Introduction
CRKII is a central signal adapter protein in
signaling pathways that propagate extracellular activation from
oncogenic tyrosine kinases to cellular targets (1,2). CRKII
contains a SH2 domain, which binds the focal adhesion components
p130Cas and paxillin (3), growth
factor receptors and signaling scaffold proteins (1) and two SH3 domains, which interacts with
guanine nucleotide exchange factors, including DOCK180 (4) and C3G, to mediate the cell
proliferation and adhesion by activating Rap1 and R-Ras (3,5).
Deregulation of CRK expression is associated a variety of cancer
types, including lung cancer, breast cancer, glioblastoma and
hepatocarcinoma (6–8). CRKII may be a potential tumor marker
(9–11); however, the role and action mechanism
of CRKII in tumor lymphatic metastasis are unclear.
As an early step of cancer malignancy (12), lymphatic metastasis is a major
mechanism of tumor metastasis (13–17). A
previous study demonstrated that lymph node metastasis (LNM) in
cancer contributes to early metastasis detection and cancer
progression (9,12,13–17). The
initial LNM of epithelial carcinoma commonly results in poor
survival and prognosis of patients with cancer (16,18).
Hepatocarcinoma is one of the leading causes of cancer-associated
mortalities globally due to its high recurrence, high metastasis
and poor prognosis (19–21). Therefore, investigating the
biological malignant ability and LNM potential of tumor cells
improves the understanding of the progression of
hepatocarcinoma.
Hca-P and Hca-F are two mouse hepatocarcinoma
ascites cell lines sharing the same genetic background (15,22–24).
Hca-F has a high LNM rate of 75% and Hca-P has a low LNM metastasis
rate of 25%. They metastasize only to lymph nodes, and do not
disseminate to other organs (25).
Hca-P cell is used as a low-LNM and LN-specific murine
hepatocarcinoma cell model for the early discovery and diagnosis of
tumor malignancies (15,22–25). Our
previous study indicated that CRK is involved in the lymphatic
metastatic potential of murine hepatocarcinoma cells (22,23).
CRKL, a member of the CRK adapter protein family, exhibited a tumor
suppressor effect on the in vitro proliferation, migration
and invasion, and in vivo tumor malignancy and LNM rate
levels of Hca-P cells (22,23). In the present study, the potential
tumor promoter role of CRKII in hepatocarcinoma was investigated.
By constructing the pcDNA3.1/V5-HisB-CRKII vector and
CRKII-overexpressing Hca-P cells, the influence of the CRKII level
on the in vitro proliferation, colony forming capacity,
migration and invasion abilities of Hca-P cells was
investigated.
Materials and methods
Cell culture
The murine hepatocarcinoma Hca-P cell line was
created by the Department of Pathology, Dalian Medical University,
and maintained in our laboratory at the Department of
Biotechnology. During the 5-day experiment, Hca-P cells were
administrated intraperitoneally, inoculated and purified in the
abdominal cavities of 3 inbred Chinese 615-mice (male; age, 6
weeks; body weight, 20±2 g) (24)
that had been acclimatized for 5 days. The volume of ascites from
three mice ranged from 0.9 to 1.2 ml (4.5–6% of body weght). The
mice were supplied by the SPF Animal Laboratory Center of Dalian
Medical University with ethical approval granted in March 2016. The
3 mice were placed in one cage, maintained at 23±0.5°C and 60–70%
humidity with a 12-h light/dark cycle (7:00 a.m.-7:00 p.m.) and
allowed access to standard rodent chow and autoclaved filtered
water ad libitum. For euthanasia, each mouse was injected with
ketamine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 100
mg/kg and with xylazine (Sigma-Aldrich; Merck KGaA) at 10 mg/kg
according to a previous study (26)
and our experience. Mice were sacrificed in a tank with 20% volume
displacement per minute by CO2 flow, followed by cervical
dislocation to confirm death. The purified Hca-P cells were then
cultured in 85% RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 15% fetal bovine serum
(PAA Laboratories GmbH; GE Healthcare Life Sciences, Little
Chalfont, UK) at 37°C in a humidified incubator with 5% CO2.
Polymerase chain reaction (PCR)
amplification
According to gene sequence (GenBank: NM_133656), the
forward (F) and reverse (R) primers of CRKII were designed by
Oligo7 as F: 5′-CCAAGCTTACAATGGCGGGCAACTTCGACTC-3′; and R:
5′-CGGGATCCATTGCTGAAGTCCTCATCGG-3′. The F primer contains a
HindIII restriction cleavage site and R primer contains a
BamHI restriction cleavage site. Total RNA was extracted
from Hca-P cells using TRIzol® reagent (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol, and
reverse transcription was conducted with a RNA PCR kit Ver.3.0
(Takara Bio, Inc., Otsu, Japan). Using PrimerScript RTase and
PrimeSTAR HS DNA Polymerase (Takara Bio, Inc.), semi-quantitative
PCR was performed on a MyCycle™ Thermal Cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The reaction was performed
initially at 98°C for 2 min, then amplified for 35 cycles using the
following conditions: 98°C for 10 sec, 65°C for 15 sec and 72°C for
1 min, and finally maintained at 72°C for 5 min. The PCR products
were analyzed by 1% agarose gel electrophoresis containing 2.5%
(v/v) ethidium bromide.
Construction of pEASY-blunt
simple-CRKII cloning vector
The specific gel band of PCR product for CRKII from
the aforementioned protocol was sliced and purified with an
Universal DNA Purification kit (Tiangen Biotech Co., Ltd., Beijing,
China). The purified PCR DNA product containing HindIII and
BamHI sites was hydrolyzed by HindIII and
BamHI enzymes, then ligated into a pEASY-blunt simple
cloning vector (Transgen Biotech Co., Ltd., Beijing, China). The
ligated product was transformed into E. coli Novablue and
screened by blue-white screening. Positive recombinant plasmid was
extracted with the alkaline-lysis method (27), digested by
HindIII/BamHI, analyzed by 1% agarose gel
electrophoresis and the DNA was sequenced.
Construction of pcDNA3.1/V5-HisB-CRKII
plasmid
The pEASY-blunt simple cloning vector containing
CRKII was digested with HindIII and BamHI. The
cleaved insert was ligated into a pcDNA3.1/V5-HisB vector (Shanghai
CPG Biotech Co., Ltd., Shanghai, China) with T4 DNA ligase (Takara
Bio, Inc.) at 16°C overnight. Subsequently, the ligation mixture
was transformed into competent E. coli Novablue and
amplified for plasmid extraction with an EZNA®Endo-Free
plasmid mini kit (Omega Bio-Tek, Inc., Norcross, GA, USA). Finally,
the plasmid extraction was measured with a Thermo Scientific
NanoDrop 2000 (Thermo Fisher Scientific, Inc.) and stored at −20°C
for further study.
CRKII transfection
Hca-P cells were seeded at the density of
1×105 cells/500 µl in a 24-well plate. Each extracted
plasmid was mixed with Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) at a dilution of 1.5 µg/4.5 µl and
mixed with RPMI-1640 medium to a final volume of 100 µl for 20 min.
The plasmid mixtures was separately added into each well containing
Hca-P cells, mixed well and transfected for 24 h at 37°C with 5%
CO2. CRKII expression and upregulation level in
pcDNA3.1/V5-HisB-CRKII-Hca-P was determined by western blotting and
compared with pcDNA3.1/V5-HisB-Hca-P cells. Experiments were
performed 24 h subsequent to transfection.
SDS-PAGE and western blotting
Protein samples were extracted from the cell pellets
of pcDNA3.1/V5-HisB-CRKII-Hca-P and pcDNA3.1/V5-HisB-Hca-P by
ultrasound sonication in 1 ml ice-cold lysis buffer (Nanjing KeyGen
Biotech Co., Ltd., Nanjing China) supplemented with l µl 100 mmol/l
phenylmethylsulfonyl fluoride, 2 µl phosphatase inhibitors and 0.2
µl protease inhibitor. Supernatants were collected by
centrifugation at 12,879 × g for 15 min at 4°C. Protein
concentration was determined using the Bradford method. Equal
amount of protein samples (20 µg) from each group were separated by
12% SDS-PAGE. Protein bands were then transferred onto a
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA),
blocked with 5% (w/v) skimmed milk for 2 h at room temperature, and
incubated with CRKII (1:1,000; sc-9004; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and GAPDH (1:5,000; 1049–1-AP; Proteintech
Group, Inc., Chicago, IL, USA) antibodies rotating at 100 rpm using
an orbital shaker at 4°C overnight. After washing with TBS with
0.5% (v/v) Tween-20 buffer for three times for 10 min each, the NC
membrane was incubated with peroxidase-conjugated goat
anti-rabbit/anti-mouse (1:5,000; TA130001/TA130015; OriGene
Technologies, Inc., Beijing, China) for 2 h at room temperature.
Immunoreactive proteins bands were visualized by enhanced
chemiluminescent WesternBright™ ECL substrate (K-12045-D50;
Adavansta; VWR, Radnor, PA, USA) and detected by Bio-Rad ChemiDoc™
MP imaging system (Bio-Rad Laboratories, Inc.) and analyzed by
densitometric analysis using the Image Lab Software 4.0.1 (Bio-Rad
Laboratories, Inc.).
Cell Counting Kit-8 (CCK-8) assay for
cell proliferation
The effect of CRKII overexpression on Hca-P
proliferation was measured using CCK-8 (Takara Bio, Inc.). The
transfected pcDNA3.1/V5-HisB-CRKII-Hca-P and pcDNA3.1/V5-HisB-Hca-P
cells were seeded into a 96-well plate with the density of 1,500
cells/well in 100 µl RPMI-1640 medium with 15% FBS at 37°C. The
relative cell viabilities were detected using a CCK-8 assay at the
time intervals 24, 48, 72 and 96 h. A total of 10 µl CCK-8 solution
was added into each well and continuously incubated at 37°C for 1.5
h. The absorbance at 450 nm was recorded to calculate the relative
cell numbers.
Soft-agar colony forming assay
The effect of CRKII overexpression on the
anchorage-independent growth of Hca-P cells was investigated. The
6-well plates were coated with two-layer soft agars. Soft agars
(Amresco, LLC, Solon, OH, USA) of 1.2% (w/v) and 0.7% (w/v) were
completely melted by autoclaving and kept in a 50°C incubator. For
the base layer, the 1.2% soft agar was mixed with equal volume of
2X medium (85% RPMI-1640/15% FBS), quickly added into a 6-well
plate at 2.0 ml mixture/well and cooled to room temperature.
Subsequently, the mixture of 0.7% soft agar solution and 2X medium
(85% RPMI-1640/15% FBS; 1:1) with 1×103 cells of each
group was pooled immediately onto the base layer, as the upper
layer. The plates were kept at 37°C with 5% CO2 for 10–14 days
until visible cell colonies appeared. Following this, the cells
were stained with crystal violet (0.05%) for 40 min at room
temperature and extensively washed with PBS buffer for 3–5 min. The
colonies were imaged using a Canon IXUS 115 HS digital camera (12
megapixels; Canon, Inc., Tokyo, Japan) fixed ~15 cm above the wells
and focused on the whole view of each well. The imaged colonies
were then counted using Photoshop CS3 software (Adobe Systems
Europe, Ltd., Maidenhead, UK) with the aid of the number counting
function.
Boyden transwell chamber assay for
cell migration and invasion
Corning Costar Transwell cell culture inserts
(Corning Inc., Corning, NY, USA) were employed to investigate the
effect of ANXA5 overexpression on the in vitro migration and
invasion properties of Hca-P cells. For the migration assay, 600 µl
RPMI-1640 with 20% FBS was loaded into each lower chamber. A total
of 10,000 cells from each group were separately loaded into one
upper chamber in 200 µl RPMI-1640 medium and incubated at 37°C with
5% CO2 for 24 h. The non-migrated cells on the upper surface of the
insert were carefully wiped off using cotton swabs. The cells that
had migrated to the lower surface of the filter were fixed in
methanol (Sigma-Aldrich; Merck KGaA) for 30 min, dried for 5 min at
room temperature, stained in 0.1% crystal violet for 40 min, washed
with PBS (200 µl) and counted by randomly selecting 5 fields per
well with a BX63 upright light microscope (Olympus Corporation,
Tokyo, Japan) at a magnification of ×200. For the invasion assay,
the Transwell surface was coated with 50 µl ice-cold ECM gel (2
mg/ml; Sigma-Aldrich; Merck KGaA) and incubated at 37°C for 6 h.
Subsequently, the Transwells were dried at room temperature for 30
min, and then hydrated with 50 µl serum-free RPMI-1640. RPMI-1640
(600 µl) with 20% FBS was then added into the lower chamber. Next,
7,500 cells suspended in 150 µl RPMI-1640 from each group were
loaded into the upper chamber. The rest of the steps were the same
as those described for the migration assay.
Statistical analysis
Data are represented as the mean ± standard
deviation of at least three independent experiments. Differences
between groups were determined by paired Student's t-test analysis.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSSx17.0
software (SPSS, Inc., Chicago, IL, USA).
Results
Gene cloning and
pcDNA3.1/V5-HisB-CRKII plasmid construction
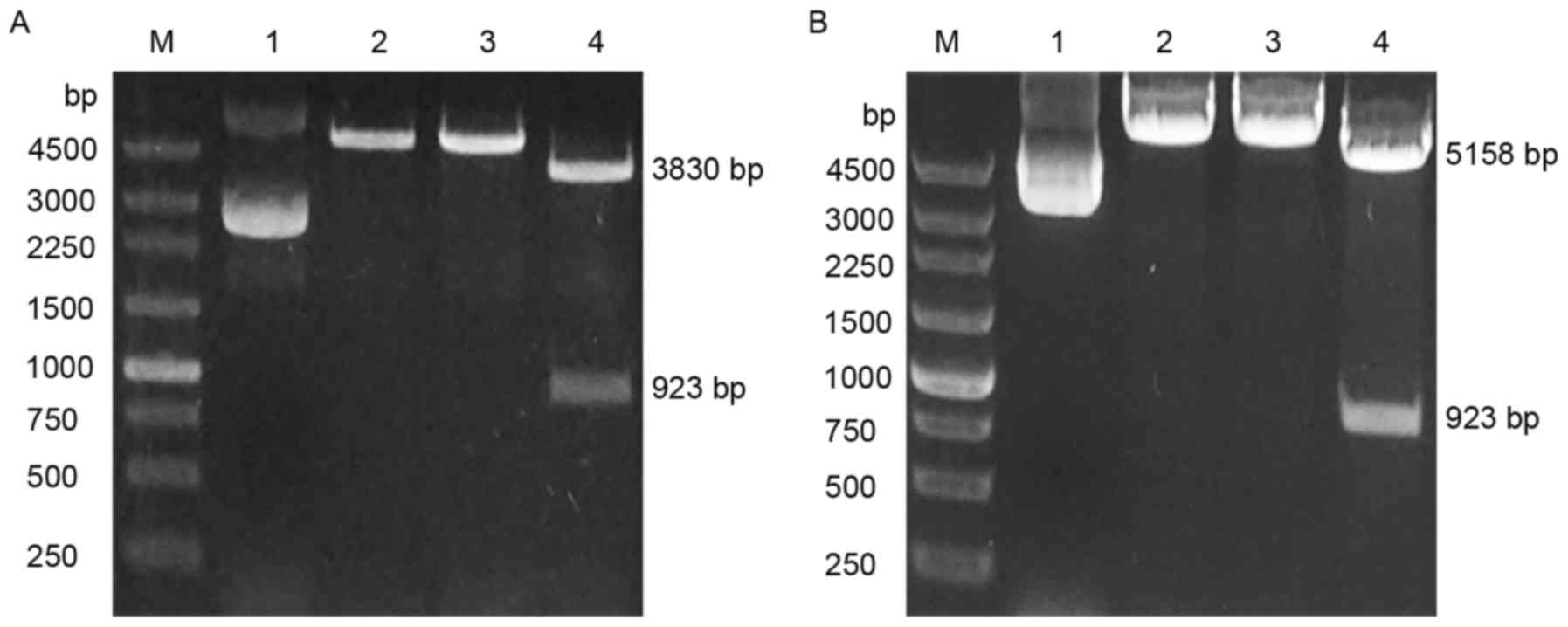
As depicted in Fig.
1A, the CRKII cloning vector was successfully constructed. The
expected size of bands for the pEASY-blunt simple vector and CRKII
following digestion with HindIII and BamHI were
~3,830 and 923 bp, as appeared in lane 4. A band of ~4,763 bp was
expected following digestion with BamHI or HindIII
alone (lanes 2 and 3). Subsequently, the pcDNA3.1/V5-HisB-CRKII
expression plasmid was established by inserting the digested
fragment from the CRKII cloning vector into the pcDNA3.1/V5-HisB
expression vector. The pcDNA3.1/V5-HisB vector is ~5,500 bp. As
shown in Fig. 1B, the band with size
of ~6,200 bp by BamHI cleavage alone or by HindIII
cleavage indicated that CRKII was inserted into the
pcDNA3.1/V5-HisB vector. The bands with sizes of ~5,200 and ~920 bp
on the gel following digestions with BamHI and
HindIII were the expected sizes for the pcDNA3.1/V5-HisB
vector and CRKII. The DNA sequencing results of the cloning and
expression vectors confirmed CRKII insertion (data not shown).
These results indicated that the pcDNA3.1/V5-HisB-CRKII plasmid was
successfully constructed.
CRKII is stably upregulated in Hca-P
cells
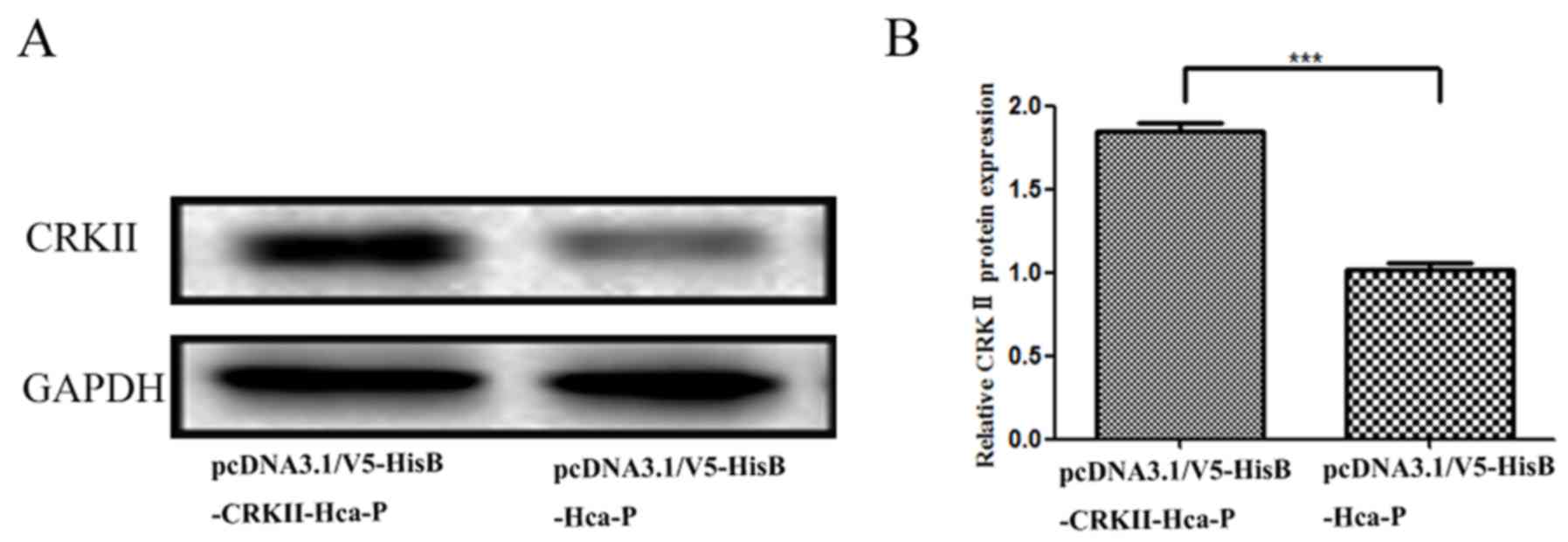
Western blotting indicated in Fig. 2A that the CRKII expression level was
upregulated in pcDNA3.1/V5-HisB-CRKII-Hca-P cells. Compared with
pcDNA3.1/V5-HisB-Hca-P, CRKII protein level in
pcDNA3.1/V5-HisB-CRKII-Hca-P cells was significantly increased by
~185% (P<0.001; Fig. 2B).
Successful overexpression of CRKII makes it feasible to investigate
its protein level change on the biological properties of Hca-P
cells.
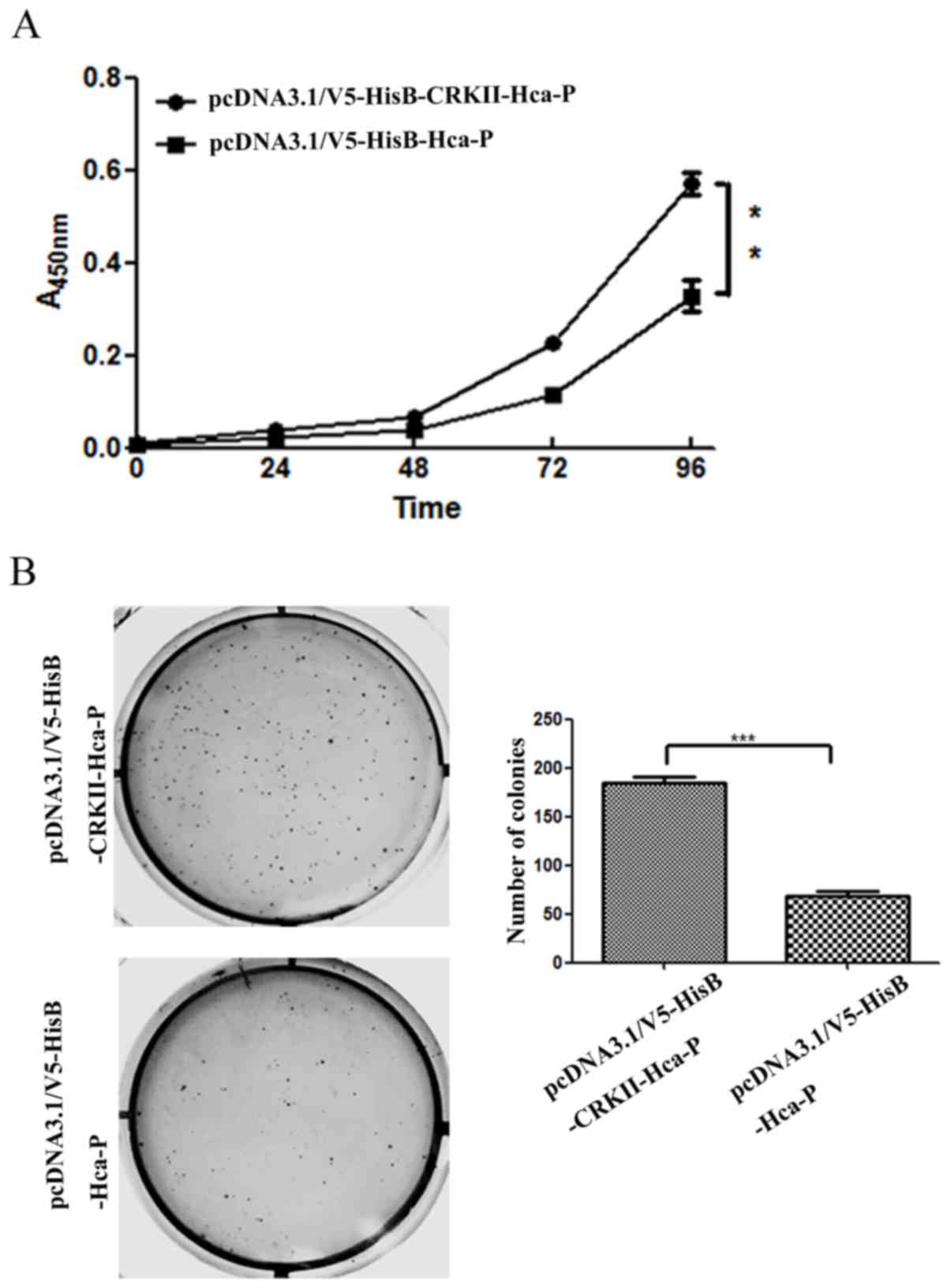
CRKII upregulation enhances the in
vitro proliferation of Hca-P cells
The impact of CRKII overexpression on the in
vitro proliferation of Hca-P was accessed with a CCK-8 assay.
As depicted in Fig. 3A, the
proliferation of pcDNA3.1/V5-HisB-CRKII -Hca-P cells was
significantly increased following CRKII overexpression, compared
with that in pcDNA3.1/V5-HisB-Hca-P cells. The relative
proliferation rates of pcDNA3.1/V5-HisB-CRKII-Hca-P cells at the
time intervals of 72 and 96 h were 246 and 150% increased, compared
with those in pcDNA3.1/V5-HisB-Hca-P cells; these differences were
statistically significant (Fig. 3A).
The aforementioned results indicate that the CRKII level is
positively associated with the in vitro proliferation of
Hca-P cells.
CRKII upregulation enhances the colony
forming ability of Hca-P
The CRKII level increase enhances the colony forming
capacity of Hca-P cells. The number of colonies formed in soft agar
was 184±12 for Hca-P cells transfected with pcDNA3.1/V5-HisB-CRKII,
and 68±8 for Hca-P cells transfected with pcDNA3.1/V5-HisB
(Fig. 3B). The relative colony
forming percentages were 18.4±1.2 and 6.8±0.8%, respectively. The
colony forming capacity of Hca-P cells increased by ~171.0%
following CRKII overexpression. Consistent with its promotion of
Hca-P proliferation, CRKII upregulation increased the colony
forming activity, indicating CRKII is a promoter of Hca-P malignant
potential.
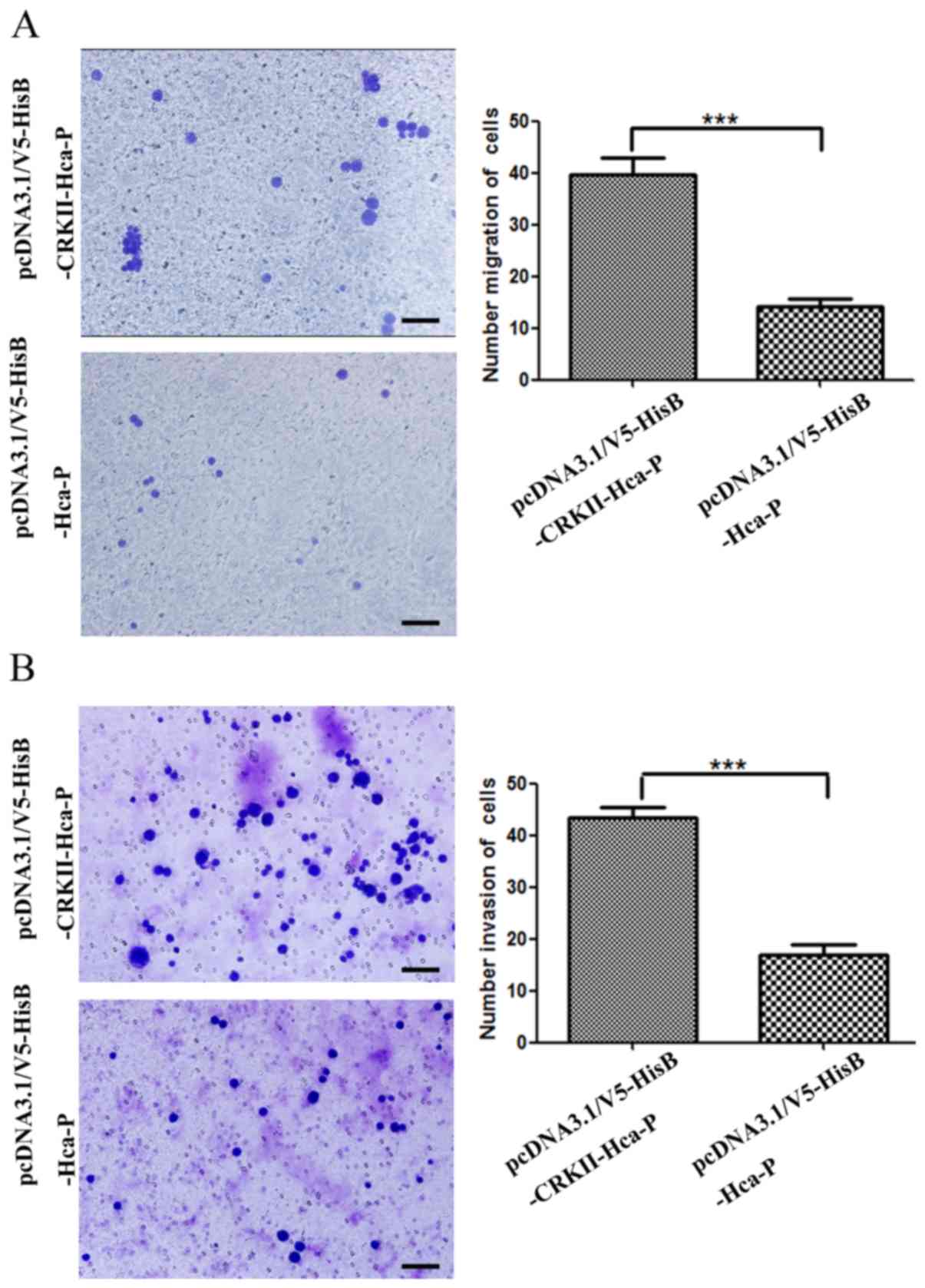
CRKII upregulation promotes migration
and invasion capacities of Hca-P
Transwell chamber assays indicated that increased
CRKII expression significantly enhanced the in vitro
migration and invasion capacities of Hca-P cells (Fig. 4).
The numbers of migrated pcDNA3.1/V5-HisB-CRKII-Hca-P
and pcDNA3.1/V5-HisB-Hca-P cells were measured as 39.7±0.8 and
14.2±0.3 per field (Fig. 4A). The
migration capacity of pcDNA3.1/V5-HisB-CRKII-Hca-P cells was
~2.8-fold increased, compared with pcDNA3.1/V5-HisB-Hca-P cells
(Fig. 4A). Therefore, CRKII
overexpression significantly increases Hca-P migration.
The invasion ability of Hca-P cells was increased
significantly following CRKII overexpression (Fig. 4B). The invaded numbers of
pcDNA3.1/V5-HisB-CRKII-Hca-P and pcDNA3.1/V5-HisB-Hca-P cells were
measured as 43.4±0.5 and 16.9±0.5 per field (Fig. 4B). The in vitro invasion
capacity of pcDNA3.1/V5-HisB-CRKII-Hca-P cells was ~2.6-fold of
that of pcDNA3.1/V5-HisB-Hca-P cells (Fig. 4B). Therefore, CRKII overexpression
results in significantly increased invasion potential of Hca-P.
Discussion
The overexpression of CRKII was associated with the
development and progression of lung tumors (6), glioblastoma (7), oral squamous cell carcinoma (28), pancreatic ductal adenocarcinoma
(29) and salivary gland tumors
(30). However, there is limited
knowledge regarding the role of CRKII in hepatocarcinoma. Results
from our previous study (31)
indicated that CRK family proteins are potentially involved in the
lymphatic metastais of hepatocarcinoma by comparative proteomics
using Hca-P and Hca-F, two murine hepatocarcinoma ascites syngeneic
cell lines with low and high LNM rates, respectively (24). It was determined that another member
of CRK family, CRKL, exhibited a tumor suppressor effect on the
in vitro proliferation, migration and invasion, and in
vivo tumor malignancy and LNM rate levels of Hca-P cells
(22,23). Therefore, in the present study, the
role of CRKII in Hca-P malignant properties were investigated by
elevating its expression level in Hca-P cells.
CRKII level affects the in vitro
proliferation of Hca-P cells. It was reported that the CRKII level
is positively correlated with the proliferation of glioblastoma
KMG4 (7) and pancreatic ductal
adenocarcinoma cell lines (29).
Consistently, the present study demonstrated that CRKII
overexpression significantly promoted the proliferation (Fig. 3A) and colony forming capacity of
Hca-P cells (Fig. 3B).
It was reported that CRKII mediates cell metastasis
and invasion via p130Cas, paxillin and Dock180 (32,33).
CRKI/II knockdown inhibits the migration and invasion of human
cancer cell lines MDA-231, MDA-435s, H1299, KB and HeLa (10). Additionally, CRKII overexpression
increases the migration and invasiveness of oral squamous cell
carcinoma (28). Consistently, the
present study indicated that overexpression of CRKII significantly
increased the migration and invasion capacities of Hca-P cells by
~179% (Fig. 4A) and ~156% (Fig. 4B), respectively. CRKII expression
level is positively associated with the migration and invasion of
Hca-P cells. The expression level change of CRKII does not affect
the expression levels of p130Cas and Dock180 (data not shown);
while notably, the expression changes of CRKI and CRKL induced the
levels of p130Cas and Dock180 (unpublished data). Therefore, the
detailed mechanism of CRKII action in tumorigenesis deserves
further investigation.
In conclusion, CRKII expression level was positively
associated with the in vitro proliferation, migration and
invasion properties of Hca-P cells. The present study demonstrates
the association of CRKII with the malignant behaviors of
hepatocarcinoma cells and lymphatic metastasis mechanism of
hepatocarcinoma.
Acknowledgements
The authors would like to thank Professor Jianwu
Tang (Department of Pathology, Dalian Medical University, Dalian,
China) for providing the Hca-P cell line.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81672737, 81272186 and
81171957) and the Provincial Natural Science Foundation of Liaoning
(nos. 2015020266 and 2014023047).
Availability of data and materials
All data used and analyzed in current study are
available from corresponding authors on reasonable request.
Authors' contributions
ZZ and XS performed the experiments and wrote the
draft. CG contributed in data analysis. MS and SL designed and
supervised the study, and corrected the manuscript.
Ethics approval
The current study was approved by the Ethical
Committee of Dalian Medical University (Permit Number:
L20160029).
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feller SM: Crk family adaptors-signalling
complex formation and biological roles. Oncogene. 20:6348–6371.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Birge RB, Kalodimos C, Inagaki F and
Tanaka S: Crk and CrkL adaptor proteins: Networks for physiological
and pathological signaling. Cell Commun Signal. 7:132009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bos JL, Rehmann H and Wittinghofer A: GEFs
and GAPs: Critical elements in the control of small G proteins.
Cell. 129:865–877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hasegawa H, Kiyokawa E, Tanaka S,
Nagashima K, Gotoh N, Shibuya M, Kurata T and Matsuda M: DOCK180, a
major CRK-binding protein, alters cell morphology upon
translocation to the cell membrane. Mol Cell Biol. 16:1770–1776.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka S, Morishita T, Hashimoto Y,
Hattori S, Nakamura S, Shibuya M, Matuoka K, Takenawa T, Kurata T,
Nagashima K, et al: C3G, a guanine nucleotide-releasing protein
expressed ubiquitously, binds to the Src homology 3 domains of CRK
and GRB2/ASH proteins. Proc Natl Acad Sci USA. 91:3443–3447. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller CT, Chen G, Gharib TG, Wang H,
Thomas DG, Misek DE, Giordano TJ, Yee J, Orringer MB, Hanash SM and
Beer DG: Increased C-CRK proto-oncogene expression is associated
with an aggressive phenotype in lung adenocarcinomas. Oncogene.
22:7950–7957. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Tabu K, Kimura T, Tsuda M, Linghu
H, Tanino M, Kaneko S, Nishihara H and Tanaka S: Signaling adaptor
protein Crk is indispensable for malignant feature of glioblastoma
cell line KMG4. Biochem Biophys Res Commun. 362:976–981. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sriram G and Birge RB: Emerging roles for
crk in human cancer. Genes Cancer. 1:1132–1139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fathers KE, Bell ES, Rajadurai CV, Cory S,
Zhao H, Mourskaia A, Zuo D, Madore J, Monast A, Mes-Masson AM, et
al: Crk adaptor proteins act as key signaling integrators for
breast tumorigenesis. Breast Cancer Res. 14:R742012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodrigues SP, Fathers KE, Chan G, Zuo D,
Halwani F, Meterissian S and Park M: CrkI and CrkII function as key
signaling integrators for migration and invasion of cancer cells.
Mol Cancer Res. 3:183–194. 2005.PubMed/NCBI
|
|
11
|
Bell ES and Park M: Models of crk adaptor
proteins in cancer. Genes Cancer. 3:341–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Y, Li S, Qu J, Wang S, Dang Y, Fan J,
Yu S and Zhang J: MiR-34a inhibits lymphatic metastasis potential
of mouse hepatoma cells. Mol Cell Biochem. 354:275–282. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Wang Q, Du Y, Bai L, Jin F, Zhang
J, Fan S, Wang H, Song L, Gao Y, et al: Inhibition of annexin A7
gene and protein induces the apotosis and decreases the invasion,
migration of the hepatocarcinoma cell line. Biomed Pharmacother.
68:819–824. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang YH, Wang SQ, Sun CR, Wang M, Wang B
and Tang JW: Inhibition of JNK1 expression decreases migration and
invasion of mouse hepatocellular carcinoma cell line in vitro. Med
Oncol. 28:966–972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Meng J, Du Y, Huang Y, Jin Y, Zhang
J, Wang B, Zhang Y, Sun M and Tang J: RACK1 promotes the
proliferation, migration and invasion capacity of mouse
hepatocellular carcinoma cell line in vitro probably by PI3K/Rac1
signaling pathway. Biomed Pharmacother. 67:313–319. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sleeman JP: The lymph node as a bridgehead
in the metastatic dissemination of tumors. Recent Results Cancer
Res. 157:55–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawada K and Taketo MM: Significance and
mechanism of lymph node metastasis in cancer progression. Cancer
Res. 71:1214–1218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kabilova TO, Kovtonyuk LV, Zonov EV,
Ryabchikova EI, Popova NA, Nikolin VP, Kaledin VI, Zenkova MA,
Vlassov VV and Chernolovskaya EL: Immunotherapy of hepatocellular
carcinoma with small double-stranded RNA. BMC Cancer. 14:3382014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdel-Rahman O: Revisiting
oxaliplatin-based regimens for advanced hepatocellular carcinoma.
Curr Oncol Rep. 16:3942014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quetglas IM, Moeini A, Pinyol R and Llovet
JM: Integration of genomic information in the clinical management
of HCC. Best Pract Res Clin Gastroenterol. 28:831–842. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chuang SC, La Vecchia C and Boffetta P:
Liver cancer: Descriptive epidemiology and risk factors other than
HBV and HCV infection. Cancer Lett. 286:9–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi J, Meng L, Sun MZ, Guo C, Sun X, Lin Q
and Liu S: CRKL knockdown promotes in vitro proliferation,
migration and invasion, in vivo tumor malignancy and lymph node
metastasis of murine hepatocarcinoma Hca-P cells. Biomed
Pharmacother. 71:84–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin Q, Sun MZ, Guo C, Shi J, Chen X and
Liu S: CRKL overexpression suppresses in vitro proliferation,
invasion and migration of murine hepatocarcinoma Hca-P cells.
Biomed Pharmacother. 69:11–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu S, Guo C, Wang J, Wang B, Qi H and Sun
MZ: ANXA11 regulates the tumorigenesis, lymph node metastasis and
5-fluorouracil sensitivity of murine hepatocarcinoma Hca-P cells by
targeting c-Jun. Oncotarget. 7:16297–16310. 2016.PubMed/NCBI
|
|
25
|
Hou L, Li Y, Jia YH, Wang B, Xin Y, Ling
MY and Lü S: Molecular mechanism about lymphogenous metastasis of
hepatocarcinoma cells in mice. World J Gastroenterol. 7:532–536.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levin-Arama M, Abraham L, Waner T,
Harmelin A, Steinberg DM, Lahav T and Harlev M: Subcutaneous
compared with intraperitoneal KetamineXylazine for anesthesia of
mice. J Am Assoc Lab Anin Sci. 55:794–800. 2016.
|
|
27
|
Feliciello I and Chinali G: A modified
alkaline lysis method for the preparation of highly purified
plasmid DNA from Escherichia coli. Anal Biochem.
212:394–401. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamada S, Yanamoto S, Kawasaki G,
Rokutanda S, Yonezawa H, Kawakita A and Nemoto TK: Overexpression
of CRKII increases migration and invasive potential in oral
squamous cell carcinoma. Cancer Lett. 303:84–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu R, Wang Q, Xu G, Li K, Zhou L and Xu
B: The adaptor protein CrkII regulates IGF-1-induced biological
behaviors of pancreatic ductal adenocarcinoma. Tumour Biol.
37:817–822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Askari M and Darabi M, Jahanzad E,
Mostakhdemian Hosseini Z, Musavi Chavoshi M and Darabi M:
Immunohistochemichal assessment of the CrkII proto-oncogene
expression in common malignant salivary gland tumors and
pleomorphic adenoma. J Dent Res Dent Clin Dent Prospects. 9:29–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun MZ, Liu S, Tang J, Wang Z, Gong X, Sun
C and Greenaway F: Proteomics analysis of two mice hepatocarcinoma
ascites syngeneic cell lines with high and low lymph node
metastasis rates provide potential protein markers for tumor
malignancy attributes to lymphatic metastasis. Proteomics.
9:3285–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klemke RL, Leng J, Molander R, Brooks PC,
Vuori K and Cheresh DA: CAS/Crk coupling serves as a ‘molecular
switch’ for induction of cell migration. J Cell Biol. 140:961–972.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kiyokawa E, Hashimoto Y, Kurata T,
Sugimura H and Matsuda M: Evidence that DOCK180 up-regulates
signals from the CrkII-p130(Cas) complex. J Biol Chem.
273:24479–24484. 1998. View Article : Google Scholar : PubMed/NCBI
|