Introduction
Lung cancer is the leading cause of
cancer-associated mortality in China (1). Two major types of lung cancer exist:
Non-small cell lung cancer (NSCLC), which accounts for between 80
and 85% of lung cancer cases, and small cell lung cancer (SCLC),
which accounts for between 10 and 15% of lung cancer cases
(2). NSCLCs include adenocarcinoma,
large cell carcinoma, bronchioloalveolar carcinoma and squamous
cell carcinoma (3,4).
It has been reported that the high mobility group
box 1 (HMGB1) protein is involved in the immune response of tumors
(5). The HMGB1 protein was first
identified as a highly conserved nuclear protein that serves a
pivotal function in chromatin organization and transcriptional
regulation (6,7). In addition, HMGB1 also serves important
functions in the immune response (8). Preclinical data revealed that dying
tumor cells emit certain danger molecular signals, known as
damage-associated molecular patterns (DAMPs) (9–14). In
brief, DAMPs are intracellular sequestered biomolecules that evade
recognition by the immune system under normal physiological
conditions (15). However, under
conditions of cellular stress or injury, these molecules are
actively secreted by stressed immune cells or released from dying
cells, and trigger a non-infectious inflammatory response (16). HMGB1 is a typical DAMP biomolecule,
as HMGB1 is released from dying cells and can trigger an immune
response (17). A previous study
demonstrated that, during chemotherapy or radiotherapy, HMGB1 was
released from dying cells and stimulated dendritic cells (DCs) via
Toll-like receptor 4 (TLR4) (5). DCs
require signaling through TLR4 and its adaptor myeloid
differentiation primary response 88 (MyD88) for efficient
processing and cross-presentation of antigens from dying tumor
cells (5).
MicroRNAs (miRNAs/miRs) serve multiple functions,
including promoting cellular proliferation and apoptosis, in the
pathogenesis of lung cancer (18–24).
HMGB1 may be a target for miRNAs. A previous study demonstrated
that miR-218 is able to suppress cell proliferation and invasion,
and promotes the apoptosis of pancreatic cancer cells by targeting
HMGB1 (25).
In the present study, the function of miR-129-5p in
lung cancer was examined and it was demonstrated that miR-129-5p
targets HMGB1. The results of the present study may provide novel
insights into the molecular mechanism for lung cancer
progression.
Patients and methods
Tissue samples
A total of 10 NSCLC tissue samples were collected
from The Pulmonary Department at Sichuan Cancer Center (Chengdu,
China). Of the ten patients included in the study, 4 were male and
6 were female, with a mean age of 60 years (range, 43–77 years).
The 10 NSCLC tissue samples were collected between January 2011 and
May 2012. The exclusion criteria was any patient who had been
diagnosed with another type of cancer. The protocol for the
collection and use of human tissues in the present study was
evaluated and approved by the Ethics Committee of Sichuan Cancer
Hospital (Chengdu, China). All patients enrolled in the present
study provided written informed consent, and all specimens were
handled and blinded as required by the legal standards of China.
All NSCLC tissue samples were evaluated and confirmed by a senior
pathologist at Sichuan Cancer Center. The clinical information are
presented in Table I.
 | Table I.Characteristics of the 10 patients
with non-small cell lung cancer. |
Table I.
Characteristics of the 10 patients
with non-small cell lung cancer.
| Patient number | Age, years | Sex | TNM stage |
|---|
| 1 | 65 | Female | I |
| 2 | 55 | Female | II |
| 3 | 56 | Female | II |
| 4 | 43 | Female | III |
| 5 | 59 | Male | III |
| 6 | 50 | Male | IV |
| 7 | 56 | Male | III |
| 8 | 77 | Female | IV |
| 9 | 66 | Male | II |
| 10 | 73 | Female | II |
Cell culture
The NSCLC cell lines A549 and SPC-A-1 were obtained
from the Cell Bank of Sichuan University (Sichuan, China). All
cells were cultured in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
incubator with 5% CO2.
Detection of miR-129-5p in tissues and
cells
The levels of miR-129-5p in tissues and cells were
determined using the reverse transcription-quantitative polymerase
chain reaction (RT-qPCR). Briefly, the total RNA from the tissue
samples or A549 and SPC-A-1 cells was extracted using
TRIzol® reagent, according to the manufacturer's
protocol (Invitrogen; Thermo Fisher Scientific, Inc.). The
corresponding cDNA was obtained using the reverse transcription kit
(cat. no. 12574018, Thermo Fisher Scientific. Inc.). The expression
of miR-129-5p was determined using TaqMan miRNA assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: 95°C for 5 min, 40 cycles of 95°C for
15 sec, 58°C for 30 sec and 74°C for 30 sec. U6 small nuclear RNA
was used as an internal loading control. RT-qPCR analysis was
performed on an ABI 7900HT instrument (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The relative expression levels were
calculated with the 2−ΔΔCq method (26). The primers were synthesized and
tested by the Sangon Biotech Co., Ltd. (Shanghai, China) (27–30). The
following primer sequences were used: miR-129-5p forward,
5′-GGGGGCTTTTTGCGGTCTGG-3′ and reverse, 5′-AGTGCGTGTCGTGGAGTC-3;
and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
Overexpression and downregulation of
miR-129-5p in NSCLC cells
The miR-129-5p levels in the A549 and SPC-A-1 cells
were increased and decreased by miR-129-5p mimic and miR-129-5p
antisense oligonucleotide (ASO), respectively. The miR-129-5p mimic
and were purchased from Sangon Biotech Co., Ltd. Prior to
transfection with 500 ng mimic or ASO, the cells were cultured
overnight (1×106 cells/well) at 37°C. The cell
transfection was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The sequences of miR-129-5p mimic and
miR-129-5p ASO were as follows: miR-129-5p mimic,
5′-GGAUCUUUUUGCGGUCUGGGCUUGCUGUUCCUCUCAACAGUAGUCAGGAAGCCCUUACCCCAAAAAGUAUCU-3′,
and miR-129-5p ASO,
5′-CCUAGAAAAACGCCAGACCCGAACGACAAGGAGAGUUGUCAUCAGUCCUUCGGGAAUGGGGUUUUUCAUAGAAA-3′.
Cell proliferation assay
Cell proliferation was assessed using the MTT assay.
Briefly, A549 and SPC-A-1 cells were seeded into 96-well plates at
a density of 5×105 cells/well. The MTT reagent was added
to the medium at a final concentration of 0.1 mg/ml. A total of 100
µl DMSO was added to each well to dissolve the formazan. The
optical density was determined using a microplate reader at 570
nm.
Cell apoptosis analysis
A549 or SPC-A-1 cell suspensions (5×105
cells/ml) and Annexin V-FITC (Abcam, Shanghai, China) binding
buffer was prepared. An Annexin V-FITC Apoptosis Detection kit
(cat. no. APOAF-20TST; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was used according the manufacturer's protocol. Annexin
V-fluorescein isothiocyanate (FITC; 0.5 mg/ml) was added to the
cell mixture and incubated at room temperature for 15 min.
Subsequently, propidium iodide (PI; 0.1 µg/ml; Abcam) was added for
5 min at room temperature, and samples were analyzed on a
fluorescence-activated cell sorting analyzer instrument using the
488 nm excitation wavelength (argon-ion laser or solid state laser)
and emission detected was at 530 nm (green, FITC) and 575–610 nm
(orange, PI) (28). Data were
analyzed with BD FACSuite™ software (23–12943; BD Biosciences, San
Jose, CA, USA).
Prediction of the possible targets of
miR-129-5p
TargetScan software (www.targetscan.org) was used to predict the possible
targets of miR-129-5p.
Dual-luciferase reporter assays
A549 cells were seeded at 1×105
cells/well and serum-starved for 6 h pre-transfection. The
3′-untranslated region (3′-UTR) of HMGB1 and mutated controls were
cloned and inserted into a reporter plasmid (500 ng) and the
pGL3-control (100 ng) (Promega Corporation, Madison, WI, USA).
miR-129-5p mimics were then transfected into A549 cells containing
wild-type or mutant 3′-UTR plasmids with Lipofectamine 2000. Cells
were harvested and luciferase activity was analyzed after 24 h
using the Dual-Luciferase Reporter assay system (Promega
Corporation). Luciferase activity was normalized to Renilla
luciferase activity. Mutants of HMGB1 3′-UTR were generated using a
Site-Directed Mutagenesis kit (Promega Corporation).
Western blotting
A549 cells were frozen and lysed in lysis buffer
(150 mM NaCl, 50 mM Tris/HCl, 1% Triton X-100 and 0.1% SDS) with
protease inhibitors cocktail (cat. no. ab65621; Abcam). Protein
concentration was determined using a BCA protein assay (cat. no.
ab146331; Abcam). Subsequently, 20 µg total protein from the cell
lysate was loaded onto a 10% SDS-PAGE gel. The protein was then
transferred to PVDF membrane and the membrane was blocked with 5%
milk for 1 h at room temperature. For the HMGB1 western blot
analysis, anti-HMGB1 antibodies (cat. no. ab79823; Abcam) were used
at a dilution of 1:1,000 at 4°C overnight. β-actin was used as an
internal reference (cat. no. ab179467; 1:1,000; Abcam). The
membrane was then incubated with horseradish peroxidase-conjugated
anti-rabbit IgG (1:2,000; cat. no. ab150077; Abcam) at room
temperature for 2 h. Proteins were detected by Enhanced
Chemiluminescence Western Blotting Detection Reagents (GE
Healthcare, Little Chalfont, UK). Images were analyzed using ImageJ
1.8.0 software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All experiments were performed three times. Results
are presented as the mean ± standard deviation. A two-tailed
Student's t-test was used to analyze the mean value between two
groups; One-way analysis of variance was used to test the mean
value among three or more groups with post hoc contrasts by
Student-Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference. All calculations were
performed using SPSS software (version 16.0; SPSS, Inc., Chicago,
IL, USA).
Results
miR-129-5p is expressed at low levels
in lung cancer tissues
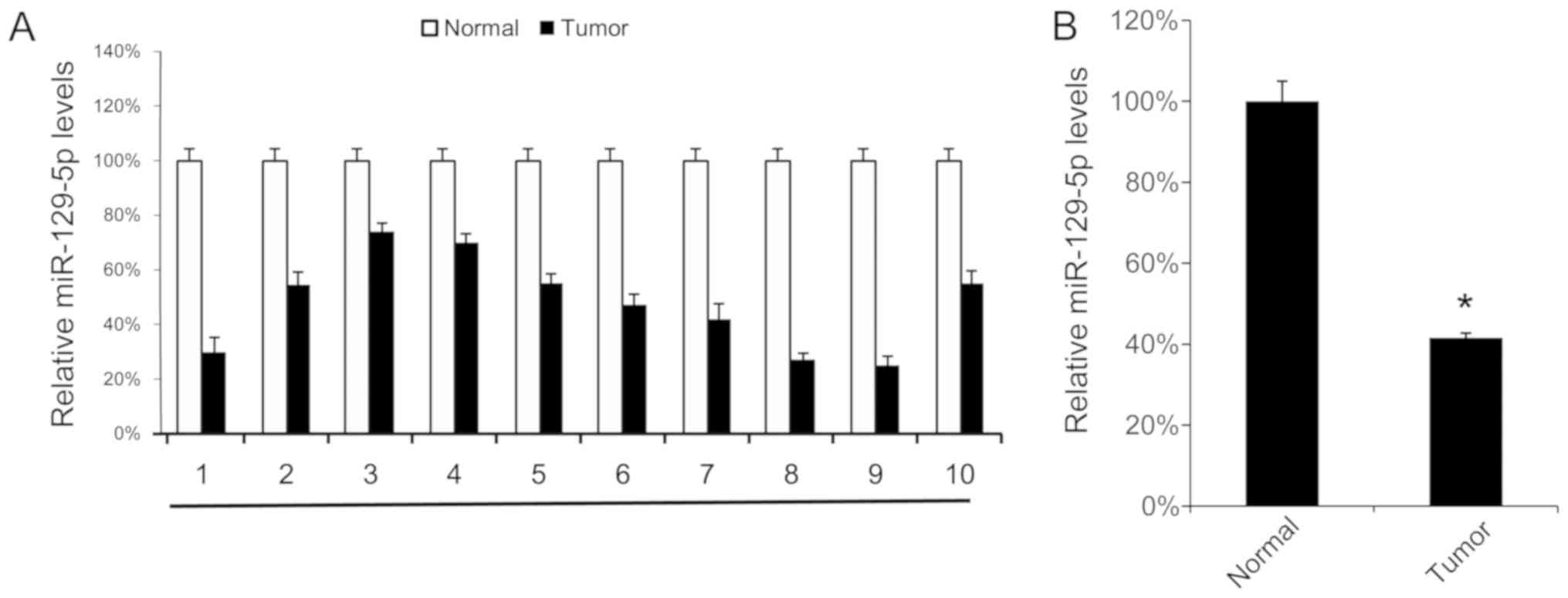
First, the miR-129-5p expression levels in 10 NSCLC
tissue samples and their corresponding normal adjacent tissue
samples were analyzed using RT-qPCR. Tumor tissue samples exhibited
lower miR-129-5p levels compared with their corresponding normal
adjacent tissues (Fig. 1A). The mean
expression levels of miR-129-5p in all 10 tumor tissue samples and
normal tissue samples were determined, and were revealed to be
significantly lower in tumor tissue samples compared with in normal
tissue samples (Fig. 1B). These
results suggest that miR-129-5p may exhibit a tumor suppressor
function in NSCLC.
Overexpression of miR-129-5p inhibits
cell proliferation and promotes cell apoptosis
To investigate the function of miR-129-5p in
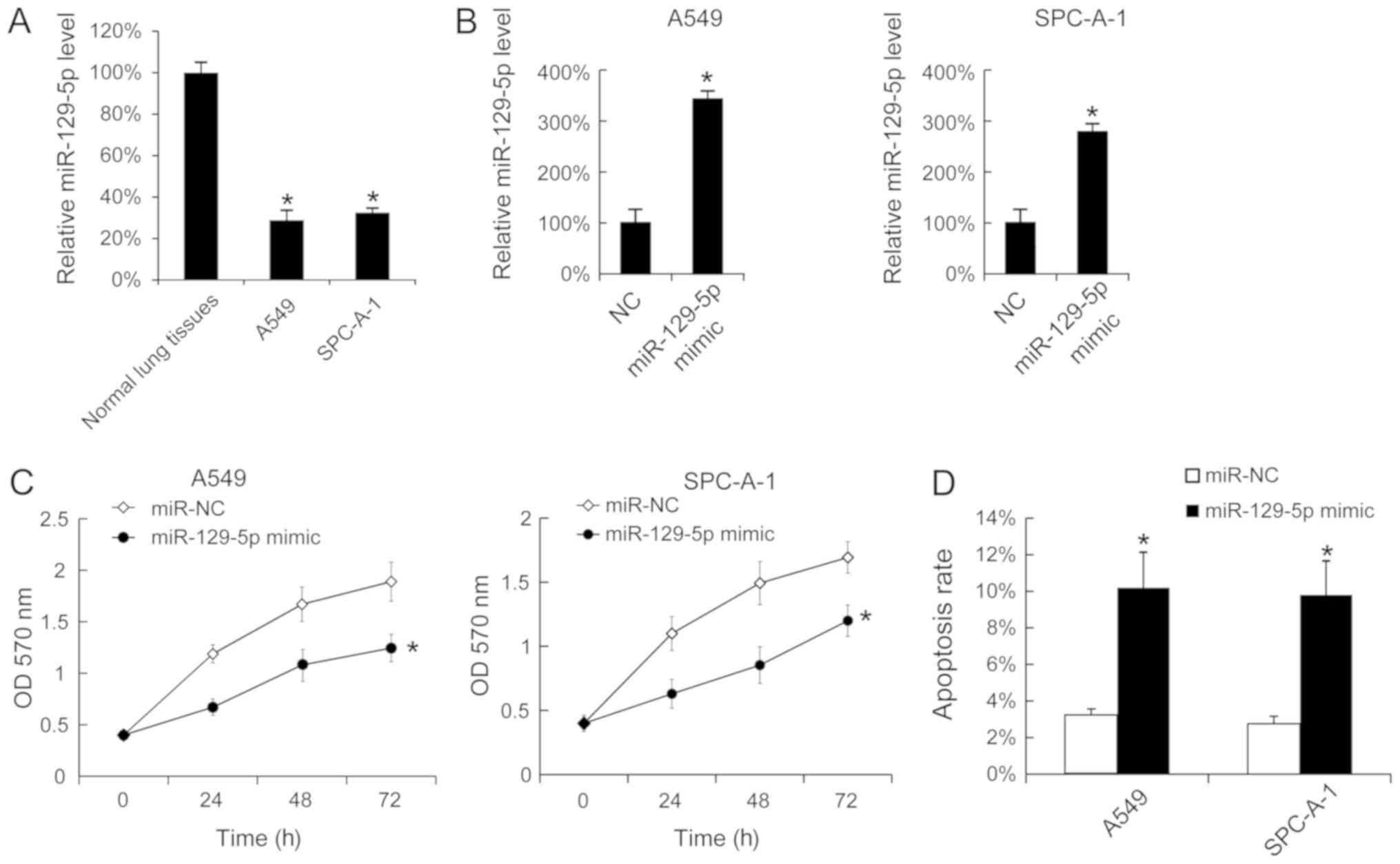
vitro, the levels of miR-129-5p in A549 and SPC-A-1 cells were
analyzed using RT-qPCR, normal lung tissues were used as control.
A549 and SPC-A-1 cells expressed significantly lower levels of
miR-129-5p compared with normal tissues (Fig. 2A). Additionally, miR-129-5p mimic was
transfected into A549 and SPC-A-1 cells, and it was identified that
the miR-129-5p mimic effectively upregulated the miR-129-5p levels
in A549 and SPC-A-1 cells (Fig. 2B).
As the miR-129-5p mimic was able to increase the miR-129-5p level
in vitro, cell proliferation following transfection with the
miR-129-5p mimic was determined, and it was identified that the
upregulation of miR-129-5p inhibited cell proliferation in A549 and
SPC-A-1 cells (Fig. 2C). The effect
of the miR-129-5p mimic on cell apoptosis was investigated and
transfection of the miR-129-5p mimic was identified to increase the
rate of apoptosis (Fig. 2D).
 | Figure 2.Overexpression of miR-129-5p inhibited
the proliferation of A549 and SPC-A-1 cells and promoted cell
apoptosis. (A) The miR-129-5p levels in normal lung tissues, A549
and SPC-A-1 cells were determined using RT-qPCR. The miR-129-5p
levels in the tumor adjacent normal lung tissues were arbitrarily
defined as 100%. (B) A549 and SPC-A-1 cells were seeded and then
were transfected with miR-129-5p mimic. After 24 h, the miR-129-5p
expression was determined using RT-qPCR. (C) Following miR-129-5p
mimic transfection, cell proliferation was determined using an MTT
assay. (D) At 48 h after miR-129-5p mimic transfection, SPC-A-1 and
A549 cells were stained with Annexin V-fluorescein isothiocyanate
and propidium iodide, and the cell apoptosis rate was evaluated
using fluorescence-activated cell sorting analysis. Results are
presented as the mean ± standard deviation. The experiments were
performed at least three times. *P<0.05 vs. normal lung tissues,
NC or miR-NC. RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; NC, negative control; miR, microRNA; OD,
optical density. |
Downregulation of miR-129-5p promotes
cell proliferation
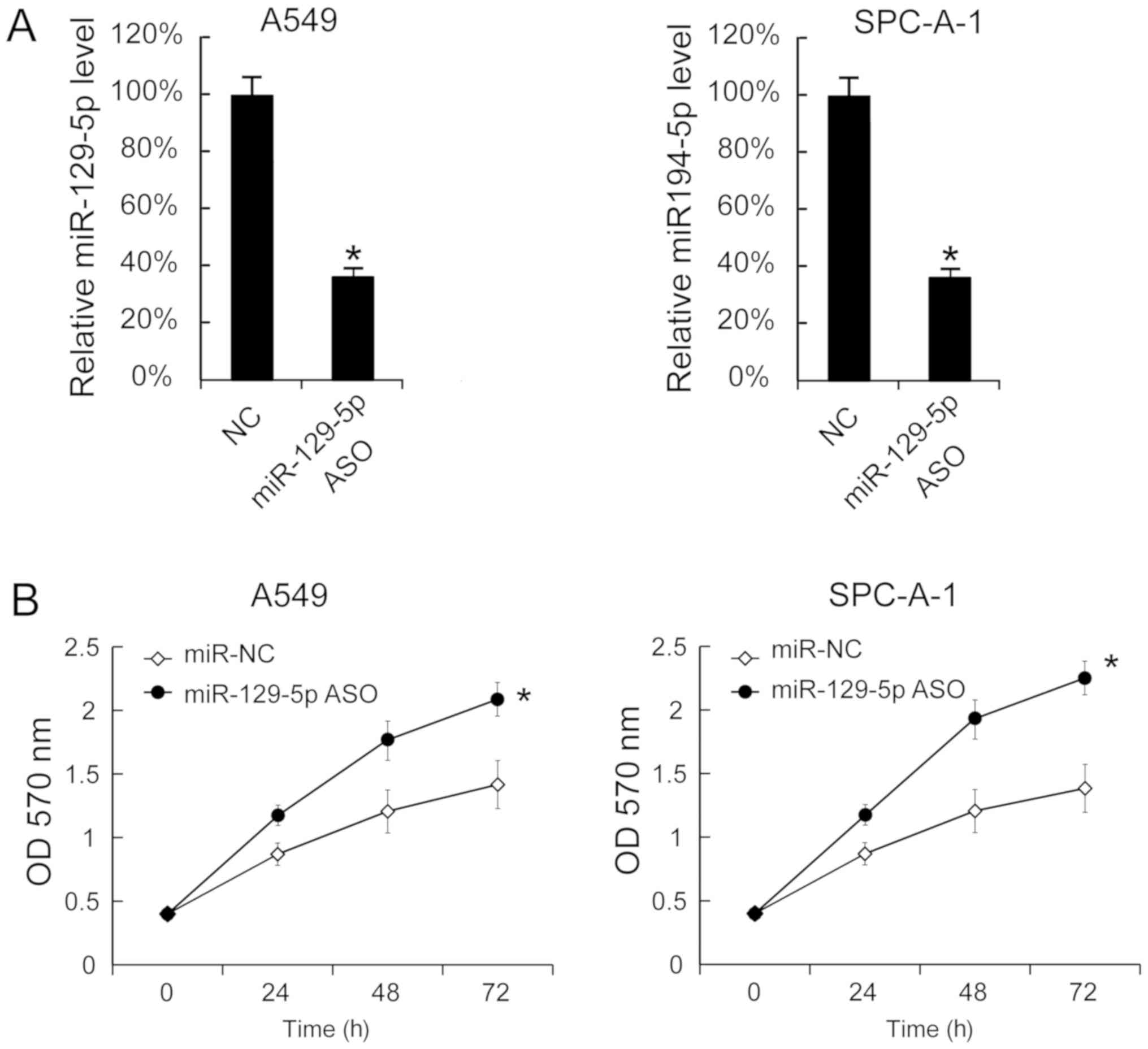
miR-129-5p ASO was transfected into A549 and SPC-A-1
cells to decrease the miR-129-5p levels. The miR-129-5p levels in
A549 and SPC-A-1 cells 24 h after miR-129-5p ASO transfection
revealed that miR-129-5p were significantly inhibited by miR-129-5p
ASO (Fig. 3A). Additionally, cell
proliferation was determined using the MTT assay and it was
identified that miR-129-5p ASO transfection significantly increased
cell proliferation (Fig. 3B).
miR-129-5p inhibits HMGB1 expression
in lung cancer cells
HMGB1 is an important immune factor and one of the
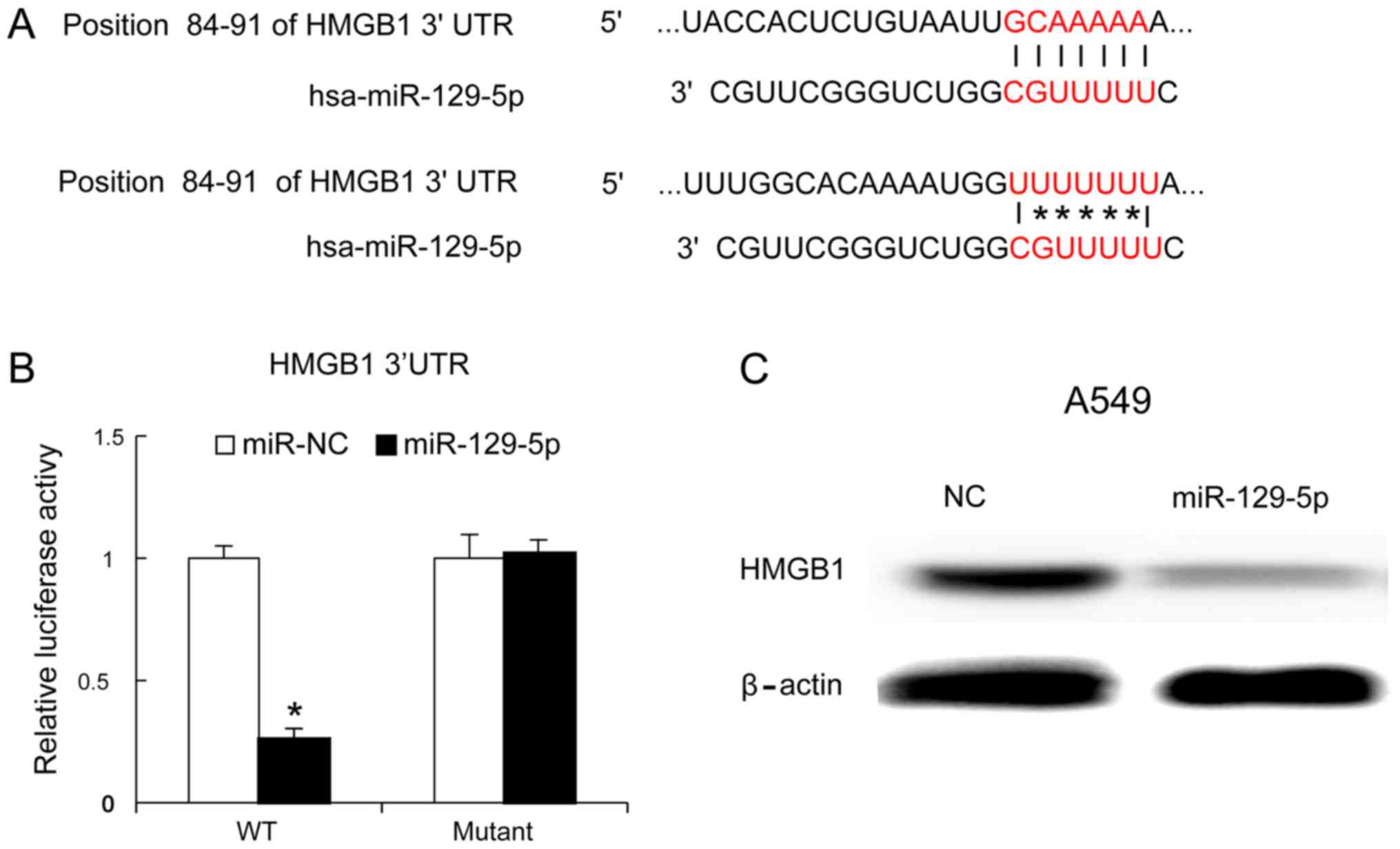
genes targeted by miR-129-5p (1,6–8). The present study identified the binding
site of miR-129-5p on the HMGB1 gene using TargetScan software
(Fig. 4A). To confirm whether
miR-129-5p targeted HMGB1, a mutant of the 3′-UTR of HMGB1 was
generated and the mutant and wild-type versions were cloned into
luciferase reporter plasmids. The miR-129-5p mimic and mutant
version were co-transfected into A549 cells. At 24 h later, it was
identified that the miR-129-5p mimic decreased the luciferase
values of the 3′UTR of HMGB1 (wild-type version), but not that of
the mutated version of the 3′UTR of HMGB1 (Fig. 4B). Furthermore, the miR-129-5p mimic
was transfected into A549 cells and, 48 h later, the HMGB1 protein
levels were evaluated using western blot analysis. The results
identified that the miR-129-5p mimic inhibited HMGB1 protein
expression (Fig. 4C).
Discussion
The present study investigated the function of
miR-129-5p in NSCLC and identified that miR-129-5p exhibited tumor
suppressor activity against NSCLC via HMGB1. The function of
miR-129-5p has previously been investigated in various types of
cancer. In gastric cancer, miR-129-5p was identified to be
downregulated and involved in the migration and invasion of gastric
cancer cells by targeting interleukin-8 (31). In laryngeal cancer, the upregulation
of miR-129-5p inhibited laryngeal cancer cell proliferation,
invasiveness and migration by affecting signal transducer and
activator of transcription 3 expression (32), and in breast cancer, miR-129-5p
attenuates irradiation-induced autophagy and decreases
radioresistance of breast cancer cells by targeting HMGB1 (33). The results also indicated that
miR-129-5p serves a function as an inhibitor of tumor growth.
HMGB1 may activate and induce the maturation of DCs
(34). Accordingly, HMGB1 is one of
the links between tumor cells and the immune system of the host
(35). Recent studies have
identified that, in NSCLC, DCs are in contact with tumor cells
(36), and tumor-infiltrating
lymphocytes (TILs) are observed in the peritumoral zones (37). HMGB1 produced by tumor cells recruits
DCs, which associate with an increased number of TILs (38). Furthermore, HMGB1 may be released
from dying cells and stimulate DCs (5). DCs required signaling through TLR4 and
its adaptor MyD88 for the efficient processing and
cross-presentation of antigens from dying tumor cells (5). We hypothesize that HMGB1 may serve an
important function in the immune response against tumors and
therefore represents a potential immune therapy target in cancer.
Accordingly, miR-129-5p may serve a function in the NSCLC immune
therapy via HMGB1.
A limitation of the present study was that only ten
NSCLC tissue samples were analyzed. The number of patients involved
in the study limited the patient survival analysis. Therefore, a
larger number of patients should be included in further
investigations.
In conclusion, the results of the present study
revealed the suppressive function of miR-129-5p in NSCLC and offer
a novel therapeutic target for the treatment of NSCLC that is
worthy of further investigation.
Acknowledgements
The authors wish to thank Dr Tao Huang (Department
of Cancer, West China Hospital, Chengdu, China) for discussion
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GL collected patient data and performed the cell
experiments. JX performed reverse transcription-quantitative
polymerase chain reaction analysis, western blotting and other
molecular experiments. JW contributed to the study design and
manuscript writing.
Ethics approval and consent to
participate
The protocol for the collection and use of human
tissues in the present study was evaluated and approved by the
Ethics Committee of Sichuan Cancer Hospital. All patients enrolled
in the present study provided written informed consent, and all
specimens were handled and blinded as required by the legal
standards of China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wen C and Dehnel T: China wrestles with
lung cancer. Lancet Oncol. 12:152011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wakelee HA, Chang ET, Gomez SL, Keegan TH,
Feskanich D, Clarke CA, Holmberg L, Yong LC, Kolonel LN, Gould MK
and West DW: Lung cancer incidence in never-smokers. J Clin Oncol.
25:472–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujii T, Dracheva T, Player A, Chacko S,
Clifford R, Strausberg RL, Buetow K, Azumi N, Travis WD and Jen J:
A preliminary transcriptome map of non-small cell lung cancer.
Cancer Res. 62:3340–3346. 2002.PubMed/NCBI
|
|
5
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bianchi ME: HMGB1 loves company. J Leukoc
Biol. 86:573–576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agresti A and Bianchi ME: HMGB proteins
and gene expression. Curr Opin Genet Dev. 13:170–178. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seong SY and Matzinger P: Hydrophobicity:
An ancient damage-associated molecular pattern that initiates
innate immune responses. Nat Rev Immunol. 4:469–478. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Foell D, Wittkowski H, Vogl T and Roth J:
S100 proteins expressed in phagocytes: A novel group of
damage-associated molecular pattern molecules. J Leukoc Biol.
81:28–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaczmarek A, Vandenabeele P and Krysko DV:
Necroptosis: The release of damage-associated molecular patterns
and its physiological relevance. Immunity. 38:209–223. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krysko DV, Agostinis P, Krysko O, Garg AD,
Bachert C, Lambrecht BN and Vandenabeele P: Emerging role of
damage-associated molecular patterns derived from mitochondria in
inflammation. Trends Immunol. 32:157–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahrens S, Zelenay S, Sancho D, Hanč P,
Kjær S, Feest C, Fletcher G, Durkin C, Postigo A, Skehel M, et al:
F-actin is an evolutionarily conserved damage-associated molecular
pattern recognized by DNGR-1, a receptor for dead cells. Immunity.
36:635–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rubartelli A and Lotze MT: Inside,
outside, upside down: Damage-associated molecular-pattern molecules
(DAMPs) and redox. Trends Immunol. 28:429–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alvarez K and Vasquez G: Damage-associated
molecular patterns and their role as initiators of inflammatory and
auto-immune signals in systemic lupus erythematosus. Int Rev
Immunol. 36:259–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Land WG: The role of damage-associated
molecular patterns (DAMPs) in human diseases: Part II: DAMPs as
diagnostics, prognostics and therapeutics in clinical medicine.
Sultan Qaboos Univ Med J. 15:e157–e170. 2015.PubMed/NCBI
|
|
17
|
Laursen TL, Stoy S, Deleuran B, Vilstrup
H, Gronbaek H and Sandahl TD: The damage-associated molecular
pattern HMGB1 is elevated in human alcoholic hepatitis, but does
not seem to be a primary driver of inflammation. APMIS.
124:741–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fabbri M, Garzon R, Cimmino A, Liu Z,
Zanesi N, Callegari E, Liu S, Alder H, Costinean S,
Fernandez-Cymering C, et al: MicroRNA-29 family reverts aberrant
methylation in lung cancer by targeting DNA methyltransferases 3A
and 3B. Proc Natl Acad Sci USA. 104:15805–15810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esquela-Kerscher A, Trang P, Wiggins JF,
Patrawala L, Cheng A, Ford L, Weidhaas JB, Brown D, Bader AG and
Slack FJ: The let-7 microRNA reduces tumor growth in mouse models
of lung cancer. Cell Cycle. 7:759–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chin LJ, Ratner E, Leng S, Zhai R, Nallur
S, Babar I, Muller RU, Straka E, Su L, Burki EA, et al: A SNP in a
let-7 microRNA complementary site in the KRAS 3′ untranslated
region increases non-small cell lung cancer risk. Cancer Res.
68:8535–8540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wiggins JF, Ruffino L, Kelnar K, Omotola
M, Patrawala L, Brown D and Bader AG: Development of a lung cancer
therapeutic based on the tumor suppressor microRNA-34. Cancer Res.
70:5923–5930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Markou A, Tsaroucha EG, Kaklamanis L,
Fotinou M, Georgoulias V and Lianidou ES: Prognostic value of
mature microRNA-21 and microRNA-205 overexpression in non-small
cell lung cancer by quantitative real-time RT-PCR. Clin Chem.
54:1696–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Z, Xu Y, Long J, Guo K, Ge C and Du R:
microRNA-218 suppresses the proliferation, invasion and promotes
apoptosis of pancreatic cancer cells by targeting HMGB1. Chin J
Cancer Res. 27:247–257. 2015.PubMed/NCBI
|
|
26
|
Zhang P, Li J, Song Y and Wang X:
MiR-129-5p inhibits proliferation and invasion of chondrosarcoma
cells by regulating SOX4/Wnt/β-catenin signaling pathway. Cell
Physiol Biochem. 42:242–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li D, Liu X, Lin L, Hou J, Li N, Wang C,
Wang P, Zhang Q, Zhang P, Zhou W, et al: MicroRNA-99a inhibits
hepatocellular carcinoma growth and correlates with prognosis of
patients with hepatocellular carcinoma. J Biol Chem.
286:36677–36685. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song B, Zhang C, Li G, Jin G and Liu C:
MiR-940 inhibited pancreatic ductal adenocarcinoma growth by
targeting MyD88. Cell Physiol Biochem. 35:1167–1177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Q and Yu Y: Upregulated CDK16
expression in serous epithelial ovarian cancer cells. Med Sci
Monit. 21:3409–3414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Xu Y, Qiu W, Zhao D and Zhang Y:
Tissue miR-193b as a novel biomarker for patients with ovarian
cancer. Med Sci Monit. 21:3929–3934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang Z, Wang H, Li Y, Hou Z, Ma N, Chen
W, Zong Z and Chen S: MiR-129-5p is down-regulated and involved in
migration and invasion of gastric cancer cells by targeting
interleukin-8. Neoplasma. 63:673–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen N, Huang X and Li J: Upregulation of
miR-129-5p affects laryngeal cancer cell proliferation,
invasiveness, and migration by affecting STAT3 expression. Tumor
Biol. 37:1789–1796. 2016. View Article : Google Scholar
|
|
33
|
Luo J, Chen J and He L: mir-129-5p
attenuates irradiation-induced autophagy and decreases
radioresistance of breast cancer cells by targeting HMGB1. Med Sci
Monit. 21:4122–4129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dumitriu IE, Bianchi ME, Bacci M, Manfredi
AA and Rovere-Querini P: The secretion of HMGB1 is required for the
migration of maturing dendritic cells. J Leukoc Biol. 81:84–91.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang R, Xie Y, Zhang Q, Hou W, Jiang Q,
Zhu S, Liu J, Zeng D, Wang H, Bartlett DL, et al: Intracellular
HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res.
27:916–932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perrot I, Blanchard D, Freymond N, Isaac
S, Guibert B, Pachéco Y and Lebecque S: Dendritic cells
infiltrating human non-small cell lung cancer are blocked at
immature stage. J Immunol. 178:2763–2769. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kusume A, Sasahira T, Luo Y, Isobe M,
Nakagawa N, Tatsumoto N, Fujii K, Ohmori H and Kuniyasu H:
Suppression of dendritic cells by HMGB1 is associated with lymph
node metastasis of human colon cancer. Pathobiology. 76:155–162.
2009. View Article : Google Scholar : PubMed/NCBI
|