Introduction
Prostate cancer (PCa) is a very common tumor in the
male urinary system, and is a clinically complex multifactorial
disease. Approximately 22,000 American men are diagnosed with PCa
each year (1). In recent years, with
the growth of aging population and improvement of living
conditions, both the incidence and mortality rates due to PCa have
increased (2). PCa is a malignant
tumor of prostate epithelium, with slow development of tumor cells,
insidious pathogenesis and no obvious symptoms in early stage
(3). The cancer is often in the late
stage when there are obvious symptoms such as dysuria, hematuria,
impotence and urodynia. The cancer cells have undergone distant
metastasis and local infiltration, and invaded organs and tissues
other than prostate capsule, therefore missing the best time for
treatment (4). Studies have shown
that timely and effective detection of early cancer with well-timed
treatment can reduce PCa mortalities, which can be conducive to the
prognosis of patients with PCa and reduce the recurrent rate of PCa
patients (5). Therefore, finding
effective and convenient diagnostic methods and indicators can
improve the early diagnosis rate of PCa patients, also it is the
key to improve the cure and survival rates of PCa patients.
Claudins is a transmembrane with connexin protein
and is currently found in approximately 30 family members with
molecular weights between 20 and 27 kDa (6). Changes in the expression levels of mRNA
and protein are often associated with a variety of disease
pathogenesis in the body. Medical scientists have confirmed that
elevated levels of Claudin-4 can lead to the development and spread
of cancer cells in patients with pancreatic cancer and breast
cancer (7,8). Claudin-1 is overexpressed in colon
cancer patients (9). Claudin-3 is a
member of the Claudins connexin protein and is closely associated
with the transmembrane protein. It has an important effect in the
transmission and transportation of cells. Some studies have
suggested that the abnormal expression of Claudin-3 is closely
related to the occurrence and development of tumors (10).
Phosphatase and tensin homolog deleted on chromosome
10 (PTEN) is composed of 9 exogenous factors and 8 inclusive
factors, 1212 base pairs located at zone 2 and band 3 of the long
arm in human chromosome 10, with a total length of 200 kb. The
functional protein encoded is a bispecific phosphate synthase, a
polypeptide chain consisting of 403 amino acid residues (11). PTEN is the first tumor suppressor
gene with phosphatase activity, which can inhibit tumors by
promoting cell apoptosis (12). This
gene has a high frequency of deletions and mutations in prostate
cancer (13) and has received
extensive attention in recent years. PTEN can mainly inhibit the
growth, invasion and metastasis of cells and the pathologic
adhesion of tumor cells (14). It is
involved in cell differentiation, cell attachment, cell migration
and apoptosis, it can maintain the body's immune system stability
and has an important effect in a variety of physiological
activities (15). The abnormal
expression of PTEN can induce the growth of cells, which benefits
the occurrence of tumors. Also induces cell invasiveness and
adhesion ability, which benefits the metastasis of tumor cells
(16).
Clinically, PCa examination methods mainly include
prostate biopsy guided by rectal ultrasound, digital rectal
examination and nuclear magnetic resonance detection (17). With the advancement of medical
science, the diagnostic rate of PCa in the discovery of PCa tumor
markers such as prostate specific antigen and prostate specific
membrane antigen has also improved. However, some studies have
found that in the autopsy of males who died normally over the age
of 70, >80% of the patients with PCa were not fully detected
when they were alive (18). This
suggests that further investigations into the pathogenesis of PCa
are required in order to find more accurate examination methods for
tumor markers.
This study explored the blood expression levels and
significance of PTEN and Claudin-3 in patients, investigated the
expression and significance of PTEN and Claudin-3 in the blood of
patients with PCa to provide a basis for clinical practice.
Patients and methods
General information
Retrospective analysis of 84 cases of PCa patients
confirmed by pathological diagnosis, and the medical records were
the experiment group. Moreover, the physical examination data of 84
healthy volunteers examined in the Affiliated Hospital of Beihua
University (Jilin, China) were the control group. The average age
in the experiment group was 67.65 years of age, according to the
Gleason scoring methods, the experiment group was divided into
different subgroups. There were 3 cases in the high differentiation
group (Gleason 2–4 unit), 16 cases in the medium differentiation
group (Gleason 5–6 unit) and 65 cases in the low differentiation
group (7–10 unit). According to the TNM staging of prostate, the
experiment group was divided into 26 cases of T1-T2 stage, treated
as T1-T1 subgroup, and 58 cases of T3-T4 stage, treated as T3-T4
subgroup.
The study was approved by the ethics committee of
Affiliated Hospital of Beihua University. All the subjects were
informed and agreed to participate in the clinical study, and
informed consents were obtained.
Inclusion and exclusion criteria
Inclusion criteria
Patients with PCa diagnosed by Clinical Pathology in
the Affiliated Hospital of Beihua University; age ≥18 years; tumor
grading and clear stage. No radiotherapy, chemotherapy and other
anticancer treatments were used before serum was taken. No
congenital genetic disease; perfect clinical medical records for
patients were available.
Exclusion criteria
Patients who had taken antibiotics within three
months before sampling; patients with liver dysfunction; autoimmune
system defects, and suffering from other tumors; PCa for recurrence
at admission and patients with urinary system diseases.
Main reagents and instruments
Automatic washing machine (model: RT-3100; Shanghai
Tiancheng Technology Co., Ltd., Shanghai, China); automatic
quantitative enzyme-labeling instrument (model: Bole 680; Shanghai
Dingqian Biotechnology Co., Ltd., Shanghai, China); Claudin-3 ELISA
kit (item no. PRE8808; Beijing Huaxia Ocean Technology Co., Ltd.,
Beijing, China); PTEN ELISA kit (item no. YD2717; Shanghai Yudu
Biotechnology Co., Ltd., Shanghai, China); UV-visible
spectrophotometer (model: UV1700; Shanghai Jeanqi Instrument
Technology Co., Ltd., Shanghai, China); and a low speed normal
temperature centrifuge (model: 3-5N; Hunan Hengnuo Instrument
Equipment Co., Ltd., Hunan, China) were used in the present
study.
Collection of specimens
All the participants in the experiment were fasted
for >8 h the night before blood collection. The next morning, 5
ml of elbow venous blood was taken on an empty stomach. After
standing at 30°C for 25 min, the serum was separated in a
centrifuge of 2,300 × g at 20°C and centrifugation time was
approximately 15 min. After the end of the centrifugation, it was
let to stand for 10 min. After the specimens were layered, the
supernatant was carefully collected and stored in a refrigerator at
−20°C until re-use.
Detection of PTEN and Claudin-3
expression levels
In this experiment, the expression levels of PTEN
and Claudin-3 in the blood between the experiment group and the
control group were determined by enzyme-linked immunosorbent assay
(ELISA).
The specimen and kit were taken out of the
refrigerator and melted at 30°C. Then 20-fold concentration of 30
ml of washing liquid and extra 570 ml of distilled water were added
prior to dilution in the original washing solution. Subsequently,
the ELISA plate was removed. Standard solution (50 µl) was poured
into the wells, then 10 µl of sample and 60 µl of sample diluent
were added to the sample wells. Chromogenic reagent (100 µl) was
added to each well except the blank one; the ELISA plate was
removed after 60 min in a 37°C water tank and 50 µl of washing
solution was added to each well. After leaving it for 1 min, the
liquid in the well was removed and the plate was washed 5 times.
Enzyme standard solution (50 µl) was added to each well (except for
the blank control well). The chromogenic reagent was added after 15
min in a 37°C reciprocal shaking bath and avoid colored-light for
15 min. Then the ELISA plate was taken out and termination solution
was added to stop the reaction. After the reaction was terminated
for 10 min, the absorption value of each well was measured at a
wavelength of 450 nm. The R-value was calculated according to the
absorption value corresponding to the concentration of the standard
product, and the product is a good when the R-value has an accuracy
of 99% or more. The linear regression equation was calculated using
a fully automatic enzyme-labeling instrument, and the sample
concentration was calculated based on the measurement of absorption
values.
Statistical analysis
Data analysis was performed using SPSS 17.0
(Shanghai Yuchuang Network Technology Co., Ltd., Shanghai, China)
statistical software. The measurement data were expressed as mean ±
standard deviation (Means Network). The measurement data between
groups were compared by t-test. ANOVA was used for the comparison
of multiple groups with LSD post hoc test. The receiver operating
characteristic (ROC) curve was used to evaluate the diagnostic
efficacy of serum PTEN and Claudin-3 expression on PCa. When
P<0.05, the difference was statistically significant.
Results
Expression levels of PTEN and
Claudin-3 in the experiment group and the control group
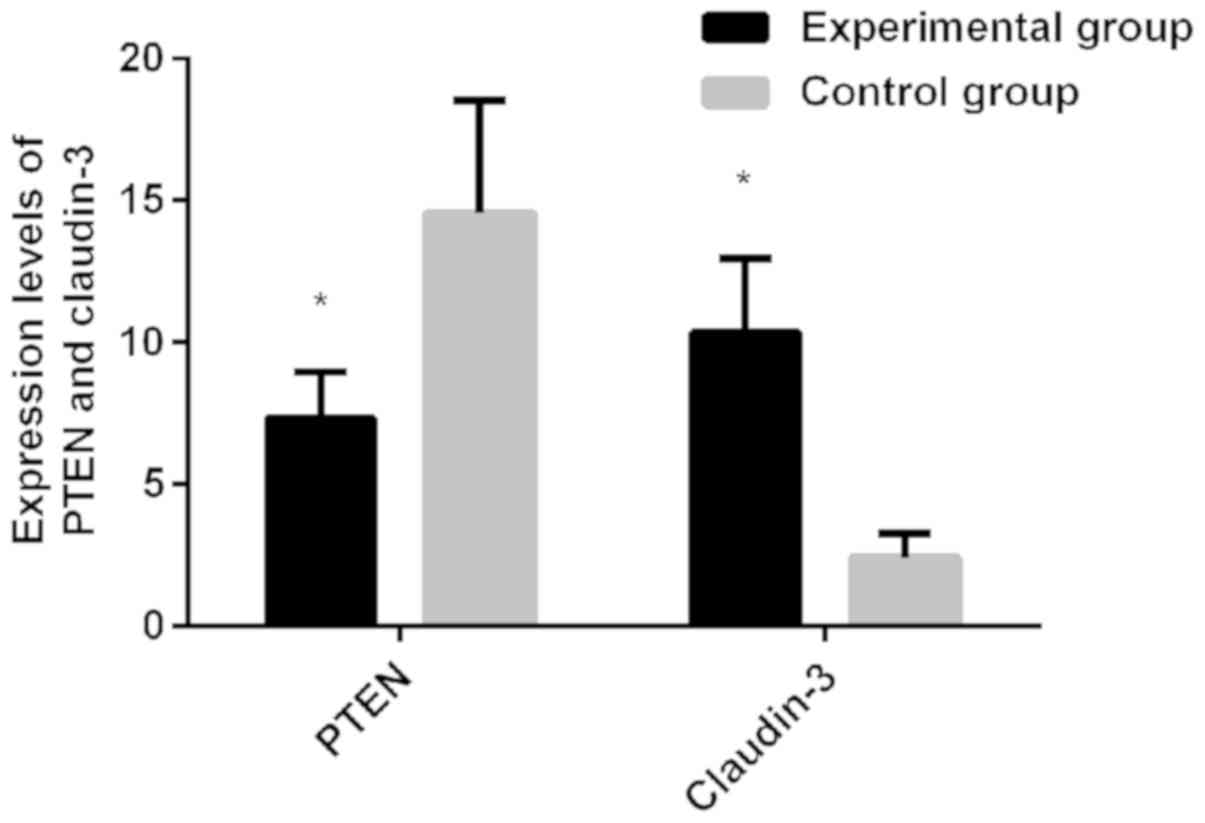
The experimental results showed that the expression
level of PTEN in the experiment group was 7.32, which was lower
than the control group (14.58), (t=15.560, P<0.01). The
expression level of Claudin-3 in the experiment group was 10.36,
which was higher than the control group (expression level of 2.43),
(t=26.790, P<0.01) (Fig. 1).
The expression levels of PTEN and
Claudin-3 of different baseline data in the experiment group
The results showed that the expression levels of
PTEN and Claudin-3 in the experiment group were not significantly
associated with age, smoking, alcoholism, body mass index,
preoperative blood glucose, preoperative Hb, preoperative Alb and
preoperative CRP (P>0.05) (Table
I).
 | Table I.The expression levels of PTEN and
Claudin-3 of different baseline data in the experiment group (mean
± SD). |
Table I.
The expression levels of PTEN and
Claudin-3 of different baseline data in the experiment group (mean
± SD).
| Type | No. of cases | PTEN | t-test | P-value | Claudin-3 | t-test | P-value |
|---|
| Age (year) |
|
| 0.936 | 0.352 |
| 1.809 | 0.074 |
|
<65 | 37 | 7.61±1.35 |
|
| 10.88±2.06 |
|
|
| ≥65 | 47 | 7.30±1.62 |
|
| 10.02±2.24 |
|
|
| Smoking |
|
| 0.438 | 0.662 |
| 0.504 | 0.616 |
|
Included | 69 | 7.45±1.51 |
|
| 10.46±2.46 |
|
|
|
Excluded | 15 | 7.26±1.58 |
|
| 10.11±2.33 |
|
|
| Alcoholism |
|
| 0.792 | 0.430 |
| 0.470 | 0.640 |
|
Included | 32 | 7.14±1.46 |
|
| 10.54±2.40 |
|
|
|
Excluded | 52 | 7.41±1.55 |
|
| 10.28±2.50 |
|
|
| BMI (km/m) |
|
| 0.516 | 0.607 |
| 0.608 | 0.545 |
|
<24 | 58 | 7.38±1.58 |
|
| 10.27±2.49 |
|
|
| ≥24 | 26 | 7.19±1.51 |
|
| 10.62±2.32 |
|
|
| Preoperative blood
glucose (mmol/l) |
|
| 1.166 | 0.247 |
| 0.464 | 0.644 |
|
<4.5 | 47 | 7.66±1.30 |
|
| 10.22±2.44 |
|
|
|
≥4.5 | 37 | 7.29±1.61 |
|
| 10.47±2.47 |
|
|
| Preoperative CRP
(mg/l) |
|
| 0.711 | 0.479 |
| 0.364 | 0.717 |
|
<100 | 66 | 7.24±1.56 |
|
| 10.27±2.49 |
|
|
|
≥100 | 18 | 7.53±1.43 |
|
| 10.51±2.43 |
|
|
| Preoperative Hb
(g/l) |
|
| 0.750 | 0.456 |
| 0.579 | 0.565 |
|
<140 | 36 | 7.43±1.53 |
|
| 10.55±2.39 |
|
|
|
≥140 | 48 | 7.18±1.50 |
|
| 10.24±2.46 |
|
|
| Preoperagive Alb
(g/l) |
|
| 0.782 | 0.437 |
| 0.894 | 0.374 |
|
<35 | 12 | 7.59±1.37 |
|
| 11.02±1.92 |
|
|
|
≥35 | 72 | 7.22±1.54 |
|
| 10.33±2.55 |
|
|
Relationship between the expression
levels of PTEN, Claudin-3 and the clinicopathological features
The results showed that the expression levels of
PTEN and Claudin-3 in the experiment group were significantly
associated with distant metastasis of cancer cells, preoperative
prostate specific antigen level, tumor diameter and pathological
stage (P<0.01) (Table II).
 | Table II.Comparison of expression levels of
PTEN and Claudin-3 in different clinicopathological features (mean
± SD). |
Table II.
Comparison of expression levels of
PTEN and Claudin-3 in different clinicopathological features (mean
± SD).
| Type | No. of cases | PTEN | T/F | P-value | Claudin-3 | t-test | P-value |
|---|
| Distant
metastasis |
|
| 10.600 | <0.01 |
| 13.380 | <0.01 |
|
Included | 41 | 6.03±0.35 |
|
| 11.6±1.28 |
|
|
|
Excluded | 43 | 7.89±1.07 |
|
| 8.57±0.79 |
|
|
| Preoperative
prostate specific antigen level |
|
| 2.110 | 0.04 |
| 3.245 | <0.01 |
| <10
ng/ml | 8 | 8.03±0.93 |
|
| 8.24±0.46 |
|
|
| ≥10
ng/ml | 76 | 7.01±1.33 |
|
| 10.7±2.18 |
|
|
| Tumor
diameters |
|
| 4.749 | <0.01 |
| 4.715 | <0.01 |
| <1.5
cm | 31 | 8.12±0.84 |
|
| 9.43±1.65 |
|
|
| ≥1.5
cm | 53 | 6.92±1.25 |
|
| 11.2±1.71 |
|
|
| Pathological
stages |
|
| 12.330 | <0.01 |
| 29.410 | <0.01 |
| Organ
limitation | 8 | 8.01±0.95 |
|
| 8.33±0.55 |
|
|
| Capsule
invasion | 13 | 7.59±1.35 |
|
| 9.12±1.24 |
|
|
| Seminal
vesicle invasion | 21 |
6.99±1.31a |
|
| 11.03±
1.91a,b |
|
|
| Lymph
node metastasis | 42 |
6.23±0.53a–c |
|
|
11.97±0.93a–c |
|
|
Expression levels of PTEN and
Claudin-3 in different clinical graded experiment groups
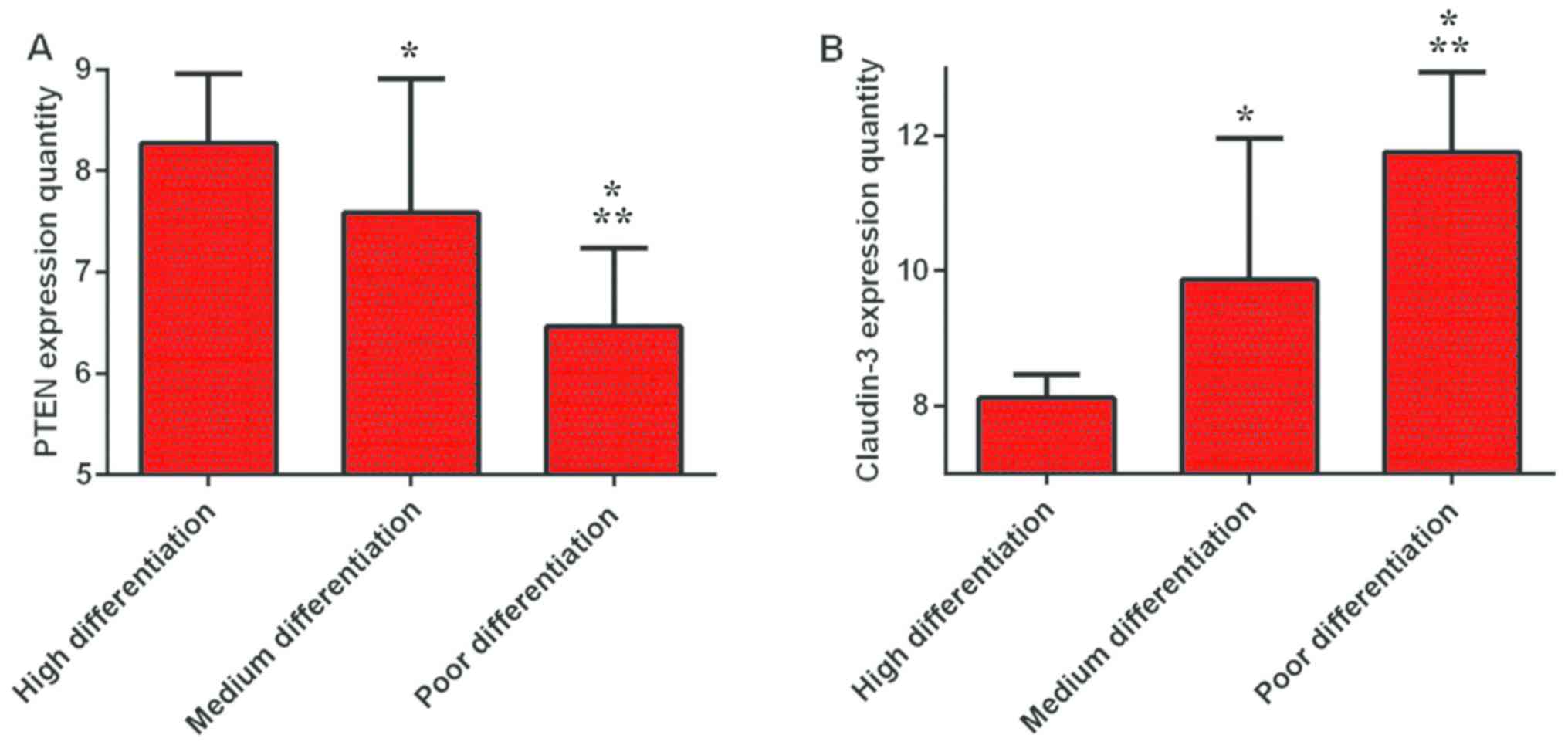
The results showed that the lowest expression level
of PTEN in the poor differentiation group was 6.46±0.78, which was
lower than the medium and high differentiation groups (7.59±1.32
and 8.27±0.69, respectively) (P<0.01). The expression level of
PTEN in the medium differentiation group was lower than that in
high differentiation group (P<0.01). Claudin-3 had the highest
expression level in the low differentiation group (11.75±1.19), and
was higher than both the medium and high differentiation groups
(9.87±2.09 and 8.12±0.34, respectively) (P<0.01). The expression
level of Claudin-3 in the medium differentiation group was higher
than that in high differentiation group (P<0.01) (Fig. 2).
Expression levels of PTEN and
Claudin-3 in different TNM stages of the experiment groups
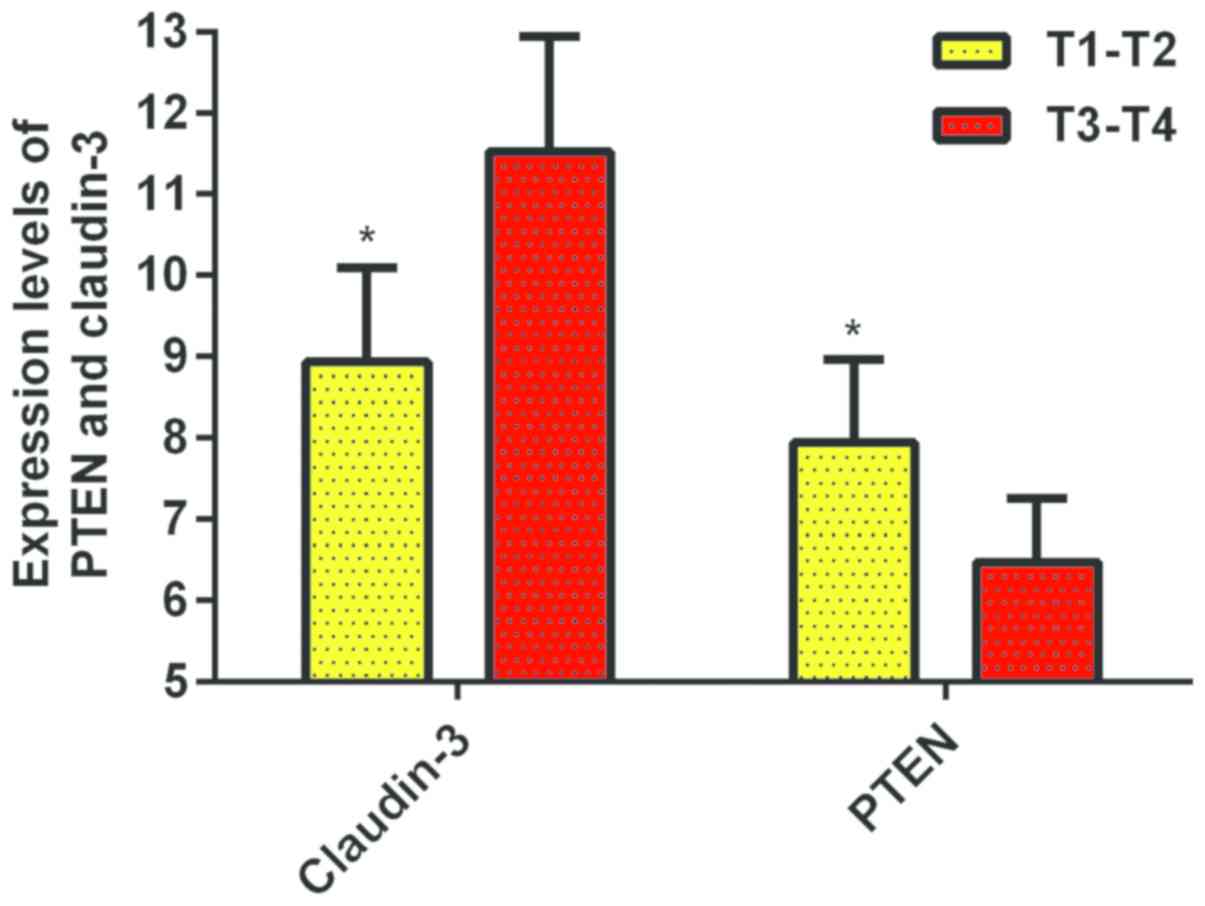
The results showed that the expression level of PTEN
in T1-T2 group was 7.94±1.02, which was higher than the T3-T4 group
(6.474±0.79), there was a significant difference between the groups
(P<0.01). The expression level of Claudin-3 in the T1-T2 group
was 8.94±1.16, which was lower than the T3-T4 group at 11.52±1.42.
There was a significant difference between the groups (P<0.01)
(Fig. 3).
Diagnostic value of PCa in the
expression levels of PTEN and Claudin-3
The expression levels of PTEN and Claudin-3 in the
blood of PCa and the normal blood of healthy volunteers and the ROC
curve diagram of the expression level of PTEN and Claudin-3 were
evaluated. The AUC of PTEN expression level for diagnosis of PCa
was 0.7943 (95% CI, 0.7243–0.9643), the diagnostic specificity was
88%, sensitivity was 64% and the optimal cut-off point for
diagnosing PCa was 8.895. The AUC of Claudin-3 expression level for
diagnosis of PCa was 0.7375 (95% CI, 0.6617–0.8133), the diagnostic
specificity was 85%, sensitivity was 57% and the optimal cut-off
point for diagnosing PCa was 3.310. Further combined with PTEN and
Claudin-3 to map the ROC curve diagram of PCa, the AUC of both PTEN
and Claudin-3 was 0.8576 (95% CI, 0.8018–0.9134), and the optimal
cut-off point for diagnosing PCa was 0.5697, the specificity was
69% and sensitivity was 88% (Fig.
4).
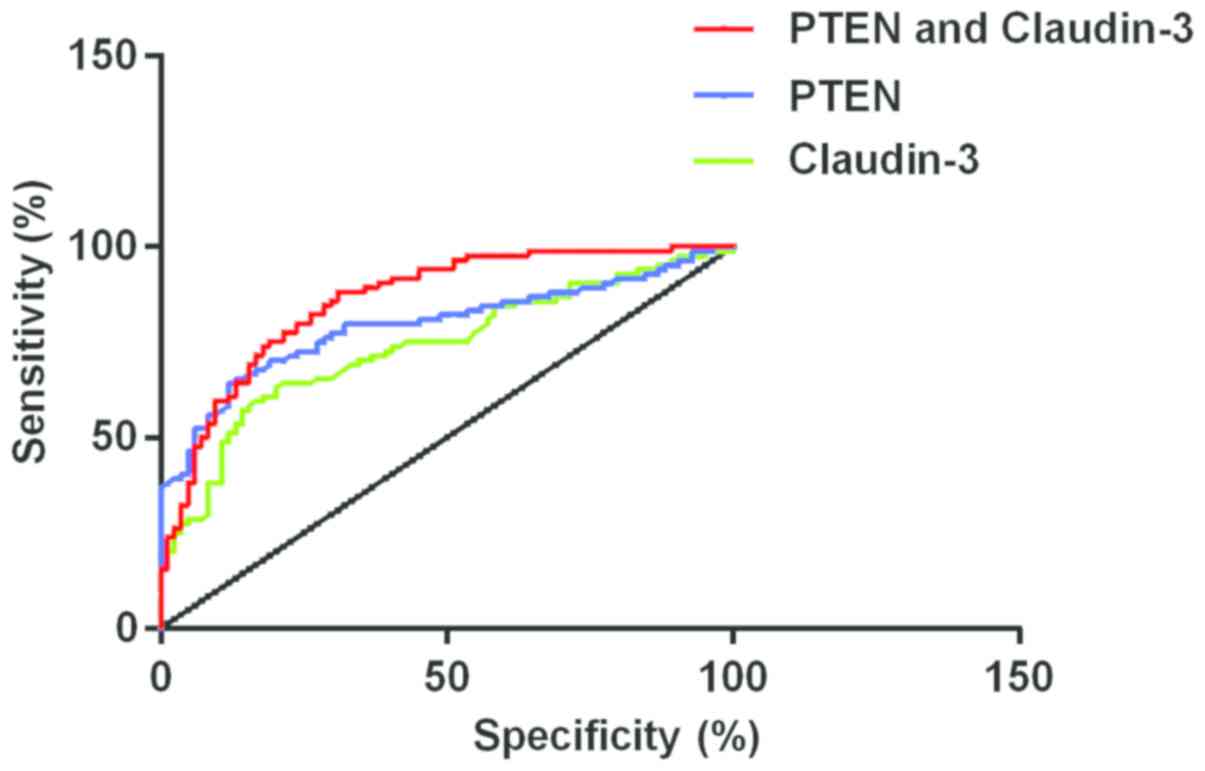 | Figure 4.The AUC of PCa in PTEN was 0.7943 (95%
CI, 0.7243–0.9643), specificity was 88%, sensitivity was 64% and
the optimal cut-off point was 8.895. The AUC of PCa in Claudin-3
was 0.7375 (95% CI, 0.6617–0.8133), specificity was 85%,
sensitivity was 57% and the optimal cut-off point was 3.310. The
AUC of PCa in combined detection of PTEN and Claudin-3 was 0.8576
(95% CI, 0.8018–0.9134), optimal cut-off point was 0.5697,
specificity was 69% and sensitivity was 88%. PTEN, phosphatase and
tensin homolog deleted on chromosome 10; PCa, prostate cancer. |
Discussion
PCa is a unique and common tumor that occurs in
males, and has the second highest incidence rate worldwide,
exceeded only by lung cancer (19).
The causes of PCa include inheritance, pathogenic microorganism,
drugs and diet. It occurs very often in middle-aged and elderly
males, and also is one of the main causes of death among them
(20). The pathogenesis of PCa is
relatively insidious, which is prone to early metastasis, and there
is often no obvious specificity in the early stage. It is often at
the late stage of cancer when patients have symptoms such as
impotence, premature ejaculation, blood essence, dysuria and
ejaculation pain. Therefore, it usually fails to achieve the
desired therapeutic effect (21).
PCa has a poor chemosensitivity and complicated treatment process,
most of the patients are elderly, and their physical functions and
organ functions have decreased to some extent. In addition, the
treatment is more difficult; the side effects of late-staged cancer
radiotherapy and chemotherapy are greater; older patients have
poor-tolerance and poorer living conditions (22). The biological behavior of PCa is
quite complicated, with the current development of PCa; the
mechanism is still unclear, and finding more accurate PCa tumor
markers is a focus for international research.
Claudin-3 is a member of the Claudins protein and is
one of the most important structural molecules that make up the
tight junction of epithelial cells. It mainly has an effect in
maintaining intercellular barrier function and cell polarity in the
body (23). Abnormal expression of
Claudin-3 often leads to loss of cell polarity, and the adhesion of
intercellular force is reduced to varying degrees, destroyed the
epithelial permeability barrier, thereby promoting the occurrence
and development of tumors. Studies have shown that in a variety of
tumor patients, the expression of Claudin-3 is significantly higher
than the adjacent normal tissues (10), indicating that the Claudin-3 is
closely related to the occurrence and development of tumors. PTEN
is a tumor suppressor gene, which relates to the growth,
development and stability of cells. Irregular expression of this
type of gene leads to abnormal regulation of cellular metabolism
and cell cycle. Therefore, it promoted cell migration, invasion and
enhanced adhesion of cells (24).
There are phosphorylase activities within the PTEN, and it has the
effect of inhibiting the growth and occurrence of tumors. In the
occurrence and development of PCa cancer cells, it promotes
apoptosis of cancer cells, inhibits the growth of cancer cells, and
cancer cell adhesion and metastasis and regulates the cycles of
cancer cells (25).
We have found that the expression levels of PTEN in
the blood of patients with PCa is significantly lower than in
normal people, and the difference was statistically significant
(P<0.05). The results of this study are similar to the research
of Yue et al (26), and they
found that irregular expression of PTEN in PCa patients of human
can cause abnormal accumulation of esterified cholesterol,
cholesterol esterification promotes cancer cell invasion. The
expression levels of Claudin-3 in the blood of patients with PCa
was higher than in normal people, and the difference was
statistically significant (P<0.01). The results of this study
are similar to those of Chinni et al (27), who found that Claudin-3 and Claudin-4
were overexpressed in PCa patients. The expression levels of PTEN
and Claudin-3 in the blood of PCa patients were significantly
correlated with distant metastasis in cancer cells, preoperative
prostate specific antigen levels, tumor diameter and pathological
stage (P<0.01). Phattarataratip and Sappayatosok (28) found that family members of the
Claudin proteins have an important effect in oral squamous cell
carcinoma. The irregular expression of Claudin-7 was associated
with pathological grade, late-staged TNM grade, tumor size,
fibropathopathy, vascular invasion and involvement of regional
lymph nodes. Moreover, Koperek et al (29) studied the expression of PTEN protein
in papillary thyroid cancer and found that the expression of PTEN
is related to sex, metastasis of lymph nodes and pathological
stages. This can be used as a prognostic factor for the evaluation
of papillary thyroid tumors. There are some similarities between
the research stated above and our studies, therefore it is
confirmed that both PTEN and Claudin-3 have important effects in
the growth, development and metastasis of tumors. In our further
studies, we found that the lowest expression levels of PTEN in the
blood of PCa patients in the low differentiation group were lower
than both the medium and high differentiation groups (P<0.01).
The highest expression levels of Claudin-3 in the low
differentiation group were higher than both the medium and high
differentiation groups (P<0.01). The expression levels of PTEN
in T1-T2 group were higher than the T3-T4 group, and the difference
between both groups were significant (P<0.01). The expression
levels of Claudin-3 in T1-T2 group were lower than the T3-T4 group,
and the difference between both groups were significant
(P<0.01). By examining the ROC curve diagram of PCa between both
PTEN and Claudin, we have found that the AUC of PCa was 0.8576 (95%
CI, 0.8018–0.9134); the optimal cut-off point was 0.5697;
specificity was 69% and the sensitivity was 88%; The ROC curve of
PCa was diagnosed by plotting the expression levels of PTEN and
Claudin-3; The combined diagnosis can improve the sensitivity
levels of PCa.
This study strictly selected the research objects
according to the inclusion and exclusion criteria, which ensured
the reliability of the study results. However, this study still has
certain defects. We only included a few patients in this study, and
more PCa patients with different pathological types should be
collected for research. The present study did not conduct an
in-depth study of the association between PTEN, Claudin-3 and other
clinical symptoms of PCa patients. Therefore, there are certain
limitations.
PTEN is lowly expressed in the blood of PCa patients
and Claudin-3 is highly expressed in the blood of PCa patients. The
expression levels of PTEN and Claudin-3 are closely related to the
distant metastasis of cancer cells, preoperative prostate specific
antigen level, tumor diameter and pathological stage. Combined
detection of PTEN and Claudin-3 can improve the specificity of PCa
for diagnosis, which has an important diagnostic value for PCa. It
can be used as a biological indicator for PCa diagnosis, disease
severity analysis and efficacy evaluation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY was involved in writing the manuscript. XY and LZ
performed ELISA. XY and JK collected the patient general data and
specimens. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Affiliated Hospital of Beihua University (Jilin, China). Patients
who participated in this research had complete clinical data.
Signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Robinson D, Van Allen EM, Wu YM, Schultz
N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC,
Attard G, et al: Integrative clinical genomics of advanced prostate
cancer. Cell. 161:1215–1228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song W and Jeon HG: Incidence of kidney,
bladder, and prostate cancers in Korea: An update. Korean J Urol.
56:422–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Nunzio C, Albisinni S, Freedland SJ,
Miano L, Cindolo L, Finazzi Agrò E, Autorino R, De Sio M, Schips L
and Tubaro A: Abdominal obesity as risk factor for prostate cancer
diagnosis and high grade disease: A prospective multicenter Italian
cohort study. Urol Oncol. 31:997–1002. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krupski TL, Stukenborg GJ, Moon K and
Theodorescu D: The relationship of palliative transurethral
resection of the prostate with disease progression in patients with
prostate cancer. BJU Int. 106:1477–1483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu L, Tian Z, Zhang Z and Fei B:
Computer-aided detection of prostate cancer with MRI: Technology
and applications. Acad Radiol. 23:1024–1046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koval M: Claudin heterogeneity and control
of lung tight junctions. Annu Rev Physiol. 75:551–567. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neesse A, Hahnenkamp A, Griesmann H,
Buchholz M, Hahn SA, Maghnouj A, Fendrich V, Ring J, Sipos B,
Tuveson DA, et al: Claudin-4-targeted optical imaging detects
pancreatic cancer and its precursor lesions. Gut. 62:1034–1043.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mosley M, Knight J, Neesse A, Michl P,
Iezzi M, Kersemans V and Cornelissen B: Claudin-4 SPECT imaging
allows detection of aplastic lesions in a mouse model of breast
cancer. J Nucl Med. 56:745–751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bezdekova M, Brychtova S, Sedlakova E,
Langova K, Brychta T and Belej K: Analysis of Snail-1, E-cadherin
and claudin-1 expression in colorectal adenomas and carcinomas. Int
J Mol Sci. 13:1632–1643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li JY, Xie F, Xu XP, Ma JJ, Zhou DC, Liao
Y, Tang J, Xie Q, Bai L and Nan QZ: Claudin-3 expression in
colorectal carcinoma and its significance. Nan Fang Yi Ke Da Xue
Xue Bao. 37:63–67. 2017.(In Chinese). PubMed/NCBI
|
|
11
|
Jones N, Bonnet F, Sfar S, Lafitte M,
Lafon D, Sierankowski G, Brouste V, Banneau G, Tunon de Lara C,
Debled M, et al: Comprehensive analysis of PTEN status in breast
carcinomas. Int J Cancer. 133:323–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Feng J, Fu H, Liu C, Yu Z, Sun Y,
She X, Li P, Zhao C, Liu Y, et al: Coagulation factor X regulated
by CASC2c recruited macrophages and induced M2 polarization in
glioblastoma multiforme. Front Immunol. 9:15572018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wise HM, Hermida MA and Leslie NR:
Prostate cancer, PI3K, PTEN and prognosis. Clin Sci (Lond).
131:197–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang LL, Liu J, Lei S, Zhang J, Zhou W
and Yu HG: PTEN inhibits the invasion and metastasis of gastric
cancer via downregulation of FAK expression. Cell Signal.
26:1011–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang J, Yuan SX, Wang DX, Wu QX, Wang X,
Pi CJ, Zou X, Chen L, Ying LJ, Wu K, et al: The role of COX-2 in
mediating the effect of PTEN on BMP9 induced osteogenic
differentiation in mouse embryonic fibroblasts. Biomaterials.
35:9649–9659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu M, Mu Y, Qi Y, Qin S, Qiu Y, Cui R and
Zhong M: Odontogenic ameloblast-associated protein (ODAM) inhibits
human colorectal cancer growth by promoting PTEN elevation and
inactivating PI3K/AKT signaling. Biomed Pharmacother. 84:601–607.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stamatiou K, Alevizos A, Karanasiou V,
Mariolis A, Mihas C, Papathanasiou M, Bovis K and Sofras F: Impact
of additional sampling in the TRUS-guided biopsy for the diagnosis
of prostate cancer. Urol Int. 78:313–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Albert RH and Clark MM: Cancer screening
in the older patient. Am Fam Physician. 78:1369–1374.
2008.PubMed/NCBI
|
|
19
|
Coakley FV, Oto A, Alexander LF, Allen BC,
Davis BJ, Froemming AT, Fulgham PF, Hosseinzadeh K, Porter C, Sahni
VA, et al Expert Panel on Urologic Imaging, : ACR Appropriateness
criteria® prostate cancer-pretreatment detection,
surveillance and staging. J Am Coll Radiol. 14:S245–S257. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bailey DE Jr and Wallace M: Critical
review: Is watchful waiting a viable management option for older
men with prostate cancer? Am J Men Health. 1:18–28. 2007.
View Article : Google Scholar
|
|
21
|
de Oliveira Barros EG, Palumbo A Jr, Mello
PL, de Mattos RM, da Silva JH, Pontes B, Viana NB, do Amaral RF,
Lima FR, da Costa NM, et al: The reciprocal interactions between
astrocytes and prostate cancer cells represent an early event
associated with brain metastasis. Clin Exp Metastasis. 31:461–474.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sinibaldi VJ: Docetaxel treatment in the
elderly patient with hormone refractory prostate cancer. Clin
Interv Aging. 2:555–560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yokoyama M, Narita T, Sakurai H,
Katsumata-Kato O, Sugiya H and Fujita-Yoshigaki J: Maintenance of
claudin-3 expression and the barrier functions of intercellular
junctions in parotid acinar cells via the inhibition of Src
signaling. Arch Oral Biol. 81:141–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang XD, Zhao SF, Zhang Q, Li W, Wang YX,
Hong XW and Hu QG: PTEN gene polymorphisms and susceptibility to
oral squamous cell carcinoma in a Chinese Han population. Tumour
Biol. 37:577–582. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Guo JX and Shao ZQ: miR-21 targets
and inhibits tumor suppressor gene PTEN to promote prostate cancer
cell proliferation and invasion: An experimental study. Asian Pac J
Trop Med. 10:87–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song
B, Cheng L, Masterson TA, Liu X, Ratliff TL, et al: Cholesteryl
ester accumulation induced by PTEN loss and PI3K/AKT activation
underlies human prostate cancer aggressiveness. Cell Metab.
19:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chinni SR, Li Y, Upadhyay S, Koppolu PK
and Sarkar FH: Indole-3-carbinol (I3C) induced cell growth
inhibition, G1 cell cycle arrest and apoptosis in prostate cancer
cells. Oncogene. 20:2927–2936. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Phattarataratip E and Sappayatosok K:
Expression of claudin-5, claudin-7 and occludin in oral squamous
cell carcinoma and their clinico-pathological significance. J Clin
Exp Dent. 8:e299–e306. 2016.PubMed/NCBI
|
|
29
|
Koperek O, Kornauth C, Capper D, Berghoff
AS, Asari R, Niederle B, von Deimling A, Birner P and Preusser M:
Immunohistochemical detection of the BRAF V600E-mutated protein in
papillary thyroid carcinoma. Am J Surg Pathol. 36:844–850. 2012.
View Article : Google Scholar : PubMed/NCBI
|