Introduction
Hepatocellular carcinoma (HCC) is one of the most
aggressive malignant tumors, causing more than one million deaths
annually (worldwide) and representing the second leading cause of
death from tumors (1,2). It is highly correlated with hepatitis B
or C virus (HBV or HCV) infection-associated chronic hepatitis
(3), and exhibits a high prevalence
rate (24.5 per 100,000) in Korea (4). Various local therapies, including
resection, transplantation, and carotid chemoembolization, have
been optimized for the treatment of HCC, but prognosis remains poor
in patients with late-stage or relapsing disease (4). The majority of HCC patients are treated
with chemoembolization or Nexavar® (sorafenib) (5). However, Nexavar® treatment
is limited by high cost and an unfavorable adverse effect profile,
and-while it has been reported to inhibit the growth of HCC-it is
known to be not induce necrosis (6).
Thus, new approaches for HCC treatment are required (4). One recent significant advance in tumor
therapy is the targeting of genes associated with important immune
system checkpoints (7). The most
notable of these is programmed cell death ligand 1 (PD-L1), which
was thought to be expressed only by inactivated immune cells, but
was then also found to be expressed by various activated immune
cell types (including T cells, B cells, natural killer cells,
dendritic cells, and monocytes) (8,9). PD-L1
is a ligand for programmed cell death protein 1 (PD-1), which is
expressed by various human cell types-including antigen-presenting
cells, parenchymal cells, and endothelial cells-and this
ligand/receptor pair plays an important role in inhibiting
antitumor immunity.
Signal transduction initiated by PD-1/PD-L1 bonding
mediates dephosphorylation of key molecules, thereby inhibiting T
cell function, reducing production of inflammatory cytokines such
as IFN-γ, and inhibiting T cell proliferation (9). The PD-1/PD-L1 signaling pathway is
reportedly activated in various types of carcinoma, including renal
cell carcinoma, gastric cancer, ovarian cancer, and hematological
malignancies (10–15). Activation of this pathway is
associated with an unfavorable prognosis in a variety of malignant
tumors, including ovarian epithelial malignancy, pulmonary
non-small cell carcinoma, gastric cancer, and nasopharyngeal cancer
(16–21).
C-MET is a known receptor for Hepatocyte Growth
Factor/Scatter Factor (HGF/SF), and has been reported to be
directly involved in mediating invasive and metastatic capacity of
non HCC cancer cells through the activation of various
intracellular pathways; it is thus is a potential target for the
development of novel anticancer drugs (22). HGF-induced C-MET activation occurs
during activation of the PD-1/PD-L1 signaling pathway, and C-MET
has various regulatory effects on this pathway (22). C-MET binds to HGF to form a dimer,
leading to C-MET carboxy-terminus tyrosine phosphorylation, which
in turn results in activation of MAPK and PI3K, ultimately
impacting cell proliferation, survival, and angiogenesis. In cancer
cells, the C-MET/HGF signaling pathway becomes hyper-activated,
leading to uncontrolled cell proliferation and angiogenesis
(23,24). One drug targeting C-MET is
cabozantinib, which has been reported to reduce tumor reactivation
and α-fetoprotein (AFP) levels in HCC patients. Another anti-HCC
MET-inhibitor, Tivantinib, is currently undergoing clinical trials
and may increase overall survival rate when administered to
patients exhibiting high levels of C-MET expression (25,26).
In Korea, immunotherapeutic agents have been
approved for use in small cell lung cancer, bladder carcinoma, and
metastatic melanoma. In order to develop and approve novel
HCC-appropriate immunotherapies, an improved understanding of PD-L1
and C-MET expression patterns in HCC patients, as well as of their
involvement in the mechanisms of growth and inhibition in HCC, is
required.
The current study analyzed and compared the
expression pattern and level of PD-L1 in HCC patient-derived
samples. This was achieved using the anti-PD-L1 monoclonal Abs
(MAbs) SP263 and SP142, which are employed in immunohistochemical
assays to determine PD-L1 positivity as part of standard treatment
guidelines (in order to determine whether treatment with
Opdivo® (nivolumab; targeting tumor cells (TC)) or and
Ticentriq® (atezolizumab; targeting immune cells (IC)
and TC) is indicated.) Additionally, the correlation of PD-L1
expression with various clinicopathologic factors was analyzed.
Finally, because C-MET inhibition-via modulation of the PD-L1
pathway-is expected to have an anticancer effect, we performed a
preliminary study examining the correlation between expression
patterns of C-MET and PD-L1 in HCC.
The present study provides important data which will
contribute towards the development of anticancer drugs and
immunotherapeutic agents for improved treatment of HCC, and towards
determination of future Korean prescription standards.
The study was approved by the ethics committee of
Chosun University Hospital (Institutional review Board of Chosun
university hospital, Gwangju, Korea), who waived the requirement
for written informed consent due to the nature of the study (IRB
no: 2018-04-003-001).
Materials and methods
Case selection
We evaluated the 70 cases of HCC using paraffin
blocks and medical records, retrospectively. Among HCC patients who
underwent lobectomy or segmentectomy at Chosun University Hospital
during the period from February 2013 to December 2017, 70 patients
with well-documented medical records were discontinuously
selected.
Histopathology
Microscopic examination
Clinical records and tissue slides were
retrospectively analyzed. Patient age and sex were confirmed, and
the presence/absence of the HBV surface antigen (HBsAg, indicating
current infection) and antibodies to the HBV surface antigen
(HBsAb) were serologically confirmed. Slides were reviewed to
select representative tissue sites corresponding to the study
purpose. Paraffin-embedded tissues fixed in 10% neutral formalin
buffer were cut into 4~5 µm-thick sections prior to hematoxylin and
eosin (H&E) staining. The sections were examined under a light
microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan). By
review of the H&E slides, Tumor stage (pT), histological
classification, tumor number, Edmonson-Steiner (ES) grade, and the
presence of portal vein invasion, bile duct invasion, and
background sclerotic lesions were re-evaluated.
Immunohistochemistry (IHC)
Expression of PD-L1 and C-MET were evaluated by
immunohistochemical staining. For detection of PD-L1, we used two
rabbit MAbs directed against PD-L1: SP-142 (cat. no. 740-4859;
Ventana Medical Systems, Inc., Tucson, AZ, USA) and SP-263 (cat.
no. 740-4907; Ventana Medical Systems). Similarly, detection of
C-MET (cat. no. 790-4430; Ventana Medical Systems) was achieved
using a rabbit MAb. All MAb assays were conducted according to the
manufacturer's instructions. Immunohistochemical staining was
performed using a Benchmark ULTRA (Ventana Medical Systems)
slide-processing instrument. Expression and amplification of
anti-PD-L1 MAb SP-142 was performed using an OptiView DAB IHC
detection kit (cat. no. 760-700/0639650000) and an OptiView
amplification kit (cat. no. 760-099/06396518001).
Immunolocalization of PD-L1 (using SP-263) was performed using a
haptenated secondary antibody and a multimeric
anti-hapten-horseradish peroxidase (HRP) conjugate, and Ab-enzyme
complexes were visualized via consequent production of a
fluorescent enzyme reaction product (Optiview DAB IHC detection
kit; cat. no. 760-700). C-MET was stained and detected using a
commercial detection kit (Ventana Medical Systems). Ab-stained
non-HCC tissues acted as expression controls: Normal tonsil
(SP-142-detected PD-L1 expression negative control), placenta
(SP-263-detected PD-L1 expression positive control), and colon
cancer (C-MET expression positive control).
Briefly, staining proceeded as follows:
Paraffin-embedded fixed tissue was cut into 4~5 µm-thick sections,
adhered to X-tra™ slides (Surgipath, Richmond, USA), deparaffinized
with xylene, treated with anhydrous alcohol (90, 75 and 50%), and
stained using a standard labeled streptavidin biotin (LSAB) method.
To recover antigenicity, slides were boiled in citrate buffer (10
mM, pH 6.0) for 15 min in an electronic oven, cooled for 20 min at
room temperature, and washed with 50 mM Tris buffer (TBS, pH 7.5).
In order to inhibit the activity of endogenous peroxidases, slides
were treated with 0.3% hydrogen peroxide-methanol solution for 10
min, washed with distilled water, reacted with blocking antibody
for 10 min at room temperature, and coated with Ab (SP-142, SP-263,
and anti-C-MET) for 32 min. Contrast staining was performed with
hematoxylin (catalog no. 760-2021; Ventana Medical Systems) and
tissue sections were sealed with Clearmount TM Mounting solution
(Zymed Laboratories; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Determination of immunohistochemical
staining
Staining results were interpreted by a pathologist
blinded to the clinical course of the corresponding patient. Tissue
sections were read as positive for PD-L1 (as detected by SP-142 or
SP-263) staining if ≥5% of ICs (intra- and peritumoral lymphocytes,
macrophages, dendritic cells, and granulocytes) exhibited a dark
brown punctate staining pattern in the cell membrane. TC are
stained with dark brown cell membrane pattern, and read as positive
when ≥5% of TCs are stained. Positive C-MET staining appeared as
yellow to dark brown staining in the cell membrane and/or cytoplasm
of TC, and was graded according to intensity (0: Negative, 1: Weak,
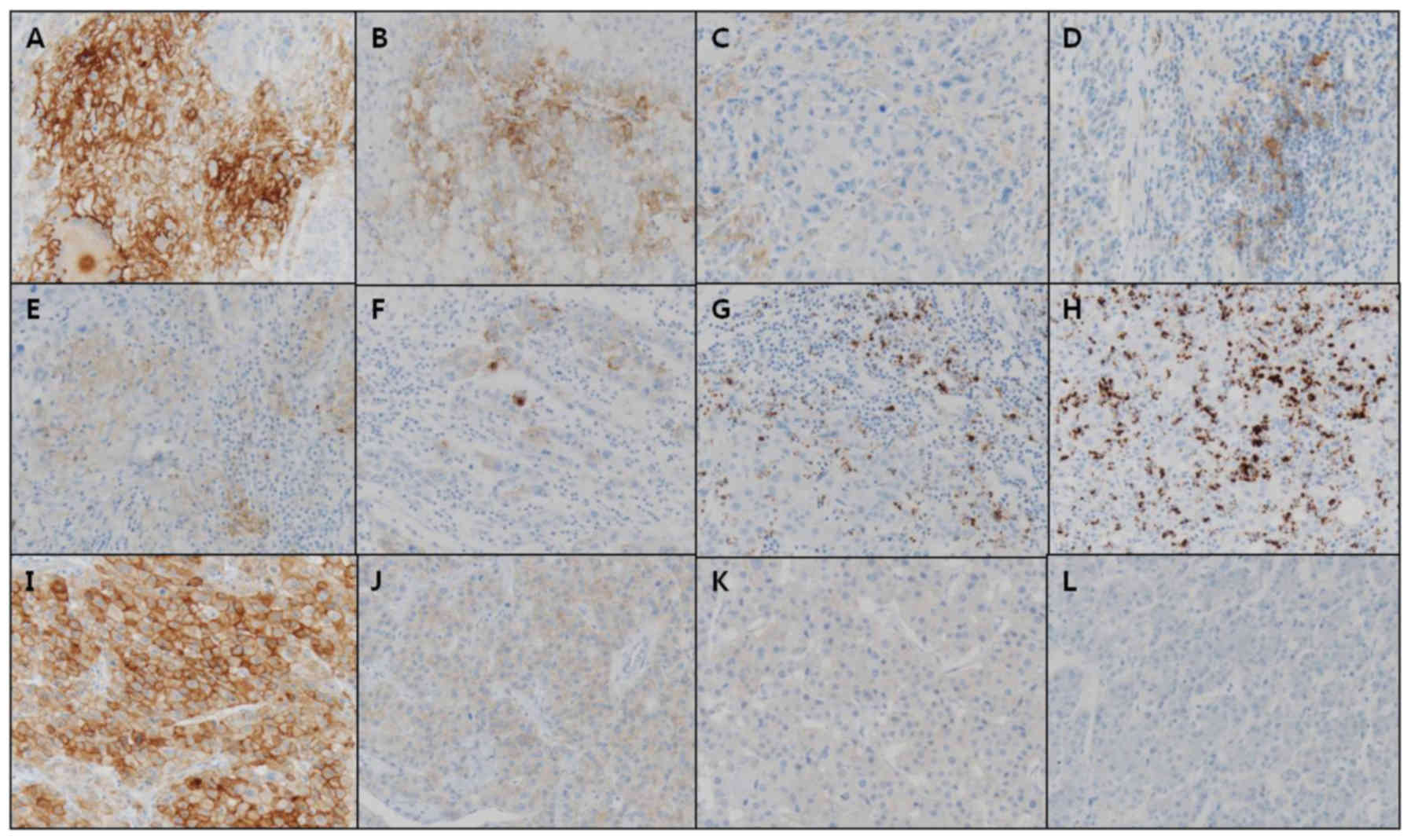
2: Moderate, and 3: Intense; Fig.
1).
Statistical analysis
Statistical analysis was performed using the STAT
View software package (Abacus Concepts, Piscataway, NJ, USA). We
examined expression levels of PD-L1 (as detected by SP-142), PD-L1
(as detected by SP-263), and C-MET protein in HCC. Correlation
between the expression of each protein, as well as between the
expression of each protein and various clinicopathologic factors,
was analyzed by the χ2-test, respectively. Additionally,
the following comparisons were made: Stage T1 with T2-4, low
(grades 1 and 2) with high (grades 3 and 4) ES grade, negative
(scores 0 and 1) with positive (scores 2 and 3). PD-L1 expression,
and low-grade (scores 0 and 1) with high-grade (scores 2 and 3)
C-MET expression. P<0.05 was considered to indicate a
statistically significant difference.
Results
Distribution of clinicopathologic
factors
We examined the distribution of various
clinicopathologic factors among a total of 70 HCC patients
(Table I). Age distribution ranged
from 33 to 80 years (mean age 61 years). Overall ratio of males to
females was 6:1 (85.7:14.3%). Regarding tumor size, 31 cases
(44.3%) exhibited tumors of less than <2 cm diameter, and 39
cases (55.7%) exhibited tumors of >2 cm diameter. Regarding T
stage distribution, 54 cases (77.1%) were staged as T1, 11 cases
(15.7%) were staged as T2, 3 cases (4.3%) were staged as T3, and 2
cases (2.9%) were staged as T4. Portal vein involvement was
observed in 3 cases (4.3%). Regarding histological classification
of the tumor, 54 cases (77.1%) exhibited a tumor of trabecular
type, 4 cases (5.7%) exhibited a tumor of pseudoglandular type, and
12 cases (17.1%) exhibited a tumor of mixed type. Bile duct
invasion was observed in 1 case (1.4%). Regarding ES grade, 45
cases (64.3%) were low-grade (grades 1 and 2), and 25 cases (35.7%)
were high-grade (grades 3 and 4). The presence of HBsAg or HBsAb
was noted in 43 cases (61.4%) and 13 cases (18.6%), respectively.
In 53 (75.7%) of tissue samples, a cirrhotic background was
evident.
 | Table I.Clinicopathologic factors. |
Table I.
Clinicopathologic factors.
| Factors | N (%) |
|---|
| Age (year) |
|
|
<62 | 33 (47.1) |
|
≥62 | 37 (52.9) |
| Sex |
|
|
Male | 60 (85.7) |
|
Female | 10 (14.3) |
| Tumor size
(cm) |
|
| ≤2 | 31 (44.3) |
|
>2 | 39 (55.7) |
| T stage (pT) |
|
|
pT1 | 54 (77.1) |
|
pT2-4 | 16 (22.9) |
| Portal vein
invasion |
|
|
Absent | 67 (95.7) |
|
Present | 3 (4.3) |
| Cirrhosis |
|
|
Absent | 17 (24.3) |
|
Present | 53 (75.7) |
| Tumor
histology |
|
|
Trabecular | 54 (77.1) |
|
Glandular | 4 (5.7) |
|
Mixed | 12 (17.1) |
| Edmonson-Steiner
grade |
|
| 1 and
2 | 45 (64.3) |
| 3 and
4 | 25 (35.7) |
| Bile duct
invasion |
|
|
Absent | 69 (98.6) |
|
Present | 1 (1.4) |
| HBsAg |
|
|
Absent | 27 (38.6) |
|
Present | 43 (61.4) |
| HBsAb |
|
|
Absent | 57 (81.4) |
|
Present | 13 (18.6) |
Association between PD-L1 expression
and clinicopathologic factors
We examined the expression patterns of PD-L1 using
two MAbs (SP263 and SP142), and the relationship between these and
various clinicopathologic factors (Tables II, III, and IV). Fig. 2
is representative staining pattern of each antibodies. (Fig. 2) As detected by SP263, PD-L1 was
expressed in 20% (14/70) of TC and 70% (49/70) of IC. As detected
by SP142, PD-L1 was expressed in 2.9% (2/70) of TC and 42.9%
(30/70) of IC. In TC, PD-L1 expression detected by the different
antibodies overlapped in both cases. In IC, PD-L1 expression
detected by the different antibodies overlapped with the exception
of 2 cases. Expression of PD-L1 (as detected by SP263) in TC
exhibited a statistically significant (positive) correlation only
with ES grade (among all clinicopathologic factors) (Table II, P<0.01). The high-grade
(grades 3 and 4) ES group accounted for 71.4% (10/14) of the
PD-L1-expressing group and only 26.8% (15/56) of the non-PD-L1
expressing group. Although no significant correlation existed
between TC PD-L1 expression (as detected by SP263) and other
clinical factors, pseudoglandular and mixed type tumors showed a
higher frequency of PD-L1 expression than trabecular type tumors
(P=0.09). Neither TC expression of PD-L1 (as detected by SP142) nor
IC expression of PD-L1 (as detected by SP263) correlated with any
clinicopathologic factors.
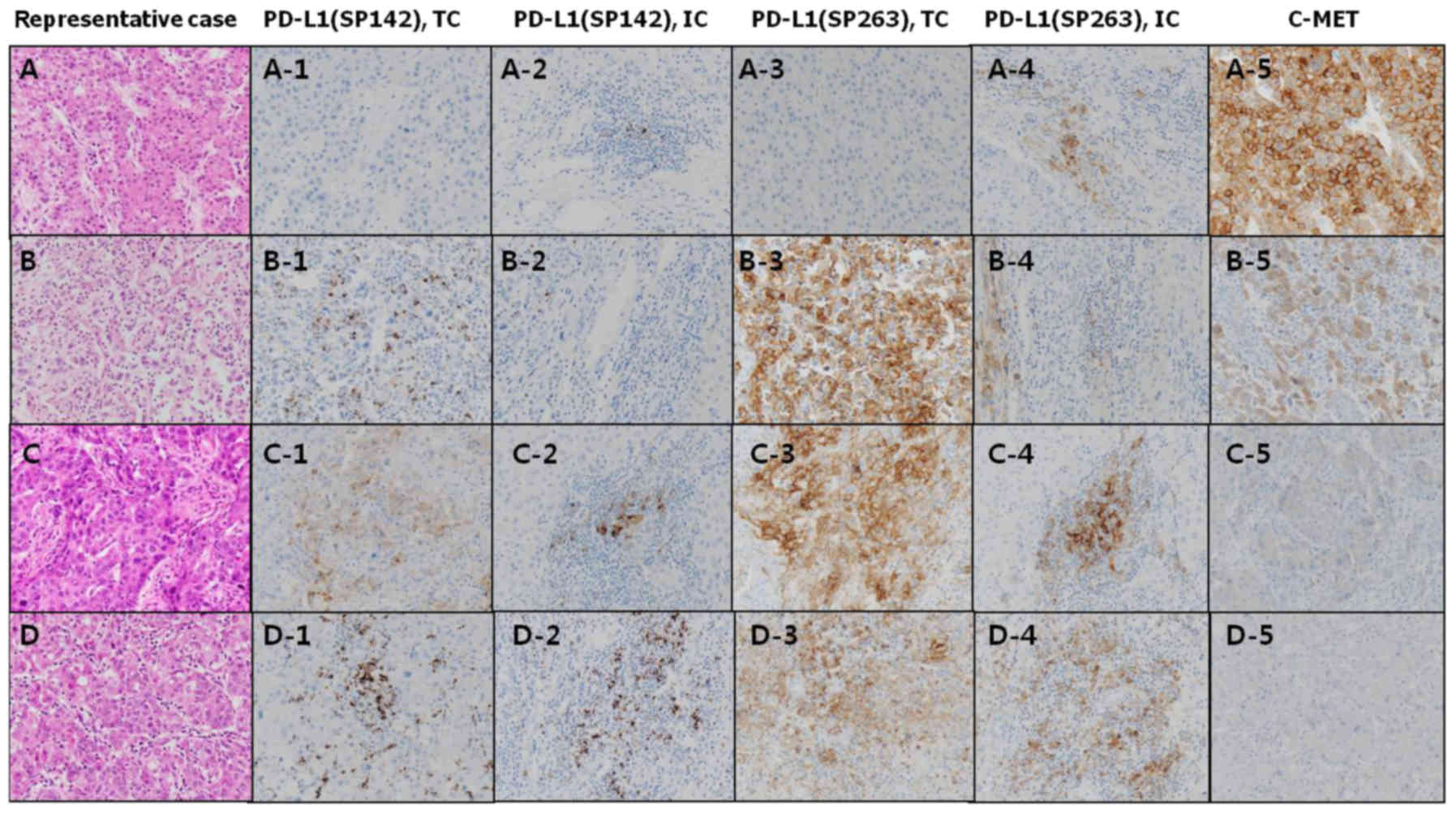 | Figure 2.Immunostaining scores of each
antibodies in representative cases. (A) [N,N,N,N,3+], (B)
[N,N,P,N,2+], (C) [P,N,P,P,1+], (D) [N,P,P,P,N]. 1, expression of
PD-L1 (SP142) in TCs; 2, expression of PD-L1 (SP142) in ICs; 3,
expression of PD-L1 (SP263) in TCs; 4, expression of PD-L1 (SP263)
in ICs; and 5, expression of C-MET in TCs. N, negative; P,
positive; TCs, tumor cells; ICs, immune cells; PD-L1, Programmed
cell death-ligand 1. |
 | Table III.Association of clinicopathologic
factors with SP142-detected expression of PD-L1 in TCs and immune
cells. |
Table III.
Association of clinicopathologic
factors with SP142-detected expression of PD-L1 in TCs and immune
cells.
|
| PD-L1 (SP142), TC
(n=70) | PD-L1 (SP142), IC
(n=70) |
|---|
|
|
|
|
|---|
| Factors | (−) n (%) | (+) n (%) | P-value | (−) n (%) | (+) n (%) | P-value |
|---|
| Age (year) |
|
<62 | 33 (48.5) | 0 (0.0) | 0.49 | 21 (52.5) | 12 (40.0) | 0.34 |
|
≥62 | 35 (51.5) | 2 (100.0) |
| 19 (47.5) | 18 (60.0) |
|
| Sex |
|
Male | 9 (13.2) | 1 (50.0) | 0.27 | 6 (15.0) | 4 (13.3) | 1.00 |
|
Female | 59 (86.8) | 1 (50.0) |
| 34 (85.0) | 26 (86.7) |
|
| Tumor Size
(cm) |
| ≤2 | 30 (44.1) | 1 (50.0) | 1.00 | 14 (35.0) | 17 (56.7) | 0.09 |
|
>2 | 38 (55.9) | 1 (50.0) |
| 26 (65.0) | 13 (43.3) |
|
| T stage (pT) |
|
pT1 | 52 (76.5) | 2 (100.0) | 1.00 | 31 (77.5) | 23 (76.7) | 1.00 |
|
pT2-4 | 16 (23.5) | 0 (0.0) |
| 9 (22.5) | 7 (23.3) |
|
| PV invasion |
|
Absent | 65 (95.6) | 2 (100.0) | 1.00 | 39 (97.5) | 28 (93.3) | 0.57 |
|
Present | 3 (4.4) | 0 (0.0) |
| 1 (2.5) | 2 (6.7) |
|
| Cirrhosis |
|
Absent | 15 (22.1) | 2 (100.0) | 0.06 | 8 (20.0) | 9 (30.0) | 0.40 |
|
Present | 53 (77.9) | 0 (0.0) |
| 32 (80.0) | 21 (70.0) |
|
| Histology |
|
Trabecular | 53 (77.9) | 1 (50.0) | 0.85 | 32 (80.0) | 22 (73.3) | 0.53 |
|
Glandular | 3 (4.4) | 1 (50.0) |
| 2 (5.0) | 2 (6.7) |
|
|
Mixed | 12 (17.6) | 0 (0.0) |
| 6 (15.0) | 6 (20.0) |
|
| ES grade |
| 1 and
2 | 45 (66.2) | 0 (0.0) | 0.12 | 26 (65.0) | 19 (63.3) | 1.00 |
| 3 and
4 | 23 (33.8) | 2 (100.0) |
| 14 (35.0) | 11 (36.7) |
|
| BD invasion |
|
Absent | 67 (98.5) | 2 (100.0) | 1.0 | 40 (100.0) | 29 (96.7) | 0.43 |
|
Present | 1 (1.5) | 0 (0.0) |
| 0 (0.0) | 1 (3.3) |
|
| HBsAg |
|
Absent | 27 (39.7) | 0 (0.0) | 0.52 | 14 (35.0) | 13 (43.3) | 0.62 |
|
Present | 41 (60.3) | 2 (100.0) |
| 26 (65.0) | 17 (56.7) |
|
| HBsAb |
|
Absent | 55 (80.9) | 2 (100.0) | 1.00 | 33 (82.5) | 24 (80.0) | 1.00 |
|
Present | 13 (19.1) | 0 (0.0) |
| 7 (17.5) | 6 (20.0) |
|
 | Table IV.Comparison of PD-L1 expression as
detected by 2 MAbs (SP263 and SP142). |
Table IV.
Comparison of PD-L1 expression as
detected by 2 MAbs (SP263 and SP142).
|
| SP263, TC | SP263, IC |
|---|
|
|
|
|
|---|
| Variables | (−) n (%) | (+) n (%) | P-value | (−) n (%) | (+) n (%) | P-value |
|---|
| SP142, TC |
|
|
|
|
|
|
| − | 56 (100.0) | 12 (85.7) | 0.04a | 21 (100.0) | 47 (95.9) | 1.00 |
| + | 0 (0.0) | 2 (14.3) |
| 0 (0.0) | 2 (4.1) |
|
| SP142, IC |
|
|
|
|
|
|
| − | 37 (66.1) | 3 (21.4) |
<0.01a | 19 (90.5) | 21 (42.9) |
<0.001a |
| + | 19 (33.9) | 11 (78.6) |
| 2 (9.5) | 28 (57.1) |
|
 | Table II.Association of clinicopathologic
factors with SP263-detected expression of PD-L1 in TCs and immune
cells. |
Table II.
Association of clinicopathologic
factors with SP263-detected expression of PD-L1 in TCs and immune
cells.
|
| PD-L1 (SP263), TC
(n=70) | PD-L1 (SP263), IC
(n=70) |
|---|
|
|
|
|
|---|
| Factors | (−) n (%) | (+) n (%) | P-value | (−) n (%) | (+) n (%) | P-value |
|---|
| Age (year) |
|
<62 | 28 (50.0) | 5 (35.7) | 0.38 | 12 (57.1) | 21 (42.9) | 0.31 |
|
≥62 | 28 (50.0) | 9 (64.3) |
| 9 (42.9) | 28 (57.1) |
|
| Sex |
|
Male | 48 (85.7) | 12 (85.7) | 1.00 | 2 (9.5) | 8 (16.3) | 0.71 |
|
Female | 8 (14.3) | 2 (14.3) |
| 19 (90.5) | 41 (83.7) |
|
| Tumor Size
(cm) |
| ≤2 | 22 (39.3) | 9 (64.3) | 0.13 | 9 (42.9) | 22 (44.9) | 1.00 |
|
>2 | 34 (60.7) | 5 (35.7) |
| 12 (57.1) | 27 (55.1) |
|
| T stage (pT) |
|
pT1 | 43 (76.8) | 11 (78.6) | 1.00 | 18 (85.7) | 36 (73.5) | 0.36 |
|
pT2-4 | 13 (23.2) | 3 (21.4) |
| 3 (14.3) | 13 (26.5) |
|
| PV invasion |
|
Absent | 55 (98.2) | 12 (85.7) | 0.10 | 21 (100.0) | 46 (93.9) | 0.55 |
|
Present | 1 (1.8) | 2 (14.3) |
| 0 (0.0) | 3 (6.1) |
|
| Cirrhosis |
|
Absent | 14 (25.0) | 3 (21.4) | 1.00 | 5 (23.8) | 12 (24.5) | 1.00 |
|
Present | 42 (75.0) | 11 (78.6) |
| 16 (76.2) | 37 (75.5) |
|
| Histology |
|
Trabecular | 46 (82.1) | 8 (57.1) | 0.09 | 17 (81.0) | 37 (75.5) | 0.42 |
|
Glandular | 2 (3.6) | 2 (14.3) |
| 2 (9.5) | 2 (4.1) |
|
|
Mixed | 8 (14.3) | 4 (28.6) |
| 2 (9.5) | 10 (20.4) |
|
| ES grade |
| 1 and
2 | 41 (73.2) | 4 (28.6) |
<0.01a | 15 (71.4) | 30 (61.2) | 0.59 |
| 3 and
4 | 15 (26.8) | 10 (71.4) |
| 6 (28.6) | 19 (38.8) |
|
| BD invasion |
|
Absent | 56 (100) | 13 (92.9) | 0.20 | 21 (100.0) | 48 (98.0) | 1.00 |
|
Present | 0 (0) | 1 (7.1) |
| 0 (0.0) | 1 (2.0) |
|
| HBsAg |
|
Absent | 21 (37.5) | 6 (42.9) | 0.76 | 8 (38.1) | 19 (38.8) | 1.00 |
|
Present | 35 (62.5) | 8 (57.1) |
| 13 (61.9) | 30 (61.2) |
|
| HBsAb |
|
Absent | 46 (82.1) | 11 (78.6) | 0.72 | 18 (85.7) | 39 (79.6) | 0.74 |
|
Present | 10 (17.9) | 3 (21.4) |
| 3 (14.3) | 10 (20.4) |
|
Association between C-MET expression
and clinicopathologic factors
High-grade C-MET expression was low overall (10/70
cases, 14.3%) (Table V), and was
found to be significantly positively correlated only with higher T
stage (P=0.042). No other statistically significant association
with clinicopathologic features was observed (Table V).
 | Table V.Association of clinicopathologic
factors with C-MET expression. |
Table V.
Association of clinicopathologic
factors with C-MET expression.
|
| C-MET (n=70) |
|---|
|
|
|
|---|
| Factors | Low n (%) | High n (%) | P-value |
|---|
| Age (year) |
|
| 1.00 |
|
<62 | 28 (46.7) | 5 (50.0) |
|
|
≥62 | 32 (53.3) | 5 (50.0) |
|
| Sex |
|
| 1.00 |
|
Male | 9 (15.0) | 1 (10.0) |
|
|
Female | 51 (85.0) | 9 (90.0) |
|
| Tumor Size
(cm) |
|
| 0.32 |
| ≤2 | 25 (41.7) | 6 (60.0) |
|
|
>2 | 35 (58.3) | 4 (40.0) |
|
| T stage (pT) |
|
| 0.04a |
|
pT1 | 49 (81.7) | 5 (50.0) |
|
|
pT2-4 | 11 (18.3) | 5 (50.0) |
|
| PV invasion |
|
| 0.38 |
|
Absent | 58 (96.7) | 9 (90.0) |
|
|
Present | 2 (3.3) | 1 (10.0) |
|
| Cirrhosis |
|
| 0.43 |
|
Absent | 16 (26.7) | 1 (10.0) |
|
|
Present | 44 (73.3) | 9 (90.0) |
|
| Histology |
|
| 0.18 |
|
Trabecular | 3 (5.0) | 1 (10.0) |
|
|
Glandular | 9 (15.0) | 3 (30.0) |
|
|
Mixed | 48 (80.0) | 6 (60.0) |
|
| ES grade |
|
| 0.15 |
| 1 and
2 | 41 (68.3) | 4 (40.0) |
|
| 3 and
4 | 19 (31.7) | 6 (60.0) |
|
| BD invasion |
|
| 1.00 |
|
Absent | 59 (98.3) | 10 (100.0) |
|
|
Present | 1 (1.7) | 0 (0) |
|
| HBsAg |
|
| 0.17 |
|
Absent | 21 (35.0) | 6 (60.0) |
|
|
Present | 39 (65.0) | 4 (40.0) |
|
| HBsAb |
|
| 1.00 |
|
Absent | 49 (81.7) | 8 (80.0) |
|
|
Present | 11 (18.3) | 2 (20.0) |
|
Comparison of PD-L1 expression as
measured by SP263 and SP142
When comparing PD-L1 expression as measured by two
MAbs, SP263-mediated detection reported higher levels of PD-L1
expression than SP142-mediated detection. Most cases (51/70, 72.9%)
exhibited positive IC PD-L1 expression, and in all of these this
expression was detected in peritumoral IC (SP263-detected: 49
cases, SP142-detected: 30 cases). Interestingly, 28/30 PD-L1
positive cases (as detected by SP142) also exhibited high PD-L1
expression as detected by SP263, and only two cases exhibited IC
PD-L1 expression detectable only by SP142. When comparing PD-L1
expression levels of TC and IC, the latter exhibited significantly
higher expression. Overall, PD-L1 expression was as follows: IC
(49/70, 70%) as detected by SP263, IC (30/70, 42.9%) as detected by
SP142, TC (14/70, 20%) as detected by SP263, and TC (2/70, 2.86%)
as detected by SP142. Thus, the frequency of PD-L1 expression in IC
was high (and was comparably detected by both MAbs). However, the
frequency of PD-L1 expression in TC was low (14/70, 20%), and only
two of these also exhibited PD-L1 expression as detected by SP142.
In summary, IC PD-L1 expression results-as detected by both
MAbs-significantly corresponded to each other: Most
SP263-detectable PD-L1 expression was also detectable by SP142.
Furthermore, statistically significant positive correlations
between PD-L1 expression (as detectable by either MAb, and as
detectable in either cell type) were observed (Tables IV and VI). The higher the IC PD-L1 positive
expression rate (as detected by SP263), the higher the IC PD-L1
positive expression rate (as detected by SP142) (P<0.001;
Table IV). The number of cases in
which both MAbs were able to detect PD-L1 are as follows: 2 cases
within TC (P=0038), 28 cases within IC (P<0.001), and 11 cases
between TC and IC (P=0.005). The expression of SP263 PD-L1 in the
IC and SP142 PD-L1 in the ICS were not significantly related
(P=1.000; Table IV). Expression of
PD-L1 as detected by SP263 was significantly positively associated
between cell types (IC and TC; Table
VI). The number of cases in which PD-L1 expression was positive
or negative in both cell types (IC and TC) were 14/70 and 21/70,
respectively (P=0.007; Table VI).
Expression of PD-L1 as detected by SP142 did not correlate between
IC and TC (Table VII).
 | Table VI.Agreement of SP263-detected PD-L1
expression between tumor and immune cells. |
Table VI.
Agreement of SP263-detected PD-L1
expression between tumor and immune cells.
|
| TC (SP263) |
|---|
|
|
|
|---|
| Factors | (−) n (%) | (+) n (%) | P-value |
|---|
| IC (SP263) |
|
|
|
|
(−) | 21 (37.5) | 0 (0.0) | 0.02a |
|
(+) | 35 (62.5) | 14 (100.0) |
|
 | Table VII.Agreement of SP142-detected PD-L1
expression between tumor and immune cells. |
Table VII.
Agreement of SP142-detected PD-L1
expression between tumor and immune cells.
|
| TC (SP142) |
|---|
|
|
|
|---|
| Factors | (−) n (%) | (+) n (%) | P-value |
|---|
| IC (SP142) |
|
|
|
| -
(n=40) | 39 (57.4) | 1 (50.0) | 1.00 |
| +
(n=30) | 29 (42.6) | 1 (50.0) |
|
Correlation of C-MET and PD-L1
expression
A statistically significant positive correlation was
observed between SP263-detected TC PD-L1 expression and C-MET
expression (P=0.022; Table VIII).
No statistically significant correlation existed between IC PD-L1
expression (as detected by either MAb) and C-MET expression.
 | Table VIII.Correlation of C-MET and PD-L1
expression. |
Table VIII.
Correlation of C-MET and PD-L1
expression.
|
| C-MET |
|---|
|
|
|
|---|
| Factors | n (%) | Low n (%) | High n (%) | P-value |
|---|
| IC (SP263) |
|
|
|
|
|
(−) | 21 (30.0) | 19 (31.7) | 2 (20.0) | 0.712 |
|
(+) | 49 (70.0) | 41 (68.3) | 8 (80.0) |
|
| TC (SP263) |
|
|
|
|
|
(−) | 56 (80.0) | 51 (85.0) | 5 (50.0) | 0.022a |
|
(+) | 14 (20.0) | 9 (15.9) | 5 (50.0) |
|
| IC (SP142) |
|
|
|
|
|
(−) | 68 (97.1) | 58 (96.7) | 10 (100.0) | 1.000 |
|
(+) | 2 (2.9) | 2 (3.3) | 0 (0.0) |
|
| TC (SP142) |
|
|
|
|
|
(−) | 40 (57.1) | 36 (60.0) | 4 (40.0) | 0.308 |
|
(+) | 30 (42.9) | 24 (40.0) | 6 (60.0) |
|
Discussion
In the immune system, T cells are activated via T
cell receptor-mediated recognition of MHC-antigen complexes, and
this activation is modulated through the integration of both
co-stimulatory and co-inhibitory signals. However, cancer cells are
often able to evade immunity by means of various mechanisms, such
as expression of proteins (or other molecules) that interfere with
induction of immune responses or elimination of cancer cells
(8,27). Immunologic anticancer drugs, which
are immunoprotein-based therapeutic agents that induce host IC to
selectively attack cancer cells, include immune checkpoint
inhibitors (CTLA4-, PD-1-, or PD-L1 inhibitors), cell-based
immunotherapy, and viral vector-based immunotherapy. Immune-based
anticancer therapeutics offer an improved adverse effect profile
compared to first-generation chemotherapeutic drugs, are not
subject to acquired resistance (as are second-generation targeted
anticancer drugs), and possess additional advantages such as
long-term efficacy, long-term survival, and broad-spectrum
anticancer effects (8,27,28).
Such benefits have generated increasing research interest in
immunotherapy, and since approval of the first-in-class drug
Provenge® (sipuleucel-T; an autologous tumor vaccine)
for the treatment of prostate adenocarcinoma in 2010, a number of
additional immune checkpoint inhibitors have been approved
(27). Exploitation of the
PD-1/PD-L1 pathway is one mechanism by which cancer cells evade T
cell immunity: PD-L1 expressed on the cancer cell surface ligates T
cell PD-1, thereby inhibiting T cell anti-tumor immunity (27). In recent years, PD-1 and PD-L1
inhibitors-which target the PD-1/PD-L1 signaling pathway in order
to induce T cell-mediated cancer cell apoptosis-have been developed
and approved (24).
Keytruda® (pembrolizumab), the first PD-1
inhibitor approved by the United States Food and Drug
Administration (US FDA) in 2014 (29), was also subsequently (in 2015)
approved for the treatment of metastatic melanoma and non-small
cell lung cancer (NSCLC) in Korea. Another PD-1 inhibitor,
Opdivo® (nivolumab), was approved by the US FDA in 2014
(29), and in Korea has since been
approved for the treatment of metastatic melanoma, NSCLC, lymphoma,
squamous cell carcinoma of the head and neck, and urothelial
carcinoma. The development of PD-1 inhibitors naturally led to the
development of PD-L1 inhibitors, the first of which,
Tecentriq® (atezolizumab), was approved by the US FDA in
2016 (29), and in Korea has since
(in 2017) been approved for the treatment of locally advanced or
metastatic NSCLC, and urothelial carcinoma. Also in 2017, the US
FDA approved a second PD-L1 inhibitor, Imfinzi®
(durvalumab), for the treatment of severe bladder cancer (i.e.
progressing even after surgery or chemotherapy) (29).
Immune checkpoint inhibitors targeting the
PD-1/PD-L1 pathway have achieved good clinical results in the
treatment of early melanoma, and have been approved for the
treatment of NSCLC and renal cancer, with indications now expanding
to include various additional cancers such as lymphoma and
urothelial carcinoma (29). In 2018,
the US FDA further approved Opdivo as a second-line treatment
(regardless of PD-L1 expression status) for HCC patients not
responding to standard first-line treatment with
Nexavar® (29). According
to the US Checkmate-040 clinical trial, Opdivo exhibited efficacy
against HCC regardless of PD-L1 expression status, and regardless
of the presence/absence of active hepatitis B or C (28).
As mentioned, tumor cell-expressed PD-L1 ligates
immune cell PD-1 receptors, leading to inhibition of T
cell-mediated anti-tumor immunity; high PD-L1 expression is
therefore correlated with poorer prognosis. For example, Gao et
al (25) reported that PD-L1
over-expression and tumor size correlates with tumor recurrence. In
the current study, HCC patients exhibiting high PD-L1 expression
also had a worse prognosis (relative to those with low PD-L1
expression). Furthermore, multivariate analysis demonstrated that
the PD-L1 expression status could be used as an independent marker
for postoperative HCC recurrence. Another recent study suggested
that PD-L1 expression in combination with CD4+ and
CD8+ T cells may have utility as an HCC prognostic
indicator (e.g. that positive Ab-mediated staining may indicate a
higher risk for recurrence) (30).
Chang et al (7) demonstrated that PD-L1 predicts a poorer
prognosis in patients exhibiting CD8+ tumor infiltrating
lymphocytes (TILs), and independently predicts poorer survival.
Jung et al (4) analyzed the
correlation between poor prognosis and over-expression of PD-L1 and
PD-L2 in HCC patients, and demonstrated that PD-L1 over-expression
correlated with tumor size, recurrence, and survival. However,
PD-L1 expression in esophageal cancer tissue exhibits no
correlation with prognosis (31).
Nevertheless, poor outcomes in esophageal cancer are largely due to
metastasis, and Miao et al (22) predicted that PD-L1 expression would
play an important role in this process. In light of the above
findings, PD-L1 expression is expected to be an important index for
the prediction of tumor recurrence.
Another study by Gao et al (25) demonstrated that over-expression of
PD-L1 and PD-L2 is closely linked to poorer survival, but that the
correlation with recurrence rate was not statistically significant.
Thus, inhibiting PD-1 expression may be a more effective anticancer
strategy than inhibiting expression of PD-L1 or PD-L2. In addition
to clinical prognostic studies, IHC-based examination of liver
tissue expression of PD-1 and PD-L1 in patients with hepatitis and
HCC demonstrated that while PD-L1 expression in HBV hepatitis and
HCC was high during the early (proliferative) stages of HCC, this
level became progressively lower as HCC progressed toward the
terminal stage (26). HBV infection
did not impact PD-L1 expression in liver cancer tissues. However, a
study investigating expression of PD-1 and PD-L1 in HBV-induced HCC
patients treated with cryoablation demonstrated that PD-L1
expression correlates with poor prognosis (32). Such results suggest that further
studies are required to clarify the relationship between PD-L1
expression and survival rate in HBV hepatitis-induced HCC.
The anti-PD-L1 MAb SP142 is raised against rabbit
serum, and is used when determining whether prescription of
Ticentriq is appropriate (33,34).
Similarly, it has been validated for use in determining whether
atezolizumab treatment for advanced urothelial carcinoma and NSCLC
is indicated (35–37). In these clinical trials, positive
PD-L1 expression by TC and IC was an indication for treatment
(38–39). The anti-PD-L1 MAb SP263 is used to
determine whether the PD-1 inhibitor Opdivo is indicated. MAb SP263
directly targets an intracellular portion of human PD-L1 (40). It was optimized for use in NSCLC
tissue samples, and its diagnostic value has been validated. It has
also been validated for use in clinical trials for the
establishment of nivolumab treatment guidelines (41,42).
In a study using SP142-based IHC to compare TC and
IC PD-L1 expression between NSCLC biopsies and surgical resection
specimens from 160 patients, results were inconsistent, with an
overall discordance rate (non-agreement of PD-L1 expression between
the two samples) of 48% (κ=0.218) (43). However, another NSCLC study which
retrospectively performed the same comparison using the same
technique (n=79 patients) demonstrated that 38.0% of surgical
resection specimens and 35.4% of biopsy specimens exhibited PD-L1
expression, with a concordance rate of 92.4% (κ=0.8366) (44). Although this retrospective study was
limited by a relatively small sample size and unavailability of the
entire biopsy specimen (44–46), it is significant that SP142-based
confirmation of PD-L1 expression is accurate
Many previous studies have suggested that PD-L1
expression correlates with a variety of oncogenic signaling
pathways. For example, Azuma et al (45) demonstrated that over-expression of
mutant EGFR correlates with high PD-L1 expression in surgically
resected NSCLC specimens. In addition, Tang et al (46) demonstrated that an EGFR mutation
correlated with PD-L1 expression during treatment of progressive
NSCLC with a tyrosine phosphorylase inhibitor. Gabrielson et
al (47), reported that low HCC
PD-L1 expression is significantly associated with a higher density
of tumor-infiltrating CD3+ T cells and CD8+ T
cells, consistent with an increased survival rate and a low tumor
recurrence rate (suggesting PD-L1 expression as a useful prognostic
indicator in HCC patients who have undergone tumor resection).
In the current study, HCC patient TC and IC PD-L1
expression was determined. The frequency of positive expression was
highest in IC as detected by SP263 (70%), followed by IC as
detected by SP142 (), then in TC as detected by SP263 (), followed
by TC as detected by SP142 (). In all except 2 cases, there was
concordance between positive IC PD-L1 expression as detected by
either MAb (i.e. the MAbs demonstrated positive correlation between
detected IC PD-L1 expression patterns). In addition, both MAbs
demonstrated a higher frequency of positive PD-L1 expression in IC
than TC, with SP263 exhibiting higher sensitivity than SP142. If
these results accurately represent positive PD-L1 expression
frequencies, SP263 may be a better candidate for IC PD-L1
expression detection, and such PD-L1 detection in IC (rather than
TC) may be a better prognostic indicator candidate. These data are
expected to contribute towards selection of the ideal MAb for PD-L1
detection, as well as determination of the PD-L1+ case
rate in HCC, which will facilitate development of HCC immunotherapy
prescription standards in Korea.
Although Jung et al (4) have reported that PD-L1 expression,
histological findings, and overall tumor size are predictors of
poor prognosis in HCC patients, to date no study has examined TC
and IC PD-L1 and C-MET expression patterns, and their correlation
with prognostic factors in HCC. C-MET is a type of tyrosine kinase
which becomes mutated and/or over-expressed on the surface of
cancer cells. It has been reported that C-MET is over-expressed in
HCC, gastric cancer, rectal cancer, and breast cancer, which are
common human carcinomas (48,49).
Activation of C-MET is known to promote tumor cell survival,
proliferation, invasion, and metastasis (50). Ligation of C-MET by hepatocyte growth
factor (HGF) triggers initiation of signaling (51) which culminates in cancer cell growth
and proliferation. Overactive C-MET/HGF signaling has been shown to
be associated with invasion and proliferation of small cell lung
cancer (30). Many studies have
demonstrated that activation of C-MET-mediated intracellular
signaling pathways may be associated with poor prognosis in lung
cancer and other solid tumors (52–55).
Several clinical NSCLC studies have reported that C-MET
over-expression correlates with poor survival (56–59).
Miao et al (22) studied PD-1
and C-MET expression relative to survival in small cell lung cancer
patients and suggested that C-MET over-expression is an important
prognostic factor during the early stages. Activated C-MET
signaling has also been identified as a potential therapeutic
target, given its involvement in cancer cell proliferation and
invasion (60,61).
Similarly, many studies have demonstrated C-MET
expression in HCC lesions. However, the value of C-MET expression
as a prognostic factor in this context remains unclear. In a recent
meta-analysis of 1,480 HCC patients undergoing surgical resection
it was suggested that C-MET over-expression (when comparing high-
and low-expression groups) is a prognostic indicator of recurrence
and survival (33). It is likely
that tumor PD-L1 and/or C-MET expression is correlated with poor
prognosis due to involvement of these proteins in mechanisms of
immune evasion. Over-expression of C-MET in HCC is reportedly
associated with tumor progression (49,62),
central venous invasion or thrombosis (49,51),
intrahepatic metastasis (63,64),
tumor recurrence (63,64) and survival (63). Wang et al (63) found that high C-MET expression in HCC
patients (with lesions of less than 5 cm) undergoing surgical
resection was independently correlated with shorter survival
intervals. Although some studies have shown that C-MET
over-expression is of prognostic value in early-stage HCC in
patients who have undergone a partial hepatectomy, C-MET
over-expression has also been shown to be of limited prognostic
value since it does not appear to be an obvious indicator of
end-stage HCC (62,64).
The current study investigated the correlation
between C-MET and PD-L1 expression (the latter as determined by two
MAb types), and clinicopathologic factors. Expression of TC PD-L1
(as detected by SP263) positively correlated with C-MET expression,
indicating that C-MET-mediated regulation of PD-L1 pathways may be
involved in HCC. Furthermore, statistically significant
correlations were observed between TC PD-L1 expression (as detected
by SP263) and ES grade, as well as between C-MET expression and T
stage. Such correlation between PD-L1 and C-MET expression and
clinicopathological parameters suggests that expression of these
proteins may be of utility as potential prognostic factors in HCC.
The enrolled patient had surgery recently (2013~2017), so we cannot
analyzed survival of patients. I will do survival analysis later.
And, molecular study will be the best and I also have the plan
about the molecular experiment of PD-L1 sub-molecules after the IHC
experiment.
Results of the current study are expected to be of
use in the future approval of immunotherapeutic agents and
determination of a prescription standard for HCC. Data also
demonstrate the value of PD-L1 and C-MET as prognostic factors
through their correlation with clinicopathologic factors.
Correlation between PD-L1 (as detected by SP263) and C-MET
expression provides baseline data for future development of
C-MET-targeting immunotherapeutic interventions.
Acknowledgements
Not applicable.
Funding
The present study was supported by research funds
from Chosun University, Republic of Korea, 2016 (grant no.
2016-01).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RH designed the study, performed the experiments,
provided financial support, revised the manuscript and gave final
approval of the version to be published. HWC provided financial
support, interpreted data and wrote the paper.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Chosun University Hospital (Institutional review Board of Chosun
University Hospital, Gwangju, Korea), who waived the requirement
for written informed consent due to the nature of the study (IRB
No.: 2018-04-003-001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jung HI, Jeong D, Ji S, Ahn TS, Bae SH,
Chin S, Chung JC, Kim HC, Lee MS and Baek MJ: Overexpression of
PD-L1 and PD-L2 is associated with poor prognosis in patients with
Hepatocellular carcinoma. Cancer Res Treat. 49:246–254. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vilarinho S and Taddei T: Therapeutic
strategies for hepatocellular carcinoma: New advances and
challenges. Curr Treat Options Gastroenterol. 13:219–234. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sangro B, Palmer D and Melero I:
Immunotherapy of hepatocellular carcinoma. Hepat Oncol. 1:433–446.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang H, Jung W, Kim A, Kim HK, Kim WB,
Kim JH and Kim BH: Expression and prognostic significance of
programmed death protein 1 and programmed death ligand-1, and
cytotoxic T lymphocyte-associated molecule-4 in hepatocellular
carcinoma. APMIS. 125:690–698. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farkona S, Diamandis EP and Blasutig IM:
Cancer immunotherapy: the beginning of the end of cancer? BMC Med.
14:732016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishimura H, Agata Y, Kawasaki A, Sato M,
Imamura S, Minato N, Yagita H, Nakano T and Honjo T:
Developmentally regulated expression of the PD-1 protein on the
surface of double-negative (CD4-CD8-) thymocytes. Int Immunol.
8:773–780. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson RH, Kuntz SM, Leibovich BC, Dong
H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H,
et al: Tumor B7-H1 is associated with poor prognosis in renal cell
carcinoma patients with long-term follow-up. Cancer Res.
66:3381–3385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG and
Xu N: Immunohistochemical localization of programmed death-1
ligand-1 (PD-L1) in gastric carcinoma and its clinical
significance. Acta Histochem. 108:19–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+
T lymphocytes are prognostic factors of human ovarian cancer. Proc
Natl Acad Sci USA. 104:3360–3365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang
JY, Yang YP, Tien P and Wang FS: PD-1 and PD-L1 upregulation
promotes CD8(+) T-cell apoptosis and postoperative recurrence in
hepatocellular carcinoma patients. Int J Cancer. 128:887–896. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maine CJ, Aziz NH, Chatterjee J, Hayford
C, Brewig N, Whilding L, George AJ and Ghaem-Maghami S: Programmed
death ligand-1 over-expression correlates with malignancy and
contributes to immune regulation in ovarian cancer. Cancer Immunol
Immunother. 63:215–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Norde WJ, Hobo W, van der Voort R and
Dolstra H: Coinhibitory molecules in hematologic malignancies:
Targets for therapeutic intervention. Blood. 120:728–736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Kang S, Shen J, He J, Jiang L,
Wang W, Guo Z, Peng G, Chen G, He J and Liang W: Prognostic
significance of programmed cell death 1 (PD-1) or PD-1 ligand 1
(PD-L1) expression in epithelial-originated cancer: A
meta-analysis. Medicine (Baltimore). 94:e5152015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Qiu M, Jin Y, Ji J, Li B, Wang X,
Yan S, Xu R and Yang D: Programmed cell death ligand 1 (PD-L1)
expression on gastric cancer and its relationship with
clinicopathologic factors. Int J Clin Exp Pathol. 8:11084–11091.
2015.PubMed/NCBI
|
|
18
|
Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ,
Jin YT and Chang Y: Increase of programmed death-1-expressing
intratumoral CD8 T cells predicts a poor prognosis for
nasopharyngeal carcinoma. Mod Pathol. 23:1393–1403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muenst S, Hoeller S, Dirnhofer S and
Tzankov A: Increased programmed death-1+ tumor-infiltrating
lymphocytes in classical Hodgkin lymphoma substantiate reduced
overall survival. Hum Pathol. 40:1715–1722. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thompson RH, Dong H, Lohse CM, Leibovich
BC, Blute ML, Cheville JC and Kwon ED: PD-1 is expressed by
tumor-infiltrating immune cells and is associated with poor outcome
for patients with renal cell carcinoma. Clin Cancer Res.
13:1757–1761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swaika A, Hammond WA and Joseph RW:
Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy.
Mol Immunol. 67:4–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao L, Lu Y, Xu Y, Zhang G, Huang Z, Gong
L and Fan Y: PD-L1 and c-MET expression and survival in patients
with small cell lung cancer. Oncotarget. 8:53978–53988.
2017.PubMed/NCBI
|
|
23
|
Gherardi E, Birchmeier W, Birchmeier C and
Vande Woude G: Targeting MET in cancer: Rationale and progress. Nat
Rev Cancer. 12:89–103. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goyal L, Muzumdar MD and Zhu AX: Targeting
the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res.
19:2310–2318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M,
Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al: Overexpression
of PD-L1 significantly associates with tumor aggressiveness and
postoperative recurrence in human hepatocellular carcinoma. Clin
Cancer Res. 15:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M,
Xing MY, Zhang WG, Qi JY, Roggendorf M, Lu MJ and Yang DL:
Immunostaining of PD-1/PD-Ls in liver tissues of patients with
hepatitis and hepatocellular carcinoma. World J Gastroenterol.
17:3322–3329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kleponis KJ, Skelton R and Zheng L:
Fueling the engine and releasing the break: combinational therapy
of cancer vaccines and immune checkpoint inhibitors. Cancer Biol
Med. 12:201–208. 2015.PubMed/NCBI
|
|
28
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): an open-label, non-comparative, phase 1/2 does
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baik CS, Rubin EH, Forde PM, Mehnert JM,
Collyar D, Butler MO, Dixon EL and Chow LQM: Immuno-oncology
clinical trial design: limitations, challenges, and opportunities.
Clin Cancer Res. 23:72017. View Article : Google Scholar
|
|
30
|
Gelsomino F, Rossi G and Tiseo M: MET and
small-cell lung cancer. Cancers (Basel). 6:2100–2115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim R, Keam B, Kwon D, Ock Cy, Kim M, Kim
TM, Kim HJ, Jeon YK, Park IK, Kang CH, et al: Programmed death
ligand-1 expression and its prognostic role in esophageal squamous
cell carcinoma. World J Gastroenterol. 22:8389–8397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeng Z, Shi F, Zhou L, Zhang MN, Chen Y,
Chang XJ, Lu YY, Bai WL, Qu JH, Wang CP, et al: Upregulation of
circulating PD-L1/PD-1 is associated with poor post-cryoablation
prognosis in patients with HBV-related hepatocellular carcinoma.
PLoS One. 6:e236212011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vennapusa B, Baker B, Kowanetz M, Boone J,
Menzl I, Bruey JM, Fine G, Mariathasan S, McCaffery I, Mocci S, et
al: Development of a PD-L1 companion diagnostic
Immunohistochemistry assay (SP142) for atezolizumab. Appl
immunohistochem Mol Morphol. 27:92–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schats KA, Van Vre EA, De Schepper S,
Boeckx C, Schrijvers DM, Waelput W, Fransen E, Vanden Bempt I,
Neyns B, De Meester I and Kockx MM: Validated programmed cell death
ligand 1 immunohistochemistry assays (E1L3N and SP142) reveal
similar immune cell staining patterns in melanoma when using the
same sensitive detection system. Histopathology. 70:253–263. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Balar AV, Galsky MD, Rosenberg JE, Powles
T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J,
Perez-Gracia JL, et al: Atezolizumab as first-line treatment in
cisplatin-ineligible patients with locally advanced and metastatic
urothelial carcinoma: A single-arm, multicentre, phase 2 trial.
Lancet. 389:67–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sidaway P: Bladder cancer: Atezolizumab:
An alternative to cisplatin? Nat Rev Urol. 14:672017. View Article : Google Scholar
|
|
38
|
Mizugaki H, Yamamoto N, Murakami H,
Kenmotsu H, Fujiwara Y, Ishida Y, Kawakami T and Takahashi T: Phase
I dose-finding study of monotherapy with atezolizumab, an
engineered immunoglobulin monoclonal antibody targeting PD-L1, in
Japanese patients with advanced solid tumors. Invest New Drugs.
34:596–603. 2016. View Article : Google Scholar
|
|
39
|
Rosenberg JE, Hoffman-Censits J, Powles T,
van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rebelatto MC, Midha A, Mistry A, Sabalos
C, Schechter N, Li X, Jin X, Steele KE, Robbins PB, Blake-Haskins
JA and Walker J: Development of a programmed cell death ligand-1
immunohistochemical assay validated for analysis of non-small cell
lung cancer and head and neck squamous cell carcinoma. Diagn
Pathol. 11:952016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Diggs LP and Hsueh EC: Utility of PD-L1
immunohistochemistry assays for predicting PD-1/PD-L1 inhibitor
response. Biomark Res. 5:122017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ilie M, Long-Mira E, Bence C, Butori C,
Lassalle S, Bouhlel L, Fazzalari L, Zahaf K, Lalvée S, Washetine K,
et al: Comparative study of the PD-L1 status between surgically
resected specimens and matched biopsies of NSCLC patients reveal
major discordances: A potential issue for anti-PDL1 therapeutic
strategies. Ann Oncol. 27:147–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kitazono S, Fujiwara Y, Tsuta K, Utsumi H,
Kanda S, Horinouchi H, Nokihara H, Yamamoto N, Sasada S, Watanabe
S, et al: Reliability of small biopsy samples compared with
resected specimens for the determination of programmed death-ligand
1 expression in non-smallcell lung cancer. Clin Lung Cancer.
16:385–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Azuma K, Ota K, Kawahara A, Hattori S,
Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, et
al: Association of PD-L1 overexpression with activating EGFR
mutations in surgically resected nonsmall-cell lung cancer. Ann
Oncol. 25:1935–1940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang Y, Fang W, Zhang Y, Hong S, Kang S,
Yan Y, Chen N, Zhan J, He X, Qin T, et al: The association between
PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced
non-small cell lung cancer patients treated with EGFR-TKIs.
Oncotarget. 6:14209–14219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gabrielson A, Wu Y, Wang H, Jiang J,
Kallakury B, Gatalica Z, Reddy S, Kleiner D, Fishbein T, Johnson L,
et al: Intratumoral CD3 and CD8 T-cell densities associated with
relapse-free survival in HCC. Cancer Immunol Res. 4:419–430. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim JH, Kim HS, Kim BJ, Jang HJ and Lee J:
Prognostic value of c-Met overexpression in hepatocellular
carcinoma: A meta-analysis and review. Oncotarget. 8:90351–90357.
2017.PubMed/NCBI
|
|
49
|
Kondo S, Ojima H, Tsuda H, Hashimoto J,
Morizane C, Ikeda M, Ueno H, Tamura K, Shimada K, Kanai Y and
Okusaka T: Clinical impact of c-MET expression and its gene
amplification in hepatocellular carcinoma. Int J Clin Oncol.
18:207–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Takeuchi H, Bilchik A, Saha S, Turner R,
Wiese D, Tanaka M, Kuo C, Wang HJ and Hoon DS: c-MET expression
level in primary colon cancer: A predictor of tumor invasion and
lymph node metastases. Clin Cancer Res. 9:1480–1488.
2003.PubMed/NCBI
|
|
51
|
Ueki T, Fujimoto J, Suzuki T, Yamamoto H
and Okamoto E: Expression of hepatocyte growth factor and its
receptor, the c-met proto-oncogene, in hepatocellular carcinoma.
Hepatology. 25:862–866. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sawada K, Radjabi AR, Shinomiya N, Kistner
E, Kenny H, Becker AR, Turkyilmaz MA, Salgia R, Yamada SD, Vande
Woude GF, et al: c-Met overexpression is a prognostic factor in
ovarian cancer and an effective target for inhibition of peritoneal
dissemination and invasion. Cancer Res. 67:1670–1679. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shattuck DL, Miller JK, Carraway KL III
and Sweeney C: Met receptor contributes to trastuzumab resistance
of Her2overexpressing breast cancer cells. Cancer Res.
68:1471–1477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Arriola E, Cañadas I, Arumí-Uría M, Dómine
M, Lopez-Vilariño JA, Arpí O, Salido M, Menéndez S, Grande E,
Hirsch FR, et al: MET phosphorylation predicts poor outcome in
small cell lung carcinoma and its inhibition blocks HGF-induced
effects in MET mutant cell lines. Br J Cancer. 105:814–823. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Park S, Choi YL, Sung CO, An J, Seo J, Ahn
MJ, Ahn JS, Park K, Shin YK, Erkin OC, et al: High MET copy number
and MET overexpression: Poor outcome in non-small cell lung cancer
patients. Histol Histopathol. 27:197–207. 2012.PubMed/NCBI
|
|
56
|
Nakamura Y, Niki T, Goto A, Morikawa T,
Miyazawa K, Nakajima J and Fukayama M: c-Met activation in lung
adenocarcinoma tissues: An immunohistochemical analysis. Cancer
Sci. 98:1006–1013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ichimura E, Maeshima A, Nakajima T and
Nakamura T: Expression of c-met/HGF receptor in human non-small
cell lung carcinomas in vitro and in vivo and its prognostic
significance. Jpn J Cancer Res. 87:1063–1069. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Takanami I, Tanaka F, Hashizume T, Kikuchi
K, Yamamoto Y, Yamamoto T and Kodaira S: Hepatocyte growth factor
and c-Met/hepatocyte growth factor receptor in pulmonary
adenocarcinomas: An evaluation of their expression as prognostic
markers. Oncology. 53:392–397. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Siegfried JM, Weissfeld LA, Singh-Kaw P,
Weyant RJ, Testa JR and Landreneau RJ: Association of
immunoreactive hepatocyte growth factor with poor survival in
resectable non-small cell lung cancer. Cancer Res. 57:433–439.
1997.PubMed/NCBI
|
|
60
|
Danilkovitch-Miagkova A and Zbar B:
Dysregulation of Met receptor tyrosine kinase activity in invasive
tumors. J Clin Invest. 109:863–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Boccaccio C and Comoglio PM: Invasive
growth: A MET-driven genetic programme for cancer and stem cells.
Nat Rev Cancer. 6:637–645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu F, Wu L, Zheng S, Ding W, Teng L, Wang
Z, Ma Z and Zhao W: The clinical value of hepatocyte growth factor
and its receptor-c-met for liver cancer patients with hepatectomy.
Dig Liver Dis. 38:490–497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang ZL, Liang P, Dong BW, Yu XL and Yu
DJ: Prognostic factors and recurrence of small hepatocellular
carcinoma after hepatic resection or microwave ablation: A
retrospective study. J Gastrointest Surg. 12:327–337. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kelley PK, Verslype C, Cohn AL, Yang TS,
Su WC, Burris H, Braiteh F, Vogelzang N, Spira A, Foster P, et al:
Carbozantinib in hepatocellular carcinoma: Results of a phase 2
placebo-controlled randomized discontinuation study. Ann Oncol.
28:528–534. 2017. View Article : Google Scholar : PubMed/NCBI
|