Introduction
Nasopharyngeal carcinoma (NPC) is a squamous cell
malignant tumor arising from the nasopharynx. The incidence of NPC
in Southeast Asia reached 20 cases per 100,000 people in 2003
(1). Currently, the standard
treatment strategy for patients with NPC is chemotherapy and
radiotherapy (2). Despite advances
in the treatment of NPC, the rate of disease recurrence remains
high, with a 5-year recurrence rate of 10.8%. Disease recurrence
typically occurs within a few years of treatment completion and is
associated with poor prognosis (3).
The molecular mechanisms underlying the development
of NPC are complex have not yet been fully elucidated. Previous
studies have revealed that a number of genes and signaling pathways
may be involved in the genesis and progression of NPC. For example,
high expression levels of Janus kinase 2, signal transducer and
activator of transcription 3 and vascular endothelial growth factor
may be associated with the clinicopathological characteristics and
prognosis of patients with NPC (4).
Overexpression of the downstream mediator of protein kinase B
(AKT), NUAK family kinase 1, may also be a prognostic factor for
NPC (5). Furthermore, increased
expression of the transcription factor, SRY-box 2 (SOX2) has been
observed in NPC, and may be associated with the prognosis of
patients with NPC; in vitro assays have revealed that SOX2
recruits the nuclear transcription factor, Kruppel like factor 4,
to bind to the phosphatidylinositol-4,5-bisphosphate 3-kinase
catalytic subunit α promoter and upregulate its expression, thus
enhancing phosphoinositide 3-kinase (PI3K)/AKT signaling and NPC
tumorigenesis (6).
microRNAs (miRNAs/miRs) are a class of short
non-coding RNA sequences that bind to the 3′-untranslated region of
mRNAs and inhibit their translation (7). As such, miRNAs may modulate various
biological processes by regulating gene expression. miRNAs have
been implicated in the genesis and/or development of a variety of
disorders, particularly in cancer (7). Previous studies have revealed that
miRNAs may serve roles in the development of NPC, and may provide
novel therapeutic targets. miR-185 negatively targets homeobox C6
and promotes cell apoptosis by inhibiting the transforming growth
factor β1/mechanistic target of rapamycin axis in NPC (8). Similarly, miR-379 negatively regulates
the growth and migration of NPC cells by suppressing tumor protein
D52 expression (9). Modulating the
expression levels of the aforementioned miRNAs may be a potential
therapeutic strategy for NPC. However, studies investigating the
roles of miRNAs in NPC remains limited. Therefore, the
identification of miRNAs that serve important roles in the genesis
and development of NPC is required.
The present study aimed to screen miRNAs which may
serve important roles in the development of NPC, and to further
assess their diagnostic and prognostic values. Differentially
expressed miRNAs (DEMs) between NPC tissues and benign
nasopharyngeal tissues were identified by analyzing public
microarray-based data. Their target genes were subsequently
predicted and their potential functions were annotated. The
diagnostic and prognostic values of the miRNAs in NPC were then
further assessed.
Materials and methods
Data source
To obtain DEMs between NPC tissues and benign
controls, microarray-based datasets were retrieved from the Gene
Expression Omnibus database (ncbi.nlm.nih.gov/geo). Datasets that met the following
criteria were selected for inclusion in the study: i) Datasets
obtained from Homo sapiens; ii) datasets that contained
normalized data regarding healthy adjacent tissues from the
patients with NPC or from healthy controls; iii) datasets amenable
to analysis by online tools or/and other software including dCHIP
(version no. 2011.01; www.hsph.harvard.edu) and R.
Screening of DEMs
The selected miRNA expression profiles were analyzed
using the GEO2R web tool (ncbi.nlm.nih.gov/geo/geo2r/), based on the limma R
package from the Bioconductor project. The results were downloaded
in text format, in which the miRNAs that met the cut-off criteria
of P<0.05 and a |log fold-change|>1.0 were considered as
DEMs. dCHIP was used for datasets that could not be analyzed by
GEO2R as the Matrix data required manual filtering. A paired
Student's t-test was used to compare the two groups (NPC vs.
controls).
Determining the intersection of
DEGs
If there were ≥2 datasets that met the
aforementioned cut-off criteria, the overall range of the DEMs may
be large as different datasets may generate different ranges of
DEMs. To narrow the scope of the DEMs, the intersecting DEMs from
each dataset were identified using Venn diagram analysis (10).
miRNA target gene prediction and
functional annotation
Several computational miRNA-target prediction tools
have been developed to predict target mRNAs of DEMs. The mirDIP
database integrates a number of tools that can reduce the
weaknesses of individual tools (11). Therefore, target genes of the DEMs in
the current study were predicted using the mirDIP database. An
integrative score, which was statistically inferred from the
obtained predictions and assigned to each unique miRNA-target
interaction to provide a unified measure of confidence, was
introduced. Candidate mRNAs with integrated scores of >0.8 were
selected as target mRNAs in this database. To annotate the
functions of the predicted target genes, Gene Ontology (GO)
(12) and Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway enrichment analysis (13) was performed using the Kobas tool
(version 3.0) (14). P<0.05 was
considered to indicate a statistically significant difference.
Association between DEMs and clinical
features, diagnosis and prognosis of NPC
To evaluate the association between the expression
levels of DEMs and the clinical features of patients, and to
further determine their diagnostic and prognostic values, datasets
containing a cohort of patients with NPC with sufficient
information were retrieved and selected for analysis. The
expression levels of the screened DEMs and the associated clinical
data were extracted from the datasets. The diagnostic and
prognostic values of the DEMs were subsequently assessed as
mentioned below.
Statistical analysis
Chi-squared values were calculated to evaluate the
association between the expression of the DEMs and
clinicopathological features. The diagnostic accuracy of miRNAs was
measured using receiver operating characteristic (ROC) curves and
the area under the ROC curve (AUC). The optimal diagnostic point of
the signature was assessed at cut-off values with the largest
Youden's index (sensitivity + specificity −1). The probability of
survival and its significance were evaluated by using the
Kaplan-Meier method and Cox proportional hazard models,
respectively. Log-rank test was used to compare the survival rates
between low and high expression groups. Two-tailed P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using MedCalc software (version
11.1.1.0; MedCalc Software, Mariakerke, Belgium).
Results
Identification of DEMs from the miRNA
expression profiles
Two miRNA expression profiles, GSE22587 (no
available reference) and GSE46172 (15), met the aforementioned inclusion
criteria and were selected for the purposes of the current study.
These datasets comprised non-coding RNA profiling data generated by
array analysis. GSE22587 was deposited by Li et al. The
dataset was generated using the Illumina Human Beta-version
microRNA expression BeadChip (Illumina Inc., San Diego, CA, USA)
and contained 8 NPC tissue specimens and 4 benign nasopharyngeal
tissue specimens. GSE46172 was based on the Agilent-031181
Unrestricted_Human_miRNA_V16.0_Microarray (miRBase release 16.0
miRNA ID version; Agilent Technologies, Inc., Santa Clara, CA, USA)
and contained 4 NPC specimens and 4 controls.
GEO2R analysis identified 18 upregulated and 20
downregulated miRNAs from the GSE22587 dataset, and 30 upregulated
and 36 downregulated miRNAs from the GSE46172 dataset. In this
context, upregulated miRNAs were downregulated in NPC tissues
relative to the controls, while downregulated miRNAs were
upregulated in NPC specimens compared with the controls.
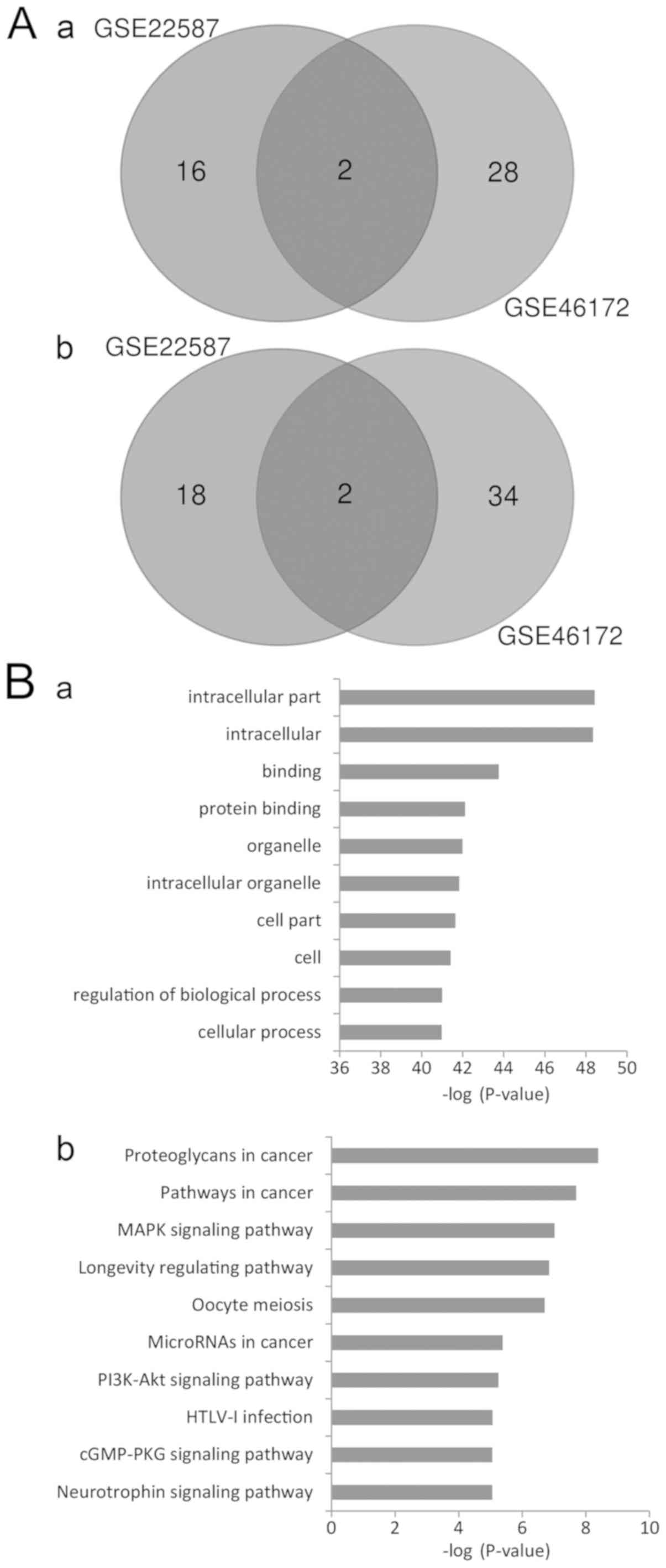
Intersection of DEMs
To narrow the range of the DEMs identified, Venn
diagram analysis was used to identify the intersecting DEM
profiles. A total of 2 upregulated and 2 downregulated DEMs were
identified in the intersection (Fig.
1A). The upregulated DEMs were miR-204, and miR-497, and the
downregulated DEMs were miR-135b and miR-18a.
Prediction of miRNA target genes and
annotation of their functions
A total of 417 genes with integrated scores of
>0.8 were considered as predicted target genes. The Kobas tool
was used to annotate the putative functions of these target genes.
GO analysis revealed that the targeted genes were enriched in 2,340
GO items. The results revealed that these genes may be associated
with a number of biological processes, including regulation of gene
expression, cell metabolism, structure development and compound
binding. The top significant items were ‘intracellular part’,
‘protein binding’, ‘intracellular organelle’, ‘regulation of
biological process’ and ‘cellular process’ (Fig. 1B-a). The results indicated that
various aspects of biological processes may be involved in the
genesis and development of NPC.
KEGG analysis revealed that the target genes may be
enriched in 108 signaling pathways. The most significant pathways
were ‘proteoglycans in cancer’, ‘pathways in cancer’, ‘MAPK
signaling pathway’, ‘longevity regulating pathway’, ‘oocyte
meiosis’, ‘microRNAs in cancer’ and ‘PI3K-AKT signaling pathway’
(Fig. 1B-b). These results suggest
that multiple signaling pathways may mediate the onset and
progression of NPC.
Diagnostic value of DEMs in NPC
To further assess the roles of the DEMs in NPC, the
GSE36682 dataset was downloaded for analysis. The GSE36682 dataset
includes non-coding RNA profiling results generated using the Human
miRNA 1K array. The data were deposited by Wei et al (no
available reference). This dataset contained 62 NPC tissue
specimens and 6 nasopharyngitis tissue specimens.
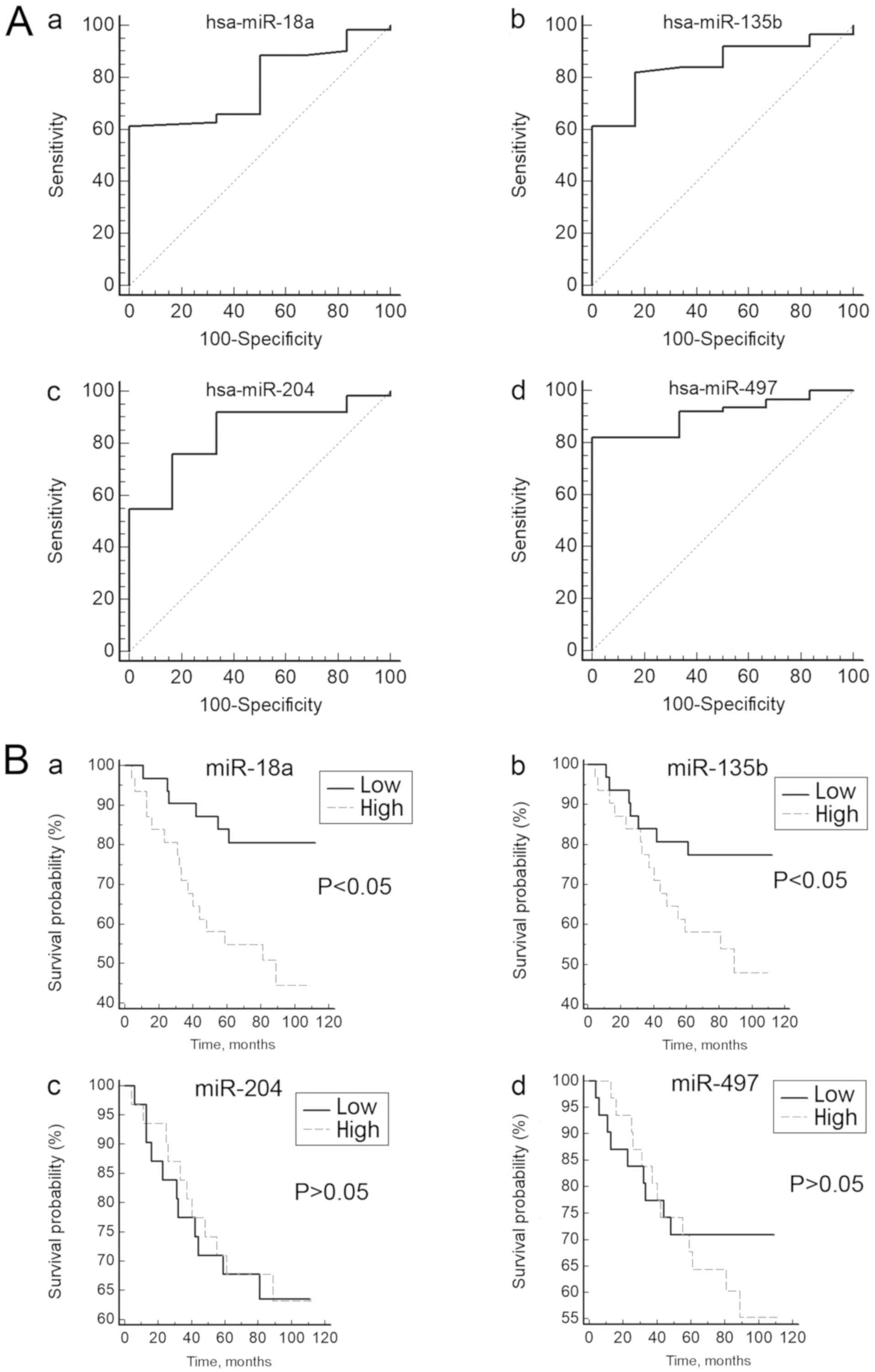
To test whether the expression of these DEMs can
distinguish patients with NPC from the controls, ROC curves were
constructed. The range of the AUCs of these 4 miRNAs (miR-204,
miR-497, miR-18a and miR-135b) was 0.778–0.911 (Table I; Fig.
2A), suggesting that any of these miRNAs may be used as a
potential biomarker to distinguish patients with NPC from healthy
controls.
 | Table I.Diagnostic accuracy of the selected
differentially expressed miRNAs in patients with nasopharyngeal
carcinoma. |
Table I.
Diagnostic accuracy of the selected
differentially expressed miRNAs in patients with nasopharyngeal
carcinoma.
| miRNA | Sensitivity, % | Specificity, % | AUC | Standard error | 95% confidence
interval of AUC |
|---|
| miR-18a | 61.29 | 100.00 | 0.778 | 0.0767 | 0.661–0.870 |
| miR-135b | 82.26 | 83.33 | 0.848 | 0.0618 | 0.740–0.924 |
| miR-204 | 75.81 | 83.33 | 0.841 | 0.0732 | 0.733–0.919 |
| miR-497 | 82.26 | 100.00 | 0.911 | 0.0404 | 0.733–0.919 |
Association between DEM expression
levels and clinical features
The expression levels of the DEMs from the GSE36682
dataset were classified as high or low according to their median
expression levels. The association between the expression levels of
these miRNAs and the clinicopathologic parameters are presented in
Table II. Details of four
confounding factors, including age, gender, metastasis status and
maximum diameters of lymph nodes, were available in the
dataset.
 | Table II.Association of miR-18a, miR-135b,
miR-204 and miR-497 expression with clinicopathological
factors. |
Table II.
Association of miR-18a, miR-135b,
miR-204 and miR-497 expression with clinicopathological
factors.
|
|
| miR-18a |
| miR-135b |
| miR-204 |
| miR-497 |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Variable | Total no. of
patients | High | Low | P-value | High | Low | P-value | High | Low | P-value | High | Low | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Male | 11 | 6 | 5 | 0.740 | 6 | 5 | 0.740 | 4 | 7 | 0.319 | 3 | 8 | 0.965 |
|
Female | 51 | 25 | 26 |
| 25 | 26 |
| 27 | 24 |
| 28 | 23 |
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
≥50 | 26 | 14 | 12 | 0.607 | 13 | 13 | 0.797 | 14 | 12 | 0.607 | 15 | 11 | 0.303 |
|
<50 | 36 | 17 | 19 |
| 18 | 18 |
| 17 | 19 |
| 16 | 20 |
|
| Metastasis
status |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 17 | 15 | 2 | <0.001 | 14 | 3 | 0.002 | 8 | 9 | 0.776 | 9 | 8 | 0.776 |
| No | 45 | 16 | 29 |
| 17 | 28 |
| 23 | 22 |
| 22 | 23 |
|
| Maximum diameter of
lymph nodes, mm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
≥10 | 43 | 25 | 18 | 0.054 | 24 | 19 | 0.168 | 19 | 24 | 0.168 | 22 | 21 | 0.783 |
|
<10 | 19 | 6 | 13 |
| 7 | 12 |
| 12 | 7 |
| 9 | 10 |
|
No association between the expression levels of the
4 miRNAs and 3 factors, including age, gender and maximum diameters
of lymph nodes, was observed (P>0.05). However, for miR-18a and
miR-135b, a significant association with metastasis status was
observed (P<0.05), indicating that high expression of miR-18a
and miR-135b expression may be associated with metastasis.
Prognostic value of the DEMs in
NPC
Survival curves were generated to assess the
association between the expression levels of the DEMs and the
prognosis of patients with NPC. The log-rank test did not reveal
any association between the expression levels of miR-204 and
miR-497 and the overall survival rate of patients with NPC
(Fig. 2B). However, the survival
curves revealed that the low miR-18a-expression group had a
significantly longer survival rate compared with the high
miR-18a-expression group (P<0.05). Similar results were observed
for miR-135b (P<0.05; Fig. 2B).
These results indicated that high expression of miR-18a or miR-135b
may be associated with a poor prognosis for patients with NPC.
Cox proportional hazard regression analysis was used
to analyze the effects of the confounding factors on the survival
time of patients with NPC. Univariate analysis revealed that
metastasis [hazard ratio (HR), 13.343; 95% confidence interval
(CI), 5.138–34.648], miR-18a (HR, 3.405; 95% CI, 1.334–8.693), and
miR-135b (HR, 2.482; 95% CI, 1.014–6.076) may be prognostic
indicators for patients with NPC (Table III). However, multivariate analysis
revealed that only metastasis (HR, 12.140; 95% CI, 4.355–33.839)
may be an independent prognostic indicator for patients with NPC.
Thus, although miR-18a and miR-135b may be prognostic factors, they
are not independent prognostic indicators for NPC patients.
 | Table III.Cox proportional hazard
regression. |
Table III.
Cox proportional hazard
regression.
|
| Univariate
analysis | Multivariate
analysis |
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥50 vs. <50
years) | 2.146
(0.921–5.002) | 0.0785 |
|
|
| Sex (male vs.
female) | 0.183
(0.025–1.344) | 0.0967 |
|
|
| Metastasis (yes vs.
no) | 13.343
(5.138–34.648) | <0.001 | 12.140
(4.355–33.839) | <0.001 |
| Max diameter of
lymph nodes (≥10 vs. <10 mm) | 2.340
(0.795–6.889) | 0.1246 |
|
|
| miR-18a | 3.405
(1.334–8.693) | 0.0108 | 2.203
(0.430–11.298) | 0.3460 |
| miR-135b | 2.482
(1.014–6.076) | 0.0477 | 0.616
(0.126–3.013) | 0.5517 |
| miR-204 | 0.945
(0.411–2.171) | 0.8938 |
|
|
| miR-497 | 1.424
(0.611–3.321) | 0.4151 |
|
|
Discussion
The incidence rate of NPC is high in South Asia
(1). A previous study investigated
the roles of miRNAs in the development of NPC (16). However, the molecular mechanisms
underlying the development of this disease have not yet been fully
elucidated. The current study identified several DEMs that may be
involved in NPC by analyzing microarray-based data. The predicted
target genes of the identified DEMs were enriched in a number of GO
items and signaling pathways. The results identified 4 DEMs that
may be used as potential biomarkers to distinguish patients with
NPC from healthy controls. In addition, miR-18a and miR135b were
identified as potential prognostic indicators for patients with
NPC.
miRNAs exert their influence on biological processes
by targeting specific mRNAs. Thus, the functions of the target
genes may reflect the roles of miRNAs in the biological processes
involved. In the present study, pathway enrichment analysis
suggested that the mechanisms by which the 4 DEMs contribute to NPC
progression are complex. A pathway crosstalk network may be used to
determine how different biological processes coordinate with each
other. However, the primary aim of the present study was to
annotate the possible functions of the target genes. Therefore, bar
charts instead of a crosstalk network were used to present the
pathway items.
A previous study revealed that miR-18a may be highly
expressed in lung cancer and may function as an oncogene in the
development of the disease (17).
Increased circulating miR-18a may also be a prognostic factor for
lung cancer (18). A meta-analysis
revealed that miR-18a may be a promising biomarker for the
diagnosis of patients with gastric carcinoma (19). The results obtained in the current
study are in accordance with the aforementioned studies. However,
an additional study demonstrated that miR-18a may serve a role as a
tumor suppressor by inhibiting cell proliferation and inducing
apoptosis in ovarian cancer (20).
Thus, miRNAs may serve different roles in different types of cancer
or in the same type of cancer under different conditions. In
vitro overexpression of miR-135b promoted the progression and
migration of gastric cancer and by targeting CKLF like MARVEL
transmembrane domain containing 3 (21). miR-135b promoted the migration,
invasion and epithelial-mesenchymal transition of pancreatic cancer
cells by targeting nuclear receptor subfamily 3 group C member 2
(22). The results obtained in the
aforementioned studies are consistent with the results of the
present study, suggesting that upregulation of miR-135b may
contribute to NPC progression. In the current study, miR-204
expression was downregulated in NPC tissues when compared with
controls. A previous study revealed that miR-204 may exert
antitumor effects on melanoma cells (23). miR-204 may decrease the growth,
migration and invasion of colon cancer cells by deactivating the
PI3K/AKT/mTOR signaling pathway and targeting C-X-C motif chemokine
ligand 8 expression (24).
Therefore, miR-204 may function as tumor suppressor in NPC.
In the present study, miR-497 was observed to be
downregulated in NPC tissues compared with healthy controls.
miR-497 may inhibit renal cancer cell proliferation and migration
by targeting the CD274 molecule (25). Furthermore, miR-497 may suppress the
proliferation and invasion of thyroid cancer cells by targeting Yes
associated protein 1 (26).
Therefore, miR-497 may also function as a tumor suppressor in NPC.
Taken together, the results suggest that miR-18a and miR135b may
function as oncogenes, while miR-204 and miR497 may function as
tumor suppressors in NPC.
The occurrence of metastasis affects the prognosis
of patients with NPC (27). In the
present study, univariate analysis revealed that metastasis,
miR-18a and miR-135b were prognostic indicators for NPC.
Multivariate analysis revealed that only metastasis was an
independent prognostic factor for NPC. Since miR-18a and miR-135b
may be associated with metastasis, these miRNAs may target genes
that serve roles in the invasion and migration of NPC cells.
Although miR-18a and miR-135b were not independent prognostic
factors for NPC, they may be useful biomarkers for monitoring NPC
prognosis. However, the current study did not investigate how these
miRNAs may affect the prognosis of patients with NPC. Future
laboratory experiments investigating the association of these
miRNAs and their target genes are required to elucidate the
underlying molecular mechanisms of NPC.
The present study had a number of limitations.
Firstly, confounding factors only included age, gender, metastasis
status and maximum diameter of the lymph nodes. Other confounding
factors that may influence the results, including Epstein-Barr
virus infection status, clinical stage of the tumor, and smoking
and drinking status, were not evaluated in the present study, as
this information was not available from the datasets. Secondly,
only microarray-based data were analyzed in this study. Further
experiments using cell models and clinical specimens are required
to substantiate the results obtained in the current study. Thirdly,
imaging modalities, including computed tomography and magnetic
resonance imaging, may be used for the detection of cancer
metastasis in patients. However, comparing the expression levels of
the identified miRNAs with the results of imaging for predicting
metastasis of NPC could not be assessed because the relevant data
was not included in the datasets.
Despite these limitations, the present study
revealed 4 miRNAs (miR-18a, miR-135b, miR-204 and miR-497) as key
miRNAs that may serve roles in the genesis and development of NPC.
The target genes of these miRNAs were enriched in multiple
biological processes and signaling pathways. Among these miRNAs,
miR-18a and miR-135b, may be associated with metastasis and may be
useful prognostic indicators for NPC. Future studies using cell and
animal models and clinical specimens are required to elucidate the
molecular mechanisms in NPC.
Acknowledgements
Not applicable.
Funding
This study was partly supported by the Fund of
Chongqing Health and Family Planning Commission (grant no.
20141020) and funding from the China Science Foundation for
Postdoctoral Researchers (grant no. 2015T80962).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the National Center for Biotechnology
Information (www.ncbi.nlm.nih.gov/gds/).
Authors' contributions
XZ and QZ designed the experiments. WZ, HY, DL, and
AC performed the experiments. XZ, WZ, and YW analyzed the data. XZ
and QZ wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perri F, Della Vittoria Scarpati G,
Caponigro F, Ionna F, Longo F, Buonopane S, Muto P, Di Marzo M,
Pisconti S and Solla R: Management of recurrent nasopharyngeal
carcinoma: Current perspectives. Onco Targets Ther. 12:1583–1591.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He Y, Guo T, Guan H, Wang J, Sun Y and
Peng X: Concurrent chemoradiotherapy versus radiotherapy alone for
locoregionally advanced nasopharyngeal carcinoma in the era of
intensity-modulated radiotherapy: A meta-analysis. Cancer Manag
Res. 10:1419–1428. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Guo Y, Xu J, Chen Z, Jiang X,
Zhang L, Huang S, He X and Zhang Y: Clinical analysis of recurrence
patterns in patients with nasopharyngeal carcinoma treated with
intensity-modulated radiotherapy. Ann Otol Rhinol Laryngol.
126:789–797. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng JZ, Chen JJ, Xue K, Wang ZG and Yu
D: Clinicopathologic and prognostic significance of VEGF, JAK2 and
STAT3 in patients with nasopharyngeal carcinoma. Cancer Cell Int.
18:1102018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Tang G, Huang H, Li H, Zhang P and
Xu L: Expression level of NUAK1 in human nasopharyngeal carcinoma
and its prognostic significance. Eur Arch Otorhinolaryngol.
275:2563–2573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang J, Zhong G, Wu J, Chen H and Jia Y:
SOX2 recruits KLF4 to regulate nasopharyngeal carcinoma
proliferation via PI3K/AKT signaling. Oncogenesis. 7:612018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dragomir M, Mafra ACP, Dias SMG, Vasilescu
C and Calin GA: Using microRNA networks to understand cancer. Int J
Mol Sci. 19:E18712018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng JZ, Chen JJ, Wang ZG and Yu D:
MicroRNA-185 inhibits cell proliferation while promoting apoptosis
and autophagy through negative regulation of TGF-β1/mTOR axis and
HOXC6 in nasopharyngeal carcinoma. Cancer Biomark. 23:107–123.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao X and Chu J: MicroRNA-379 suppresses
cell proliferation, migration and invasion in nasopharyngeal
carcinoma by targeting tumor protein D52. Exp Ther Med.
16:1232–1240. 2018.PubMed/NCBI
|
|
10
|
Yu JW, Mai W, Cui YL and Kong LY: Genes
and pathways identified in thyroid carcinoma based on
bioinformatics analysis. Neoplasma. 63:559–568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tokar T, Pastrello C, Rossos AEM, Abovsky
M, Hauschild AC, Tsay M, Lu R and Jurisica I: mirDIP
4.1-integrative database of human microRNA target predictions.
Nucleic Acids Res. 46:D360–D370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al-Mubaid H: Gene multifunctionality
scoring using gene ontology. J Bioinform Comput Biol.
16:18400182018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong
S, Kong L, Gao G, Li CY and Wei L: KOBAS 2.0: A web server for
annotation and identification of enriched pathways and diseases.
Nucleic Acids Res. 39:W316–W322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Plieskatt JL, Rinaldi G, Feng Y, Levine
PH, Easley S, Martinez E, Hashmi S, Sadeghi N, Brindley PJ, Bethony
JM and Mulvenna JP: Methods and matrices: Approaches to identifying
miRNAs for nasopharyngeal carcinoma. J Transl Med. 12:32014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee KT, Tan JK, Lam AK and Gan SY:
MicroRNAs serving as potential biomarkers and therapeutic targets
in nasopharyngeal carcinoma: A critical review. Crit Rev Oncol
Hematol. 103:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang C, Zhang X, Wang HM, Liu XM, Zhang
XJ, Zheng B, Qian GR and Ma ZL: MicroRNA-18a-5p functions as an
oncogene by directly targeting IRF2 in lung cancer. Cell Death Dis.
8:e27642017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu X, Zhu S, Tao Z and Ye S: High
circulating miR-18a, miR-20a, and miR-92a expression correlates
with poor prognosis in patients with non-small cell lung cancer.
Cancer Med. 7:21–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang Q, Zhang G, Wang J and Sheng S:
Diagnostic value of MicroRNA-18a for gastric cancer: A
meta-analysis. Clin Lab. 64:177–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu P, Qi X, Bian C, Yang F, Lin X, Zhou
S, Xie C, Zhao X and Yi T: MicroRNA-18a inhibits ovarian cancer
growth via directly targeting TRIAP1 and IPMK. Oncol Lett.
13:4039–4046. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu M, Huang Y, Sun W, Li P and Li L:
miR-135b-5p promotes gastric cancer progression by targeting CMTM3.
Int J Oncol. 52:589–598. 2018.PubMed/NCBI
|
|
22
|
Zhang Z, Che X, Yang N, Bai Z, Wu Y, Zhao
L and Pei H: miR-135b-5p promotes migration, invasion and EMT of
pancreatic cancer cells by targeting NR3C2. Biomed Pharmacother.
96:1341–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Palkina N, Komina A, Aksenenko M, Moshev
A, Savchenko A and Ruksha T: miR-204-5p and miR-3065-5p exert
antitumor effects on melanoma cells. Oncol Lett. 15:8269–8280.
2018.PubMed/NCBI
|
|
24
|
Shuai F, Wang B and Dong S: microRNA-204
inhibits the growth and motility of colorectal cancer cells by
downregulation of CXCL8. Oncol Res. 15:8269–8280. 2018.
|
|
25
|
Qu F, Ye J, Pan X, Wang J, Gan S, Chu C,
Chu J, Zhang X, Liu M, He H and Cui X: MicroRNA-497-5p
down-regulation increases PD-L1 expression in clear cell renal cell
carcinoma. J Drug Target. 27:1–15. 2018.PubMed/NCBI
|
|
26
|
Cheng H, Dong H, Feng J, Tian H, Zhang H
and Xu L: miR-497 inhibited proliferation, migration and invasion
of thyroid papillary carcinoma cells by negatively regulating YAP1
expression. Onco Targets Ther. 11:4711–4721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng Y, Cao C, Hu Q and Chen X: Prognostic
value and staging classification of lymph nodal necrosis in
nasopharyngeal carcinoma after intensity-modulated radiotherapy.
Cancer Res Treat. 2018. View Article : Google Scholar
|