Introduction
Lung cancer is a globally widespread disease and a
leading cause of cancer-associated mortality, with ~1.6 million
cases of lung cancer-associated mortality per year (1). Non-small cell lung cancer (NSCLC)
accounts for ~85% of all primary cases, the majority of which
present with advanced and unresectable disease at diagnosis, which
is associated with a poor prognosis (2,3). In the
past few years, targeted therapy and immunotherapy have led to
promising results in patients with advanced NSCLC (4). However, the presence of distant
metastasis (DM) remains a cause of high mortality in the majority
of patients with NSCLC (5,6).
Out of all newly diagnosed NSCLC cases, ~40% present
with metastatic disease at diagnosis (7), and the majority of these patients have
a low 5-year survival rate (8). A
previous study reported that patients with NSCLC with multi-site
metastases exhibit worse outcomes than patients with a single
metastatic site (9). Additionally,
some studies have observed poor survival in patients with liver
metastasis (10,11). However, for the majority of these
studies, the main limitation was that the sample size was too
small.
The most frequent metastatic site of NSCLC is bone,
followed by the lung, brain, liver and adrenal glands (5). Sex, age at diagnosis and histological
subtypes can effectively influence the metastasis of NSCLC
(6). However, the clinical
associations and prognostic values of site-specific metastases have
not been well studied. Only a limited number of studies have been
conducted to investigate the various metastatic patterns, and their
occurrence rate and prognosis in NSCLC and its subtypes (5,6).
Therefore, studying the metastatic patterns is
crucial for the management and treatment of clinical NSCLC cases.
In the present study, which was based on the Surveillance,
Epidemiology and End Results (SEER) database, different metastatic
patterns, and their incidence rates and influence on survival of
patients with NSCLC were analyzed. The aim of the present study was
to assess the occurrence patterns and prognostic value of
site-specific DM for NSCLC.
Patients and methods
Patient selection
Specific data were collected from the SEER-18
registry of the US National Cancer Institute (12). The clinical information of ~34.6% of
patients with cancer within the US are precisely collected and
organized in the SEER database. The diagnosis time of selected
patients was limited to the period between 2010 and 2014, since
information regarding metastatic sites was not available prior to
that period. The eligible patients were selected using the
SEER*Stat v8.3.5 software (https://seer.cancer.gov).
To identify patients with NSCLC, cases with a
primary site of ‘lung and bronchus’ were selected. Diagnosis was
confirmed microscopically and only one primary tumor was
identified. According to histological type, NSCLC cases were
classified as: Adenocarcinoma (AD; histological codes 8140, 8230,
8250–8255, 8260, 8310, 8333, 8470, 8480, 8481, 8490 and 8550),
squamous cell carcinoma (SQCC; histological codes 8052, 8070–8073,
8083 and 8084), adenosquamous carcinoma (ASC; histological code
8560), large cell carcinoma (LCC; histological codes 8012–8014,
8082, 8123 and 8310) and others (histological codes 8022, 8031,
8032, 8200, 8240, 8249, 8430, 8562, 8972 and 8980). All patients
without information regarding cause of mortality and survival
months were excluded. Additionally, patients with ‘blanks’ for
metastatic site (n=6,952) and unknown American Joint Committee on
Cancer stage T/N (n=9) were excluded (13).
Statistical analysis
In the present study, patients with metastatic NSCLC
were sorted according to metastatic site, including bone, brain,
liver and lung. Overall survival (OS; defined as the time from
diagnosis to mortality due to any reason) and cancer-specific
survival (CSS; defined as the time from diagnosis to
NSCLC-associated mortality) were set as primary endpoints of the
present study. Comparison of the associations between
clinicopathological characteristics and different metastatic sites
was achieved using a χ2 test. Curve plotting and
analysis of survival were accomplished by the Kaplan-Meier method
and log-rank tests, respectively. Multivariate analyses and hazard
ratios with corresponding 95% confidence interval (CI) on behalf of
the prognostic factors affecting OS and CSS were carried out using
a Cox proportional hazard model. All tests were performed using
SPSS Statistics v21.0 software (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
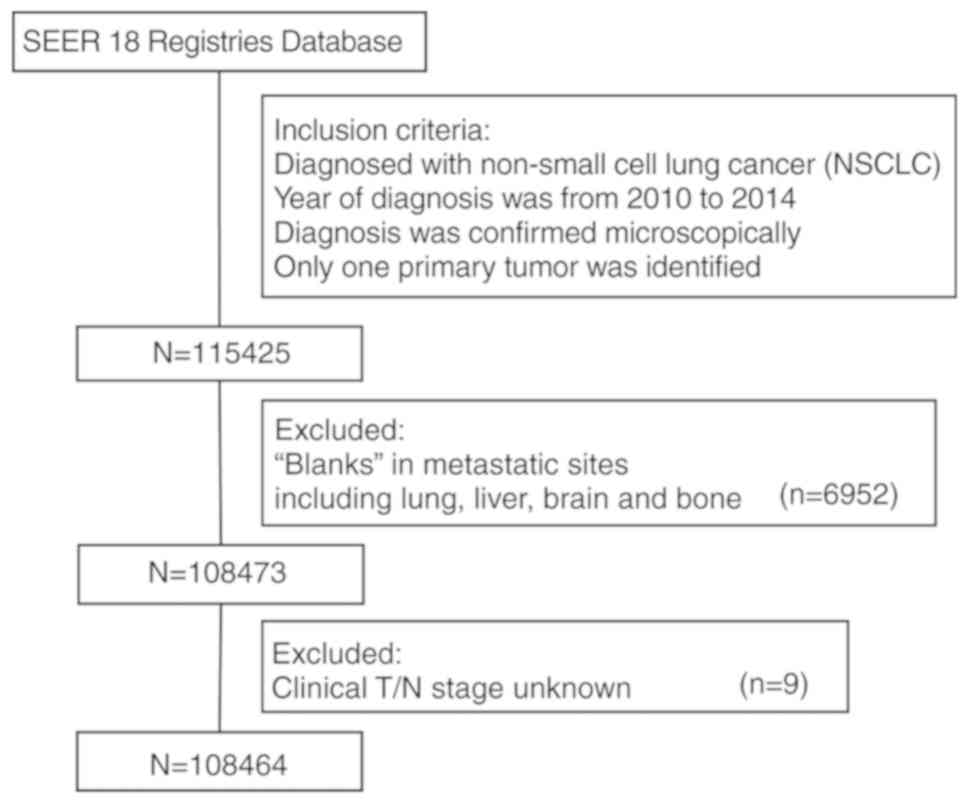
From the SEER database, 108,464 patients diagnosed
with NSCLC between 2010 and 2014 were identified. Details of the
selection procedure are shown in Fig.
1. Within the identified group, 51,788 patients (47.7%)
exhibited DM at diagnosis. Table I
summarizes the clinical characteristics of patients with and
without DM at diagnosis. Patients with the characteristics of male,
African descent, higher clinical T stage, positive nodes or
adenocarcinoma histological type were more likely to exhibit
metastasis at diagnosis (all P<0.001). Patients with metastatic
disease at diagnosis were less likely to undergo surgery and more
likely to undergo chemotherapy and/or radiotherapy (all
P<0.001). For the whole cohort, mean and median follow-up were
17.2 and 12 months, respectively. The mean follow-up for patients
with and without metastatic disease was 8.7 and 21.8 months,
respectively.
 | Table I.Patient characteristics, stratified by
presence of metastases at time of diagnosis. |
Table I.
Patient characteristics, stratified by
presence of metastases at time of diagnosis.
| Characteristic | No metastases at
diagnosis, n=56,676 (%) | Metastases at
diagnosis, n=51,788 (%) | P-value |
|---|
| Age (years) |
|
| <0.001 |
|
<50 | 2,419 (4.27) | 3,020 (5.83) |
|
|
≥50 | 54,257 (95.73) | 48,768 (94.17) |
|
| Sex |
|
| <0.001 |
|
Male | 27,863 (49.16) | 28,364 (54.77) |
|
|
Female | 28,813 (50.84) | 23,424 (45.23) |
|
| Ethnicity |
|
| <0.001 |
|
Caucasian | 46,098 (81.34) | 39,986 (77.21) |
|
|
African
descent | 6,441
(11.36) | 6,964
(13.45) |
|
|
Others | 4,137 (7.30) | 4,838 (9.34) |
|
| T stage |
|
| <0.001 |
|
T0, T1, T2 | 37,435 (66.05) | 17,341 (33.48) |
|
|
T3, T4 | 16,585 (29.27) | 28,142 (54.34) |
|
|
TX | 2,656 (4.69) | 6,305 (12.17) |
|
| N stage |
|
| <0.001 |
|
N0 | 32,690 (57.68) | 12,543 (24.22) |
|
|
N positive | 23,047 (40.67) | 36,311 (70.12) |
|
|
NX |
939 (1.66) | 2,934 (5.67) |
|
| Tumor grade |
|
| <0.001 |
|
I | 6,385 (11.27) | 1,379 (2.66) |
|
|
II | 16,862 (29.75) | 6,561 (12.67) |
|
|
III | 16,913 (29.84) | 13,090 (25.28) |
|
|
IV |
597 (1.05) |
545 (1.05) |
|
|
Unknown | 15,919 (28.09) | 30,213 (58.34) |
|
| Histology |
|
| <0.001 |
|
AD | 30,592 (53.98) | 36,942 (71.33) |
|
|
SQCC | 21,399 (37.76) | 12,150 (23.46) |
|
|
ASC | 1,110 (1.96) |
836 (1.61) |
|
|
LCC | 1,158 (2.04) | 1,390 (2.68) |
|
|
Others | 2,417 (4.26) |
470 (0.91) |
|
| Chemotherapy |
|
| <0.001 |
|
Yes | 21,141 (37.30) | 27,966 (54.00) |
|
|
No/Unknown | 35,535 (62.70) | 23,822 (46.00) |
|
| Radiotherapy |
|
| <0.001 |
|
Yes | 21,167 (37.35) | 22,658 (43.75) |
|
|
No/Unknown | 35,509 (62.65) | 29,130 (56.25) |
|
| Surgery |
|
| <0.001 |
|
Yes | 27,789 (49.03) | 1,923
(3.71) |
|
|
No/Unknown | 28,887 (50.97) | 49,865 (96.28) |
|
Metastatic patterns
At the time of diagnosis, stage IV cases accounted
for 47.75% (51,788/108,464) of all patients with NSCLC. The
database only included information for liver, lung, bone and brain
metastasis. Patients who exhibited metastasis to any of the four
sites accounted for 74.84% (38,756/51,788) of stage IV cases. The
clinical characteristics of all included patients with different
metastatic sites are listed in Table
II. For patients with and without bone metastasis, the
distribution of age (P=0.002) and ethnicity (P<0.001) were
significantly different. The same phenomenon was observed for
patients with brain and lung metastases (all P<0.01) but not for
patients with liver metastasis (P>0.05). In addition, the
distribution of sex, clinical T/N stage, tumor grade and histology
were significantly associated with metastasis at these four
metastatic sites (all P<0.001).
 | Table II.Clinical features and metastatic
sites. |
Table II.
Clinical features and metastatic
sites.
|
| Liver metastasis
(%) |
| Brain metastasis
(%) |
| Bone metastasis
(%) |
| Lung metastasis
(%) |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Characteristic | No | Yes | P-value | No | Yes | P-value | No | Yes | P-value | No | Yes | P-value |
|---|
| Age (years) |
|
| 0.246 |
|
| <0.001 |
|
| 0.002 |
|
| 0.003 |
|
<50 | 1,859 (78.51) | 509 (21.49) |
| 1,331 (56.21) | 1,037 (43.79) |
| 1,107 (46.75) | 1,261 (53.25) |
| 1,451 (61.28) | 917 (38.72) |
|
|
≥50 | 28,193 (77.48) | 8,195 (22.52) |
| 24,341 (66.89) | 12,047 (33.11) |
| 18,180 (49.96) | 18,208 (50.04) |
| 21,161 (58.15) | 15,227 (41.85) |
|
| Sex |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
Male | 16,347 (76.84) | 4,926 (23.16) |
| 14,548 (68.39) | 6,725 (31.61) |
| 10,125 (47.60) | 11,148 (52.40) |
| 12,612 (59.29) | 8,661 (40.71) |
|
|
Female | 13,705 (78.39) | 3,778 (21.61) |
| 11,124 (63.63) | 6,359 (36.37) |
| 9,162 (52.41) | 8,321 (47.59) |
| 10,000 (57.20) | 7,483 (42.80) |
|
| Ethnicity |
|
| 0.321 |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
Caucasian | 23,204 (77.37) | 6,787 (22.63) |
| 19,982 (66.63) | 10,009 (33.37) |
| 14,902 (49.69) | 15,089 (50.31) |
| 17,816 (59.40) | 12,175 (40.60) |
|
|
African | 3,979 (78.07) | 1,118 (21.93) |
| 3,375 (66.22) | 1,722 (33.78) |
| 2,668 (52.34) | 2,429 (47.66) |
| 2,902 (56.94) | 2,195 (43.06) |
|
| descent |
|
|
|
|
|
|
|
|
|
|
|
|
|
Others | 2,869 (78.22) | 799 (21.78) |
| 2,315 (63.11) | 1,353 (36.89) |
| 1,717 (46.81) | 1,951 (53.19) |
| 1,894 (51.64) | 1,774 (48.36) |
|
| T stage |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
T0, T1, T2 | 9,738 (78.12) | 2,727 (21.88) |
| 7,568 (60.71) | 4,897 (39.29) |
| 5,829 (46.76) | 6,636 (53.24) |
| 9,981 (80.07) | 2,484 (19.93) |
|
|
T3, T4 | 17,166 (77.94) | 4,858 (22.06) |
| 15,209 (69.06) | 6,815 (30.94) |
| 11,546 (52.42) | 10,478 (47.58) |
| 9,482 (43.05) | 12,542 (56.95) |
|
|
TX | 3,148 (73.78) | 1,119 (26.22) |
| 2,895 (67.85) | 1,372 (32.15) |
| 1,912 (44.81) | 2,355 (55.19) |
| 3,149 (73.80) | 1,118 (26.20) |
|
| N stage |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
N0 | 7,283 (82.31) | 1,565 (17.69) |
| 5,982 (67.61) | 2,866 (32.39) |
| 4,880 (55.15) | 3,968 (44.85) |
| 5,451 (61.61) | 3,397 (38.39) |
|
|
N positive | 21,210 (76.23) | 6,612 (23.77) |
| 18,197 (65.41) | 9,625 (34.59) |
| 13,402 (48.17) | 14,420 (51.83) |
| 15,881 (57.08) | 11,941 (42.92) |
|
|
NX | 1,559 (74.74) | 527 (25.26) |
| 1,493 (71.57) | 593 (28.43) |
| 1,005 (48.18) | 1,081 (51.82) |
| 1,280 (61.36) | 806 (38.64) |
|
| Tumor grade |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
I | 918 (85.71) | 153 (14.29) |
| 828 (77.31) | 243 (22.69) |
| 678 (63.31) | 393 (36.69) |
| 408 (38.10) | 663 (61.90) |
|
|
II | 4,064 (83.86) | 782 (16.14) |
| 3,294 (67.97) | 1,552 (32.03) |
| 2,573 (53.10) | 2,273 (46.90) |
| 2,631 (54.29) | 2,215 (45.71) |
|
|
III | 7,727 (78.04) | 2,174 (21.96) |
| 6,284 (63.47) | 3,617 (36.53) |
| 5,331 (53.84) | 4,570 (46.16) |
| 5,852 (59.11) | 4,049 (40.89) |
|
|
IV | 312 (79.59) | 80 (20.41) |
| 238 (60.71) | 154 (39.29) |
| 195 (49.74) | 197 (50.26) |
| 252 (64.29) | 140 (35.71) |
|
|
Unknown | 17,031 (75.54) | 5,515 (24.46) |
| 15,028 (66.65) | 7,518 (33.35) |
| 10,510 (46.62) | 12,036 (53.38) |
| 13,469 (59.74) | 9,077 (40.26) |
|
| Histology |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
AD | 22,149 (78.80) | 5,959 (21.20) |
| 17,754 (63.16) | 10,354 (36.84) |
| 13,451 (47.85) | 14,657 (52.15) |
| 16,471 (58.60) | 11,637 (41.40) |
|
|
SQCC | 6,449 (75.04) | 2,145 (24.96) |
| 6,617 (77.00) | 1,977 (23.00) |
| 4,736 (55.11) | 3,858 (44.89) |
| 4,815 (56.03) | 3,779 (43.97) |
|
|
ASC | 494 (77.67) | 142 (22.33) |
| 414 (65.09) | 222 (34.91) |
| 270 (42.45) | 366 (57.55) |
| 400 (62.89) | 236 (37.11) |
|
|
LCC | 709 (66.08) | 364 (33.92) |
| 623 (58.06) | 450 (41.94) |
| 618 (57.60) | 455 (42.40) |
| 749 (69.80) | 324 (30.20) |
|
|
Others | 251 (72.75) | 94 (27.25) |
| 264 (76.52) | 81 (23.48) |
| 212 (61.45) | 133 (38.55) |
| 177 (51.30) | 168 (48.70) |
|
Frequency differences among different
metastatic patterns
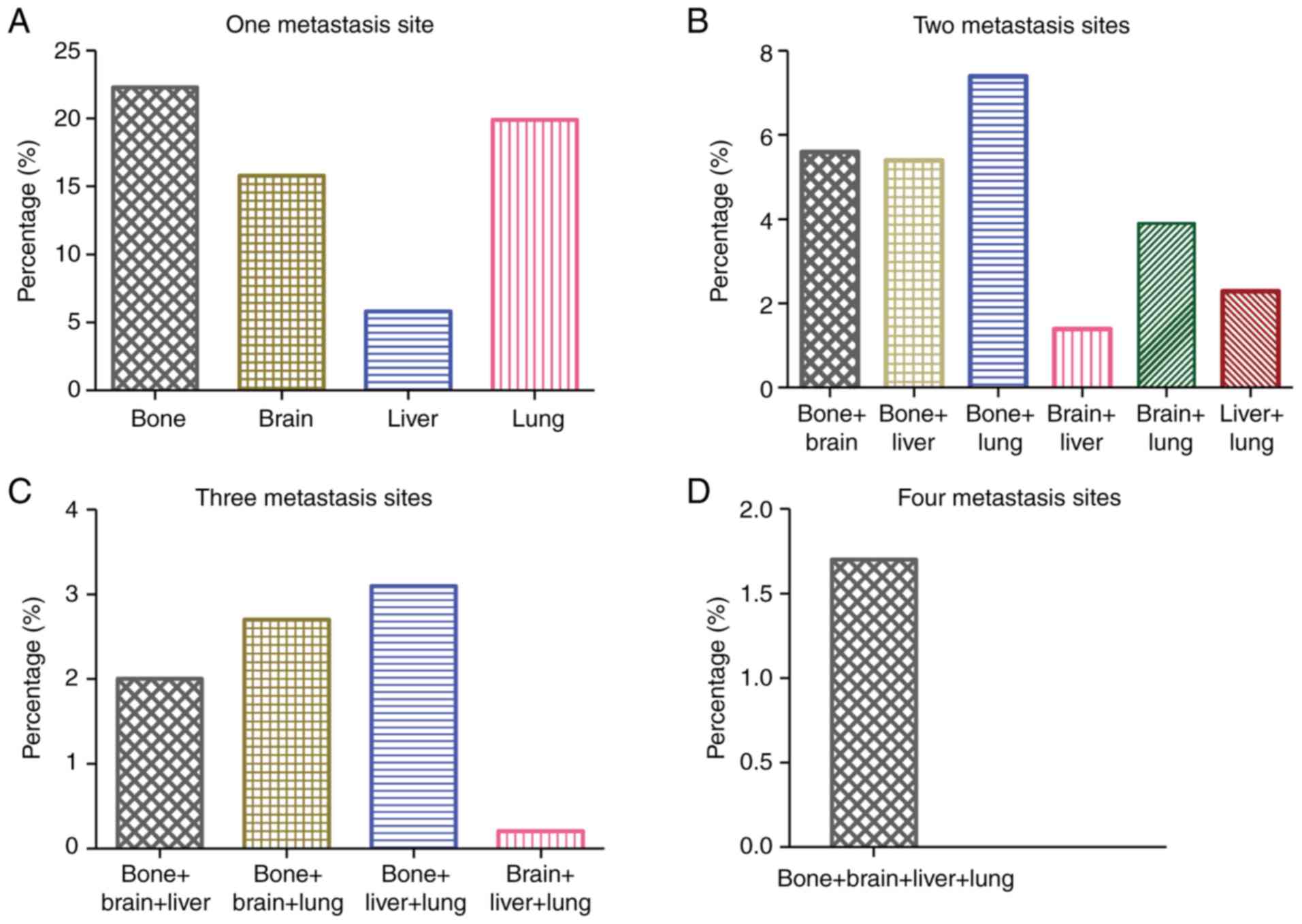
Fig. 2 shows the
proportion of different metastatic combination patterns for the
included patients with site-specific metastasis. A total of 8,654
(22.3%), 7,699 (19.9%), 6,109 (15.8%) and 2,264 (5.8%) patients
presented with isolated bone, lung, brain and liver metastasis at
the time of diagnosis, respectively (Fig. 2A). Among patients with two metastatic
sites, bone and lung metastasis was the most common combination,
and brain and liver metastasis was the least common combination,
accounting for 7.4 and 1.4% of all metastatic cases, respectively
(Fig. 2B). The frequency of
three-site metastasis combination was low. The most common
three-site metastasis combination comprised bone, liver and lung,
accounting for 3.1% of all metastatic cases (Fig. 2C). Four-site metastasis was
relatively rare, and was diagnosed in 652 (1.7%) patients (Fig. 2D). Notably, patients with NSCLC who
presented with bone metastasis were more likely to exhibit
multi-site metastases.
Metastatic features based on different
NSCLC subtypes
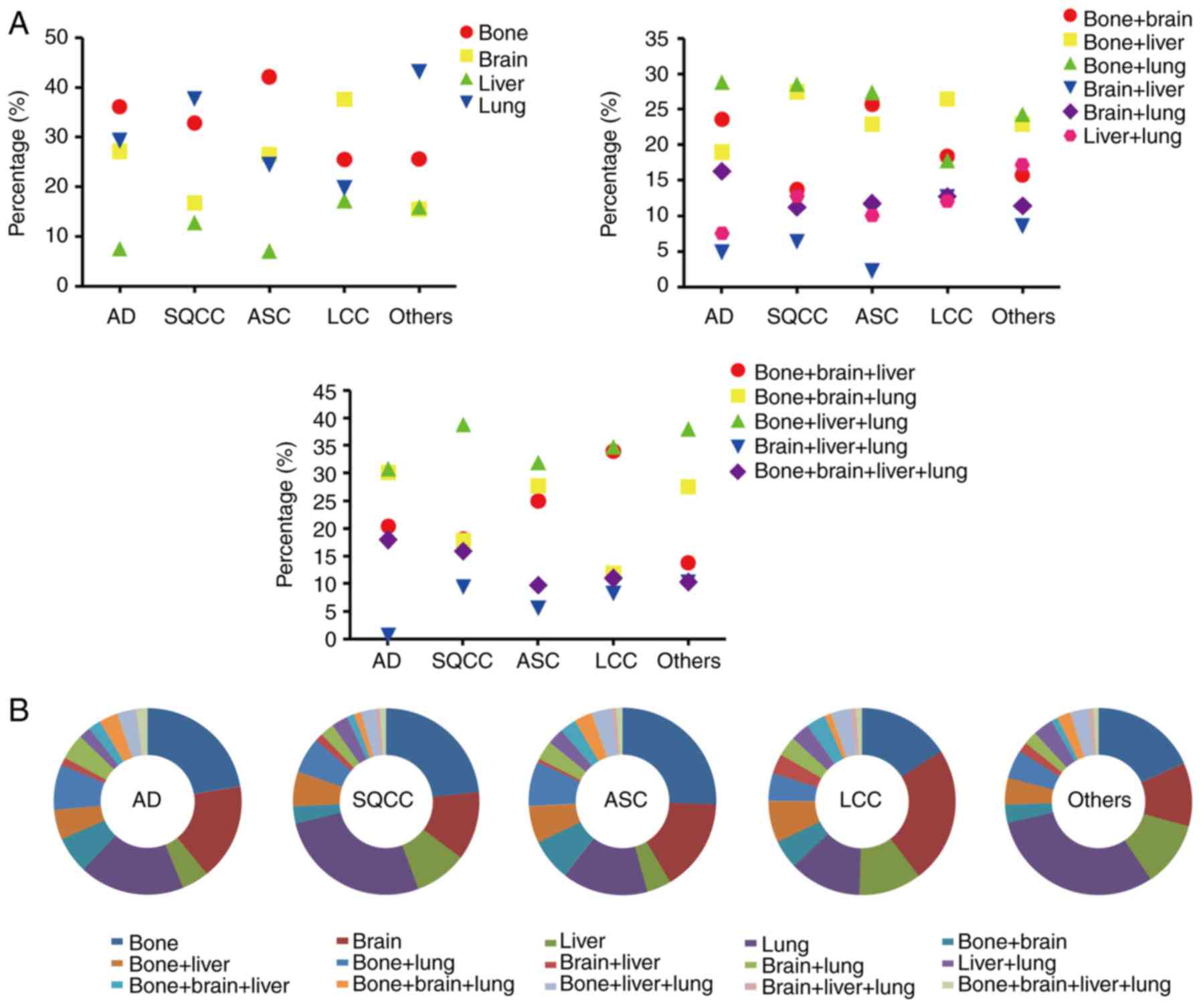
Subsequently, the metastatic characteristics of
patients with different NSCLC subtypes were investigated (Fig. 3). The results revealed that the most
common single metastatic site was bone for patients with AD and
ASC, accounting for 36.13 and 42.08%, respectively. For patients
with SQCC and other subtypes, single lung metastasis was most
common, accounting for 37.68 and 43.09%, respectively. Notably, in
patients with LCC the most common single metastatic site was the
brain, accounting for 37.57%. The most common two-site metastasis
combination was bone and lung, accounting for 28.79% of patients
with AD, 28.51% of patients with SQCC, 27.37% of patients with ASC
and 24.29% of patients with other subtypes. However, the most
common two-site metastasis combination for patients with LCC was
bone and liver, accounting for 26.42%. For three-site metastasis,
bone, liver and lung was the most common combination for all
subtypes, accounting for 30.77 (AD), 38.85 (SQCC), 31.94 (ASC),
34.86 (LCC) and 37.93% (other subtypes).
Survival analysis for different
metastatic patterns of NSCLC
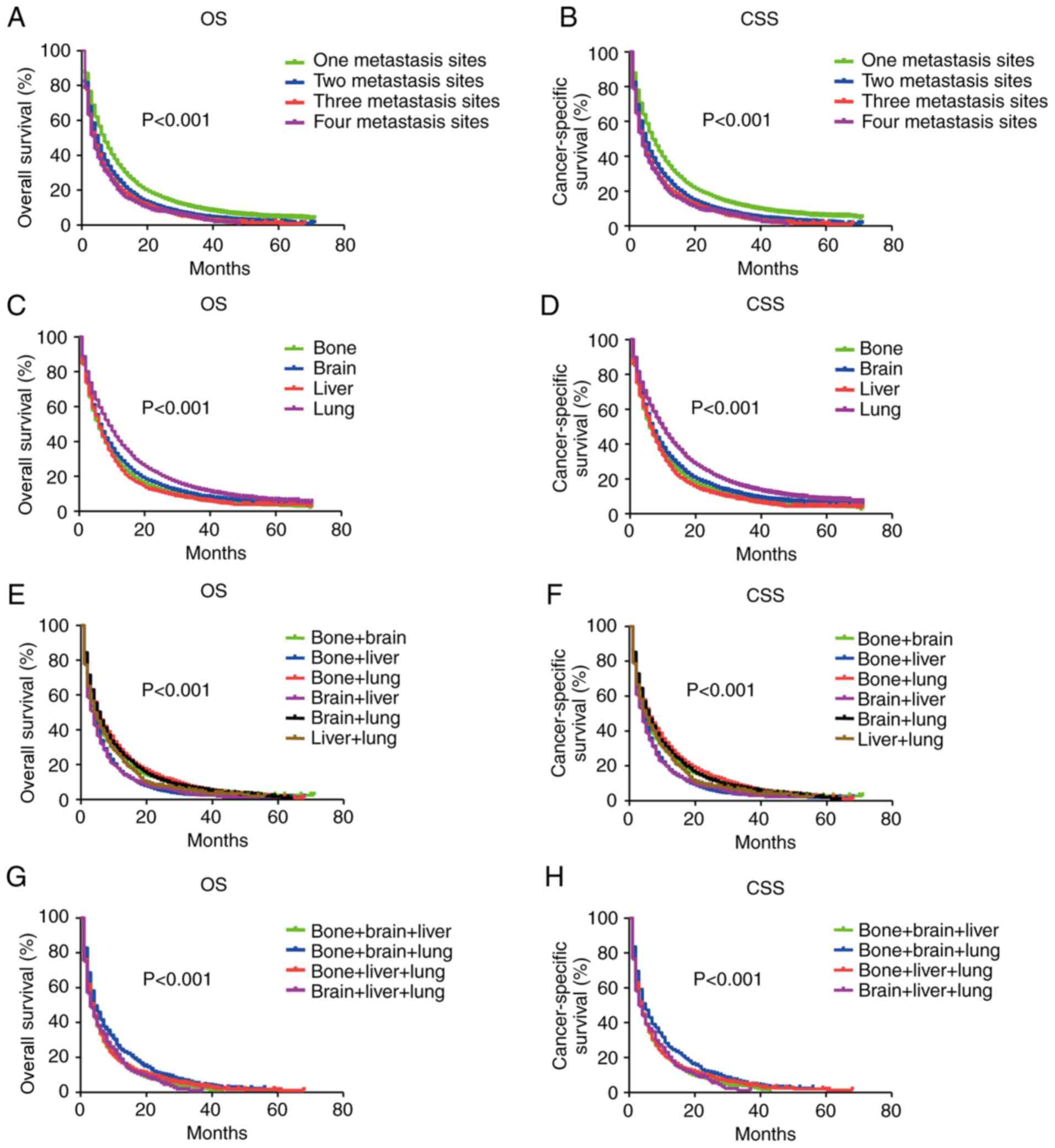
Differences in the prognosis of patients with
different metastatic patterns were evaluated by Kaplan-Meier
analysis. OS and CSS were compared among patients with NSCLC with
different metastatic sites (Fig. 4).
Patients with two-site metastasis exhibited better OS and CSS than
those with three- or four-site metastasis, but exhibited worse OS
and CSS than patients with single metastasis (P<0.001; Fig. 4A and B). There was no significant
difference in survival identified between patients with three- and
four-site metastasis (P=0.380). Among patients with a single
metastatic site, patients with isolated lung metastasis exhibited
the best outcome, followed by single brain and bone metastasis (all
P<0.001). Isolated liver metastasis was associated with the
worst outcome (P<0.001; Fig. 4C and
D). For two-site metastasis, patients with liver-combined
metastasis exhibited worse OS and CSS compared with patients with
other metastatic patterns (all P<0.001). By contrast, no
significant differences in survival among patients with bone and
lung, bone and brain, and brain and lung metastases were identified
(all P>0.05; Fig. 4E and F).
Additionally, no significant differences among patients with bone
and liver, brain and liver, and liver and lung metastases were
identified (all P>0.05; Fig. 4E and
F). Similar findings were observed in patients with three-site
metastasis. Patients with bone, brain and lung metastasis exhibited
improved OS and CSS compared with those with liver-combined
three-site metastatic patterns (all P<0.001; Fig. 4G and H). However, log-rank tests
identified no significantly different effect on prognosis among
patients with liver-combined three-site metastatic patterns.
Therefore, patients with isolated liver or liver-combined
metastasis exhibited a poorer prognosis than those with other
metastatic patterns.
Cox regression analysis based on OS
and CSS
In multivariate analysis, increased age, being male,
positive nodes, higher tumor grade and more metastatic sites were
associated with worse outcomes. Additionally, patients receiving
appropriate treatments, including chemotherapy, radiotherapy or
surgery, had a significantly lower risk of mortality than those
without treatment (Table III).
 | Table III.Multivariate analysis of OS and CSS
in patients with metastatic non-small cell lung cancer. |
Table III.
Multivariate analysis of OS and CSS
in patients with metastatic non-small cell lung cancer.
|
| OS | CSS |
|---|
|
|
|
|
|---|
| Feature | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age (years) |
|
|
|
|
|
<50 | 1.000
(reference) |
| 1.000
(reference) |
|
|
≥50 | 1.236
(1.183–1.292) | <0.001 | 1.282
(1.223–1.344) | <0.001 |
| Sex |
|
|
|
|
|
Female | 1.000
(reference) |
| 1.000
(reference) |
|
|
Male | 1.166
(1.141–1.191) | <0.001 | 1.183
(1.157–1.209) | <0.001 |
| Ethnicity |
|
|
|
|
|
Caucasian | 1.000
(reference) |
| 1.000
(reference) |
|
| African
descent | 0.984
(0.954–1.015) | 0.306 | 0.970
(0.939–1.002) | 0.063 |
|
Others | 0.770
(0.743–0.799) | <0.001 | 0.690
(0.663–0.718) | <0.001 |
| T stage |
|
|
|
|
| T0, T1,
T2 | 1.000
(reference) |
| 1.000
(reference) |
|
| T3,
T4 | 1.055
(1.031–1.081) | <0.001 | 1.061
(1.035–1.087) | <0.001 |
| TX | 1.149
(1.106–1.193) | <0.001 | 1.149
(1.105–1.195) | <0.001 |
| N stage |
|
|
|
|
| N0 | 1.000
(reference) |
| 1.000
(reference) |
|
| N
positive | 1.252
(1.220–1.285) | <0.001 | 1.281
(1.247–1.316) | <0.001 |
| NX | 1.225
(1.163–1.298) | <0.001 | 1.243
(1.178–1.311) | <0.001 |
| Tumor grade |
|
|
|
|
| I | 1.000
(reference) |
| 1.000
(reference) |
|
| II | 1.176
(1.097–1.260) | <0.001 | 1.233
(1.142–1.331) | <0.001 |
|
III | 1.405
(1.315–1.502) | <0.001 | 1.514
(1.407–1.629) | <0.001 |
| IV | 1.537
(1.356–1.743) | <0.001 | 1.655
(1.455–1.883) | <0.001 |
|
Unknown | 1.356
(1.271–1.447) | <0.001 | 1.440
(1.341–1.547) | <0.001 |
| Histology |
|
|
|
|
| AD | 1.000
(reference) |
| 1.000
(reference) |
|
|
SQCC | 1.187
(1.156–1.218) | <0.001 | 1.200
(1.168–1.232) | <0.001 |
|
ASC | 1.123
(1.033–1.221) | 0.006 | 1.135
(1.043–1.235) | 0.003 |
|
LCC | 1.227
(1.149–1.310) | <0.001 | 1.287
(1.206–1.374) | <0.001 |
|
Other | 0.605
(0.541–0.677) | <0.001 | 0.477
(0.415–0.548) | <0.001 |
| Chemotherapy |
|
|
|
|
|
No/Unknown | 1.000
(reference) |
| 1.000
(reference) |
|
|
Yes | 0.405
(0.396–0.414) | <0.001 | 0.387
(0.378–0.396) | <0.001 |
| Radiotherapy |
|
|
|
|
|
No/Unknown | 1.000
(reference) |
| 1.000
(reference) |
|
|
Yes | 0.923
(0.904–0.943) | <0.001 | 0.937
(0.917–0.958) | <0.001 |
| Surgery |
|
|
|
|
|
No/Unknown | 1.000
(reference) |
| 1.000
(reference) |
|
|
Yes | 0.597
(0.561–0.635) | <0.001 | 0.523
(0.487–0.562) | <0.001 |
| Number of distant
metastases |
|
|
|
|
| One
metastatic site | 1.000
(reference) |
| 1.000
(reference) |
|
| Two
metastatic sites | 1.299
(1.267–1.332) | <0.001 | 1.336
(1.302–1.371) | <0.001 |
| Three
metastatic sites | 1.572
(1.511–1.635) | <0.001 | 1.631
(1.567–1.696) | <0.001 |
| Four
metastatic sites | 1.674
(1.541–1.818) | <0.001 | 1.745
(1.607–1.896) | <0.001 |
Additionally, multivariate analyses indicated that
SQCC, ASC and LCC were significantly associated with decreased OS
and CSS compared with AD. Among them, LCC had the highest risk of
mortality referring to AD [OS: Hazard ratio (HR), 1.227; 95% CI,
1.149–1.310; CSS: HR, 1.287; 95% CI, 1.206–1.374; P<0.001 for
the two endpoints; Table III].
Notably, patients with isolated liver metastasis
exhibited the worst outcomes (OS: HR, 1.385; 95% CI, 1.318–1.455;
CSS: HR, 1.507; 95% CI, 1.432–1.585; P<0.001 for the two
endpoints). As for multi-site metastasis, patients with
liver-combined metastases exhibited a significantly higher risk of
mortality than those without liver metastasis (Table IV).
 | Table IV.Multivariate analysis of the
association among different metastatic patterns and OS and CSS in
patients with metastatic non-small cell lung cancer. |
Table IV.
Multivariate analysis of the
association among different metastatic patterns and OS and CSS in
patients with metastatic non-small cell lung cancer.
|
| OS | CSS |
|---|
|
|
|
|---|
| Metastatic
pattern | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| One metastatic
site |
|
|
|
|
|
Lung | 1.000
(reference) |
| 1.000
(reference) |
|
|
Brain | 1.111
(1.072–1.151) | <0.001 | 1.162
(1.119–1.206) | <0.001 |
|
Bone | 1.218
(1.179–1.258) | <0.001 | 1.295
(1.252–1.340) | <0.001 |
|
Liver | 1.385
(1.318–1.455) | <0.001 | 1.507
(1.432–1.585) | <0.001 |
| Two metastatic
sites |
|
|
|
|
| Bone +
lung | 1.000
(reference) |
| 1.000
(reference) |
|
| Brain +
lung | 1.001
(0.973–1.068) |
0.981 | 1.018
(0.953–1.088) | 0.595 |
| Bone +
brain | 1.050
(0.990–1.114) |
0.102 | 1.059
(0.997–1.125) | 0.061 |
| Liver +
lung | 1.277
(1.179–1.382) | <0.001 | 1.279
(1.180–1.387) | <0.001 |
| Brain +
liver | 1.357
(1.233–1.493) | <0.001 | 1.358
(1.232–1.496) | <0.001 |
| Bone +
liver | 1.328
(1.251–1.409) | <0.001 | 1.379
(1.299–1.464) | <0.001 |
| Three/four
metastatic sites |
|
|
|
|
| Bone +
brain + lung | 1.000
(reference) |
| 1.000
(reference) |
|
| Bone +
liver + lung | 1.228
(1.127–1.339) | <0.001 | 1.282
(1.175–1.399) | <0.001 |
| Bone +
brain + liver | 1.189
(1.078–1.311) |
0.001 | 1.249
(1.132–1.378) | <0.001 |
| Brain +
liver + lung | 1.214
(1.055–1.397) |
0.007 | 1.273
(1.108–1.464) |
0.001 |
| Bone +
brain + liver + lung | 1.176
(1.061–1.303) |
0.002 | 1.215
(1.096–1.347) | <0.001 |
Discussion
Postmus et al (14) reported that the median OS for
patients with NSCLC with DM is only 6 months. The incidence of DM
of NSCLC at diagnosis is ~40%, and the most common metastatic site
is the bone, followed by the lung, brain, liver and adrenal glands
(5,8). The present retrospective study,
investigating patients with NSCLC, demonstrated major differences
in the frequency of metastases to one, two, three or four organs,
and further identified the prognostic influence of different
site-specific metastatic combinations.
Only a small number of studies have investigated the
incidence of different metastatic patterns in patients with
metastatic NSCLC (6,15). A retrospective study reported that
among the 729 patients with metastatic NSCLC, 250 (34.3), 234
(32.1), 207 (28.4), 122 (16.7), 98 (13.4) and 69 (9.5%) exhibited
bone, lung, brain, adrenal gland, liver and distant lymph node
metastasis, respectively (5).
Another study based on the Swedish Family Cancer Database
demonstrated that ~38% of all deceased patients with lung cancer
had one metastatic site, and 19% had two or more reported
metastases (6). However, in the
present study, ~63.8% of all metastatic cohorts exhibited
metastasis to one site. Ren et al (11) reported that the most common
combination for two-site metastasis for AD was bone and brain
(11.4%), and that for SQCC was bone and liver (11.8%). However, in
the present study, the most common two-site metastatic combination
was bone and lung for AD (28.79), SQCC (28.51), ASC (27.37), and
other subtypes (24.29%). Bone and liver was the most common
two-site metastatic combination for patients with LCC, and
accounted for 26.42%. A previous study identified that the
incidence of bone metastasis is as high as 34.3% in patients with
metastatic NSCLC (5). Notably, the
present study revealed that patients with bone metastasis accounted
for more than a half of all patients with metastatic NSCLC.
However, the patients with isolated liver metastasis at the initial
diagnosis accounted for 5.8%, which was lower than the 13–24%
reported previously (5,16). In addition, the present study
revealed that bone in combination with other sites accounted for
most of the multi-site metastases.
In the present study, LCC exhibited specific
metastatic characteristics, which were entirely different from
those of other histological subtypes. The most common single
metastatic site was the brain for LCC. Notably, the most common
two-site metastatic combination was bone and liver, which did not
involve the most common single metastatic site. This result may be
associated with the averaged incidence of metastasis to each site
in LCC. Analysis of single metastatic sites revealed that LCC was
more frequently associated with brain and liver metastases than
other histological subtypes, whereas bone metastasis was less
common in LCC. Similar findings have been confirmed in another
study (17). Additionally, a
previous study reported an association between liver and bone
metastasis in major histological types of NSCLC, which was
particularly evident in LCC (18).
This may explain why the most common two-site metastasis
combination in LCC identified in the present study was bone and
liver.
Only a small number of studies have reported that
the differences in survival among patients with metastatic NSCLC
may be associated with different metastatic patterns (5,19). A
previous study reported that patients with NSCLC with liver
metastasis exhibit worse prognosis (5). Another study demonstrated that AD
patients with liver metastasis at diagnosis have a shorter
progression-free survival (PFS) and OS than those without liver
metastasis (2.5 and 6.3 months, respectively) (20). As expected, the results of the
present study also confirmed that liver metastasis was associated
with the worst outcomes in patients with metastatic NSCLC, which
was consistent with the findings from other types of cancer
(21,22). Notably, in the present study, the
hazard ratio for isolated liver metastasis was 1.385-fold and
1.507-fold higher than that for isolated lung metastasis in terms
of OS and CSS, respectively. Additionally, patients with
liver-combined metastases exhibited worse survival rates than those
with other metastatic patterns. Although liver metastasis accounted
for the smallest number of patients with metastatic NSCLC, it is a
metastatic type that is of great concern due to its poor prognosis.
Previous studies have demonstrated that patients with liver
metastasis gain limited therapeutic benefit with
checkpoint-inhibitor monotherapy (23–25).
However, a recent phase 3 randomized trial revealed that a benefit
in regard to PFS was observed with atezolizumab plus bevacizumab
plus carboplatin plus paclitaxel (ABCP) in patients with NSCLC with
liver metastasis subgroups (median, 7.4 months with ABCP vs. 4.9
months with bevacizumab plus carboplatin plus paclitaxel;
unstratified HR, 0.42; 95% CI, 0.26–0.66) (26). Notably, liver metastasis became a
stratified factor for improved PFS with ABCP, suggesting that
patients with NSCLC with liver metastasis may be a more special
subgroup and may require a specific treatment strategy. In
addition, patients with isolated lung metastasis possessed the best
prognosis, which was consistent with the findings of a previous
study (27). Therefore, knowledge of
the prognostic effects of different metastatic sites may be
valuable for classifying patients with advanced NSCLC and may serve
as a reference for individualized precise treatment.
Additionally, the present study investigated the
prognosis of patients with metastatic NSCLC with single or multiple
metastatic sites. A recent study demonstrated that metastasis to a
single site is associated with significantly improved OS compared
with multiple sites in patients with NSCLC (28). It has been previously reported that
the most common two-site metastatic combinations were nervous
system and bone, bone and liver, and nervous system and liver, but
differences in survival rates among them are unknown (6). However, in the present study, the most
common two-site metastatic combination involved bone and lung.
Notably, among patients with two-site metastasis, those with
liver-combined two-site metastasis exhibited worse OS and CSS than
those with other metastatic patterns. Similar findings were
observed in three-site metastasis. Combined two- or three-site
metastasis involving liver was associated with worse OS and CSS in
patients with metastatic NSCLC. Therefore, identifying patients
with multi-site DM involving the liver is crucial to improve
outcomes or treatment value in these specific cohorts. In addition,
men and elderly patients had a shorter survival, which is
consistent with previously published retrospective studies
(22,29).
There were several limitations in the present study.
First, there was lack of information regarding details of systemic
treatment administered; in particular, no information regarding
targeted therapy and immunotherapy was available. During the past
decade, through improved understanding of the molecular and
immunological features of cancer, novel targeted therapies and
immunotherapies have been available to treat patients with
metastatic NSCLC and have brought unprecedented survival benefits
in selected cohorts (30,31). Second, there was a lack of
information regarding co-morbidities, performance status and gene
mutations. This information will be discussed in future studies.
Finally, the present study only included metastatic sites
associated with the bone, brain, liver and lung. Metastasis to
other sites, including the adrenal glands, may also influence the
outcomes of patients with NSCLC.
In conclusion, the present study demonstrated that
in patients with NSCLC, bone was the most commonly targeted site
for single- or multi-organ metastases. As for NSCLC subtypes, the
most common single metastatic site was bone for AD and ASC, and
lung for SQCC and other subtypes. Additionally, bone and lung was
the most common combination for two-site metastasis for AD, SQCC,
ASC and other subtypes. Notably, for LCC, the brain was the most
common single metastatic site, and bone and liver were most
commonly involved in two-site metastasis. The present study
demonstrated that metastasis to the liver alone or in combination
with other organs was a factor for poor prognosis of patients with
metastatic NSCLC, while isolated lung metastasis was associated
with the best outcomes. Knowledge regarding the prognostic value of
different sites of DM may be valuable for classifying patients with
advanced NSCLC, laying a foundation for individualized precise
treatment.
Acknowledgements
The authors would like to thank Professor Rongxia
Liao (Medical English Department, Army Medical University) for
language editing and critically reading the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81773245 and
8160111873) and Chongqing Natural Science Foundation (grant no.
cstc2016jcyjA2013).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZX, JS, XC and QYa designed the study. ZX, LuZ and
MC participated in data selection and assembly. LiZ, YY and QYo
performed the data analysis. ZX, QYa and XC were involved in
drafting the manuscript and revising it critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crinò L, Weder W, van Meerbeeck J and
Felip E; ESMO Guidelines Working Group, : Early stage and locally
advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21 (Suppl 5):v103–v115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zago G, Muller M, van den Heuvel M and
Baas P: New targeted treatments for non-small-cell lung cancer-role
of nivolumab. Biologics. 10:103–117. 2016.PubMed/NCBI
|
|
4
|
Lo RG, Imbimbo M and Garassino MC: Is the
chemotherapy era in advanced non-small cell lung cancer really
over? Maybe not yet. Tumori. 2016:223–225. 2016.PubMed/NCBI
|
|
5
|
Tamura T, Kurishima K, Nakazawa K,
Kagohashi K, Ishikawa H, Satoh H and Hizawa N: Specific organ
metastases and survival in metastatic non-small-cell lung cancer.
Mol Clin Oncol. 3:217–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riihimäki M, Hemminki A, Fallah M, Thomsen
H, Sundquist K, Sundquist J and Hemminki K: Metastatic sites and
survival in lung cancer. Lung Cancer. 86:78–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morgensztern D, Ng SH, Gao F and Govindan
R: Trends in stage distribution for patients with non-small cell
lung cancer: A national cancer database survey. J Thorac Oncol.
5:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eberhardt WE, Mitchell A, Crowley J, Kondo
H, Kim YT, Turrisi AR III, Goldstraw P and Rami-Porta R;
International Association for Study of Lung Cancer Staging and
Prognostic Factors Committee, Advisory Board Members, Participating
Institutions, : The IASLC lung cancer staging project: Proposals
for the revision of the M descriptors in the forthcoming eighth
edition of the TNM classification of lung cancer. J Thorac Oncol.
10:1515–1522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang YP, Chen YM, Lai CH, Lin CY, Fang
WF, Huang CH, Li SH, Chen HC, Wang CC and Lin MC: The impact of de
novo liver metastasis on clinical outcome in patients with advanced
non-small-cell lung cancer. PLoS One. 12:e1786762017.
|
|
11
|
Ren Y, Dai C, Zheng H, Zhou F, She Y,
Jiang G, Fei K, Yang P, Xie D and Chen C: Prognostic effect of
liver metastasis in lung cancer patients with distant metastasis.
Oncotarget. 7:53245–53253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Surveillance, Epidemiology, and End
Results (SEER) Program, . About the SEER program. http://www.seer.cancer.govJanuary
20–2017
|
|
13
|
Abdel-Rahman O: Validation of the
prognostic value of new sub-stages within the AJCC 8th edition of
non-small cell lung cancer. Clin Transl Oncol. 19:1414–1420. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Postmus PE, Brambilla E, Chansky K,
Crowley J, Goldstraw P, Patz EJ Jr and Yokomise H; International
Association for the Study of Lung Cancer International Staging
Committee; Cancer Research and Biostatistics; Observers to the
Committee, Participating Institutions, : The IASLC lung cancer
staging project: Proposals for revision of the M descriptors in the
forthcoming (seventh) edition of the TNM classification of lung
cancer. J Thorac Oncol. 2:686–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Griffioen GH, Toguri D, Dahele M, Warner
A, de Haan PF, Rodrigues GB, Slotman BJ, Yaremko BP, Senan S and
Palma DA: Radical treatment of synchronous oligometastatic
non-small cell lung carcinoma (NSCLC): Patient outcomes and
prognostic factors. Lung Cancer. 82:95–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuchuk M, Kuchuk I, Sabri E, Hutton B,
Clemons M and Wheatley-Price P: The incidence and clinical impact
of bone metastases in non-small cell lung cancer. Lung Cancer.
89:197–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Derks JL, Hendriks LE, Buikhuisen WA,
Groen HJ, Thunnissen E, van Suylen RJ, Houben R, Damhuis RA, Speel
EJ and Dingemans AM: Clinical features of large cell neuroendocrine
carcinoma: A population-based overview. Eur Respir J. 47:615–624.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Milovanovic IS, Stjepanovic M and Mitrovic
D: Distribution patterns of the metastases of the lung carcinoma in
relation to histological type of the primary tumor: An autopsy
study. Ann Thorac Med. 12:191–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gibson A, Li H, D'Silva A, Tudor RA,
Elegbede AA, Otsuka SM, Bebb DG and Cheung WY: Impact of number
versus location of metastases on survival in stage IV M1b non-small
cell lung cancer. Med Oncol. 35:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu KL, Tsai MJ, Yang CJ, Chang WA, Hung
JY, Yen CJ, Shen CH, Kuo TY, Lee JY, Chou SH, et al: Liver
metastasis predicts poorer prognosis in stage IV lung
adenocarcinoma patients receiving first-line gefitinib. Lung
Cancer. 88:187–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong F, Shen Y, Gao F, Xu T, Wang X, Zhang
X, Zhong S, Zhang M, Chen S and Shen Z: Prognostic value of
site-specific metastases and therapeutic roles of surgery for
patients with metastatic bladder cancer: A population-based study.
Cancer Manag Res. 9:611–626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai H, Wang H, Li Z, Lin J and Yu J: The
prognostic analysis of different metastatic patterns in
extensive-stage small-cell lung cancer patients: A large
population-based study. Future Oncol. 14:1397–1407. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paz-Ares LG, Shen K, Higgs BW, Morehouse
C, Rizvi NA, Segal NH, Jin X, Zheng Y, Narwal R, Gupta AK, et al:
Association of liver metastases (LM) with survival in NSCLC
patients treated with durvalumab (D) in two independent clinical
trials. J Clin Oncol. 15:30382017. View Article : Google Scholar
|
|
24
|
Pillai RN, Kamphorst AO, Owonikoko TK,
Behera M, Behera S, Khuri FR, Ahmed R and Ramalingam SS: Liver
metastases and sensitivity to checkpoint inhibition in patients
with non-small cell lung cancer (NSCLC). J Clin Oncol.
15:e206652016. View Article : Google Scholar
|
|
25
|
Tumeh PC, Hellmann MD, Hamid O, Tsai KK,
Loo KL, Gubens MA, Rosenblum M, Harview CL, Taube JM, Handley N, et
al: Liver metastasis and treatment outcome with anti-PD-1
monoclonal antibody in patients with melanoma and NSCLC. Cancer
Immunol Res. 5:417–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu B, Wei S, Tian J, Song X, Hu P and Cui
Y: Comparison of the survival time in the non-small cell lung
cancer patients with different organ metastasis. Zhongguo Fei Ai Za
Zhi. 22:105–110. 2019.(In Chinese). PubMed/NCBI
|
|
28
|
Yang J, Zhang Y, Sun X, Gusdon AM, Song N,
Chen L, Jiang G and Huang Y: The prognostic value of multiorgan
metastases in patients with non-small cell lung cancer and its
variants: A SEER-based study. J Cancer Res Clin Oncol.
144:1835–1842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He YY, Zhang XC, Yang JJ, Niu FY, Zeng Z,
Yan HH, Xu CR, Guan JL, Zhong WZ, Yang LL, et al: Prognostic
significance of genotype and number of metastatic sites in advanced
non-small-cell lung cancer. Clin Lung Cancer. 15:441–447. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blumenthal GM, Zhang L, Zhang H,
Kazandjian D, Khozin S, Tang S, Goldberg K, Sridhara R, Keegan P
and Pazdur R: Milestone analyses of immune checkpoint inhibitors,
targeted therapy, and conventional therapy in metastatic non-small
cell lung cancer trials: A meta-analysis. JAMA Oncol.
3:e1710292017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|