Introduction
Gastric cancer has a poor prognosis that is largely
attributable to early and frequent metastasis (1–3). Surgery
and combined radio-chemotherapeutic regimens are associated with
modest survival benefits in advanced gastric cancer, with an
overall 5-year survival rate of <24% (3). At present, the mechanisms underlying
the initiation and progression of gastric cancer remain unclear and
molecular markers for gastric cancer remain to be identified. It is
imperative to identify the biological and molecular changes that
frequently occur during gastric carcinogenesis, in order to
elucidate cancer pathology and identify new diagnostic markers, in
order to individualize treatment for patients with gastric
cancer.
Interleukin 1 receptor-like 1 (ST2/IL1RL1), also
known as T1, DER4 or FIT-1, which was originally identified as a
primary responsive gene, is a member of the interleukin (IL)-1
receptor family and is highly induced by growth stimulation and
oncogenic Ras-induced signaling (4).
Based on alternative splicing and processing of mRNA, ST2 exists as
4 isoforms, including a transmembrane full-length form (ST2L), also
known as IL1RL1-b, a soluble secreted form (sST2), also known as
IL1RL1-a, a variant without the third immunoglobulin motif and
splicing in the C-terminal (ST2v) and a fourth that has yet to be
fully characterized (4–6). ST2 is produced by various types of
immune cells produce ST2 including mast cells, macrophages and
dendritic cells, and non-immune cells, including endothelial,
epithelial, smooth muscle and fibroblast cells (7). The widespread expression of the ST2
gene in different cell types indicates that it may have important
functions across a broad spectrum of biological systems. A recent
functional study demonstrated that ST2 is the ligand-binding
component of the interleukin-33 (IL-33) receptor, whereas sST2 is
considered as a decoy receptor preventing IL-33 signaling (8). ST2L stimulated by IL-33 was
demonstrated to activate the transcription factor through common
signaling molecules to interleukin-1 receptor (IL-1R), including
myeloid differentiation primary response protein MyD88 (MyD88),
MyD88-adapter-like, the Toll/IL-1R domain of the cytoplasmic region
of IL1RL1, IL-1R-associated kinase, tumor necrosis factor
receptor-associated factor 6 and NF-κB, and the mitogen-activated
protein kinase family (8,9). ST2/IL-33 signaling has also been
identified to be involved in T-cell mediated immune responses,
particularly Th2 cells and Th2-associated cytokines (9). IL-33 binds to ST2 and induces the
expression of interferon, IL-4, IL-5 and IL-13, thereby leading to
severe pathological changes in mucosal organs. ST2L has previously
been used as a marker for Th2 cells. In addition, sST2 may also
serve a key role in downregulating the inflammatory response
(10). It has also been suggested
that ST2L/IL-33 participates in a number of inflammatory immune
response processes associated with asthma, allergic diseases,
autoimmune diseases, cardiovascular diseases and other conditions
associated with acute heart failure, myocardial infarction,
respiratory failure and acute trauma (11,12).
Previous studies revealed that ST2 was involved in the pathogenesis
and prognosis of multiple types of cancer, including glioblastoma,
breast and pancreatic cancer, and leukemia (13–16).
However, to the best of our knowledge, the role of ST2 in gastric
cancer remains to be elucidated. The aim of the present study was
to investigate the expression status of the 2 primary splice
variants, sST2 and ST2v, by western blot analysis, and to evaluate
ST2 protein expression in gastric cancer using immunohistochemistry
and determine its clinicopathological significance.
Patients and methods
Patient information and tissue
specimens
A microarray chip containing 178 samples of gastric
cancer tissues from Beijing Hua Nuo Aomei Biotechnology, Inc. was
utilized. A second cohort of 52 formalin-fixed paraffin-embedded
tumor specimens was collected from patients who underwent surgery
at the Xijing Hospital Affiliated to The Fourth Military Medical
University (Xi'an, China) between September 2006 and January 2012.
There were 230 patients in total, including 69 females and 161
males (median age, 54 years; range, 31–75 years) included in the
present study. The patients were diagnosed as follows: 104 cases of
gastric antrum carcinoma; 76 cases of carcinoma of the gastric
cardia; and 50 cases of gastric body carcinoma. A total of 116
adjacent non-cancerous tissue specimens were also collected as
controls. Tumor grade and stage were classified in accordance with
the International Union against Cancer/American Joint Committee on
Cancer pathological Tumor-Node-Metastasis (TNM) classification, 7th
edition (2010) (17). In addition,
12 independent primary gastric cancer tissues and matched adjacent
non-cancerous samples were frozen and stored in liquid nitrogen for
western blot analysis. Signed informed consent was obtained from
the patients prior to tissue sample collection. The study protocol
conformed to the ethical guidelines outlined in the Declaration of
Helsinki and was approved by The Institutional Review Board
(approval no. 07-170) of Ningxia Hui Autonomous Region People's
Hospital (Ningxia Hui Autonomous Region, China).
Western blot analysis
This procedure was performed as described previously
(18–21). Frozen tissue samples, including
gastric cancer tissues and paired adjacent non-cancerous tissues
obtained from 12 patients were prepared in radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology). A total
of 20 µg proteins were separated by 8% SDS-PAGE and transferred to
a polyvinylidene fluoride membrane (EMD Millipore). The membranes
were incubated in blocking buffer (TBS with 0.1% Tween and 5%
non-fat dry milk) for 1 h at 37°C and then incubated with a rabbit
polyclonal anti-ST2 antibody (cat. no. 11920-1-AP; dilution, 1:500;
ProteinTech Group, Inc.) in blocking buffer overnight at 4°C,
followed by a horseradish peroxidase-conjugated secondary antibody
against rabbit IgG (cat. no. sc-2004; dilution, 1:2,000; Santa Cruz
Biotechnology, Inc.) for 1 h at 37°C. Signals were visualized with
the enhanced chemiluminescence system according to the
manufacturer's protocol (Amersham; GE Healthcare). The blots were
probed with an anti-β-actin monoclonal antibody (cat. no. ab8226;
dilution, 1:2,000; Abcam) as the control. Densitometric analyses of
protein expression levels were performed using Bio-Rad Quantity One
software (version 4.5.2; Bio-Rad Laboratories, Inc.). Expression
was considered to be decreased when the ratio of expression in
tumor and paired non-cancerous tissue was <2.
Immunofluorescence microscopy
Immunofluorescence microscopy was performed as
described previously (18,19). Sections (4 µm) cut from tissue
specimens fixed in 10% buffered formalin and embedded in paraffin
were deparaffinized, rehydrated (anhydrous ethanol gradient, 100,
95, 80 and 70%) and incubated with 0.3% H2O2
for 20 min at 37°C. Non-specific blocking was performed by using
10% goat serum (Sigma-Aldrich; Merck KGaA) containing 0.05%
Tweeen-20 for 30 min at 37°C. Glass slides were coated with a
rabbit polyclonal anti-ST2 antibody (cat. no. 11920-1-AP; dilution,
1:50; ProteinTech Group, Inc.) overnight at 4°C. The tissue
sections were washed with PBS containing 0.05% Tween-20 and then
incubated with a fluorescein isothiocyanate-conjugated goat
anti-rabbit secondary antibody (cat. no. sc-2012; dilution,
1:2,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The sections were counterstained with propidium iodide
(PI; Sigma-Aldrich; Merck KGaA) for 5 min at 37°C in the dark
(final concentration of PI, 50 µg/ml). Finally, the slides were
washed twice with PBS and were examined under an Olympus Flouview
FV1000 confocal laser scanning microscope (Olympus Corporation) at
×400 magnification.
Immunohistochemistry and
evaluation
Immunohistochemical analysis was performed as
described previously (20,21). Sections (4 µm thick) were cut from
tissue specimens fixed in 10% buffered formalin and embedded in
paraffin. After deparaffinization (xylene, 2 times and 10 min each
at 37°C) and rehydration (alcohol gradient, 100, 95, 80 and 70%),
endogenous peroxidase activity was blocked with 0.3% hydrogen
peroxide for 30 min at room temperature. For antigen retrieval,
tissue sections were autoclaved at 121°C in citrate buffer (10 mM,
pH 6.0) for 10 min. The sections were blocked for 1 h in 5% bovine
serum albumin (Sigma-Aldrich; Merck KGaA) in PBS at 37°C, and were
subsequently incubated with a rabbit polyclonal anti-ST2 antibody
(cat. no. 11920-1-AP; dilution, 1:50; ProteinTech Group, Inc.)
overnight at 4°C. Staining was visualized using an EnVision
antibody complex method. An EnVision kit (OriGene Technologies,
Inc.) was used and 3,3′-diaminobenzidine was used as the chromogen.
Nuclei were counterstained with 0.5% hematoxylin for 2 min at room
temperature. Sections immunostained with the normal rabbit IgG
(cat. no. PP501P; dilution, 1:50; OriGene Technologies, Inc.) as
the primary antibody were used as negative controls. A total of 10
random microscopic fields/slide, were evaluated by 2 independent
observers who were blinded to the clinical information, at a
magnification of ×400 using a light microscope (Carl Zeiss AG,
Oberkochen, Germany). ST2 staining was assessed using a
semi-quantitative approach, which combined the staining intensity
and proportion of positive cells. The mean percentage of
positively-stained cells was scored as follows: 0, 0–5%; 1, 5–25%;
2, 26–50%; 3, 51–75%; and 4, 76–100%. Staining intensity was
categorized as follows: 0, Absent; 1, weak; 2, moderate; and 3,
strong. The multiplication of staining intensity and percentage of
positive cells was used as the final staining score. For
statistical evaluation, the tumor samples with final staining
scores of <3 were classed as negative ST2 expression and those
with scores ≥3 as positive ST2 expression.
Statistical analysis
Statistical analysis was performed using the SPSS
17.0 statistical software package (SPSS, Inc., Chicago, IL, USA).
The association between the expression levels of ST2 with different
clinical variables was assessed using Fisher's exact test or
Pearson's χ2 test as appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
Protein expression levels of ST2 in
cancer and non-cancerous tissues
In light of the data indicating that ST2 is a
frequently silenced candidate tumor suppressor gene during
tumorigenesis (14), the present
study first examined whether the expression of ST2 was altered at
the protein level in tumors compared with normal tissues. In 12
pairs of gastric cancer tissues and their adjacent non-cancerous
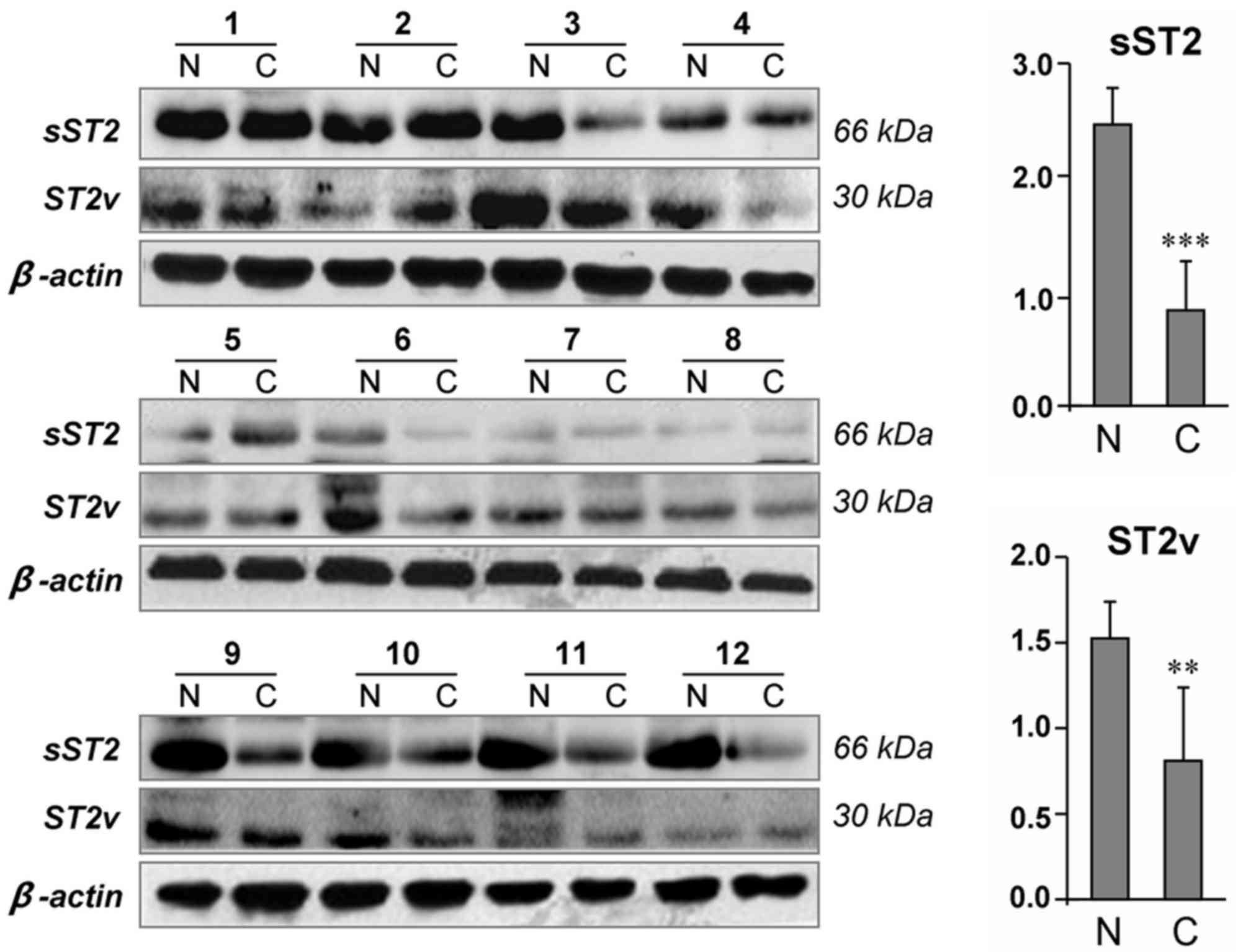
tissues, 2 bands were observed with molecular weights corresponding
to the size of sST2 (30 kDa) and ST2v (66 kDa) (Fig. 1). Notably, 75% (9/12) of gastric
cancer tissues exhibited decreased sST2 expression compared with
the adjacent non-cancerous tissues (P<0.001). Similarly,
decreased ST2v expression levels were also identified in 66.7%
(8/12) of the tumors compared with the adjacent non-cancerous
tissues (P<0.01).
The expression profile of ST2 in a cohort of 230
primary gastric cancer tissues and 116 adjacent non-cancerous
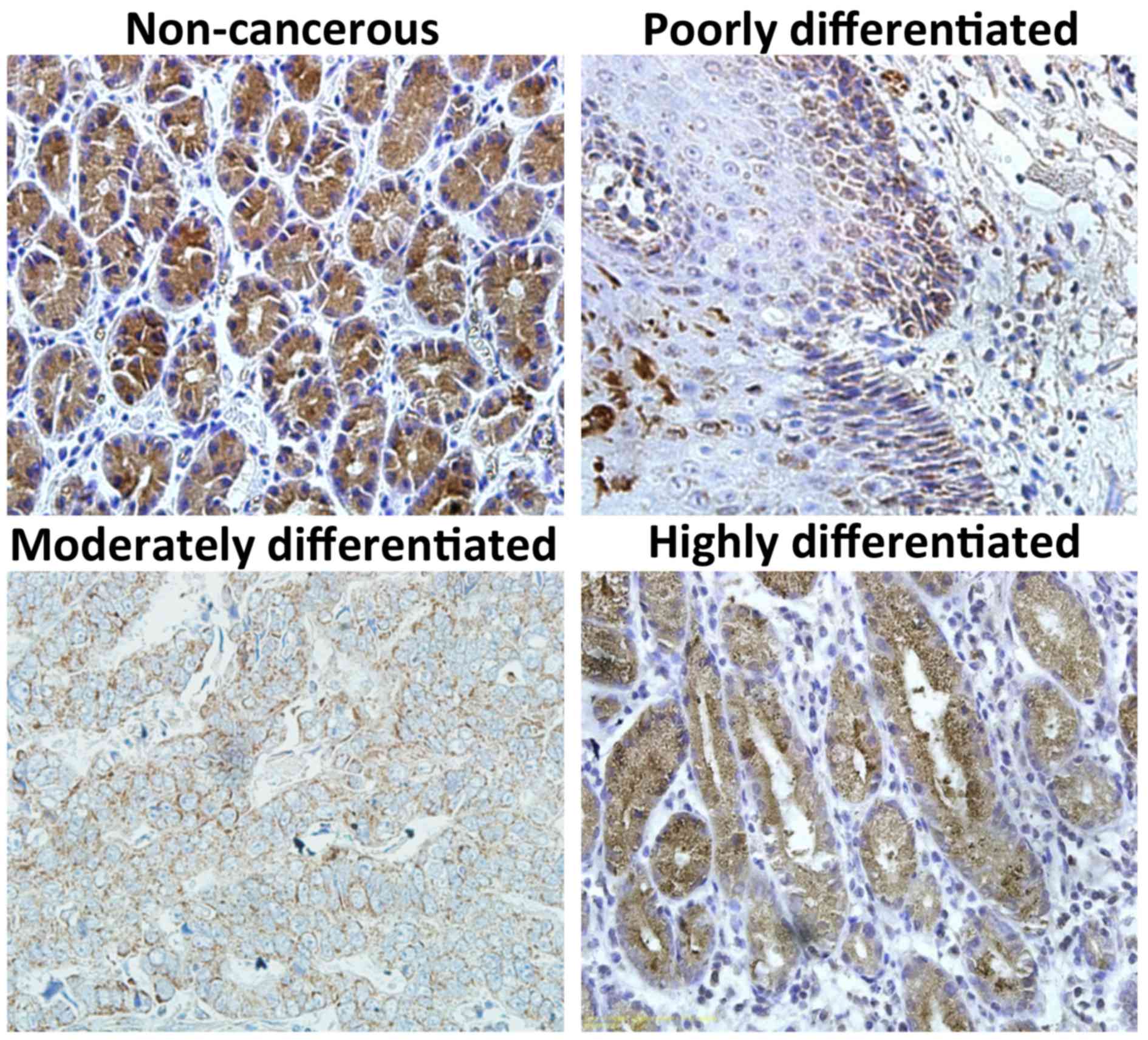
tissues was additionally investigated by immunohistochemistry. ST2
expression was observed primarily in the cytoplasm of neoplastic
cells, although it was also observed to a certain extent in the
cell membrane (Fig. 2). Positive
expression of the ST2 protein was observed in 39.1% (90/230) of
tumor specimens and in 60.7% (54/89) of adjacent non-cancerous
tissues (P<0.05). Taken together, these results clearly indicate
that ST2 expression is frequently downregulated in gastric cancer
tissues compared with normal controls.
Cellular localization of ST2
expression in gastric cancer
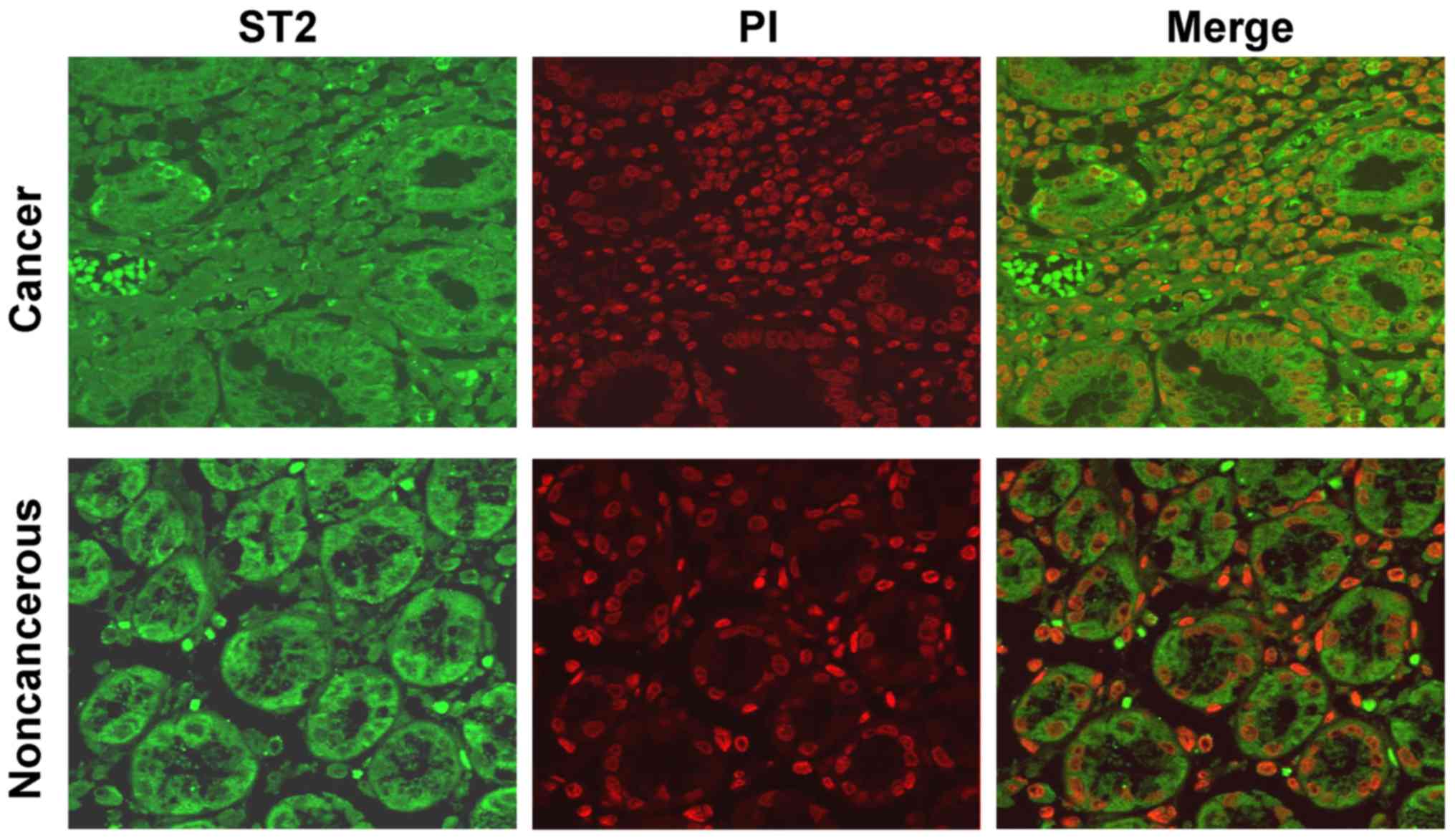
To determine the cellular localization of ST2
expression, immunofluorescence analysis was performed. Consistent
with the results of the immunohistochemistry analysis, positive
immunostaining for ST2 was observed in the cytoplasm and the
membrane of cancer cells, whereas marked ST2 immunoreactivity was
predominantly identified in the cytoplasm of epithelial cells in
non-cancerous tissues (Fig. 3).
Clinicopathological implications of
ST2 expression
The potential associations between ST2 protein
levels and the clinicopathological characteristics of 230 gastric
cancer specimens were next assessed. As demonstrated in Table I, decreased ST2 expression was
identified to be significantly associated with decreased
differentiation (P<0.001) and advanced TNM stage (P<0.001).
No significant association was observed between ST2 expression and
the other clinicopathological factors, including patient age and
sex. In summary, these data indicated that decreased ST2 expression
is closely associated with parameters implicated in gastric cancer
progression and pathogenesis.
 | Table I.Associations between ST2 expression
and clinicopathological variables. |
Table I.
Associations between ST2 expression
and clinicopathological variables.
|
|
| ST2 expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Patients, n | Negative, n | Positive, n | P-value |
|---|
| Sex |
|
|
|
|
| Male | 161 | 101 | 60 | 0.38 |
|
Female | 69 | 39 | 30 |
|
| Age, years |
|
|
|
|
| ≤61 | 134 | 85 | 15 | 0.44 |
|
>61 | 96 | 55 | 20 |
|
|
Differentiationa |
|
|
|
|
| Well | 37 |
9 | 28 | <0.001 |
|
Moderate | 72 | 38 | 34 |
|
| Poor | 94 | 71 | 23 |
|
| TNM staging |
|
|
|
|
|
I/II | 143 | 76 | 67 | <0.001 |
|
III/IV | 87 | 64 | 23 |
|
Discussion
ST2 has been identified as a regulatory effector for
Th2-type responses, and as a negative feedback regulator in
pro-inflammatory responses (4). ST2
also has a significant effect on the pathogenesis of several
diseases, including inflammation, allergies, fibrillation, cardiac
hypertrophy and rheumatoid arthritis (22). Numerous studies have suggested that
ST2 expression is associated with carcinogenesis and tumor
progression in multiple cancer types (13–16,22). ST2
has been shown to regulate innate and acquired immunity in tumors.
The biological functions of ST2 in cancer growth and progression
are primarily mediated by the IL-33/ST2 signaling pathway, which
compromises the integrity of the intestinal barrier and promotes
the production of pro-tumorigenic IL-6 by immune cells (22).
However, the exact physiological and pathological
functions of ST2 in cancer development and progression remain to be
elucidated (23,24). In the present study, the protein
expression of the ST2 gene in human gastric cancer tissues and
their matched non-cancerous tissues was analyzed by western blot
analysis and immunohistochemistry. A total of 2 variant subtypes of
the ST2 protein were also identified in gastric cancer tissues,
sST2 and ST2v, with corresponding molecular weights of 30 and 66
kDa, respectively. Notably, the expression levels of ST2 among
normal tissues adjacent to the tumor were variable across the
patient cohort. This discrepancy may be due to the difference
between individuals. However, 75% (9/12) of gastric cancer tissues
exhibited a significantly decreased sST2 expression compared with
the adjacent non-cancerous tissues. This observation suggested that
ST2 protein expression was markedly decreased in cancer tissues
compared with non-cancerous tissues. The present analysis in
gastric cancer indicated an association between negative ST2
expression and tumor progression phenotype, including advanced
tumor stage and poor tumor differentiation. These results suggested
that ST2 may act as a tumor suppressor gene in gastric cancer,
consistent with a previous study in glioblastoma cells (24), but are inconsistent with other
studies in breast and colorectal cancer (23,25).
Notably, a previous study involving gastric cancer revealed that
IL-33 enhanced tumor cell invasion and migration via the ST2-ERK1/2
pathway, and knockdown of the IL-33 receptor ST2 attenuates the
IL-33-mediated malignant phenotypes (26). It may be concluded that this
disparity may be due to the intrinsic differences among tumor
types.
It has been demonstrated that the ST2 protein may be
involved in the immunity of the tumor microenvironment. IL-33
combined with IL-1 and other inflammatory cytokines enhance the
expression of ST2, resulting in the activation of oncogenes
(27). It has been suggested that
knockdown of ST2L in mice may lead to the inhibition of mammary
tumor growth and metastasis, followed by enhanced circulating
levels of pro-inflammatory cytokines and activation of natural
killer and CD8+ T cells (28). Accumulating evidence suggests that
ST2 may serve as an inflammatory cytokine in promoting cancer
development and the production of Th2 cell-associated cytokines,
including IL-4, IL-5 and IL-13. These cytokines have been detected
in the microenvironment of several tumors (29–31).
Taking into consideration the complexity of the tumor
microenvironment, the physiological and molecular mechanisms of
action of the ST2 protein in gastric cancer remain to be
elucidated.
There were certain limitations to the present study.
Additional validation of the results in a larger series of patients
with gastric cancer will strengthen the results of the present
study and improve the understanding of the clinical behavior of
ST2. In addition, a number of factors are able to contribute to
abnormal gene silencing in cancer, and not all aberrant gene
silencing is involved in tumor development. Therefore, candidate
tumor suppressor genes, the expression levels of which are
downregulated in tumors, require additional investigation for their
biological roles in cancer development and progression.
In the present study, several methods were used to
characterize the ST2 expression profile in gastric cancer.
Downregulated ST2 expression is a molecular signature of gastric
cancer progression and pathogenesis. Therefore, it may be concluded
that ST2 not only appears to be a promising diagnostic biomarker,
but also a potential treatment target in gastric cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Ningxia, China (grant no., 2018AAC02016), the
National Natural Science Foundation of China (grant nos. 81760440
and 81860426), the Regional Science and Technology Development
Program Conducted by the Central Government of China (grant no.
YDZX20176400004650) and the Foundation of Ningxia Medical
University (grant no. XM2016077).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding authors on reasonable
request.
Authors' contributions
FBai, YY and YN conceived, designed the experiments
and drafted the manuscript. FBa, YF, WT, CW and MJ performed the
experiments. YF and WT analyzed the data.
Ethics approval and consent to
participate
Signed informed consent was obtained from the
patients prior to tissue sample collection. The study protocol
conformed to the ethical guidelines outlined in the Declaration of
Helsinki and was approved by the Institutional Review Board
(approval no. 07-170) of Ningxia Hui Autonomous Region People's
Hospital.
Patient consent for publication
Signed informed consent was obtained from the
patients prior to tissue sample collection.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yonemura Y, Endou Y, Sasaki T, Hirano M,
Mizumoto A, Matsuda T, Takao N, Ichinose M, Miura M and Li Y:
Surgical treatment for peritoneal carcinomatosis from gastric
cancer. Eur J Surg Oncol. 36:1131–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bozzetti F, Yu W, Baratti D, Kusamura S
and Deraco M: Locoregional treatment of peritoneal carcinomatosis
from gastric cancer. J Surg Oncol. 98:273–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwahana H, Yanagisawa K, Ito-Kosaka A,
Kuroiwa K, Tago K, Komatsu N, Katashima R, Itakura M and Tominaga
S: Different promoter usage and multiple transcription initiation
sites of the interleukin-1 receptor-related human ST2 gene in UT-7
and TM12 cells. Eur J Biochem. 264:397–406. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bergers G, Reikerstorfer A, Braselmann S,
Graninger P and Busslinger M: Alternative promoter usage of the
Fos-responsive gene Fit-1 generates mRNA isoforms coding for either
secreted or membrane-bound proteins related to the IL-1 receptor.
EMBO J. 13:1176–1188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayakawa H, Hayakawa M, Kume A and
Tominaga S: Soluble ST2 blocks interleukin-33 signaling in allergic
airway inflammation. J Biol Chem. 282:26369–26380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trajkovic V, Sweet MJ and Xu D: T1/ST2-an
IL-1receptor-like modulator of immune responses. Cytokine Growth
Factor Rev. 15:87–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hentschke I, Graser A, Melichar VO, Kiefer
A, Zimmermann T, Kroß B, Haag P, Xepapadaki P, Papadopoulos NG,
Bogdan C and Finotto S: IL-33/ST2 immune responses to respiratory
bacteria in pediatric asthma. Sci Rep 7:43426. 2017. View Article : Google Scholar
|
|
9
|
Yamamoto M, Sato S, Hemmi H, Hoshino K,
Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K and
Akira S: Role of adaptor TRIF in the MyD88-independent Toll-like
receptor signaling pathway. Science. 301:640–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siede J, Fröhlich A, Datsi A, Hegazy AN,
Varga DV, Holecska V, Saito H, Nakae S and Löhning M: IL-33
receptor-expressing regulatory T cells are highly activated, Th2
biased and suppress CD4 T cell proliferation through IL-10 and TGFβ
release. PLoS One. 11:e01615072016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Vark LC, Lesman-Leegte I, Baart SJ,
Postmus D, Pinto YM, Orsel JG, Westenbrink BD, Brunner-la Rocca HP,
van Miltenburg AJM, Boersma E, Hillege HL, Akkerhuis KM; TRIUMPH
Investigators, ; et al: Prognostic value of serial ST2 measurements
in patients with acute heart failure. J Am Coll Cardiol.
70:2378–2388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ko FW, Yan BP, Lam YY, Chu JH, Chan KP and
Hui DS: Undiagnosed airflow limitation is common in patients with
coronary artery disease and associated with cardiac stress.
Respirology. 21:137–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang JF, Wang P, Yan YJ, Li Y, Guan MW,
Yu JJ and Wang XD: IL-33 enhances glioma cell migration and
invasion by upregulation of MMP2 and MMP9 via the ST2-NF-κB
pathway. Oncol Rep. 38:2033–2042. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jovanovic I, Radosavljevic G, Mitrovic M,
Juranic VL, McKenzie AN, Arsenijevic N, Jonjic S and Lukic ML: ST2
deletion enhances innate and acquired immunity to murine mammary
carcinoma. Eur J Immunol. 41:1902–1912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang Y, Zhao L, Xiao H, Cook KM, Bai Q,
Herrick EJ, Chen X, Qin C, Zhu Z, Wakefield MR and Nicholl MB:
IL-33 acts as a foe to MIA PaCa-2 pancreatic cancer. Med Oncol.
34:232017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida K, Arai T, Yokota T, Komatsu N,
Miura Y, Yanagisawa K, Tetsuka T and Tominaga S: Studies on natural
ST2 gene products in the human leukemic cell line UT-7 using
monoclonal antihuman ST2 antibodies. Hybridoma. 14:419–427. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
You Y, Bai F, Ye Z, Zhang N, Yao L, Tang Y
and Li X: Downregulated CDK10 expression in gastric cancer:
Association with tumor progression and poor prognosis. Mol Med Rep.
17:6812–6818. 2018.PubMed/NCBI
|
|
18
|
Lin C, Xin S, Qin X, Li H, Lin L and You
Y: Zoledronic acid suppresses metastasis of esophageal squamous
cell carcinoma cells through upregulating the tight junction
protein occuludin. Cytotechnology. 68:1233–1241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
You Y, Yang W, Qin X, Wang F, Li H, Lin C,
Li W, Gu C, Zhang Y and Ran Y: ECRG4 acts as a tumor suppressor and
as a determinant of chemotherapy resistance in human nasopharyngeal
carcinoma. Cell Oncol (Dordr). 38:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
You Y, Li H, Qin X, Ran Y and Wang F:
Down-regulated ECRG4 expression in breast cancer and its
correlation with tumor progression and poor prognosis-a short
report. Cell Oncol (Dordr). 39:89–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
You Y, Li H, Qin X, Zhang Y, Song W, Ran Y
and Gao F: Decreased CDK10 expression correlates with lymph node
metastasis and predicts poor outcome in breast cancer patients-a
short report. Cell Oncol (Dordr). 38:485–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mertz KD, Mager LF, Wasmer MH, Thiesler T,
Koelzer VH, Ruzzante G, Joller S, Murdoch JR, Brümmendorf T,
Genitsch V, et al: The IL-33/ST2 pathway contributes to intestinal
tumorigenesis in humans and mice. Oncoimmunology. 5:e10629662015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dominguez D, Ye C, Geng Z, Chen S, Fan J,
Qin L, Long A, Wang L, Zhang Z, Zhang Y, et al: Exogenous IL-33
restores dendritic cell activation and maturation in established
cancer. J Immunol. 198:1365–1375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haga Y, Yanagisawa K, Ohto-Ozaki H,
Tominaga S, Masuzawa T and Iwahana H: The effect of ST2 gene
product on anchorage-independent growth of a glioblastoma cell
line, T98G. Eur J Biochem. 270:163–170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jovanovic IP, Pejnovic NN, Radosavljevic
GD, Pantic JM, Milovanovic MZ, Arsenijevic NN and Lukic ML:
Interleukin-33/ST2 axis promotes breast cancer growth and
metastases by facilitating intratumoral accumulation of
immunosuppressive and innate lymphoid cells. Int J Cancer.
134:1669–1682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu XX, Hu Z, Shen X, Dong LY, Zhou WZ and
Hu WH: IL-33 promotes gastric cancer cell invasion and migration
Via ST2-ERK1/2 pathway. Dig Dis Sci. 60:1265–1272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun P, Ben Q, Tu S, Dong W, Qi X and Wu Y:
Serun interleukin-33 levels in patients with gastric cancer. Dig
Dis Sci. 56:3596–3601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmieder A, Multhoff G and Radons J:
Interleukin-33 acts as a pro-inflammatory cytokine and modulates
its receptor gene expression in highly metastatic human pancreatic
carcinoma cells. Cytokine. 60:514–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Levescot A, Flamant S, Basbous S, Jacomet
F, Féraud O, Anne Bourgeois E, Bonnet ML, Giraud C, Roy L, Barra A,
et al: BCR-ABL-induced deregulation of the IL-33/ST2 pathway in
CD34+ progenitors from chronic myeloid leukemia patients. Cancer
Res. 74:2669–2676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin L, Dominguez D, Chen S, Fan J, Long A,
Zhang M, Fang D, Zhang Y, Kuzel TM and Zhang B: Exogenous IL-33
overcomes T cell tolerance in murine acute myeloid leukemia.
Oncotarget. 7:61069–61080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bergis D, Kassis V, Ranglack A, Koeberle
V, Piiper A, Kronenberger B, Zeuzem S, Waidmann O and Radeke HH:
High serum levels of the interleukin-33 receptor soluble ST2 as a
negative prognostic factor in hepatocellular carcinoma. Transl
Oncol. 6:311–318. 2013. View Article : Google Scholar : PubMed/NCBI
|