prognosis of pulmonary
metastasis cases treated with TKI was the best, so such
introduction criteria seemed to be successfully approved so far. If
possible, surgical resection should be performed for local and
lymph node recurrence including parapharyngeal metastasis (20); however, cases recurring multiple
surgeries or those diagnosed as unresectable had previously
undergone external irradiation or additional RAI therapy. Lamartina
et al reported that the rate of CR following the first
reoperation due to persistent/recurrent DTC was 53% at the last
assessment after a median of 5 years (21). Furthermore, TKI treatment of lesions
involving the common carotid artery and mediastinal large vessels
should be administered with caution (22). An example of a lesion requiring
particular attention with TKI treatment is parapharyngeal
metastasis. Among five patients with parapharyngeal metastasis,
three demonstrated syncopal attacks due to carotid sinus syndrome
(23) based on the metastatic
lesion, which is a serious symptom to start TKI treatment. Among
patients with bone metastasis, some metastatic lesions, such as
those on the ribs, are resectable, but bone load and vertebral body
bone metastasis may cause pathologic fractures and spinal cord
paralysis. Consequently, early initiation of TKI treatment will
prolong the quality of life. In the case of spinal metastasis, if a
risk of spinal paralysis due to the withdrawal of thyroid hormone
is observed while preparing for RAI therapy, it should be preceded
by decompression surgery and external irradiation (24), and then TKI treatment should be
initiated. The therapeutic effects of TKI are shown in Table II and Fig. 3. The tumor diameter of lung
metastasis as the target lesion was relatively small, and the
therapeutic effect was most satisfactory. In contrast, bone
metastasis with a large tumor diameter had a poorer therapeutic
effect. Other lesions showed intermediate results (Table II). Both tumor size prior to TKI
treatment and metastasis site are believed to affect the
therapeutic effect.
Tg-DT as the indicator for disease
progression
On the basis of a previous report that Tg-DT
reflects the prognosis of DTC (9),
we examined the prognostic value of this variable following the
introduction of TKI treatment. The incidence of TgAb positivity was
statistically high both in the TKI and UT groups (Table II), but those Tg-DTs were not
informative. Although there were 64 of 147 informative cases
(43.5%), the calculated Tg-DT was not statistically significant
between the SD group and the TKI and UT groups (Fig. 2 and Table
II). Tg levels either decreased or insignificantly increased in
several patients in the SD group, and RAI therapy or external
irradiation was successful; such findings reflect a lack of disease
progression, making TKI treatment unnecessary. In other words, as
Tg levels do not increase with treatment success (17), it is important to measure Tg during
both TKI treatment and postoperative SD. Both reversed TgAb
positivity (25) and increased Tg
levels (26) indicate disease
progression; however, Tg-DT did not accurately reflect tumor growth
when compared with imaging assessments. Although Tg-DT is not an
indicator of disease progression in many cases, the value was <1
year in six patients in the SD group and eight patients in the TKI
group. Although Tg-DT is useful for the detection of recurrence and
distant metastases, it was not an indicator of disease progression
in cases with existing distant metastases. The frequency of TgAb
increased with disease progression, and the rate of increase of Tg
level became low for further disease progression.
TKI treatment to prolong survival of DTC
with distant metastasis
No increase in Tg levels or the number of metastatic
lesions was observed in the SD group; therefore, TKI treatment was
not required. Although it is obvious, there have been no previous
reports demonstrating that the OS of patients with distant
metastasis at the initial diagnosis for DTC is worse than that of
patients recognized with distant metastasis postoperatively
(Fig. 4C). Moreover, 10 of 14
patients (71.4%) in the UT group died, and the median OS in the TKI
group was 22.2 years. This finding is particularly significant due
to the extended SD period of this retrospective study. Although the
study population of UT group was limited, the OS of the TKI and UT
groups was significantly different, it is important to further
improve survival by starting TKI treatment at appropriate time. The
treatment outcomes of TKI included treatment failure due to AE,
three cases of regrowth phenomenon, and three cases of AE death.
Because of PD in 10/22 (45.5%) sorafenib cases and 5/49 (10.2%)
lenvatinib cases, they are considered non-responders (Fig. 4). Other cases were responders, and
tumor shrinkage was observed as shown in Fig. 3. Outcomes may be further improved by
AE management. The prevention of toxicities is necessary to allow
patients to remain on treatment as long as possible without dose or
schedule modifications (27).
However, the existence of AEs cannot be ignored
(11), as two patients with
recurrent tumors involving large vessels experienced fatal bleeding
following TKI therapy. According to the Common Terminology Criteria
for Adverse Events, version 3.0, dose reduction or treatment
withdrawal should be initiated in cases of renal dysfunction or
skin fistula. Meanwhile, three patients experienced sudden tumor
regrowth following drug withdrawal, leading to decreased treatment
outcomes. Despite the satisfactory DCR, it must be noted that no
CRs were achieved following TKI therapy. Thus, it may be necessary
to continue TKI therapy as long as possible. Because TKI treatment
is the final option, treatment failure resulted in death. As more
cases of long-term TKI treatment will be observed in the future, we
believe that proper dose reduction and drug withdrawal guidelines
are necessary; in absence of these, more patients will die from
tumor regrowth following discontinuation of TKIs. Therefore, it is
hard to predict whether TKI therapy, which is expensive and has
various AEs, contributes to long-term improvement in the treatment
outcomes for distant metastasis from DTC.
Distant metastases from DTCs comprise a
heterogeneous group of thyroid cancers with diverse histology,
disease progression, and genomic alterations. Due to the limited
treatment options, the OS is usually poor among patients with
aggressive metastatic disease (28).
In this study, we report a high Tg-Ab-positive rate in patients
with disease progression; however, Tg-DT was not informative for PD
diagnosis. The OS was poor among patients with distant metastasis
at the initial diagnosis for DTC. TKI treatment does improve OS in
distant metastases DTCs, especially in patients with pulmonary
metastasis. Management of this unique group of patients will
improve as we gain a better understanding of the optimal timing of
TKI therapy administration.
Acknowledgements
The authors thank Dr Akira Yoshida (Center Clinic of
Kanagawa Health Service Association) and Dr Yoshio Kure (Director
of Kure Clinic) for referring several patients to our hospital. An
abstract (poster 1139) covering a part of this article was
presented at the 20th European congress of Endocrinology, May
19–22, Barcelona, Spain in 2018.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HI and HY designed the study. HT and RS checked
analysis and interpretation data, especially statistical analysis.
NS, HN, SH, ST and KM contributed by performing the surgery and
caring for the patients. NS, HN, SH contributed to analysis and
interpretation data, particularly drug efficacy and clinical
assessment. ST and KM contributed to data acquisition. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The chemotherapy committee of Kanagawa Cancer Center
(Yokohama, Kanagawa, Japan) approved this regimen of lenvatinib or
sorafenib for use in patients with DTC. The cancer board of the
hospital also approved these TKI treatment, including surgery, for
patients with DTC. The study was approved by the Institutional
Review Board of Kanagawa Cancer Center.
Patient consent for publication
All patients provided a comprehensive consent form
stating that personal data may be used for academic presentation or
paper presentation while ensuring complete anonymity prior to
receiving treatment.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
HI is an endocrine surgeon working at the Kanagawa
Cancer Center and has an extensive experience of several surgeries
for advanced thyroid cancer as well as ATC treatment.
Glossary
Abbreviations
Abbreviations:
|
DTC
|
differentiated thyroid cancer
|
|
RAI
|
radioactive iodine
|
|
AE
|
adverse event
|
|
TKI
|
tyrosine kinase inhibitor
|
|
UT
|
untreated
|
|
Tg-DT
|
thyroglobulin doubling time
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
RECIST
|
response evaluation criteria in solid
tumors
|
|
Tg
|
thyroglobulin
|
|
TgAb
|
thyroglobulin antibody
|
|
CR
|
complete remission
|
|
TTF
|
time to treatment failure
|
|
OS
|
overall survival
|
|
DCR
|
disease control rate
|
|
CT
|
computed tomography
|
References
|
1
|
Carhill AA, Litofsky DR, Ross DS, Jonklaas
J, Cooper DS, Brierley JD, Ladenson PW, Ain KB, Fein HG, Haugen BR,
et al: Long-term outcomes following therapy in differentiated
thyroid carcinoma: NTCTCS registry analysis 1987–2012. J Clin
Endocrinol Metab. 100:3270–3279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scheffel RS, Zanella AB, Dora JM and Maia
AL: Timing of radioactive iodine administration does not influence
outcomes in patients with differentiated thyroid carcinoma.
Thyroid. 26:1623–1629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwong N, Marqusee E, Gordon MS, Larsen PR,
Garber JR, Kim MI and Alexander EK: Long-term, treatment-free
survival in select patients with distant metastatic papillary
thyroid cancer. Endocr Connect. 3:207–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brink JS, van Heerden JA, McIver B,
Salomao DR, Farley DR, Grant CS, Thompson GB, Zimmerman D and Hay
ID: Papillary thyroid cancer with pulmonary metastases in children:
Long-term prognosis. Surgery. 128:881–887. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goffredo P, Sosa JA and Roman SA:
Differentiated thyroid cancer presenting with distant metastases: A
population analysis over two decades. World J Surg. 37:1599–1605.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haugen BR: 2015 American thyroid
association management guidelines for adult patients with thyroid
nodules and differentiated thyroid cancer: What is new and what has
changed? Cancer. 123:372–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nixon IJ, Wang LY, Migliacci JC, Eskander
A, Campbell MJ, Aniss A, Morris L, Vaisman F, Corbo R, Momesso D,
et al: An international multi-institutional validation of age 55
years as a cutoff for risk stratification in the AJCC/UICC staging
system for well-differentiated thyroid cancer. Thyroid. 26:373–380.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Machiels JP, Henry S, Zanetta S, Kaminsky
MC, Michoux N, Rommel D, Schmitz S, Bompas E, Dillies AF, Faivre S,
et al: Phase II study of sunitinib in recurrent or metastatic
squamous cell carcinoma of the head and neck: GORTEC 2006-01. J
Clin Oncol. 28:21–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schlumberger M, Tahara M and Wirth LJ:
Lenvatinib in radioiodine-refractory thyroid cancer. N Engl J Med.
372:18682015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brose MS, Nutting CM, Jarzab B, Elisei R,
Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R,
Shong YK, et al: Sorafenib in radioactive iodine-refractory,
locally advanced or metastatic differentiated thyroid cancer: A
randomised, double-blind, phase 3 trial. Lancet. 384:319–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito Y, Suzuki S, Ito K, Imai T, Okamoto T,
Kitano H, Sugitani I, Sugino K, Tsutsui H, Hara H, et al:
Tyrosine-kinase inhibitors to treat radioiodine-refracted,
metastatic, or recurred and progressive differentiated thyroid
carcinoma (Review). Endocr J. 63:597–602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perrier ND, Brierley JD and Tuttle RM:
Differentiated and anaplastic thyroid carcinoma: Major changes in
the American Joint Committee on Cancer eighth edition cancer
staging manual. CA Cancer J Clin. 68:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gül G, Sendur MA, Aksoy S, Sever AR and
Altundag K: A comprehensive review of denosumab for bone metastasis
in patients with solid tumors. Curr Med Res Opin. 32:133–145. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyauchi A, Kudo T, Miya A, Kobayashi K,
Ito Y, Takamura Y, Higashiyama T, Fukushima M, Kihara M, Inoue H,
et al: Prognostic impact of serum thyroglobulin doubling-time under
thyrotropin suppression in patients with papillary thyroid
carcinoma who underwent total thyroidectomy. Thyroid. 21:707–716.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Werner RA, Lückerath K, Schmid JS, Higuchi
T, Kreissl MC, Grelle I, Reiners C, Buck AK and Lapa C:
Thyroglobulin fluctuations in patients with iodine-refractory
differentiated thyroid carcinoma on lenvatinib treatment-initial
experience. Sci Rep. 6:280812016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine ZH, Borchardt BR, Brandenburg NJ,
Clark CW, Muralikrishnan B, Shakarji CM, Chen JJ and Siegel EL:
RECIST versus volume measurement in medical CT using ellipsoids of
known size. Opt Express. 18:8151–8159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giordano L, Pilolli F, Toma S and Bussi M:
Parapharyngeal metastases from thyroid cancer: Surgical management
of two cases with minimally-invasive video-assisted technique. Acta
Otorhinolaryngol Ital. 35:289–292. 2015.PubMed/NCBI
|
|
21
|
Lamartina L, Borget I, Mirghani H, Al
Ghuzlan A, Berdelou A, Bidault F, Deandreis D, Baudin E, Travagli
JP, Schlumberger M, et al: Surgery for neck recurrence of
differentiated thyroid cancer: Outcomes and risk factors. J Clin
Endocrinol Metab. 102:1020–1031. 2017.PubMed/NCBI
|
|
22
|
Hui EP, Ma BB, King AD, Mo F, Chan SL, Kam
MK, Loong HH, Ahuja AT, Zee BC and Chan AT: Hemorrhagic
complications in a phase II study of sunitinib in patients of
nasopharyngeal carcinoma who has previously received high-dose
radiation. Ann Oncol. 22:1280–1287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Epstein SS and Shaw HJ: Metastatic cancer
of the larynx as a cause of carotid-sinus syndrome. Cancer.
10:933–937. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramadan S, Ugas MA, Berwick RJ, Notay M,
Cho H, Jerjes W and Giannoudis PV: Spinal metastasis in thyroid
cancer. Head Neck Oncol. 4:392012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morbelli S, Ferrarazzo G, Pomposelli E,
Pupo F, Pesce G, Calamia I, Fiz F, Clapasson A, Bauckneht M, Minuto
M, et al: Relationship between circulating anti-thyroglobulin
antibodies (TgAb) and tumor metabolism in patients with
differentiated thyroid cancer (DTC): Prognostic implications. J
Endocrinol Invest. 40:417–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Neshandar Asli I, Siahkali AS, Shafie B,
Javadi H and Assadi M: Prognostic value of basal serum
thyroglobulin levels, but not basal antithyroglobulin antibody
(TgAb) levels, in patients with differentiated thyroid cancer. Mol
Imaging Radionucl Ther. 23:54–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Resteghini C, Cavalieri S, Galbiati D,
Granata R, Alfieri S, Bergamini C, Bossi P, Licitra L and Locati
LD: Management of tyrosine kinase inhibitors (TKI) side effects in
differentiated and medullary thyroid cancer patients. Best Pract
Res Clin Endocrinol Metab. 31:349–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeung KT and Cohen EE: Lenvatinib in
advanced, radioactive iodine-refractory, differentiated thyroid
carcinoma. Clin Cancer Res. 21:5420–5426. 2015. View Article : Google Scholar : PubMed/NCBI
|