Introduction
Ovarian cancer is a malignancy of the female genital
systems. The incidence of ovarian cancer ranks third in gynecologic
malignancies, second only to cervical cancer and endometrial
cancer. However, its mortality rate ranks first in gynecologic
malignancies (1). The diagnostic
rate of ovarian cancer is relatively low due to imaging limitations
for early diagnosis and the possibility of multifocal origin
(2). There are no obvious and
reliable clinical symptoms in the early stage of ovarian cancer.
Thus, missed diagnosis and misdiagnosis can occur. Often when
clinical symptoms appear, the cancer cells have already spread to
the abdominal organs. Approximately 65% of patients diagnosed with
ovarian cancer are at advanced stage at the time of diagnosis
(3). The primary treatment of
ovarian cancer is radical resection. Good outcomes can be achieved
for most patients undergoing radical resection of ovarian cancer.
However, improper use of anesthetic drugs in ovarian cancer surgery
can cause unstable vital signs and even lead to respiratory
depression and other adverse reactions (4). Therefore, selection of appropriate
anesthetic drugs has become the key to the success of ovarian
cancer radical surgery.
Midazolam is extensively used in clinic as the
preferred anesthesia for radical resection of ovarian cancer due to
fast onset of action and rapid metabolism. However, it was reported
that multiple postoperative adverse reactions, such as delirium,
hallucination, hypotension, and even thrombophlebitis, can occur
following the use of midazolam anesthesia (5). Dexmedetomidine is a novel receptor
agonist, which causes less adverse reactions and has no respiratory
depression effect (6). According to
literature, under general anesthesia, single dose of 0.5 µg/kg
dexmedetomidine given before extubation can attenuate extubation
reflexes, stabilizing patient hemodynamics and vital signs
(7). Tumor necrosis factor α (TNF-α)
is an important cytokine. An appropriate amount of TNF-α can kill
target cells and regulate adaptive immunity, so as to protect the
body. Interleukin-6 (IL-6) is a cytokine involved in the body's
inflammatory response. In some types of cancer, IL-6 is secreted as
a growth factor to stimulate tumor growth.
The function of the ovaries is to produce steroid
hormones and egg cells to maintain female reproductive function and
endocrine homeostasis. It is an important reproductive organ in
women (8). In recent years, ovarian
tumors have become one of the common tumors affecting the quality
of life of women (9). The mortality
rate of ovarian cancer ranks first in gynecological tumors.
Therefore, it is of great significance to improve the diagnosis and
treatment of ovarian malignant tumors (10). There is no obvious symptom in the
early stage of ovarian cancer. When the pelvic tumor spreads to the
abdominal cavity, there may be signs such as abdominal distension,
abdominal pain and bulging sensation, which may be accompanied by
irregular bleeding or irregular menstruation. The symptoms are not
typical and are easily misdiagnosed or fail-diagnosed (11,12). It
has been reported (13) that if
ovarian cancer is effectively treated before it is transferred, the
survival rate of patients can be increased to up to 90%, so early
diagnosis and early treatment are important for patients with
ovarian cancer. The operation with fertility preservation has low
safety and the recurrence rate is high, therefore, most patients
with stage II or above undergo radical ovarian surgery (14).
Dexmedetomidine anesthesia use has rarely been
reported in radical resection of ovarian cancer. In this study, the
clinical records of patients undergoing laparoscopic radical
resection of ovarian cancer were retrospectively analyzed. The
hemodynamics, as well as the serum levels of TNF-α and IL-6, was
compared between patients treated with dexmedetomidine anesthesia
and midazolam anesthesia, in order to provide a reference for the
clinical procedures of ovarian cancer radical surgery.
Patients and methods
Clinical data
A total of 343 patients with ovarian cancer who
underwent radical resection from April 2013 to June 2016 in the
Affiliated Hospital of Qingdao University (Qingdao, China) were
selected for retrospective analysis. Among the patients, 169 were
treated with dexmedetomidine (dexmedetomidine group). The patient
age in this group ranged from 21 to 74 years, with an average age
of 36.62±4.15 years. The remaining 174 patients were treated with
midazolam (midazolam group). The patient age in this group ranged
from 19 to 71 years, with an average age of 38.35±5.32 years. In
order to reach accurate and reliable results, the baseline clinical
data of patients in the two groups were compared, and it was found
that all the variables were comparable (P>0.05). The baseline
clinical data are listed in Table
I.
 | Table I.Baseline clinical data of 343 patients
undergoing laparoscopic radical resection of ovarian cancer (n,
%). |
Table I.
Baseline clinical data of 343 patients
undergoing laparoscopic radical resection of ovarian cancer (n,
%).
| Variable | Dexmedetomidine group
(n=169) | Midazolam group
(n=174) | χ2 | P-value |
|---|
| Age |
|
<40 | 74
(43.79) | 81
(46.55) | 0.265 | 0.607 |
| ≥40 | 95
(56.21) | 93
(53.45) |
|
|
| Marital status |
| Yes | 141 (83.43) | 149 (85.63) | 0.318 | 0.573 |
| No | 28
(16.57) | 25
(14.37) |
|
|
| Pregnancy |
| Yes | 115 (68.05) | 113 (64.94) | 0.371 | 0.543 |
| No | 54
(31.95) | 61
(35.06) |
|
|
| Peritoneal
effusion |
| Yes | 135 (79.88) | 132 (75.86) | 0.803 | 0.37 |
| No | 34
(20.12) | 42
(24.14) |
|
|
| Pathological
classification |
|
Epithelial ovarian cancer | 152 (89.94) | 155 (89.08) | 1.901 | 0.387 |
| Ovarian
sex cord-stromal tumors | 11
(6.51) | 8
(4.60) |
|
|
|
Others | 6
(3.55) | 11
(6.32) |
|
|
| FIGO stage |
| Stage
I | 21
(12.43) | 26
(14.94) | 2.144 | 0.543 |
| Stage
II | 87
(51.48) | 76
(43.68) |
|
|
| Stage
III | 54
(31.95) | 63
(36.21) |
|
|
| Stage
IV | 7
(4.14) | 9
(5.17) |
|
|
The study was approved by the Ethics Committee of
the Affiliated Hospital of Qingdao University (Qingdao, China).
Inclusion and exclusion criteria
Patients who met the following criteria were
eligible for the study: i) Patients who were diagnosed with ovarian
cancer by pathological biopsy in the above hospital; ii) patients
who underwent laparoscopic radical resection of ovarian cancer in
the hospital after diagnosis; iii) patients who received total
intravenous anesthesia; iv) patients who had complete clinical
records; and v) patients aged >18 years; patients with ASA
physical status score 2 or 3. Patients who met the following
criteria were excluded from the study: i) Patients who were
allergic to the drugs used in this study; ii) patients who were
pregnant or in lactation; iii) patients who had acute
gastrointestinal hemorrhage or other serious diseases; and iv)
patients who had communication disorders or cognitive disorders.
The patients and their families signed an informed consent and
cooperated with medical staff to complete the study.
Methods
The patients underwent 8 h fasting and 4 h
water-deprivation before surgery. After entering the operating
room, the patient was connected to a ProSim 8 vital signs simulator
(Fluke Electronics Corp., Everett, WA, USA) for monitoring the
electrocardiogram and oxygen saturation. General anesthesia was
induced using 2–3 mg/kg of fentanyl, 1.5 mg/kg of propofol, 0.05
mg/kg of midazolam, and 0.12 mg/kg of vecuronium bromide through
i.v. injection. The pump infusion rate and dosage were adjusted
based on the patient's heart rate (HR) and blood pressure. After
that, the nasal catheter was used to take oxygen at the speed of 4
l/min until the end of the operation. A venous blood sample was
drawn before oxygen inhalation (T1), shortly after extubation (T2),
and on the morning of day 1 (T3) and day 2 (T4) after surgery,
respectively (15). The serum levels
of TNF-α and IL-6 (primary endpoint) were measured by enzyme-linked
immunosorbent assay (ELISA) using a kit manufactured by Wuhan
Sanying Biotech Inc. (Wuhan, China) with a lot no. 5200103007. The
mean arterial pressure (MAP) and heart rate (HR) (secondary
endpoint) were recorded at each time point.
Statistical analysis
Statistical analysis was performed using SPSS 19.1
(IBM Corp., Armonk, NY, USA) software system. Enumeration data were
expressed in percentage, and the χ2 test was used to compare
differences between the two groups. Measurement data were expressed
as mean ± SD, and the t-test was used to compare differences
between the two groups. A difference was statistically significant
at P<0.05.
Results
MAPs of patients in the
dexmedetomidine group and the midazolam group at different time
points
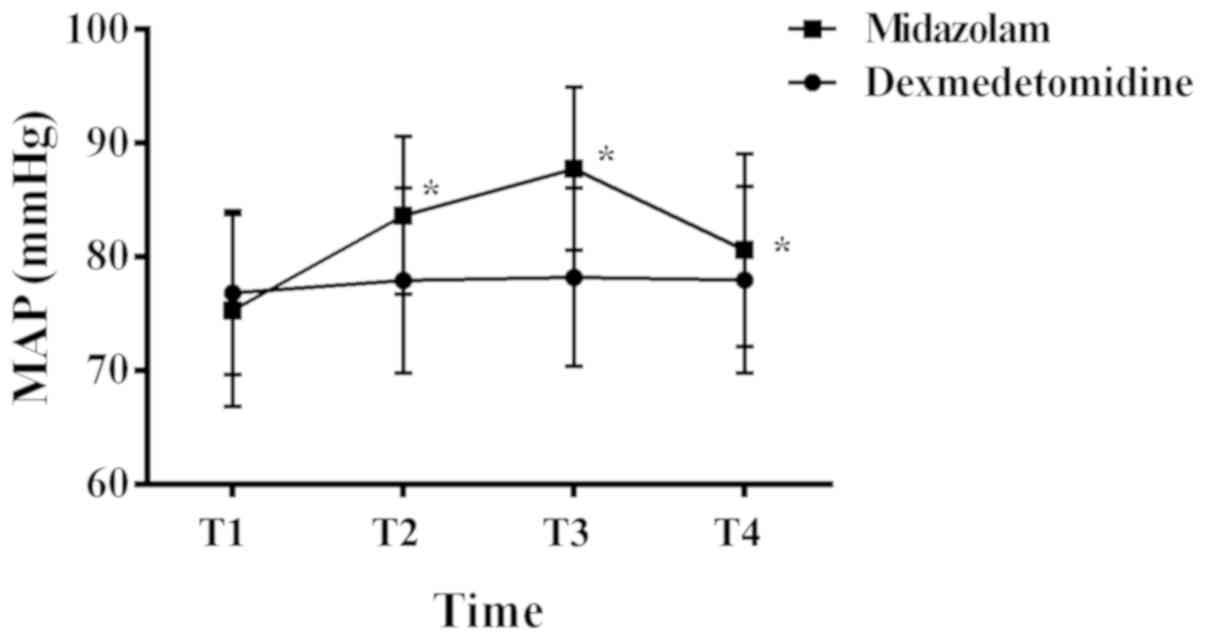
At T1, T2, T3 and T4, the MAPs of patients in the
dexmedetomidine group were (76.83±7.17) mmHg, (77.91±8.14) mmHg,
(78.19±7.83) mmHg, and (77.96±8.21) mmHg, respectively, while the
MAPs of patients in the midazolam group were (75.27±8.42) mmHg,
(83.63±6.92) mmHg, (87.71±7.16) mmHg, and (80.57±8.47) mmHg,
respectively (Fig. 1). The MAPs at
T1 in both groups were comparable, and there was no significant
difference between them (P>0.05). However, the MAPs at T2, T3
and T4 were all lower in the dexmedetomidine group than those in
the midazolam group, and the differences were statistically
significant (P<0.05). Within the dexmedetomidine group, the MAPs
at each time point were comparable, and the differences were not
statistically significant (P>0.05). Within the midazolam group,
the MAPs at T2 and T3 were higher than that at the preceding time
point, whereas the MAP at T4 was lower than that at the preceding
time point. All the differences were statistically significant
(P<0.05).
HRs of patients in the dexmedetomidine
group and the midazolam group at different time points
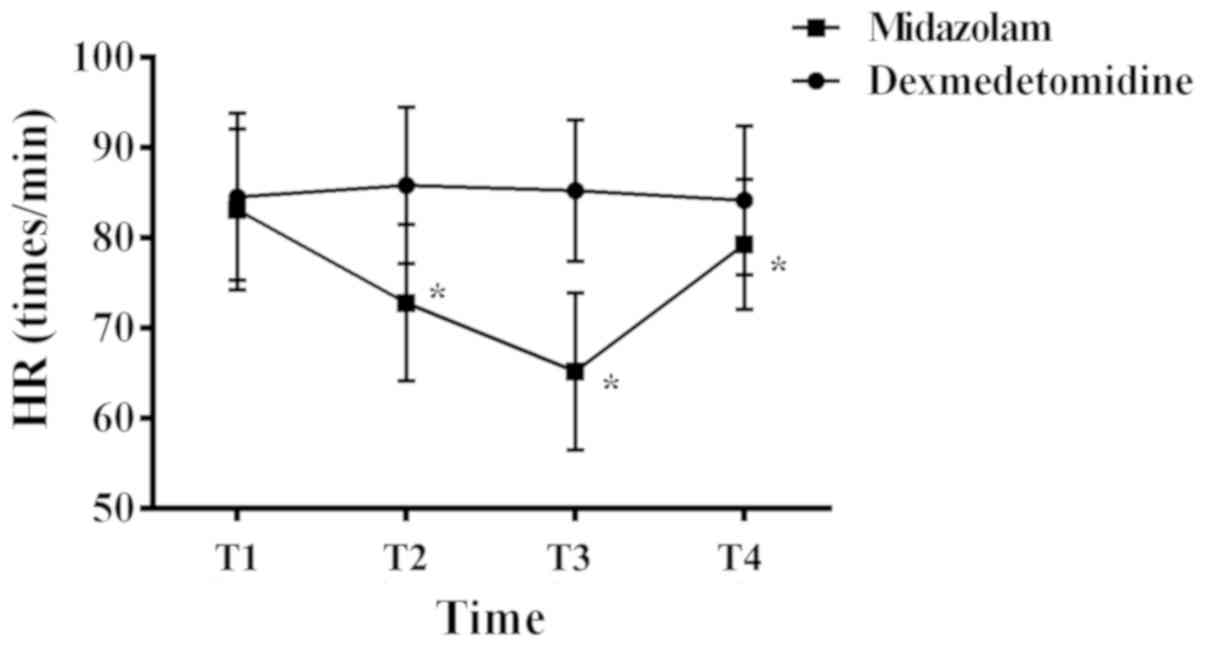
At T1, T2, T3 and T4, the HRs of patients in the
dexmedetomidine group were (84.51±9.24) bpm, (85.81±8.67) bpm,
(85.21±7.86) bpm, and (84.13±8.23) bpm, respectively, while the HRs
of patients in the midazolam group were (83.12±8.89) bpm,
(72.79±8.65) bpm, (65.19±8.71) bpm, and (79.26±7.22) bpm,
respectively (Fig. 2). The HRs at T1
in both groups were comparable, and there was no significant
difference between them (P>0.05). However, the HRs at T2, T3 and
T4 were all higher in the dexmedetomidine group than those in the
midazolam group, and the differences were statistically significant
(P<0.05). Within the dexmedetomidine group, the HRs at each time
point were comparable, and the differences were not statistically
significant (P>0.05). Within the midazolam group, the HRs at T2
and T3 were lower than that at the preceding time point, whereas
the HR at T4 was higher than that at the preceding time point. All
the differences were statistically significant (P<0.05).
Serum levels of TNF-α of patients in
the dexmedetomidine group and the midazolam group at different time
points
At T1, the serum levels of TNF-α in both groups were
comparable, and the difference between them was not statistically
significant (P>0.05). However, the serum levels of TNF-α at T2,
T3 and T4 were all lower in the dexmedetomidine group than those in
the midazolam group, and the differences were statistically
significant (P<0.05). Within each group, the serum levels of
TNF-α at T2, T3 and T4 were all higher than that at T1 (P<0.05).
The level peaked at T2, and then decreased at T3 and T4 compared to
the preceding time point (P<0.05). The details are listed in
Table II.
 | Table II.Serum levels of TNF-α of patients in
the dexmedetomidine and the midazolam group at different time
points (pg/ml). |
Table II.
Serum levels of TNF-α of patients in
the dexmedetomidine and the midazolam group at different time
points (pg/ml).
| Time point | Dexmedetomidine group
(n=169) | Midazolam group
(n=174) | t | P-value |
|---|
| T1 | 1.13±0.32 | 1.17±0.51 |
0.867 |
0.387 |
| T2 |
5.12±2.83a |
8.61±1.15a | 15.040 | <0.001 |
| T3 |
3.25±0.79a |
6.99±1.71a | 25.880 | <0.001 |
| T4 |
3.01±0.45a |
6.18±0.67a | 51.290 | <0.001 |
| F | 201.4 | 1,446 |
|
|
| P-value | <0.001 | <0.001 |
|
|
Serum levels of IL-6 of patients in
the dexmedetomidine group and the midazolam group at different time
points
At T1, the serum levels of IL-6 in both groups were
comparable, and the difference between them was not statistically
significant (P>0.05). However, the serum levels of IL-6 at T2,
T3 and T4 were all higher in the dexmedetomidine group than in the
midazolam group, and the differences were statistically significant
(P<0.05). Within each group, the serum levels of IL-6 at T2, T3
and T4 were all higher than that at T1 (P<0.05). The level
peaked at T2, and then decreased at T3 and T4 compared to the
preceding time point (P<0.05). The details are listed in
Table III.
 | Table III.Serum levels of IL-6 of patients in
the dexmedetomidine group and the midazolam group at different time
points (pg/ml). |
Table III.
Serum levels of IL-6 of patients in
the dexmedetomidine group and the midazolam group at different time
points (pg/ml).
| Time point | Dexmedetomidine group
(n=169) | Midazolam group
(n=174) | t | P-value |
|---|
| T1 | 27.83±12.72 | 28.25±11.51 | 0.321 | 0.748 |
| T2 |
88.83±12.71a |
61.26±18.36a | 6.788 | <0.001 |
| T3 |
62.28±12.63a |
49.48±12.51a | 9.429 | <0.001 |
| T4 |
55.51±11.79a |
43.89±18.98a | 16.130 | <0.001 |
| F | 682.6 | 132.7 |
|
|
| P-value | <0.001 | <0.001 |
|
|
Discussion
Our study showed that there was no significant
difference in serum TNF-α and IL-6 levels between the two groups at
T1 (P>0.05). The level of serum TNF-α in patients with
dexmedetomidine at T2, T3 and T4 was lower than that of the
midazolam group, and the serum IL-6 in patients with
dexmedetomidine at T2, T3, T3 was higher than that of the midazolam
group (P<0.05). Serum TNF-α and IL-6 levels in the
dexmedetomidine group at T2, T3 and T4 were higher than those at T1
(P<0.05). T2 was the peak of serum TNF-α and IL-6 levels. The
levels of serum TNF-α and IL-6 at T3 and T4 were lower than those
at the preceding time points (P<0.05). Serum levels of TNF-α and
IL-6 at T2, T3 and T4 in the midazolam group were significantly
higher than those at T1 (P<0.05), and T2 was the peak. The
levels of serum TNF-α and IL-6 at T3 and T4 were significantly
lower than those at the preceding time points. The results of this
study are consistent with the results of Djaiani et al
(16), suggesting that the choice of
dexmedetomidine as an anesthetic can reduce the release of
pro-inflammatory factor TNF-α and IL-6 induced by TNF-α and
macrophages.
Because midazolam is a short-acting water-soluble
benzodiazepine sedative, midazolam produces sedative, hypnotic,
anticonvulsant, and anxiolytic effects by altering the
configuration of the GABAA receptor complex and opening the
chloride channel. The effect of antegrade amnesia, combined with
opioids, etomidate, propofol drugs can produce synergistic or
additive effects, resulting in analgesic effect (17). However, the use of midazolam can
accumulate in patients with renal failure, and some patients may
also develop drug resistance; while the excessive injection dose or
use may cause blood pressure drop or respiratory depression in
patients with hypovolemia (18).
Dexmedetomidine is a highly selective novel α2 adrenergic receptor
agonist with a specific pharmacological effect (19). Dexmedetomidine mainly acts on the
locus coeruleus of the brainstem with the most concentrated α2
receptor in the central nervous system. The locus coeruleus in the
brain is mainly responsible for regulating the key parts of human
sleep and awakening. After dexmedetomidine is produced, people can
be awakened, but there are still anxiolytic effects; at the same
time, analgesic effects are produced by the synaptic and
post-synaptic membrane α2 receptors in the spinal cord
interneurons; dexmedetomidine inhibits central sympathetic nerves,
enhances vagal activity, and attenuates sympathetic nerves tension,
which brings better sedative effect (20). The anti-sympathetic effects of
dexmedetomidine can lower blood pressure, HR, myocardial
contractility, myocardial oxygen consumption, and plasma
catecholamine levels, so patients gain hemodynamic stability
without significant respiratory depression (21). Dexmedetomidine limits the
inflammatory response of endotoxin, thereby reduces the stress
response in patients (22).
There was no significant difference in MAP between
dexmedetomidine group and midazolam at T1 (P>0.05). At T2, T3
and T4, MAP index in dexmedetomidine group was significantly lower
than that in midazolam group (P<0.05). There was no significant
change in the MAP index at each time point in the dexmedetomidine
group, and the difference was not statistically significant
(P>0.05). However, the MAP index at T2 and T3 was significantly
higher in the midazolam group than that in the preceding time
point, and was significantly lower at T4 (P>0.05). There was no
significant difference in the HR between the dexmedetomidine group
and the midazolam group at T1 (P>0.05). The HR index of the
dexmedetomidine group at T2, T3, T4 was higher compared with that
in midazolam group, and the difference was statistically
significant (P<0.05). There was no significant difference in HR
in the dexmedetomidine group (P>0.05), but HR index in the
midazolam group at T2, T3, T4 was significantly lower, compared
with preceding time points. There was no significant change in the
HR index at each time point in the dexmedetomidine group
(P>0.05). However, the HR index at T2 and T3 in the midazolam
group was significantly lower compared with that of preceding time
points, while it was significantly higher at T4. It shows that
dexmedetomidine has less hemodynamic fluctuations compared with
midazolam, which can increase the safety of patients and stabilize
the life of patients. The results of MAP and HR by Barends et
al (23), when using
dexmedetomidine for surgery were similar to ours, which further
corroborated our experiment.
After trauma, pain and stress, the tissue would be
stimulated to release a large number of inflammatory factors. In
the body's reaction mechanism, macrophages, B lymphocytes and T
lymphocytes can produce TNF-α, which is an early inflammatory
reaction that further induce cytokine cascades and induce the
production of other cytokines and chemokines, stimulate the release
of IL-6, causing a series of inflammatory chain reactions (24). Our study examined the changes of
TNF-α and IL-6 levels in the perioperative period of patients
undergoing laparoscopic ovarian cancer radical surgery. Therefore,
during the radical operation of ovarian cancer, the choice of
anesthetic drugs is the key to stabilizing the patient's vital
signs and reducing the inflammatory factors in patients.
In this experiment, due to the limited medical
resources in our hospital, the number of selected subjects was
small, and there may be some contingency in the results. It is not
excluded that there are differences in the response of anesthesia
at different ages. We intend to conduct longer-term follow-up
surveys.
In summary, compared with midazolam anesthesia, the
use of dexmedetomidine anesthesia can more effectively stabilize
the vital signs of patients, improve safety, and reduce the release
of inflammatory factors. It is more suitable for ovarian cancer
laparoscopic surgery, and is worthy of clinical promotion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML conceived and designed this study. YY collected
and analyzed the data. ML, YY and MZ performed the experiments. ML
and MZ wrote the manuscript and revised it critically. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Qingdao University (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salomon-Perzyński A, Salomon-Perzyńska M,
Michalski B and Skrzypulec-Plinta V: High-grade serous ovarian
cancer: The clone wars. Arch Gynecol Obstet. 295:569–576. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramachandran SM, Liu LY and Perkins SH:
Peristomal nodule patient with ovarian cancer. JAMA. 319:1158–1159.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gallotta V, Cicero C, Conte C, Vizzielli
G, Petrillo M, Fagotti A, Chiantera V, Costantini B, Scambia G and
Ferrandina G: Robotic versus laparoscopic staging for early ovarian
cancer: A case-matched control study. J Minim Invasive Gynecol.
24:293–298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong H, Zhang Y and Xi H: The effects of
epidural anaesthesia and analgesia on natural killer cell
cytotoxicity and cytokine response in patients with epithelial
ovarian cancer undergoing radical resection. J Int Med Res.
40:1822–1829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schaller SJ, Alam SM, Mao J, Zhao Y,
Blobner M, Greenblatt DJ and Martyn JA: Pharmacokinetics cannot
explain the increased effective dose requirement for morphine and
midazolam in rats during their extended administration alone or in
combination. J Pharm Pharmacol. 69:82–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Armstrong DK: New therapies for ovarian
cancer. J Natl Compr Canc Netw 16 (5S). 632–635. 2018. View Article : Google Scholar
|
|
7
|
Park SJ, Shin S, Kim SH, Kim HW, Kim SH,
Do HY and Choi YS: Comparison of dexmedetomidine and fentanyl as an
adjuvant to ropivacaine for postoperative epidural analgesia in
pediatric orthopedic surgery. Yonsei Med J. 58:650–657. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scibelli G, Maio L, Sasso M, Lanza A and
Savoia G: Dexmedetomidine: Current role in burn ICU. Transl Med
UniSa. 16:1–10. 2017.PubMed/NCBI
|
|
9
|
Pudenz M, Roth K and Gerhauser C: Impact
of soy isoflavones on the epigenome in cancer prevention.
Nutrients. 6:4218–4272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nicula R and Costin N: Management of
endometrial modifications in perimenopausal women. Clujul Med.
88:101–110. 2015.PubMed/NCBI
|
|
11
|
Chatterjee M, Hurley LC and Tainsky MA:
Paraneoplastic antigens as biomarkers for early diagnosis of
ovarian cancer. Gynecol Oncol Rep. 21:37–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Felix AS, Bower JK, Pfeiffer RM, Raman SV,
Cohn DE and Sherman ME: High cardiovascular disease mortality after
endometrial cancer diagnosis: Results from the Surveillance,
Epidemiology, and End Results (SEER) Database. Int J Cancer.
140:555–564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zambirinis CP and Miller G: Cancer
manipulation of host physiology: Lessons from pancreatic cncer.
Trends Mol Med. 23:465–481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brand AH, DiSilvestro PA, Sehouli J and
Berek JS: Cytoreductive surgery for ovarian cancer: Quality
assessment. Ann Oncol. 28 (Suppl 8):viii25–viii29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghodki PS, Sardesai SP and Halikar SS:
Dexmedetomidine premedication in cataract surgery under topical
anaesthesia: To assess patient and surgeon satisfaction. S Afr J
Anaesthesiol Analg. 21:17–21. 2015.
|
|
16
|
Djaiani G, Silverton N, Fedorko L, Carroll
J, Styra R, Rao V and Katznelson R: Dexmedetomidine versus propofol
sedation reduces delirium after cardiac surgery: A randomized
controlled trial. Anesthesiology. 124:362–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Balk M, Hentschke H, Rudolph U, Antkowiak
B and Drexler B: Differential depression of neuronal network
activity by midazolam and its main metabolite 1-hydroxymidazolam in
cultured neocortical slices. Sci Rep. 7:3503–3515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yasoshima Y and Shimura T: Midazolam
impairs the retrieval of conditioned taste aversion via opioidergic
transmission in mice. Neurosci Lett. 636:64–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Freeman J and Buggy DJ: Modelling the
effects of perioperative interventions on cancer outcome: Lessons
from dexmedetomidine. Br J Anaesth. 120:15–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z and Sheng L: Significance of dynamic
evolution of TNF-α, IL-6 and intestinal fatty acid-binding protein
levels in neonatal necrotizing enterocolitis. Exp Ther Med.
15:1289–1292. 2018.PubMed/NCBI
|
|
21
|
Kim Y, Lim HJ, Jang HJ, Lee S, Jung K, Lee
SW, Lee SJ and Rho MC: Portulaca oleracea extracts and their active
compounds ameliorate inflammatory bowel diseases in vitro and in
vivo by modulating TNF-α, IL-6 and IL-1β signalling. Food Res Int.
106:335–343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta B, Verma RK, Kumar S and Chaudhary
G: Comparison of analgesic efficacy of dexmedetomidine and
midazolam as adjuncts to lignocaine for intravenous regional
anesthesia. Anesth Essays Res. 11:62–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barends CR, Absalom A, van Minnen B,
Vissink A and Visser A: Dexmedetomidine versus midazolam in
procedural sedation. A systematic review of efficacy and safety.
PLoS One. 12:e01695252017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Field C and Goff BA: Dermatomyositis - key
to diagnosing ovarian cancer, monitoring treatment and detecting
recurrent disease: Case report. Gynecol Oncol Rep. 23:1–3. 2017.
View Article : Google Scholar : PubMed/NCBI
|