Introduction
Prothymosin α (ProTα) is a 12.5 kDa acidic nuclear
protein, initially isolated from rat thymus as the putative
precursor of thymosin α1, and is regarded as a thymic
immunoregulatory hormone (1). The
biological function of ProTα contributes to cell cycle regulation,
transcription, proliferation and apoptosis (2–4). The
ProTα gene is upregulated by MYC proto-oncogene, BHLH transcription
factor (c-Myc), E2F transcription factor 1 and the human papilloma
virus type 16 E6 oncogene, whereas ProTα is downregulated by the
p53 tumor suppressor (5). In
addition, ProTα is present only in cells that are in the
proliferative cycle, and therefore, is not expressed in
non-proliferative cells (5). In
colon cancer cells, ProTα mRNA expression has been reported to be
positively correlated with c-myc, and its expression level
was higher in the tumor tissue compared with the adjacent normal
tissue (6). Overexpression of ProTα
has been associated with a poor prognostic outcome in urinary tract
transitional cell carcinoma, head and neck cancer, hepatocellular
carcinoma and colon cancer (7–10).
However, to the best of our knowledge, studies on the association
between ProTα and lung carcinogenesis are limited. Previous study
has indicated that the secreted thymosin-α1 in plasma from patients
with lung cancer was higher compared with healthy individuals, but
was not associated with age or pathological subtype of lung cancer
in the first human lung cancer study (11). In a urethane injection carcinogenesis
A/J mouse model, daily administration of thymosin-α1 significantly
reduced lung adenoma multiplicity, providing a different biological
perspective on ProTα (12). A study
of 20 lung cancer tissues reported that overexpression of ProTα
mRNA was associated with poor prognosis (13).
Our previous research focused on the contribution of
ProTα to the acetylation of histone and nuclear factor-κB, and
particularly on smoke exposure (14). ProTα transgenic mice are prone to
develop emphysema when exposed to cigarette smoke extract (14). However, the association of lung
cancer with ProTα, in terms of cigarette exposure and pathological
subtypes, has not been well defined (14). The aim of the present study was to
investigate the impact of ProTα on pathological subtypes and
clinical parameters in patients with lung cancer.
Materials and methods
Patient characteristics
A total of 149 patients (mean, 66; range, 28–90
years), including 87 male and 62 female patients, with a
pathological diagnosis of lung carcinoma were included in the
present study. Lung metastasis from other primary site was
excluded. The lung cancer tissues were harvested between 1997 and
2008 by surgical resection at Chi-Mei Medical Center (Yong Kang,
Taiwan). Data on parameters including age, sex, operative
procedure, recurrence, disease-free survival, pathological subtypes
of lung carcinoma and history of cigarette smoking were collected
from the patients' medical records (Table I).
 | Table I.Clinicopathological parameters of the
present study population. |
Table I.
Clinicopathological parameters of the
present study population.
| Parameter | n=149 |
|---|
| Median age (range),
years | 66 (28–90) |
| Sex (%) |
| Male | 87 (58) |
|
Female | 62 (42) |
| Pathological subtype
(%) |
|
| Squamous
cell carcinoma | 30 (20) |
|
Adenocarcinoma | 119 (80) |
| Tumor stage (%) (TNM
system) (17) |
|
| I | 79 (53) |
| II | 35 (24) |
| III | 32 (21) |
| IV | 3 (2) |
| Cigarette smoking
(%) |
|
| Yes | 23 (15) |
| No | 126 (85) |
| Intensity of ProTα
expression (%) |
|
|
Negative | 22 (15) |
| Weak | 63 (42) |
|
Moderate | 33 (22) |
|
Strong | 31 (21) |
| ProTα score (%) |
|
| ≤50 | 73 (49) |
|
>50 | 76 (51) |
Immunohistochemistry stain
Immunohistochemistry staining of 5 µm thick
paraffin-embedded sections was carried out using the 2-step
protocol Novolink Polymer Detection System (Leica Microsystems.
Ltd., Milton Keynes, UK), according to the manufacturer's
protocols. In brief, the sections were first deparaffinized in
xylene two times for 5 min to remove paraffin and subsequently
rehydrated through a gradient of ethanol for 3 min in each
concentration, 100, 100, 95, 70 and 50%, followed by de-ionized
water. Following microwave 10 mM sodium citrate buffer (pH 6.0)
boiled for 10 min, slides were washed for 5 min × 2 in PBS.
Endogenous peroxidase was neutralized using a peroxidase block
(3.5% hydrogen peroxide) for 5 min. Following incubation for 1 h at
room temperature, the sections were washed three times in PBS for 5
min each. Subsequently, the slides were treated with 1% skimmed
milk in PBS for 30 min at room temperature, and non-specific
background staining was minimized further by incubation in 0.3%
bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in 0.1 M Tris-buffered saline for 1 h at room
temperature. Sections were incubated with antibody diluent (Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) for 1 h at room
temperature and washed again in PBS in triplicate for 5 min each.
The primary monoclonal antibody used was anti-human-prothymosin α
antibody (4f4 clone; culture supernatant generated from Professor
Chao-Liang Wu's lab according to references) (15,16).
Following serial incubation with the primary antibody overnight at
4°C, the sections were washed in triplicate with PBS for 5 min
each, and incubated with goat anti-mouse IgG-HRP (115-035-003,
dilution, 1:300; Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA) for 2 h at room temperature. Following incubation,
the slides were washed five times in PBS for 5 min each. Negative
controls included sections stained with mouse universal negative
control with the same concentration of primary antibodies (Dako;
Agilent Technologies, Inc.) overnight at 4°C. Reactivity was
visualized with DAB Quanto (Thermo Fisher Scientific, Inc.) and
counterstained with hematoxylin (MUTO, 5X dilution) for 10 min at
room temperature. The sections were washed in di-H2O for
10 min prior to dehydration, clearing and mounting. Slide scorings
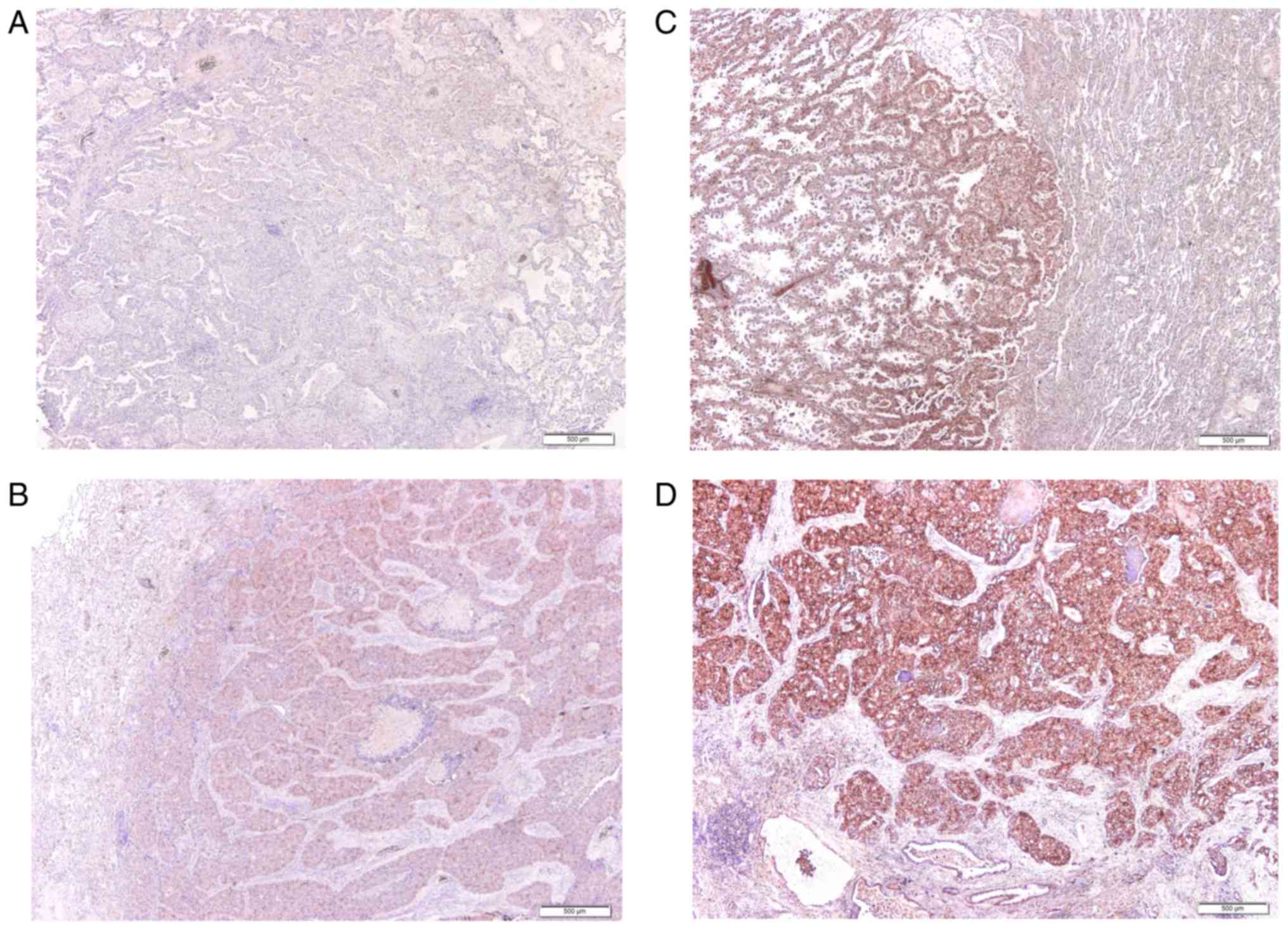
were based on intensity of stain as follows: 0, negative; 1, weak;
2, moderate; 3, strong Fig. 1) and
percentage of area stained (0–100%), with both scores multiplied to
yield the total score. The definition of a high ProTα score was
>50. The results were interpreted by light microscope under the
power of ×100.
Statistical analysis
All statistical analyses were performed using
SigmaStat 3.5 software (Systat Software, Inc., San Jose, CA, USA).
The unpaired t-test and χ2 test were used to evaluate
the differences in discrete variables and continuous variables
between the expression of ProTα and the clinicopathological
parameters. Values are presented as the mean ± standard deviation.
For disease-free survival, the Kaplan-Meier method was adapted to
generate survival curves, and the log-rank test was used to
estimate the differences. All tests were two-tailed, and P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient demography
A total of 149 patients with resected lung cancer
were enrolled for the present study between September 1998 and
September 2008. Participating patients did not receive adjuvant
chemotherapy, since adjuvant chemotherapy was not the recommended
treatment at the time of diagnosis (1998–2008) or in that physical
condition of poor performance status or significant organ
dysfunction. Patients, who had undergone peri-operative
radiotherapy were excluded. The median age of these patients was 66
years (range, 28–90); there were 87 male and 62 female patients.
Regarding pathological subtypes, 30 cases were squamous cell
carcinoma and 119 cases were adenocarcinoma. A total of 79 cases
were stage 1, 35 cases were stage 2, 32 cases were stage 3 and 3
cases were stage 4 by TNM system (based on 6th edition of cancer
staging manual, American Joint Committee on Cancer) (17). The 3 patients with stage 4 underwent
operation for primary lung tumor and distant metastasis, due to
solitary metastasis. Primary lung cancer resection with
metastasectomy was suggested in the aforementioned conditions,
based on the decision of the physicians at Chi-Mei Medical Center.
The majority of the cases, 126, had no history of cigarette
smoking, while 23 cases presented with a smoking history. A total
of two methods were used to measure the expression of ProTα:
staining intensity and the percentage of area stained. The results
of staining intensity indicated that the expression of ProTα was
negative in 22 cases, weak in 63 cases, moderate in 33 cases and
strong in 31 cases. Using the scoring system described above for
the percentage of area stained, 76 cases had a high ProTα score
(score >50; Table I). Nuclear and
nucleo-cytoplasmic staining of ProTα were regarded as positive for
ProTα expression. However, in the present study, sole nuclear stain
of ProTα was rare.
ProTα expression and
clinicopathological parameters
In order to verify the association between
clinicopathological characteristics and the expression of ProTα,
the following parameters were assessed: Age, sex, pathological
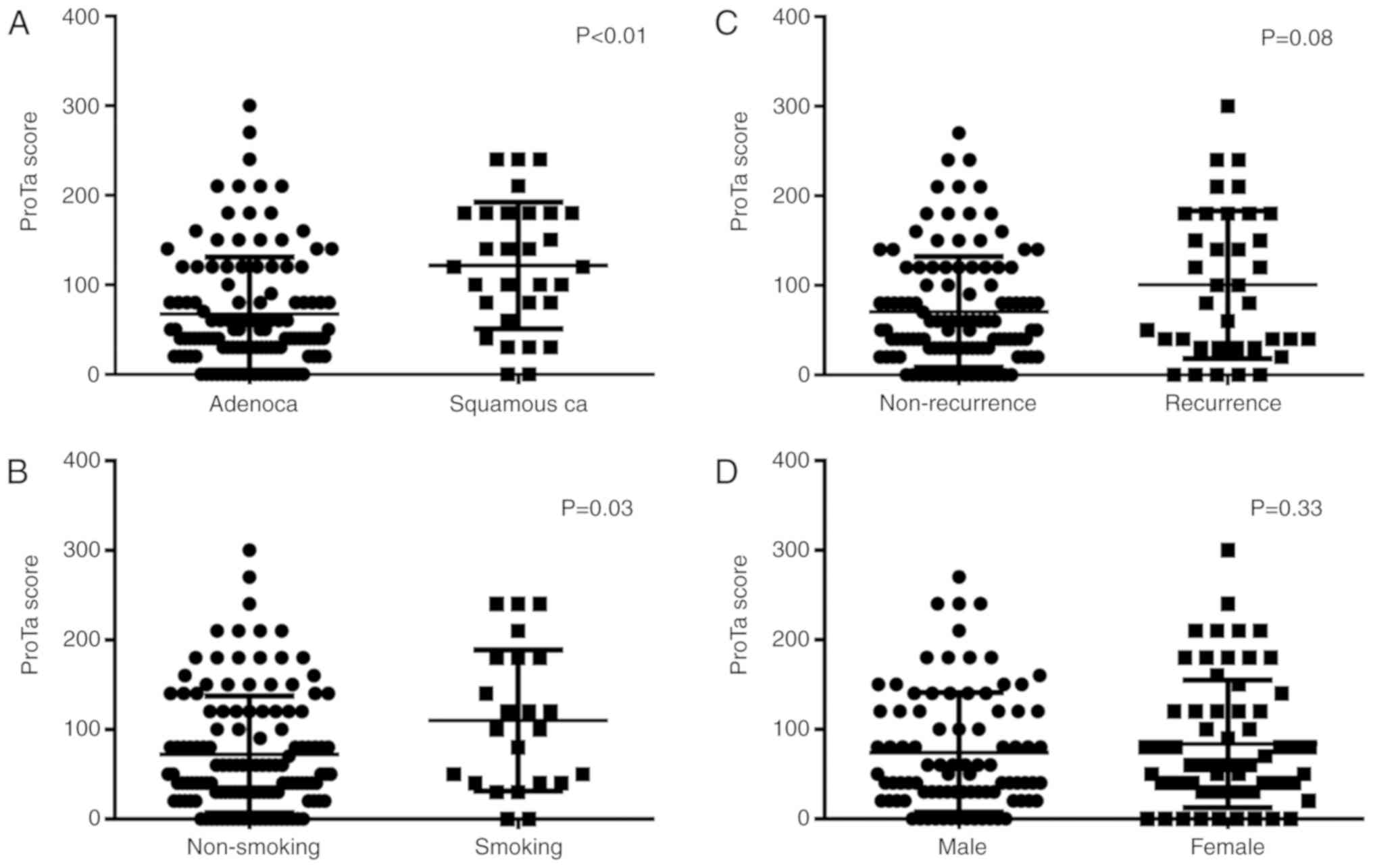
subtype, stage, disease recurrence and cigarette smoking. Using the
ProTα scoring system, squamous cell carcinoma and cigarette smoking
were the only 2 parameters that were significantly associated with
a high ProTα score (Fig. 2).
Patients with recurrence of lung cancer tended to have a higher
ProTα score, however, the result was not statistical significant.
Although cigarette smoking was associated with a high ProTα score
in the analysis of these 149 patients, only 20% (30 cases) had
squamous cell carcinoma and 18% cigarette exposure (23 cases),
which may render difficult an accurate interpretation of the
contribution of ProTα relative to cigarette smoking and
pathological subtypes. The results of the association between
clinicopathological parameters and ProTα expression are presented
in Table II. Further, the
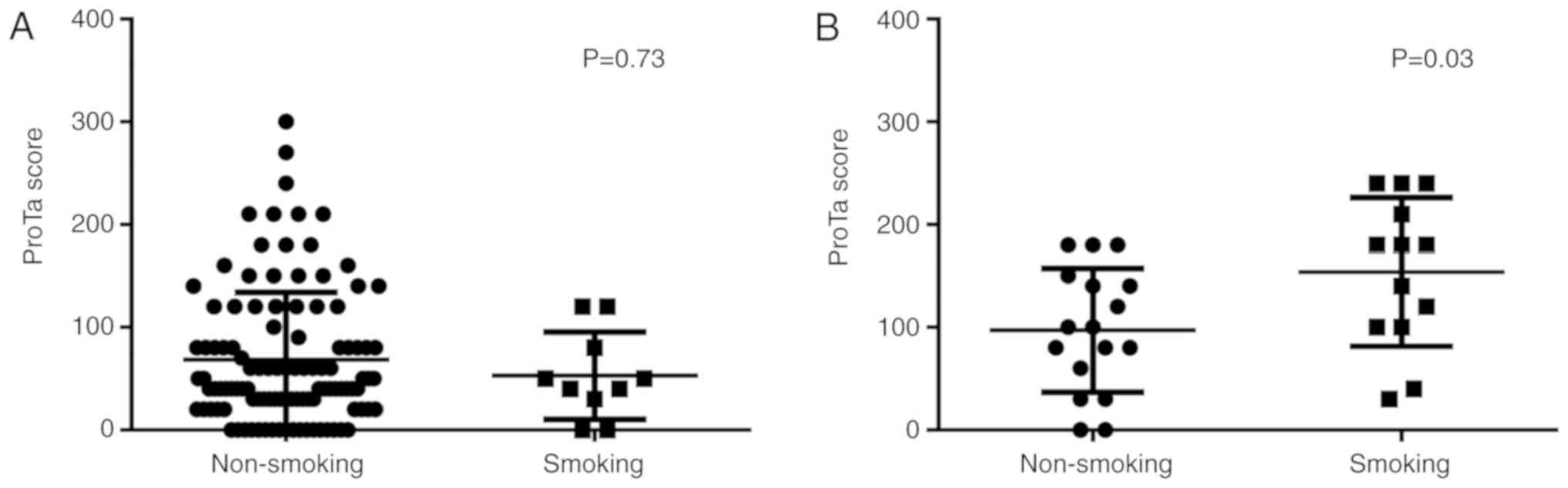
association of ProTα expression with cigarette smoking was
evaluated in patients with squamous cell carcinoma or
adenocarcinoma. It was indicated that the ProTα score was higher
among patients with exposure to cigarettes compared among patients
without exposure to cigarettes in the squamous cell carcinoma group
(P=0.03), however, this was not the case in the adenocarcinoma
group (P=0.73) (Fig. 3). These
results indicate that ProTα may serve a role in cigarette
smoking-mediated carcinogenesis.
 | Table II.Association between
clinicopathological parameters and ProTα expression. |
Table II.
Association between
clinicopathological parameters and ProTα expression.
| Parameter
(number) | Positive ProTα
expression by intensity (%) | P-value | High ProTα score
(>50) (%) | P-value |
|---|
| Age, years |
| 0.05 |
| 0.04 |
| ≤65
(67) | 34 (51) |
| 40 (60) |
|
| >65
(82) | 30 (37) |
| 35 (43) |
|
| Sex |
| 0.90 |
| 0.69 |
| Male
(87) | 37 (43) |
| 42 (48) |
|
| Female
(62) | 27 (44) |
| 34 (55) |
|
| Stage (TNM
system) |
| 0.49 |
| 0.22 |
| I
(79) | 36 (46) |
| 44 (56) |
|
|
II/III/IV (70) | 28 (40) |
| 32 (46) |
|
| Lymph node
involvement |
| 0.58 |
| 0.49 |
|
Negative (94) | 42 (45) |
| 50 (53) |
|
|
Positive (55) | 22 (40) |
| 26 (47) |
|
| Pathological
subtype |
| <0.01 |
| <0.01 |
|
Squamous cell carcinoma
(30) | 23 (77) |
| 24 (80) |
|
|
Adenocarcinoma (119) | 41 (34) |
| 52 (44) |
|
| Recurrence |
| 0.05 |
| 0.42 |
|
Negative (112) | 43 (38) |
| 55 (49) |
|
|
Positive (37) | 21 (57) |
| 21 (57) |
|
| Cigarette
smoking |
| 0.61 |
| 0.90 |
|
Negative (126) | 53 (42) |
| 64 (51) |
|
|
Positive (23) | 11 (48) |
| 12 (52) |
|
Survival analysis of patients with
lung cancer
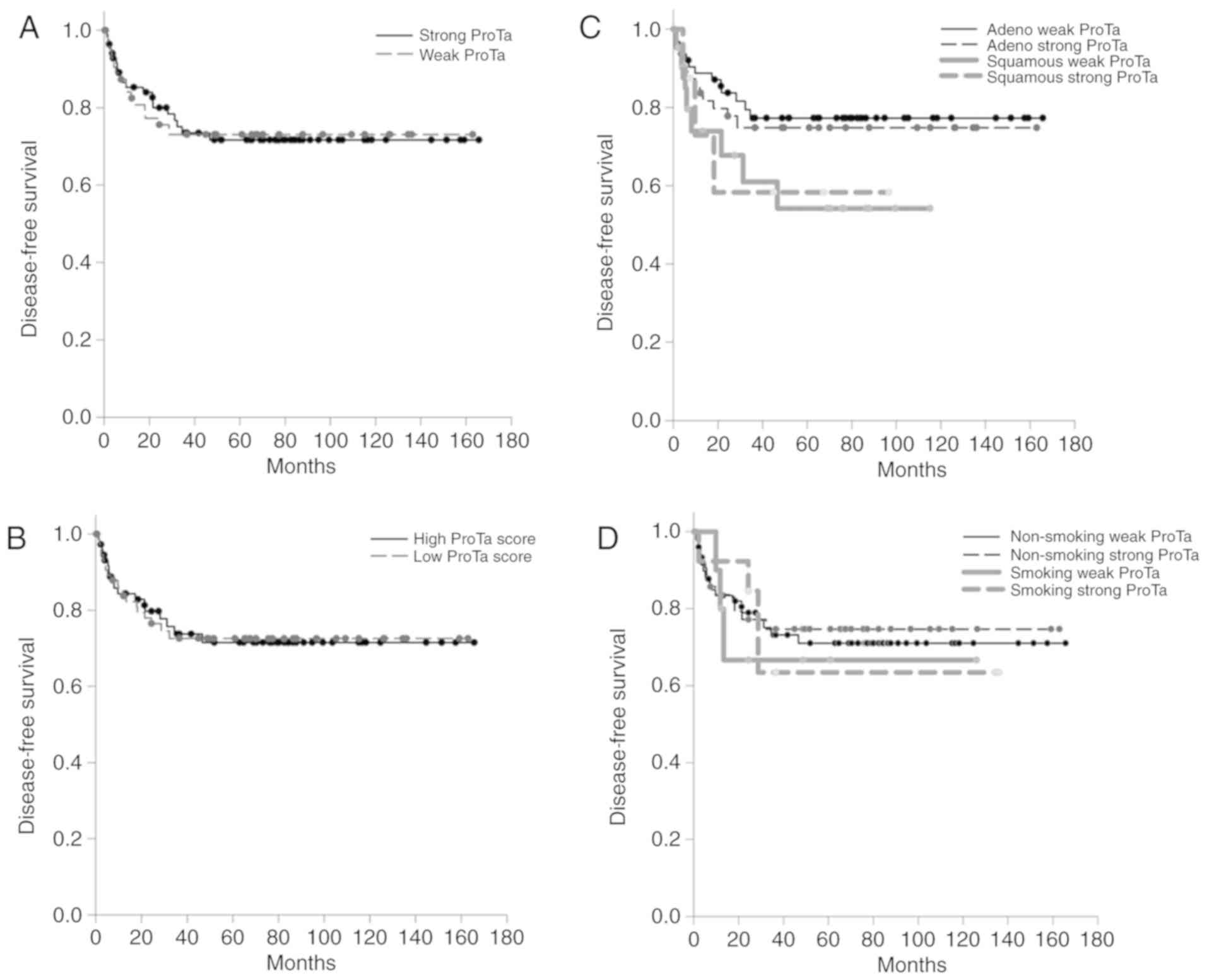
In a previous study with 20 cases of lung cancer, it
was suggested that the presence of ProTα may be a poor prognostic
factor for lung cancer (13). In the
present study, involving 149 patients with lung cancer with
operable disease, neither ProTα expression intensity nor ProTα
expression score was associated with disease-free survival
(Fig. 4A and B). Following
categorization by pathological subtypes of adenocarcinoma and
squamous cell carcinoma, squamous cell carcinoma was indicated to
be associated with poor disease-free survival compared with cases
of adenocarcinoma. However, patients who smoked and exhibited
strong ProTα expression tended to have the poorest disease-free
survival rate. However, the difference between the disease-free
survival rate of patients with different ProTα expressions and
cigarette exposure statuses was not statistically significant
(Fig. 4C and D).
Discussion
To the best of our knowledge, there have been no
previous studies that focus on the protein expression of ProTα in
human lung cancer. The mRNA expression of a small group of patients
with lung cancer has been investigated, however, the ProTα mRNA
levels were not associated with stage or pathological subtype
(13). Our previous findings
suggested that ProTα was positively correlated with the severity of
emphysema in ProTα transgenic mice and patients with emphysema
(14). ProTα transgenic mice were
susceptible to cigarette smoking extract-induced emphysema mainly
due to the inhibition of histone deacetylases and the promotion of
matrix metalloproteinase 2 and matrix metalloproteinase 9 (14). As a result, the association between
ProTα and cigarette smoking requires further attention.
Cigarette smoking has been reported to have a
stronger association with squamous cell carcinoma compared with
adenocarcinoma (18). Aside from
lung cancer, ProTα has been used to distinguish oral pre-malignant
lesions from histologically normal oral tissues by tissue proteomic
analysis (19). Overexpression of
ProTα, as detected by immunohistochemistry, has been reported to
have a positive correlation with nuclear staining of tumor at an
advanced stage, nodal involvement and inferior disease-free
survival in patients with squamous cell carcinoma of the head and
neck undergoing curative cancer surgery (8). ProTα was regarded as a poor prognostic
factor in primary breast cancer, hepatocellular carcinoma, gastric
cancer and upper urinary tract cancer, as well as in prostate
cancer (7,20–23). In
the present study the association of ProTα with squamous cell
carcinoma and cigarette smoking was defined in a small sample.
However, the underlying mechanism beyond this association requires
further examination.
In our previous report on ProTα transgenic mice,
increased Smad family member 7 and reduced tissue inhibitor of
matrix metalloproteinase-3 were indicated in mice with cigarette
smoke extract-induced emphysema (24). A proteomic profile using ProTα as 1
out of 5 biomarkers was valid in predicting the disease-free
survival of patients with oral squamous cell carcinoma undergoing
curative surgery in India and Canada (25). However, the present study did not
examine the association between ProTα and cigarette smoking in oral
squamous cell carcinoma (25). ProTα
has been revealed to protect cells against apoptosis and oxidative
stress (3). Caspase-9 activation
negatively regulated by ProTα can inhibit apoptosome formation
(26). Elimination of ProTα
expression by suppression of RNA has been reported to sensitize
cells to ultraviolet irradiation-induced apoptosis (3). In human lung adenocarcinoma A549 cells,
human PNAS4 had the ability to induce apoptosis through
downregulation of annexin A1 and ProTα. However, no detailed
information on the role of ProTα in lung adenocarcinoma was
provided in the aforementioned study (26).
In conclusion, the data of the present study
indicated that ProTα expression was higher in squamous cell
carcinoma of the lung compared with adenocarcinoma. Patients with
squamous cell carcinoma and who smoked had higher ProTα scores
compared with patients with squamous cell carcinoma and who did not
smoke. However, cigarette smoking did not contribute to a
difference in ProTα expression in adenocarcinoma of the lung. These
results indicate a potential association between ProTα and
cigarette smoking in squamous cell carcinoma. However, this result
is limited to reflect only the clinical implications of ProTα at
present. Therefore, comparing the expression of ProTα in lung
cancer and adjacent normal control tissue samples of smoking and
non-smoking patients is required to investigate smoking-associated
carcinogenesis of squamous cell carcinoma. Further investigations
of the clinical impact of ProTα in lung cancer, including a larger
sample size of patients with lung cancer, particularly patients
with squamous cell carcinoma, are required.
Acknowledgements
We appreciated the technical support from Professor
Wu CL's Lab and the collection of clinical information by Cancer
Center of Chi-Mei Medical Center.
Funding
The present study was supported by Chi Mei Medical
Center (grant nos. CMFHR 10409 and CMFHR 10516).
Availability of data and materials
The datasets used during the current study are
available from corresponding author on reasonable request.
Author's contributions
YHK and YHF were major contributors in writing the
manuscript and analyzing the patient data. CLT, YCS and CFL
performed the histological examination. ALS, PW, BHS, CJT and CLW
made substantial contributions to study design, data analysis and
interpretation, and manuscript organization. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of the Chi Mei Medical Center (approval no. 10308-002). The
condition of inform consent was a waiver documentation of consent,
based on the protection of patient identifiable information.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Haritos AA, Goodall GJ and Horecker BL:
Prothymosin alpha: Isolation and properties of the major
immunoreactive form of thymosin alpha 1 in rat thymus. Proc Natl
Acad Sci U S A. 81:1008–1011. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith MR, al-Katib A, Mohammad R,
Silverman A, Szabo P, Khilnani S, Kohler W, Nath R and Mutchnick
MG: Prothymosin alpha gene expression correlates with
proliferation, not differentiation, of HL-60 cells. Blood.
82:1127–1132. 1993.PubMed/NCBI
|
|
3
|
Jiang X, Kim HE, Shu H, Zhao Y, Zhang H,
Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S and Wang X:
Distinctive roles of PHAP proteins and prothymosin-alpha in a death
regulatory pathway. Science. 299:223–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pai CW and Chen YH: Transgenic expression
of prothymosin alpha on zebrafish epidermal cells promotes
proliferation and attenuates UVB-induced apoptosis. Transgenic Res.
19:655–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Letsas KP and Frangou-Lazaridis M: Surfing
on prothymosin alpha proliferation and anti-apoptotic properties.
Neoplasma. 53:92–96. 2006.PubMed/NCBI
|
|
6
|
Mori M, Barnard GF, Staniunas RJ, Jessup
JM, Steele GD Jr and Chen LB: Prothymosin-alpha mRNA expression
correlates with that of c-myc in human colon cancer. Oncogene.
8:2821–2826. 1993.PubMed/NCBI
|
|
7
|
Jou YC, Tung CL, Tsai YS, Shen CH, Syue-Yi
C, Shiau AL, Tsai HT, Wu CL and Tzai TS: Prognostic relevance of
prothymosin-alpha expression in human upper urinary tract
transitional cell carcinoma. Urology. 74:951–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tripathi SC, Matta A, Kaur J, Grigull J,
Chauhan SS, Thakar A, Shukla NK, Duggal R, Choudhary AR, Dattagupta
S, et al: Overexpression of prothymosin alpha predicts poor disease
outcome in head and neck cancer. PLoS One. 6:e192132011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ha SY, Song DH, Hwang SH, Cho SY and Park
CK: Expression of prothymosin alpha predicts early recurrence and
poor prognosis of hepatocellular carcinoma. Hepatobiliary Pancreat
Dis Int. 14:171–177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang M, Cui F, Lu S, Lu H, Jiang T, Chen
J, Zhang X, Jin Y, Peng Z and Tang H: Increased expression of
prothymosin-α, independently or combined with TP53, correlates with
poor prognosis in colorectal cancer. Int J Clin Exp Pathol.
7:4867–4876. 2014.PubMed/NCBI
|
|
11
|
Sasaki H, Fujii Y, Masaoka A, Yamakawa Y,
Fukai I, Kiriyama M, Saito Y and Matsui H: Elevated plasma
thymosin-alpha1 levels in lung cancer patients. Eur J Cardiothorac
Surg. 12:885–891. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moody TW, Leyton J, Zia F, Tuthill C,
Badamchian M and Goldstein AL: Thymosinalpha1 is chemopreventive
for lung adenoma formation in A/J mice. Cancer Lett. 155:121–127.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasaki H, Nonaka M, Fujii Y, Yamakawa Y,
Fukai I, Kiriyama M and Sasaki M: Expression of the prothymosin-a
gene as a prognostic factor in lung cancer. Surg Today. 31:936–938.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su BH, Tseng YL, Shieh GS, Chen YC, Shiang
YC, Wu P, Li KJ, Yen TH, Shiau AL and Wu CL: Prothymosin α
overexpression contributes to the development of pulmonary
emphysema. Nat Commun. 4:19062013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sukhacheva EA, Evstafieva AG, Fateeva TV,
Shakulov VR, Efimova NA, Karapetian RN, Rubtsov YP and Vartapetian
AB: Sensing prothymosin alpha origin, mutations and conformation
with monoclonal antibodies. J Immunol Methods. 266:185–196. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai YS, Jou YC, Lee GF, Chen YC, Shiau
AL, Tsai HT, Wu CL and Tzai TS: Aberrant prothymosin-alpha
expression in human bladder cancer. Urology. 73:188–192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: AJCC Cancer Staging Manual. 6th.
Springer Publ. Corp.; New York, NY: pp. 165–178. 2002
|
|
18
|
Pesch B, Kendzia B, Gustavsson P, Jöckel
KH, Johnen G, Pohlabeln H, Olsson A, Ahrens W, Gross IM, Brüske I,
et al: Cigarette smoking and lung cancer-Relative risk estimates
for the major histological types from a pooled analysis of
case-control studies. Int J Cancer. 131:1210–1219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ralhan R, Desouza LV, Matta A, Tripathi
SC, Ghanny S, Datta Gupta S, Bahadur S and Siu KW: Discovery and
verification of head-and-neck cancer biomarkers by differential
protein expression analysis using iTRAQ labeling, multidimensional
liquid chromatography, and tandem mass spectrometry. Mol Cell
Proteomics. 7:1162–1173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Magdalena C, Dominguez F, Loidi L and
Puente JL: Tumour prothymosin alpha content, a potential prognostic
marker for primary breast cancer. Br J Cancer. 82:584–590. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu CG, Habib NA, Mitry RR, Reitsma PH, van
Deventer SJ and Chamuleau RA: Overexpression of hepatic prothymosin
alpha, a novel marker for human hepatocellular carcinoma. Br J
Cancer. 76:1199–1204. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leys CM, Nomura S, LaFleur BJ, Ferrone S,
Kaminishi M, Montgomery E and Goldenring JR: Expression and
prognostic significance of prothymosin-alpha and ERp57 in human
gastric cancer. Surgery. 141:41–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki S, Takahashi S, Takahashi S,
Takeshita K, Hikosaka A, Wakita T, Nishiyama N, Fujita T, Okamura T
and Shirai T: Expression of prothymosin alpha is correlated with
development and progression in human prostate cancers. Prostate.
66:463–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su BH, Tseng YL, Shieh GS, Chen YC, Wu P,
Shiau AL and Wu CL: Over-expression of prothymosin-α antagonizes
TGFβ signalling to promote the development of emphysema. J Pathol.
238:412–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chauhan SS, Kaur J, Kumar M, Matta A,
Srivastava G, Alyass A, Assi J, Leong I, MacMillan C, Witterick I,
et al: Prediction of recurrence-free survival using a protein
expression-based risk classifier for head and neck cancer.
Oncogenesis. 4:e1472015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gou LT, Tong AP, Yan F, Yuan Z, He F, Wang
W, Zhou Y, Chen LJ, Tang MH and Yang JL: Altered protein-expressing
profile in hPNAS4-induced apoptosis in A549 human lung
adenocarcinoma cells. J Cell Biochem. 108:1211–1219. 2009.
View Article : Google Scholar : PubMed/NCBI
|