Introduction
Cervical sarcomas are rare and constitute <1% of
all cervical malignancies (1). A
specific subtype, Ewing sarcoma of the cervix, is an extremely rare
tumor and for this reason particularly challenging regarding the
choice of optimal therapeutic strategy (2).
Ewing sarcoma is a mesenchymal malignancy with
specific genetic and immunohistochemical characteristics, mainly
affecting the bones. However, 20–30% of Ewing sarcomas arise from
an extraosseous site (3).
Extraosseous Ewing sarcomas affect patients of any age. The
diagnosis of Ewing sarcomas of the female genital tract is
extremely rare. The identification of reciprocal translocation
t(11;22) and the subsequent formation of the fusion gene EWS/FLI
are pathognomonic for the diagnosis of this tumor (4).
Several different therapeutic modalities have been
applied to the few reported cases in the literature (5–17). ESMO
and NCCN guidelines recommend to treat these tumors like uterine
sarcomas (18,19). Nevertheless, there is no universal
consensus on the therapeutic approach of cervical Ewing sarcomas.
The coexistence of pregnancy makes the therapeutic strategy
challenging. Only 6 cases with extraosseous Ewing sarcomas during
pregnancy have been reported (13,20).
Case report
A 38 year old woman referred to our hospital in the
first trimester of her pregnancy (9th week) due to a tumor mass in
her cervix, as an incidental finding during her scheduled first
trimester abdominal ultrasound. Pelvic examination revealed that
the vulva and the vagina were normal. However, the cervix was
enlarged with smooth surface without necrotic lesions. Bimanual
examination revealed a large cervical mass measuring 8 cm in
diameter. The size of the uterus was slightly enlarged. There was
no extension of the lesion into the vagina, parametria or adjacent
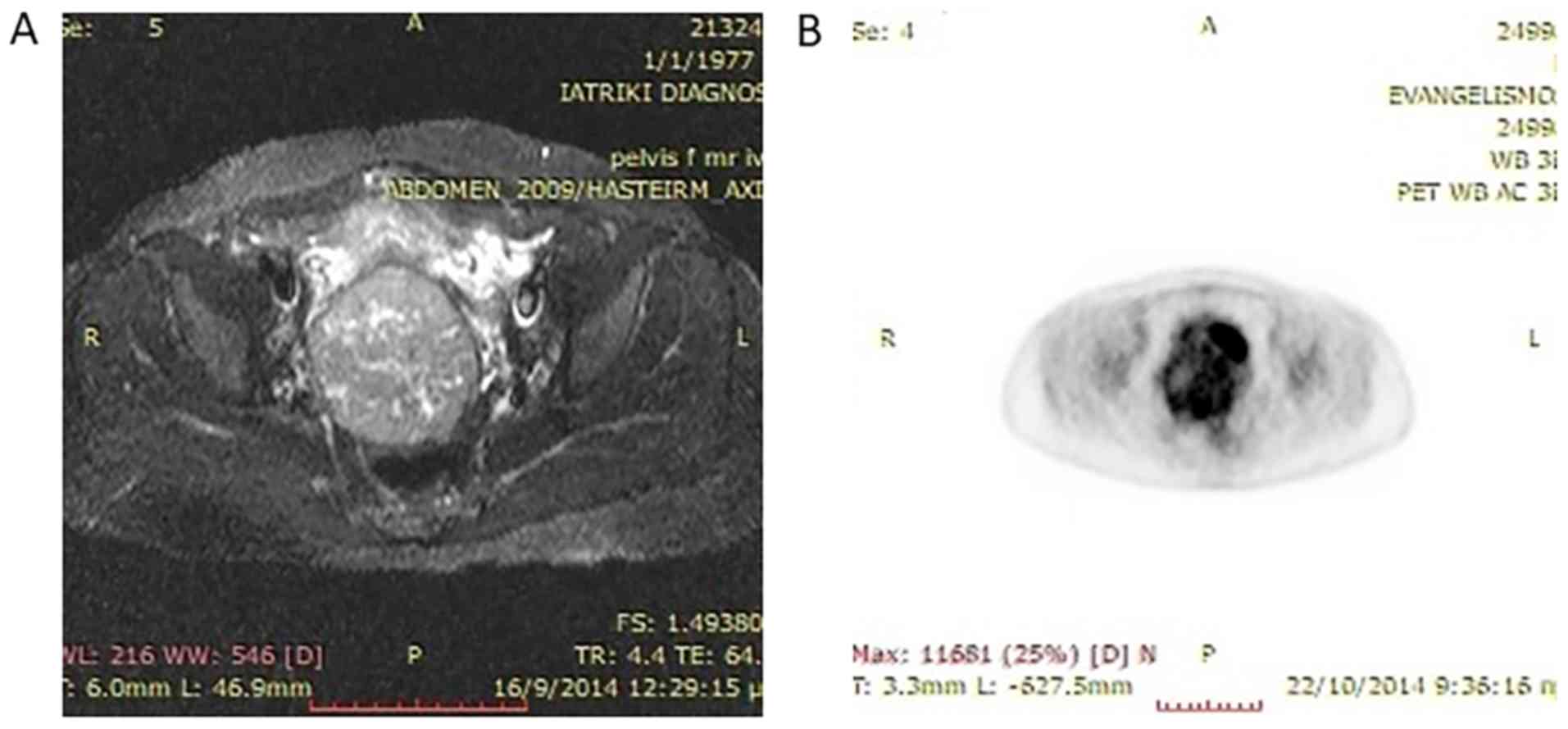
organs. MRI of her abdomen revealed a 8×7.6 cm tumor of the cervix
(Fig. 1). PET-CT verified the
locoregional extension of the tumor without any indications of
metastatic sites (Fig. 1). Tumor
biopsy was performed during termination of her pregnancy. The
patient was already the mother of 2 children and decided to end her
pregnancy. Histopathologic report of tissue specimen favored the
diagnosis of Ewing's sarcoma/PNET. Clinical Staging of the disease
according to FIGO stage system was IB2. Histopathology slides were
reviewed by an independent pathologist who confirmed the diagnosis
of Ewing's sarcoma/PNET.
The patient underwent radical hysterectomy with
bilateral salpingoophorectomy and systematic pelvic
lymphadenectomy. Pathology revealed a cervical tumor of 8.5×7.7×7
cm, which invaded the cervical wall with no extension beyond it.
Surgical margins were free of disease. Lymph nodes were free of
metastasis. The overall immunomorphologic characteristics of the
tumor favor the diagnosis of Ewing's sarcoma/PNET of the cervix
(Fig. 2). The Ethics Committee of
Alexandra Hospital (Athens, Greece) has approved this study and the
patient has signed form of consent.
To further characterize this rare case, we performed
genetic analyses to the tissue specimen. Fluoresence in situ
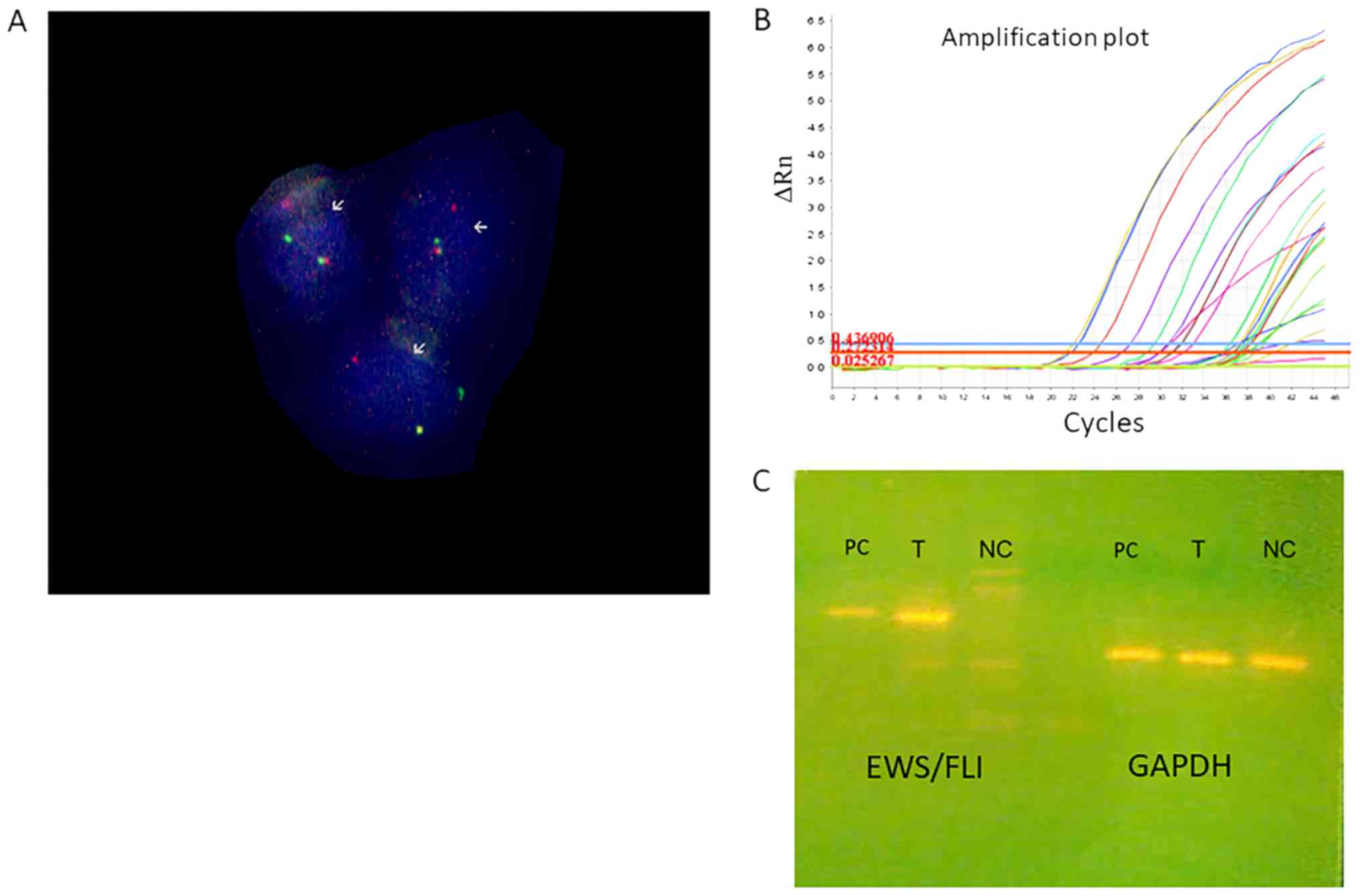
hybridization (FISH) analysis with EWS break-apart kit revealed
fusion of EWS gene (Fig. 3). RT-PCR
detected the formation of EWS-FLI1 chimeric gene (Fig. 3).
The patient performed post-operative CT scans of her
abdomen and her chest, which showed absence of residual disease or
metastases. She received 6 cycles of adjuvant chemotherapy with
VIDE (21): Vincristine 1.5
mg/m2 day 1, Ifosfamide 3 gr/m2 day 1–3,
Doxorubicin 20 mg/m2 day 1–3, Etoposide 150
mg/m2 day 1–3, Uromitexan day 1–3 (intravenous 20% of
ifosfamide dose, per os 40% of ifosfamide dose 2 and 8 h after the
infusion of ifosfamide), inj. GCSF 48MU day 7–14, and
chemoprophylaxis with fluconazole 50 mgx1 day 7–14, ciprofloxacin
250 mgx2 day 7–14 and co-trimoxazole 480 mg day 1–21. During the
chemotherapy patient presented with Grade I nausea, Grade III
anaemia, which was treated with Erythropoiesis stimulating agent
and Grade I stomatitis, which was resolved after the administration
of miconazole oral gel. After the completion of chemotherapy she
received local pelvic radiotherapy (total dose 45 Gy). Forty two
months after her diagnosis she remains free of disease.
Histopathology
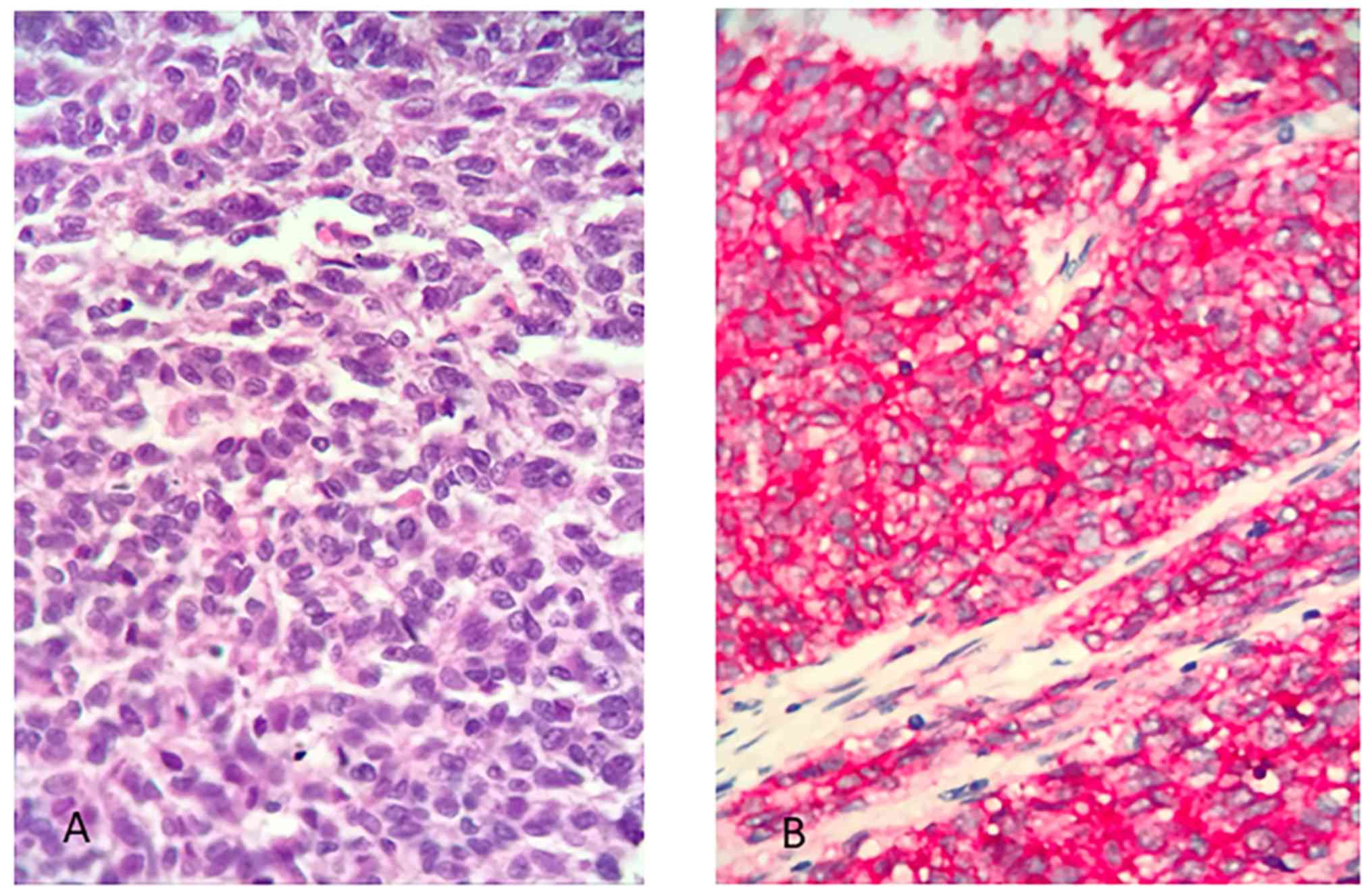
Microscopic examination of the biopsy tissue
specimen showed sheets of small to medium sized round primitive
cells with 5 mitoses/10 HPF, harboring i) immunohistochemical
positive markers: p16 and CD99 and ii) negative markers: SMA,
desmin, S100, AE1/AE3, CD56, p63, LCA, CK18, chromogranin, favoring
the diagnosis of Ewing's sarcoma/PNET. Histopathology slides were
subsequently reviewed by an independent pathologist who described
i) immunohistochemical positivity for Vimentin, Fli-1, MIC-2, EMA
and NSE and ii) negativity for TdT, Desmin, CD7, CD79a, CD10, WT1,
MyoD1, CD3, CD56, S-100, SMA, CEA, PR, ER, caldesmin, CK19, CK7,
CK18, Chromogranin and Synaptophysin, confirming the diagnosis of
Ewing's sarcoma/PNET.
Immunohistochemical staining of the tumor after
total hysterectomy was positive for CD99, CD117 and vimentin, while
it was negative for LCA, HMB-45, desmin, synaptophysin,
chromogranin, keratin5/6 and keratin7. Surgical margins were free
of disease. Lymph nodes were free of metastasis. The overall
immunomorphologic characteristics of the tumor favor the diagnosis
of Ewing's sarcoma/PNET of the cervix (Fig. 2).
Genetics
FISH
Formalin-fixed, paraffin-embedded biopsy blocks from
the specimen were analyzed for the detection of translocations
involving the EWSR gene at 22q12.2. A section of 4 µm was
cut from the selected block and applied to silinized slides.
Additional serial sections from the representative block were
stained with hematoxylin-eosin in order to confirm the presence of
tumor cells and to choose the appropriate area for the
hybridization procedures. A section from normal tissue was used as
negative control. The slides, baked at 60°C for 4 h, deparaffinized
in 2 changes of fresh xylene for 10 min at RT, dehydrated for 5 min
in 100% (twice), 90 and 70% ethanol solutions and allowed to
air-dry before application of the pretreatment kit (Zytolight
FISH-Tissue kit; Zytovision GmbH, Bremerhaven, Germany) according
to the manufacturer's instructions. For hybridization procedures,
the FISH probe ‘ZytoLight SPEC EWSR1 Dual Color Break Apart
(Zytovision GmbH) was used. Probe mixture was applied onto the
areas of interest on the slides according to the manufacturer's
instructions. Target areas were, afterwards, covered with glass
coverslips and sealed with rubber cement. Two post-hybridization
washes were performed in 2× SSC/0.3% NP40. Slides were air-dried
and counterstained using 10 µl DAPI. The prepared slides were
microscopically analyzed soon afterwards. Hybridization signals
were counted by the use of a Zeiss Axioplan fluorescence microscope
(Carl Zeiss AG, Oberkochen, Germany) equipped with the appropriate
filter combination and the ISIS digital imaging system and software
(MetaSystems Hard and Software GmbH, Altlußheim, Germany). The
evaluation of FISH signals meet the following criteria: i) No
overlapping cells are counted, ii) a probe is considered to be
split (break-a-part) when the orange and the green signals are
separated by two times distance greater than the size of one
hybridization signal, iii) a sample is determined to be positive
for EWSR gene translocation if the number of nuclei that
carried the break apart signals exceeds the cutoff of the control
sample.
RT-qPCR
Total RNA was extracted from FFPE sections using
NucleoSpin total RNA FFPE Mini Kit (Macherey-Nagel, GmbH and Co.,
Düren, Germany), according to the manufacturer's instructions.
Approximately 500 ng of total RNA was reverse transcribed using the
SuperScript II Reverse Transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and random hexamers. cDNA was
subjected to TaqMan qPCR analysis, for the detection of the
EWS/Fli-1 fusion genes both type 1 and 2, using Platinum qPCR
Supermix-UDG system (Invitrogen; Thermo Fisher Scientific, Inc.),
with specific primers and PCR conditions as previously described
(22,23).
Results
Histopathology results favor the diagnosis of
Ewing's sarcoma/PNET (Fig. 2). The
results of FISH of the counted nuclei are presented to the Table I. According to Table I, 20% nuclei of the control sample
appeared with break-a-part signals, whilst 60% of the specimen
examined nuclei, carried split signals. These results confirm the
presence EWSR1 gene translocations (Fig.
3).
 | Table I.Results of fluorescence in situ
hybridization analysis. |
Table I.
Results of fluorescence in situ
hybridization analysis.
| Cases | Ex.N | Br-Ap | NORMAL | Non Sp | % ERWR1 |
|---|
| Specimen | 213 | 129 | 35 | 49 | 60.5 |
| Control | 63 | 13 | 39 | 11 | 20.6 |
The type 1 EWS/FLI fusion gene was detected in the
examined specimen sections, which consists of the first seven exons
of EWS joined to exons 6–9 of FLI1 and accounts for approximately
60% of reported Ewing's sarcoma cases (Fig. 3).
Discussion
Extraosseus Ewing's sarcoma is an uncommon
malignancy of mesenchymal origin. Cervical Ewing's sarcoma is a
rare entity with very few cases reported (Table II). Diagnosis of Ewing's/PNET
sarcomas in many cases is a challenge. Especially, occurrence of
Ewing's sarcoma in the female genital tract makes this diagnosis
even more difficult. Herein we present a case of Ewing's sarcoma of
the cervix in a pregnant woman.
 | Table II.Reported cases of cervical Ewing
sarcomas with clinical data, treatment and outcome. |
Table II.
Reported cases of cervical Ewing
sarcomas with clinical data, treatment and outcome.
| Author | Age (years) | Stage | Surgery | RT | Chemotherapy | Outcome (follow
up) | (Refs.) |
|---|
| Horn et
al | 26 | IB1 | TAH+BSO+LND | YES | Cisplatin and 5FU on
metastases | Died 50 months | (14) |
| Cenacchi et
al | 36 | IB2 | TAH without BSO | NO | NO | Alive 18 moths | (8) |
| Pauwels et
al | 45 | IB2 | TAH | YES | NO | Alive 42
months | (7) |
| Tsao et
al | 24 | N/A | TAH+transposition
of the ovaries+LNs | YES | 2 cycles of VAC
alternating with IE | Alive 24
months | (9) |
| Malpica et
al | 35 | IB1 | TAH+BSO+LND | NO | Adjuvant
Chemotherapy not reported | Alive 5 months | (10) |
| Malpica et
al | 51 | IB2 | TAH+BSO+LND | NO | Adjuvant
Chemotherapy not reported | Alive 18
months | (10) |
| Snijders-Keilholz
et al | 21 | IB2 | TAH | NO | Neoadjuvant 6
cycles DIME, Adjuvant 5 Cycles VIA | Alive 27
months | (12) |
| Goda et
al | 19 | N/A | NO | YES | Induction VAC for
further consolidation after RT | Alive on
treatment | (31) |
| Farzaneh et
al | 45 | IB2 | Radical
Hysterectomy | NO | VAC alternating
with IE Neoadjuvant and Adjuvant | Alive 4 years | (15) |
| Arora et
al | 23 | N/A | TAH+BSO+LND | YES | Neoadjuvant 1 cycle
of VAC followed by 2 cycles of etoposide-cisplatin | Alive 4 years | (6) |
| Masoura et
al | 23 | IV | TAH+BSO | NO | Adjuvant Cisplatin
1 cycle | Died 12 days | (16) |
| Li et
al | 27 | IIIB | NO | YES | Alternating VAC
with IE | Alive 6 months | (11) |
| Khosla et
al | 28 | IB2 | TAH+BSO+LND,
Termination of pregnancy | NO | Adjuvant VAC | Alive 33
months | (13) |
| Xiao et
al | 52 | IIA | TAH+BSO+LND | N/A | PVB 2 cycles | Died 9 months | (32) |
| Xiao et
al | 59 | IVB | TAH+BSO+LND | N/A | NO | Died | (32) |
| Mashriqi et
al | 49 | IIB | TAH+BSO | YES | Adjuvant VAC
alternating with IE | Died 10 months | (2) |
| Horn et
al | 57 | IV | NO | YES | VIDE with VIA | Alive 18
months | (33) |
In our case histopathology was complemented with
genetic analysis to further confirm the diagnosis of Ewing's
sarcoma. FISH and Real time PCR revealed the characteristic fusion
gene EWS-FLI1. The presence of this fusion is associated with a
better prognosis and a more favorable clinical outcome (24). However, with the use of new
chemotherapeutic regimens, non-type 1 fusion type carriers seem to
share similar prognosis with patients harboring EWS/FLI1 chimeric
gene. In the rare occasion of Ewing sarcomas of the female genital
tract there are no data regarding molecular prognostication.
The patient remains free of disease after 42 months
of follow up. Ewing sarcoma is an aggressive tumor with generally
poor prognosis. Our case however, harbored some favorable
clinicopathological characteristics. Mitotic index of her tumor was
5 mitoses/10 HPF indicating limited proliferation status. Several
studies have shown that high mitotic index is an independent
prognostic factor for sarcomas (25–28).
Additionally, our patient presented with Figo Stage IB2 disease.
Early tumor stage is another favorable prognostic factor confirmed
in several reported studies (26,27,29).
Additionally, our patient was treated with radical hysterectomy and
lymphadenectomy due to the large size of the tumor, in order to
obtain clear margins (parametrium, upper vagina and sacro-uterine
legaments) and avoid any lymph node involvement. However, it is
difficult to support that lymphadenectomy has any added value to
the prognosis of our patient.
Literature review revealed a few cases of cervical
Ewing sarcoma which were treated with several chemotherapeutic
regimens (Table II). The
heterogeneity of the used regimens reflect the rarity of the
disease and the evolution of multiagent chemotherapy for Ewing
Sarcoma the last few decades (30–33). Our
case is the only one, which was initially treated with radical
hysterectomy and afterwards the patient has been treated with
adjuvant VIDE, a chemotherapeutic option commonly used in
extraosseus Ewing's sarcomas (21).
Our patient was pregnant and her diagnosis was an incidental
finding during her scheduled routine prenatal ultrasound. In the
current literature, that extraosseus Ewing sarcoma diagnosis during
pregnancy has been reported in 6 cases (13,20).
Only 5 of these cases received chemotherapy during pregnancy. The
chemotherapeutic regimens used were: Doxorubicin-ifosfamide,
actinomycine
D-cyclophosphamide-vincristine-bleomycin-vincristine-doxorubicin,
doxorubicin-cyclophosphamide-vincristine, VIDE scheme followed by
Vincristine Adriamycin Cyclophosphamide (VAC). The latter
combination caused the abortion due to oligohydramnion. Since our
patient decided to end her pregnancy there was no clinical dilemma
regarding the selection of the chemotherapeutic regimen. The
biological mechanism by which pregnancy might be connected with
Ewing sarcoma or cervical neoplasia is vague. However, there are
data indicating that Ewing's sarcoma precursors are highly enriched
in embryonic osteochondrogenic progenitors therefore providing
clues to the histogenesis of Ewing's sarcoma (34). In our case the patient presented to
our Department post-operatively and neoadjuvant schemes could not
be administered to her.
To conclude, we present a case of cervical Ewing
sarcoma, which was diagnosed during pregnancy. Diagnostic approach
included both immunohistochemistry and genetic characterization of
the tumor. This is a patient that was treated aggressively with
tri-modality therapy according to Ewing's sarcoma experience and
she is currently free of disease 3.5 years after her diagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AK analyzed the patient, undertook analysis of the
clinical, radiological and laboratory results and wrote the
manuscript. GT also analyzed the patient, and analyzed the
clinical, radiological and laboratory results. ML analyzed the
patient, analyzed the clinical, radiological and laboratory results
and drafted the manuscript. AP and LM analyzed and interpreted the
molecular genetics and FISH tests and drafted the manuscript. GM
and IP analyzed and interpreted the pathology tests and drafted the
manuscript. NT analyzed, and operated on the patient, and
contributed to the analysis of the clinical, radiological and
laboratory results and drafted the manuscript Finally, AB analyzed
the patient, analyzed the clinical, radiological and laboratory
results and drafted the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Alexandra Hospital has
approved this study and the patient has signed form of consent for
the analysis and publication of her data.
Patient consent for publication
The patient has provided consent for the analysis
and publication of her data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fadare O: Uncommon sarcomas of the uterine
cervix: A review of selected entities. Diagn Pathol. 1:302006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mashriqi N, Gujjarlapudi JK, Sidhu J, Zur
M and Yalamanchili M: Ewing's sarcoma of the cervix, a diagnostic
dilemma: A case report and review of the literature. J Med Case
Rep. 9:2552015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Applebaum MA, Worch J, Matthay KK, Goldsby
R, Neuhaus J, West DC and Dubois SG: Clinical features and outcomes
in patients with extraskeletal Ewing sarcoma. Cancer.
117:3027–3032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Alava E and Gerald WL: Molecular
biology of the Ewing's sarcoma/primitive neuroectodermal tumor
family. J Clin Oncol. 18:204–213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato S, Yajima A, Kimura N, Namiki T,
Furuhashi N and Sakuma H: Peripheral neuroepithelioma (peripheral
primitive neuroectodermal tumor) of the uterine cervix. Tohoku J
Exp Med. 180:187–195. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arora N, Kalra A, Kausar H, Ghosh TK and
Majumdar A: Primitive neuroectodermal tumour of uterine cervix-a
diagnostic and therapeutic dilemma. J Obstet Gynaecol. 32:711–713.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pauwels P, Ambros P, Hattinger C, Lammens
M, Dal Cin P, Ribot J, Struyk A and van den Berghe H: Peripheral
primitive neuroectodermal tumour of the cervix. Virchows Arch.
436:68–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cenacchi G, Pasquinelli G, Montanaro L,
Cerasoli S, Vici M, Bisceglia M, Giangaspero F, Martinelli GN and
Derenzini M: Primary endocervical extraosseous Ewing's
sarcoma/PNET. Int J Gynecol Pathol. 17:83–88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsao AS, Roth LM, Sandler A and Hurteau
JA: Cervical primitive neuroectodermal tumor. Gynecol Oncol.
83:138–142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malpica A and Moran CA: Primitive
neuroectodermal tumor of the cervix: A clinicopathologic and
immunohistochemical study of two cases. Ann Diagn Pathol.
6:281–287. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li B, Ouyang L, Han X, Zhou Y, Tong X,
Zhang S and Zhang Q: Primary primitive neuroectodermal tumor of the
cervix. Onco Targets Ther. 6:707–711. 2013.PubMed/NCBI
|
|
12
|
Snijders-Keilholz A, Ewing P, Seynaeve C
and Burger CW: Primitive neuroectodermal tumor of the cervix uteri:
A case report-changing concepts in therapy. Gynecol Oncol.
98:516–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khosla D, Rai B, Patel FD, Sreedharanunni
S, Dey P and Sharma SC: Primitive neuroectodermal tumor of the
uterine cervix diagnosed during pregnancy: A rare case with review
of literature. J Obstet Gynaecol Res. 40:878–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horn LC, Fischer U and Bilek K: Primitive
neuroectodermal tumor of the cervix uteri. A case report. Gen Diagn
Pathol. 142:227–230. 1997.PubMed/NCBI
|
|
15
|
Farzaneh F, Rezvani H, Boroujeni PT and
Rahimi F: Primitive neuroectodermal tumor of the cervix: A case
report. J Med Case Rep. 5:4892011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masoura S, Kourtis A, Kalogiannidis I,
Kotoula V, Anagnostou E, Angelidou S and Agorastos T: Primary
primitive neuroectodermal tumor of the cervix confirmed with
molecular analysis in a 23-year-old woman: A case report. Pathol
Res Pract. 208:245–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Gao Y, Xu Y, Liu Y and Qu P:
Primary primitive neuroectodermal tumor of the cervix: A report of
two cases and review of the literature. Mol Clin Oncol. 6:697–700.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
ESMO/European Sarcoma Network Working
Group, : Soft tissue and visceral sarcomas: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 25
(Suppl 3):iii102–iii112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Demetri GD, Baker LH, Beech D, Benjamin R,
Casper ES, Conrad EU III, DeLaney TF, Ettinger DS, Heslin MJ,
Hutchinson RJ, et al: Soft tissue sarcoma clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 3:158–194.
2005.PubMed/NCBI
|
|
20
|
Schur S, Wild J, Amann G, Kostler W,
Langer M and Brodowicz T: Sarcoma of the ewing family in pregnancy:
A case report of intrauterine fetal death after induction of
chemotherapy. Case Rep Oncol. 5:633–638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strauss SJ, McTiernan A, Driver D,
Hall-Craggs M, Sandison A, Cassoni AM, Kilby A, Michelagnoli M,
Pringle J, Cobb J, et al: Single center experience of a new
intensive induction therapy for ewing's family of tumors:
Feasibility, toxicity, and stem cell mobilization properties. J
Clin Oncol. 21:2974–2981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lewis TB, Coffin CM and Bernard PS:
Differentiating Ewing's sarcoma from other round blue cell tumors
using a RT-PCR translocation panel on formalin-fixed
paraffin-embedded tissues. Mod Pathol. 20:397–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peter M, Gilbert E and Delattre O: A
multiplex real-time pcr assay for the detection of gene fusions
observed in solid tumors. Lab Invest. 81:905–912. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Alava E, Panizo A, Antonescu CR, Huvos
AG, Pardo-Mindán FJ, Barr FG and Ladanyi M: Association of EWS-FLI1
type 1 fusion with lower proliferative rate in Ewing's sarcoma. Am
J Pathol. 156:849–855. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng W, Malpica A, Robboy SJ, Gudlaugsson
E, Hua K, Zhou X and Baak JP: Prognostic value of the diagnostic
criteria distinguishing endometrial stromal sarcoma, low grade from
undifferentiated endometrial sarcoma, 2 entities within the
invasive endometrial stromal neoplasia family. Int J Gynecol
Pathol. 32:299–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lusby K, Savannah KB, Demicco EG, Zhang Y,
Ghadimi MP, Young ED, Colombo C, Lam R, Dogan TE, Hornick JL, et
al: Uterine leiomyosarcoma management, outcome, and associated
molecular biomarkers: A single institution's experience. Ann Surg
Oncol. 20:2364–2372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pautier P, Genestie C, Rey A, Morice P,
Roche B, Lhommé C, Haie-Meder C and Duvillard P: Analysis of
clinicopathologic prognostic factors for 157 uterine sarcomas and
evaluation of a grading score validated for soft tissue sarcoma.
Cancer. 88:1425–1431. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gremel G, Liew M, Hamzei F, Hardell E,
Selling J, Ghaderi M, Stemme S, Pontén F and Carlson JW: A
prognosis based classification of undifferentiated uterine
sarcomas: Identification of mitotic index, hormone receptors and
YWHAE-FAM22 translocation status as predictors of survival. Int J
Cancer. 136:1608–1618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng W, Malpica A, Skaland I, Gudlaugsson
E, Robboy SJ, Dalen I, Hua K, Zhou X and Baak JP: Can proliferation
biomarkers reliably predict recurrence in World Health Organization
2003 defined endometrial stromal sarcoma, low grade? PLoS One.
8:e758992013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grier HE, Krailo MD, Tarbell NJ, Link MP,
Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers
PA, et al: Addition of ifosfamide and etoposide to standard
chemotherapy for Ewing's sarcoma and primitive neuroectodermal
tumor of bone. N Engl J Med. 348:694–701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goda J, Mayur BN, Pramod PK and Udayan K:
Primitive neuroectodermal tumour of the Cervix: A rare entity. Int
J Radiol. 6:2006.
|
|
32
|
Xiao C, Zhao J, Guo P, Wang D, Zhao D, Ren
T, Yang J, Shen K, Lang J, Xiang Y and Cui Q: Clinical analysis of
primary primitive neuroectodermal tumors in the female genital
tract. Int J Gynecol Cancer. 24:404–409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bílek O, Holánek M, Zvaríková M, Fabian P,
Robešová B, Procházková M and Adámková Krákorová D: Extraoseus
ewings sarcoma, primary affection of uterine cervix-case report.
Klin Onkol. 28:284–287. 2015.(In Czech). View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanaka M, Yamazaki Y, Kanno Y, Igarashi K,
Aisaki K, Kanno J and Nakamura T: Ewing's sarcoma precursors are
highly enriched in embryonic osteochondrogenic progenitors. J Clin
Invest. 124:3061–3074. 2014. View
Article : Google Scholar : PubMed/NCBI
|