Introduction
Lung cancer is one of the most devastating diseases
worldwide. Neutrophil infiltration is frequently observed in lung
cancer tissues (1). Neutrophil
extracellular traps (NETs), composed of extracellular DNA,
hypercitrullined histones and antimicrobial enzymes from
neutrophils, may increase the adhesion of cancer cells (2) and sequester lung cancer cells in the
blood (3). NET formation has
previously been described in patients with lung cancer (4). However, the mechanisms regulating the
formation of NETs associated with lung cancer are yet to be fully
elucidated.
Diverse stimuli have been suggested to initiate the
formation of NETs, ranging from pathogen components (5) to neutrophil antibodies (6) and activated platelets (7). In addition, interleukin (IL)-1β
(8), IL-8 (9) or granulocyte colony stimulating factor
(G-CSF) (10) in the tumor
microenvironment may also promote NET formation. As a
damage-associated molecular pattern protein, high mobility group
box 1 (HMGB1) serves a paradoxical role in regulating cell death
and survival in tumor development (11). HMGB1 interactions with Toll-like
receptor 4 (TLR4) have been demonstrated to induce NETs (12). Therefore, it was hypothesized that
lung cancer cells may release HMGB1, which may induce NET
formation.
Morphine is an effective analgesic for
cancer-associated pain. In the end-stages of lung cancer,
continuous morphine infusion is used to alleviate pain (13). Although pain adversely affects the
prognosis of patients with lung cancer, morphine administration
controls the pain but does not improve survival (14). Arguably, morphine may stimulate
angiogenesis and promote tumor progression (15,16).
HGMB1 and morphine are able to bind with TLR4 (17,18). The
present study aimed to evaluate the role of HMGB1 from lung cancer
cells in the formation of NETs. In addition, the effect of morphine
on HMGB1-induced NETs was investigated.
Materials and methods
Animals and ethics statement
In total, 40 wild-type female ICR mice (age matched
8–10 weeks old) weighing 29–32 g were purchased from Yangzhou
University (Yangzhou, China) and bred in the animal facility of
Nanjing Medical University (Nanjing, China) under standard
laboratory conditions (12:12 h light:dark cycle, relative humidity
60±5%, temperature 25±2°C) in individually ventilated cages without
restriction to water or food. All animal procedures were approved
by The Institutional Animal Care Committee of Nanjing Medical
University.
Lewis lung carcinoma (LLC) cell
culture and flow cytometry
The murine LLC cell line was purchased from the Cell
Bank of the Shanghai Institute for Biological Sciences, Chinese
Academy of Sciences. LLC cells were maintained in high-glucose
Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life
Sciences) supplemented with penicillin (100 U/ml), streptomycin
(100 µg/ml) and 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in
a 5% CO2 humidified atmosphere at 37°C.
Cells which had adhered to the base of the T-25
flask were dislodged by aspiration several times with culture
medium. The supernatants were used to stimulate neutrophils for
NETs as described subsequently. The LLC cells were resuspended in
PBS buffer with 1% BSA (Sigma-Aldrich; Merck KGaA). Aliquots
containing 1×105 cells in 100 µl buffer were stained
with 10-µl propidium iodide (50 mg/ml) solution and with 5 µl
fluorescein isothiocyanate-conjugated Annexin V (17.6 mg/ml; BD
Biosciences) for 5 min at 37°C. Following staining, 400 µl PBS was
added to the cells. Immediately, flow cytometry analysis was
performed using a FACScan flow cytometer (BD Biosciences). All FACS
data were analyzed using FlowJo v10 software (FlowJo LLC).
Quantification of NETs released from
neutrophils
Terminal anesthesia was performed by intraperitoneal
injection of a mixture of 10 mg/kg xylazine (MTC Pharmaceuticals)
and 100 mg/kg ketamine hydrochloride (Rogar/STB; Pfizer Canada,
Inc.) prior to sacrifice by cervical dislocation. Following
sacrifice, the femur and the tibia from the two hind legs were
removed and the extreme distal tip of each extremity was cut off.
PBS solution was forced through the bone with a 1 ml syringe.
Following ammonium chloride erythrocyte lysis, murine neutrophils
were prepared by Histopaque-based density gradient centrifugation,
as described previously (19).
Murine neutrophils were unstimulated or challenged
with LLC cell supernatants for 2, 4 or 8 h. Following incubation,
the non-cell-permeable DNA dye Sytox Green (5 µM; Invitrogen;
Thermo Fisher Scientific, Inc.) was used to quantify the released
NETs in the supernatants as described previously (20). The samples were examined with a
fluorometric reader Infiniti M200 (Tecan Group, Ltd.) using an
excitation wavelength of 488 nm and an emission wavelength of 523
nm, as described previously (4).
Concomitantly, prior to stimulation with LLC cell
supernatants, neutrophils were pretreated with the following
specific inhibitors for 30 min at 37°C: HMGB1 inhibitor
Glycyrrhizin glycyrrhizic acid (GA; 10 µM; Selleck Chemicals), TLR4
inhibitor C34 (10 µM; Tocris Bioscience), extracellular
signal-regulated kinase (ERK) inhibitor UO126 (50 µM; Cell
Signaling Technology, Inc.) and p38 mitogen-activated protein
kinases (p38 MAPKs) inhibitor SB203580 (10 µM; Selleck Chemicals).
For the morphine experiments, neutrophils were pretreated with
morphine (10 nM; Sigma-Aldrich; Merck KGaA) or naloxone (100 nM;
Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. Then, neutrophils
were incubated with LLC cell supernatants for 4 h.
Western blot analysis
Following incubation, neutrophils were collected and
centrifuged for 5 min at 500 × g and 4°C. Cell pellets were lysed
in radioimmunoprecipitation assay lysis buffer (Beyotime Institute
of Biotechnology) on ice for 5 min. Then, the cell suspensions were
centrifuged for 10 min at 12,000 × g and 4°C. The supernatant was
solubilized in 5X SDS-PAGE sample buffer and boiled for 10 min. The
protein concentration in each sample was quantified using a
NanoDrop 2000 with NanoDrop One (v1.6; both from Thermo Fisher
Scientific, Inc.) and 30 µg protein per lane was resolved using a
10% SDS-PAGE, transferred by electrophoresis onto a polyvinylidene
fluoride membrane (EMD Millipore), blocked with 5% BSA for 1 h at
room temperature and probed with the following antibodies at
1:1,000 dilution overnight at 4°C: Anti-Histone H3 (cat. no. 4499s;
Cell Signaling Technology, Inc.), anti-histone3 (cat. no. ab5103;
citrulline R2+R8+R17; Abcam), MAPK/Phospho-MAPK family antibody
(cat. no. 9926; Cell Signaling Technology, Inc.),
anti-TIR-domain-containing adapter-inducing interferon-β (TRIF)
antibody (cat. no. ab13810, Abcam), anti-HMGB1 antibody (cat. no.
10829-1-AP, ProteinTech Group, Inc., Chicago, IL, USA) and
anti-myeloid differentiation factor 88 (MyD88) antibody (cat. no.
4283; Cell Signaling Technology, Inc.). This was followed by
incubation with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG (dilution 1:10,000; cat. no. ab6721; Abcam) in PBS
with 0.5% BSA for 1 h at room temperature. Signals were developed
and analyzed using the chemiluminescent horseradish peroxidase
substrate (EMD Millipore) and the G:BOX system (Syngene Europe).
Grayscale analysis was performed using Adobe Photoshop CS6 (v13.0
×32; Adobe Systems Europe, Ltd., Maidenhead, UK).
Immunofluorescence microscopy
Neutrophils (1×106) were seeded on
glass-bottomed dishes (Shanghai Jing An biological science and
Technology Co., Ltd.). According to the aforementioned method, LLC
cell supernatants or different inhibitors were added. Following 4 h
of incubation, cells that adhered to the bottom of the glass were
carefully fixed with ice-cold acetone (≥99%) for 10 min at room
temperature. The samples were blocked with 5% goat serum (cat. no.
16210072, Gibco, Thermo Fisher Scientific, Inc.) and stained
overnight at 4°C with rabbit polyclonal antibody against Histone3
(cat. no. ab5103, citrulline R2+R8+R17; 1:300; Abcam). The samples
were washed in PBST and stained with Alexa Fluor® 555
goat anti-rabbit antibody (1:500; cat. no. A-21428; Thermo Fisher
Scientific, Inc.). DNA in the samples was stained with Sytox Green
(Invitrogen; Thermo Fisher Scientific, Inc.; 1:10,000) for 30 min
at room temperature. Images were captured using Carl Zeiss confocal
microscopes (Carl Zeiss AG) with appropriate lenses and filters
(magnification, 200×).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 (GraphPad Software, Inc.). Data are presented as the mean ±
standard error of the mean. One-way analysis of variance and a
post-hoc Tukey's honest significant difference test was used to
compare multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
HMGB1 from LLC cells induces NETs
In the complete culture medium from LLC cells, HGMB1
was detected (Fig. 1A). As expected,
in the complete medium, only a small number of tumor cells
underwent apoptosis or necrosis (Fig.
1B). Therefore, it was determined that LLC cells actively
released HGMB1 without exogenous stimulus.
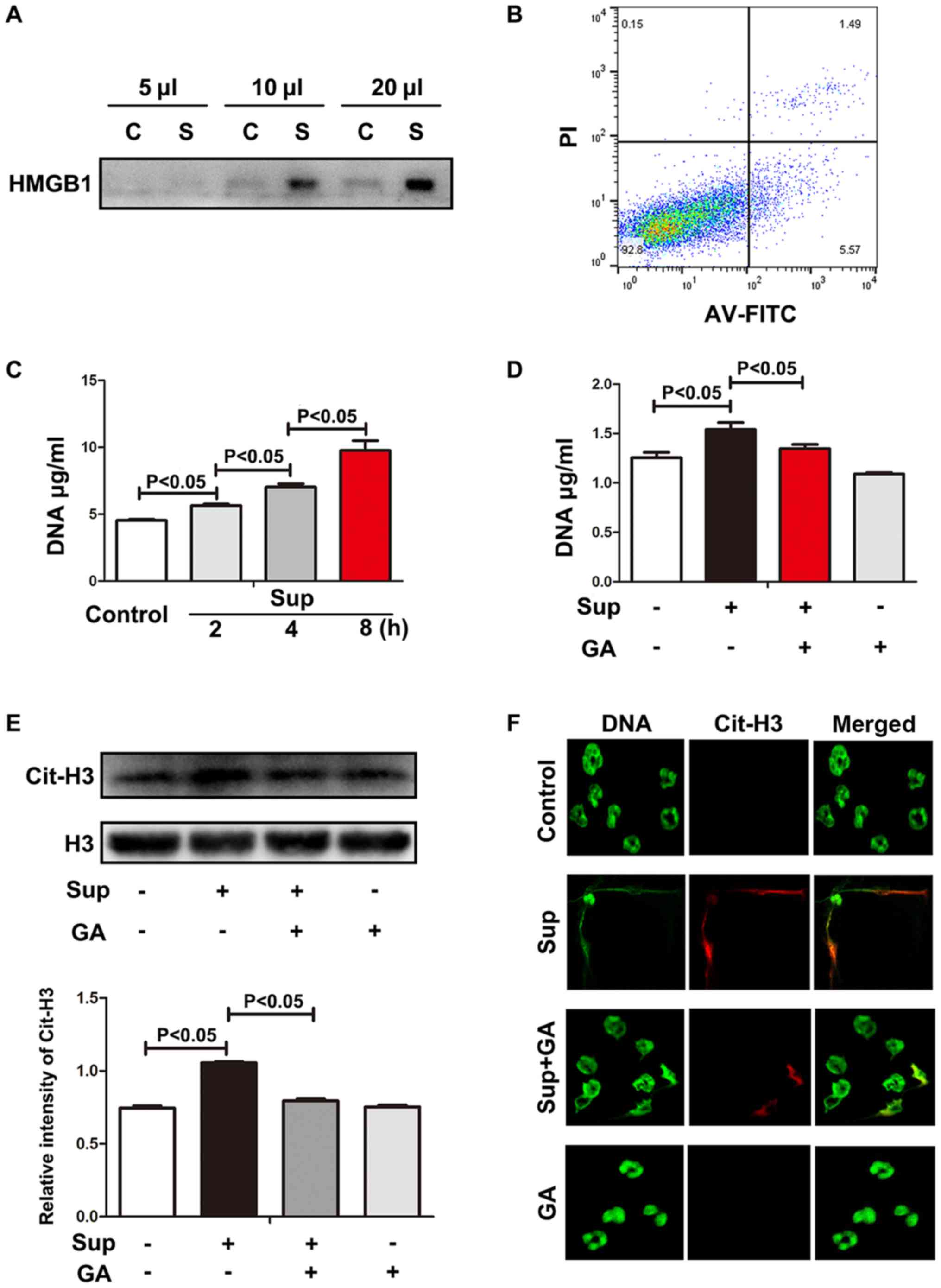 | Figure 1.HMGB1 from LLC cells induces NETs.
(A) HMGB1 from LLC supernatant was measured using western blot
analysis. (B) Cell viability was evaluated using flow cytometry.
(C) Neutrophils were treated with LLC supernatant and exDNA was
detected using Sytox Green. (D) HMGB1 inhibitor decreased exDNA.
(E) Levels of histone 3 and citrullinated histone 3 were measured
using western blot analysis. Grayscale analysis verified the
repeated results. (F) Images of NETs stained with Sytox Green and
citrullinated histone 3 were captured using confocal microscopy.
Magnification, 200×. LLC, Lewis lung cancer; HMGB1, high mobility
group box 1; MLE, Murine Lung Epithelial; C, MLE cell culture
supernatant; S/Sup, LLC culture supernatant; AV-FITC,
Annexin-V-fluorescein isothiocyanate; PI, propidium iodide; exDNA,
extracellular DNA; NETs, neutrophil extracellular traps; Cit-H3,
citrullinated histone 3; GA, glycyrrhizic acid. |
As recombinant HMGB1 is able to induce NETs
(12), it was hypothesized that LLC
cell supernatants containing HMGB1 may also trigger the formation
of NETs. Neutrophils were treated with LLC cell supernatants and
the extracellular DNA (exDNA) was measured using DNA dye Sytox
Green. As indicated in Fig. 1C,
exDNA was progressively increased, suggesting that upon LLC cell
supernatant challenge, neutrophils produced exDNA during the cell
culture for 8 h. To verify whether HMGB1 was involved with exDNA
production, HMGB1 inhibitor GA was added to the neutrophil culture.
GA significantly alleviated the exDNA production evoked by LLC cell
supernatant (Fig. 1D), suggesting
that the role of LLC cell supernatant in exDNA induction was at
least partially dependent on HMGB1.
exDNA may originate from necrotic neutrophils or
neutrophils with NETs. However, necrosis may be differentiated from
neutrophils with NETs due to the observation of histone
hypercitrullination in NETs (21).
Therefore, histone hypercitrullination was evaluated in the
neutrophils treated with LLC cell supernatant. As indicated in
Fig. 1E, hypercitrullinated histone
3 expression was significantly increased in the neutrophils treated
with LLC cell supernatant. In addition, HMGB1 inhibitor rescued the
deleterious effects of LLC cell supernatant. Under confocal
microscopy, LLC cell supernatant-treated neutrophils were observed
to produce exDNA overlaid with hypercitrullinated histone 3, which
was alleviated by treatment with HMGB1 inhibitor GA (Fig. 1F). These results indicate that lung
cancer cells actively release HMGB1, which directly promotes the
formation of NETs.
TLR4 is required for lung cancer
cell-induced NETs
As a damage-associated molecular pattern protein,
soluble HMGB1 may bind with diverse receptors, including TLR4, the
receptor for advanced glycation end products, macrophage adhesion
molecule-1, receptor-type protein-tyrosine phosphatase-ζ/β,
chemokine (C-X-C motif) ligand 4, T-cell immunoglobulin mucin-3,
cluster of differentiation 24 and syndecan 1 (11). Among these potential receptors, TLR4
is highly expressed on neutrophils (22) and closely associated with NETs. In
bacterial sepsis, platelet TLR4 detected ligands and promoted NETs
(7). Furthermore, NETs induced by
recombinant HMGB1 were dependent on TLR4 (12). Therefore, in the present study, the
role of TLR4 in LLC cell supernatant-induced NETs was explored.
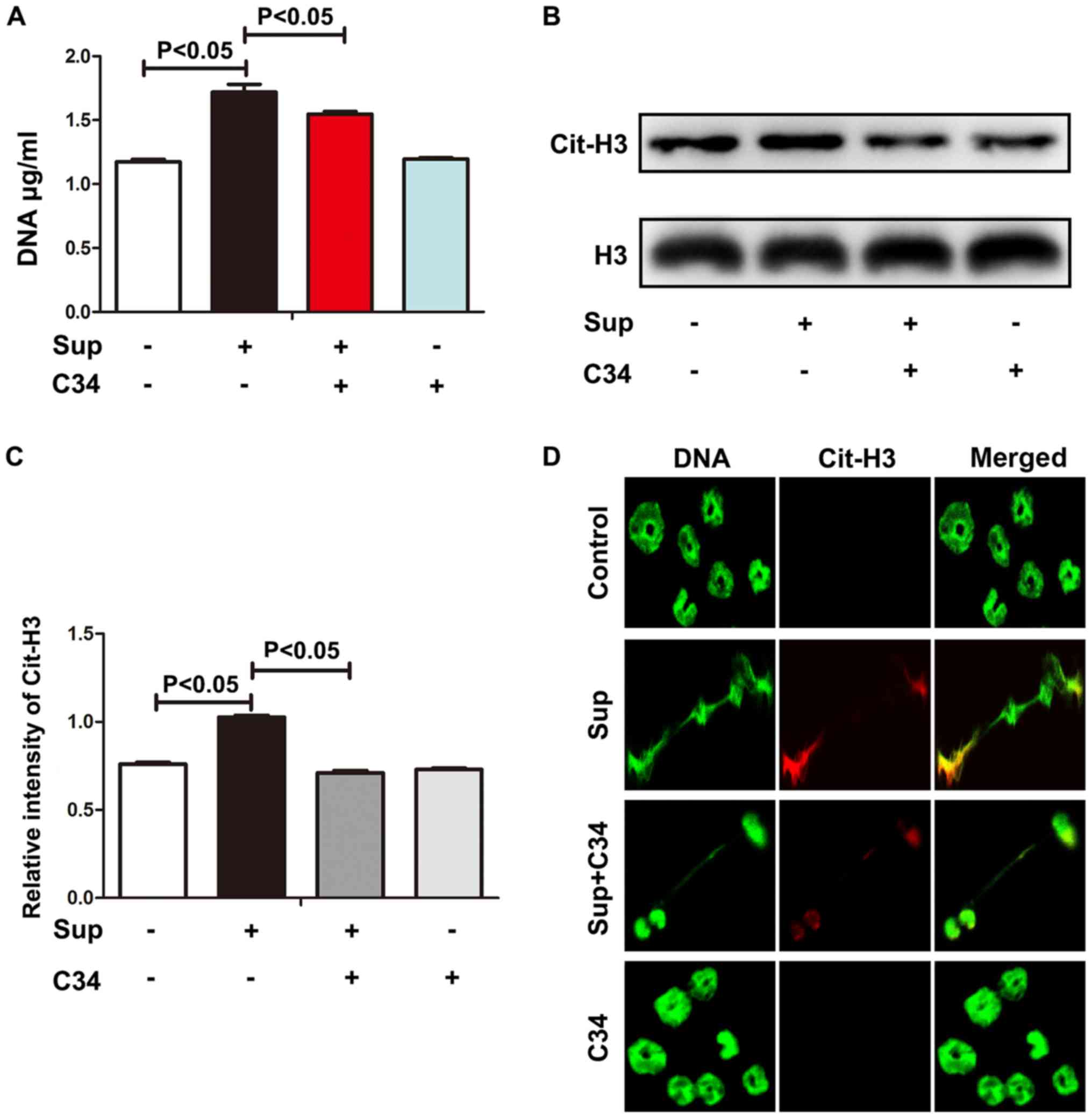
C34 is a selective TLR4 inhibitor (23). ExDNA and histone 3
hypercitrullination were significantly decreased upon C34 treatment
(Fig. 2A-C), suggesting that TLR4
may be required in LLC cell-induced NETs. Consistent with the
observations of exDNA and histone 3 hypercitrullination, C34 also
diminished NET formation as observed by confocal microscopy
(Fig. 2D). Although C34 selectively
targets TLR4 (23), TLR4 knockout
neutrophils may be required to confirm whether LLC cell-induced
NETs was via TLR4. Nevertheless, these results indicate that
TLR4-HMBG1 may be required for lung cancer cell-induced NETs.
MAPK pathway is involved in lung
cancer cell-induced NETs
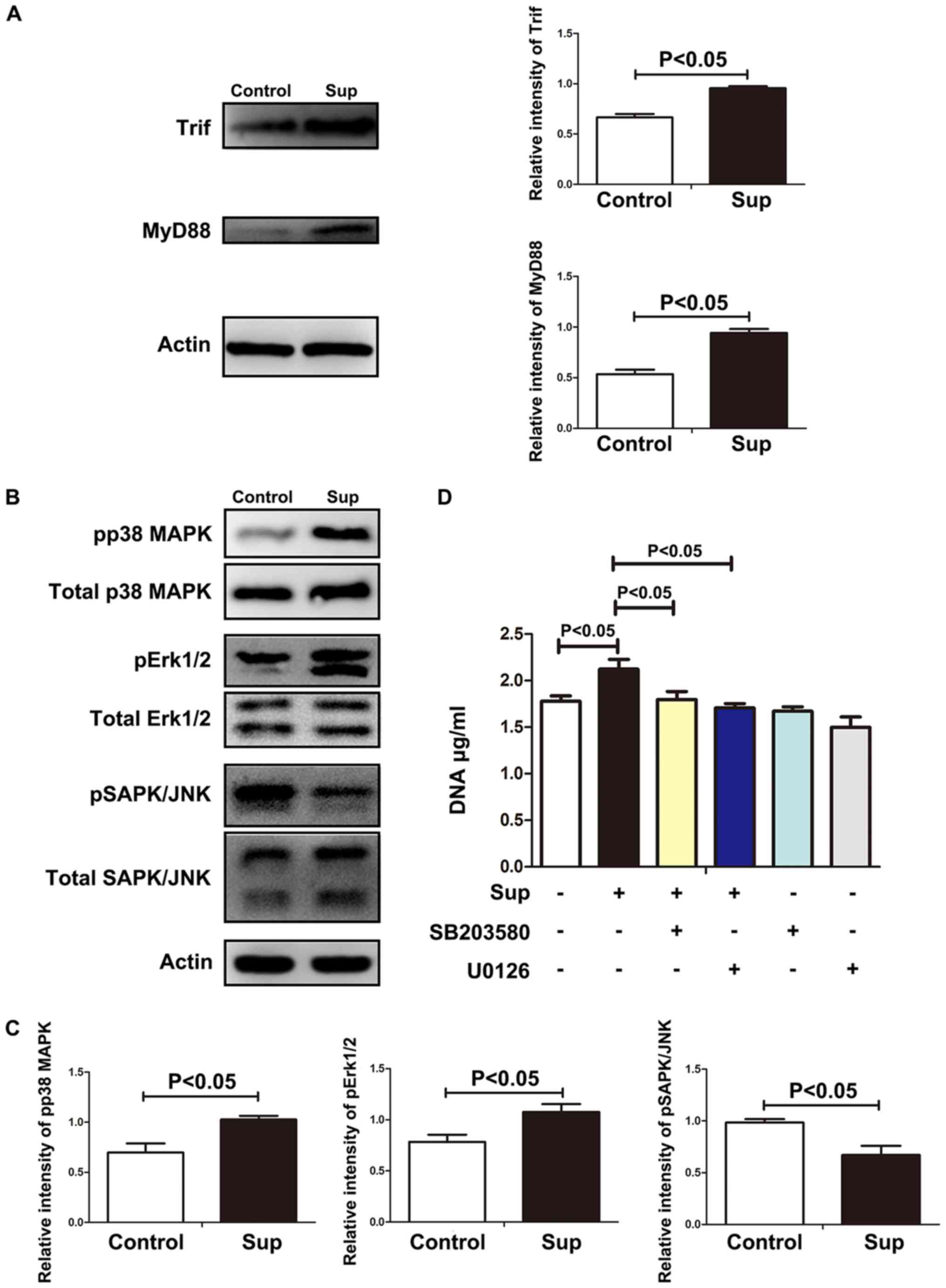
Soluble HMGB1, once bound with TLR4, may trigger
signal transduction via MyD88 or TRIF (24). As indicated in Fig. 3A, treatment with LLC cell
supernatants resulted in the significant increase of MyD88 and
TRIF. Once bound with the cytoplasmic portion of TLR4, Myd88
recruits nuclear factor-κB and MAPK (25), which have been demonstrated to be
essential in NET formation (26,27). As
indicated in Fig. 3B and C,
phosphorylation of p38 MAPKs, ERK or Janus kinase was significantly
increased in the neutrophils treated with lung cancer cell
supernatants. Furthermore, p38 MAPKs inhibitor sb203580 or ERK
inhibitor U0126 significantly decreased the level of NETs induced
by lung cancer cell supernatants (Fig.
3D), suggesting that p38 MAPKs and ERK were involved in lung
cancer cell-induced NETs. These results indicate that HMGB1 induced
NET formation via TLR4 and p38 MAPKs/ERK.
Morphine promotes lung cancer
cell-induced NETs
The aforementioned results indicate that HMGB1
released from lung cancer cells induces NETs via the TLR4/MAPK
signaling pathway. To alleviate cancer-associated pain, patients
with lung cancer may be administered morphine, which also binds
with TLR4 (17,18). Therefore, neutrophils infiltrated
into lung tissues may be stimulated by HMGB1 and morphine. To
explore the combinational effects of morphine and HMGB1 on NETs,
neutrophils were treated with morphine and LLC cell supernatants.
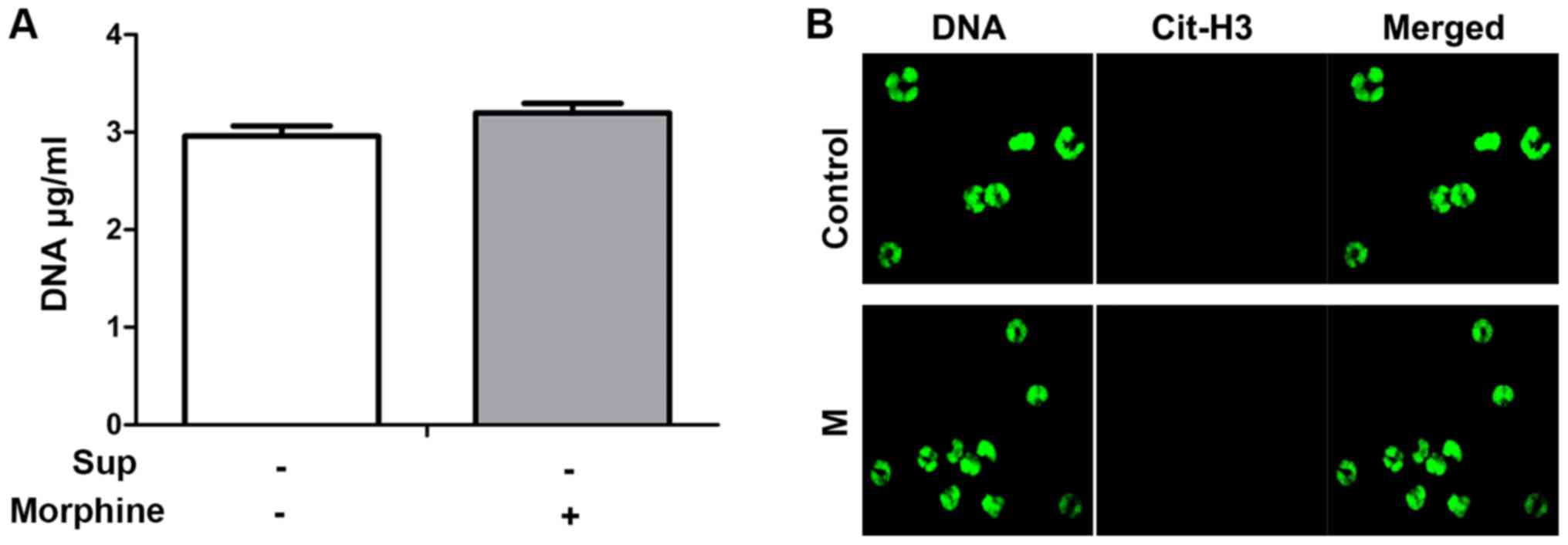
In the preliminary experiment, morphine alone did not evoke the
formation of NETs (Fig. 4). However,
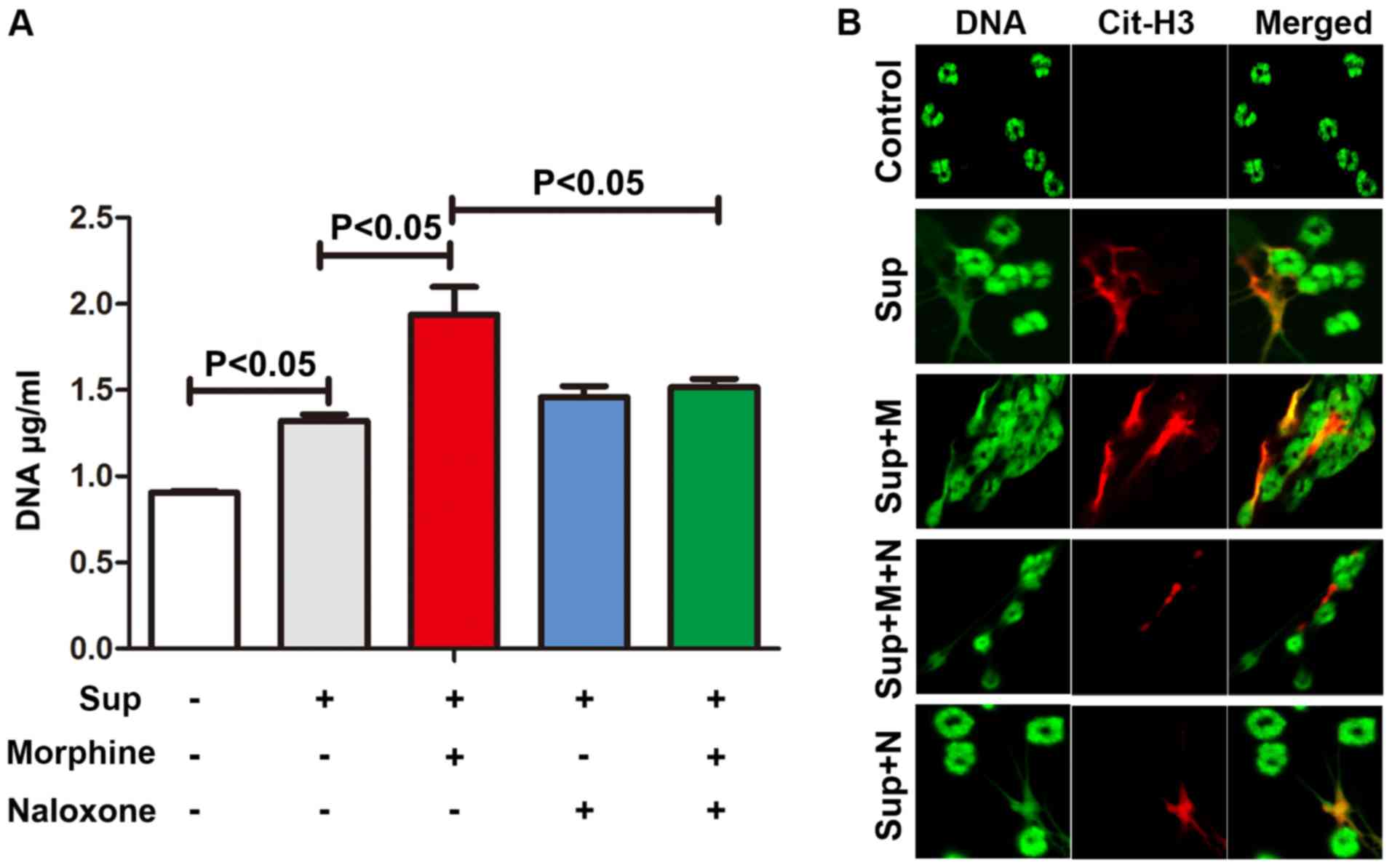
morphine augmented the formation of NETs induced by lung cancer
cell supernatants (Fig. 5).
Naloxone, an antagonist of morphine, significantly inhibited the
effect of morphine on NET induction, suggesting that opioid
receptors may also be involved. In summary, these results indicate
that morphine may promote lung cancer cell-induced NETs.
Discussion
Increased levels of HMGB1 are associated with
increased disease severity in patients with non-small cell lung
cancer (28,29). In chemotherapy, HMGB1 passively
released from necrotic cancer cells may increase invasion and
metastasis. Cancer cells may also actively secrete HMGB1 upon
exogenous and endogenous stimuli (11). Although HMGB1-stimulated NETs have
been described previously (12), the
role of this in cancer remains unclear. The present study provided
evidence that HMGB1 from cancer cells may contribute to NET
formation.
Once bound with neutrophil TLR4, HMGB1 induces the
activation of Myd88 and TRIF. Although neutrophils express TRIF, it
has been demonstrated that the TLR4 ligand lipopolysaccharide is
not able to mobilize the TRIF signaling pathway, indicating that
TRIF may not be directly involved with neutrophil TLR4 activation
(30). In ischemia-reperfusion
injury, HMGB1-TLR4-mediated acute cerebral infarct was identified
to be TRIF-independent (31).
Therefore, the present study focused on TLR4-Myd88 signal
transduction initiated by HMGB1 in lung cancer cell supernatants.
Activated platelets induced NETs in a pathway that involved TLR4
but was independent of p38 MAPKs (32). In inflammatory disease, oxidized
low-density lipoprotein triggered the activation of p38 MAPKs/ERK
and the formation of NETs through TLR2 and TLR6 (33). TLR2/TLR6 is also able to bind with
HMGB1 (34). Therefore, signal
transduction in the formation of NETs may vary depending on the
stimulus. In the present study, it was demonstrated that HMGB1 from
lung cancer cells induced NETs, which was at least partially
dependent on the TLR4 and p38 MAPKs/ERK signaling pathway.
As an analgesic for treating severe pain, morphine
may suppress the immune response, impairing the function of T cells
and macrophages (35). In addition,
neutrophils from patients with sepsis are able to release
endogenous morphine, which may inhibit inflammation (36). The present study aimed to explore
whether morphine contributed to the formation of NETs. In
combination with supernatants from lung cancer cells, morphine may
aggravate the formation of NETs. In the end-stages of lung cancer,
HMGB1 from lung cancer cells and exogenous morphine administration
may synergistically fuel the formation of NETs and cancer
progression. It would be useful to investigate whether lung cancer
cells are able to release endogenous morphine. Future studies will
investigate the association between morphine and NETs in greater
detail.
In the infiltrated inflammatory cells within the
tumor microenvironment, tumor-associated neutrophils confer a poor
prognosis (37). In breast cancer,
G-CSF-induced NETs facilitate metastasis (38). As HMGB1 from lung cancer cells and
morphine have been indicated to promote the formation of NETs, it
is postulated that targeting NETs and their initiators, including
HMGB1 and morphine, may be valuable in cancer therapy.
The present study contains certain limitations.
Firstly, the mechanisms through which lung cancer cells actively
release HMGB1 were not explored. Secondly, the effects of NETs
in vivo were not assessed. Thirdly, NETs formation in the
patients with lung cancer with or without morphine treatment was
not compared. However, the observations from the present study
clearly indicated that HMGB1 from lung cancer cells and morphine
contributed to the NETs formation, which may provide additional
information concerning the tumorigenesis of lung cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81671563), Natural Science
Foundation of Jiangsu Province (grant no. BK2015155) and Nanjing
Medical University key project (grant no. 2014NJMUZD010).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and MZ conceived and designed the study. JZ, YY,
TG and YL conducted the experiments; FH, NH, BY and MZ analyzed the
results. All authors reviewed and approved the manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the
Institutional Animal Care Committee of Nanjing Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bar-Ad V, Palmer J, Li L, Lai Y, Lu B,
Myers RE, Ye Z, Axelrod R, Johnson JM, Werner-Wasik M, et al:
Neutrophil to lymphocyte ratio associated with prognosis of lung
cancer. Clin Transl Oncol. 19:711–717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Najmeh S, Cools-Lartigue J, Giannias B,
Spicer J and Ferri LE: Simplified human neutrophil extracellular
traps (NETs) isolation and handling. J Vis Exp. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cools-Lartigue J, Spicer J, McDonald B,
Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P and Ferri L:
Neutrophil extracellular traps sequester circulating tumor cells
and promote metastasis. J Clin Invest. July 1–2013.(Epub ahead of
print). View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oklu R, Sheth RA, Wong KHK, Jahromi AH and
Albadawi H: Neutrophil extracellular traps are increased in cancer
patients but does not associate with venous thrombosis. Cardiovasc
Diagn Ther. 7 (Suppl 3):S140–S149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brinkmann V, Reichard U, Goosmann C,
Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y and Zychlinsky A:
Neutrophil extracellular traps kill bacteria. Science.
303:1532–1535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ben-Smith A, Dove SK, Martin A, Wakelam MJ
and Savage CO: Antineutrophil cytoplasm autoantibodies from
patients with systemic vasculitis activate neutrophils through
distinct signaling cascades: Comparison with conventional Fcgamma
receptor ligation. Blood. 98:1448–1455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clark SR, Ma AC, Tavener SA, McDonald B,
Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair
GD, et al: Platelet TLR4 activates neutrophil extracellular traps
to ensnare bacteria in septic blood. Nat Med. 13:463–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitroulis I, Kambas K, Chrysanthopoulou A,
Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT and
Ritis K: Neutrophil extracellular trap formation is associated with
IL-1beta and autophagy-related signaling in gout. PLoS One.
6:e293182011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta AK, Joshi MB, Philippova M, Erne P,
Hasler P, Hahn S and Resink TJ: Activated endothelial cells induce
neutrophil extracellular traps and are susceptible to
NETosis-mediated cell death. FEBS Lett. 584:3193–3197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demers M, Krause DS, Schatzberg D,
Martinod K, Voorhees JR, Fuchs TA, Scadden DT and Wagner DD:
Cancers predispose neutrophils to release extracellular DNA traps
that contribute to cancer-associated thrombosis. Proc Natl Acad Sci
USA. 109:13076–13081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang R, Zhang Q, Zeh HJ III, Lotze MT and
Tang D: HMGB1 in cancer: Good, bad, or both? Clin Cancer Res.
19:4046–4057. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tadie JM, Bae HB, Jiang S, Park DW, Bell
CP, Yang H, Pittet JF, Tracey K, Thannickal VJ, Abraham E and
Zmijewski JW: HMGB1 promotes neutrophil extracellular trap
formation through interactions with Toll-like receptor 4. Am J
Physiol Lung Cell Mol Physiol. 304:L342–L349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YH, Okuda C, Sakamori Y, Masago K,
Togashi Y and Mishima M: Continuous morphine infusion for end-stage
lung cancer patients. Oncol Lett. 5:972–974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zylla D, Kuskowski MA, Gupta K and Gupta
P: Association of opioid requirement and cancer pain with survival
in advanced non-small cell lung cancer. Br J Anaesth. 113 (Suppl
1):i109–i116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen J, Luk K, Vang D, Soto W, Vincent
L, Robiner S, Saavedra R, Li Y, Gupta P and Gupta K: Morphine
stimulates cancer progression and mast cell activation and impairs
survival in transgenic mice with breast cancer. Br J Anaesth. 113
(Suppl 1):i4–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zylla D, Gourley BL, Vang D, Jackson S,
Boatman S, Lindgren B, Kuskowski MA, Le C, Gupta K and Gupta P:
Opioid requirement, opioid receptor expression, and clinical
outcomes in patients with advanced prostate cancer. Cancer.
119:4103–4110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shah M, Anwar MA, Yesudhas D, Krishnan J
and Choi S: A structural insight into the negative effects of
opioids in analgesia by modulating the TLR4 signaling: An in silico
approach. Sci Rep. 6:392712016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shah M and Choi S: Toll-like
Receptor-Dependent Negative Effects of Opioids: A Battle between
Analgesia and Hyperalgesia. Front Immunol. 8:6422017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swamydas M, Luo Y, Dorf ME and Lionakis
MS: Isolation of mouse neutrophils. Curr Protoc Immunol.
110:3.20.1–3.15. 2015. View Article : Google Scholar
|
|
20
|
Behnen M, Leschczyk C, Moller S, Batel T,
Klinger M, Solbach W and Laskay T: Immobilized immune complexes
induce neutrophil extracellular trap release by human neutrophil
granulocytes via Fc gamma RIIIB and Mac-1. J Immunol.
193:1954–1965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brinkmann V and Zychlinsky A: Neutrophil
extracellular traps: Is immunity the second function of chromatin?
J Cell Biol. 198:773–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayashi F, Means TK and Luster AD:
Toll-like receptors stimulate human neutrophil function. Blood.
102:2660–2669. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neal MD, Jia H, Eyer B, Good M, Guerriero
CJ, Sodhi CP, Afrazi A, Prindle T Jr, Ma C, Branca M, et al:
Discovery and validation of a new class of small molecule Toll-like
receptor 4 (TLR4) inhibitors. PLoS One. 8:e657792013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reino DC, Pisarenko V, Palange D, Doucet
D, Bonitz RP, Lu Q, Colorado I, Sheth SU, Chandler B, Kannan KB, et
al: Trauma hemorrhagic shock-induced lung injury involves a
gut-lymph-induced TLR4 pathway in mice. PLoS One. 6:e148292011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lapponi MJ, Carestia A, Landoni VI,
Rivadeneyra L, Etulain J, Negrotto S, Pozner RG and Schattner M:
Regulation of neutrophil extracellular trap formation by
anti-inflammatory drugs. J Pharmacol Exp Ther. 345:430–437. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hakkim A, Fuchs TA, Martinez NE, Hess S,
Prinz H, Zychlinsky A and Waldmann H: Activation of the Raf-MEK-ERK
pathway is required for neutrophil extracellular trap formation.
Nat Chem Biol. 7:75–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma Y, Kang S, Wu X, Han B, Jin Z and Guo
Z: Up-regulated HMGB1 in the pleural effusion of non-small cell
lung cancer (NSCLC) patients reduces the chemosensitivity of NSCLC
cells. Tumori. 104:338–343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niki M, Yokoi T, Kurata T and Nomura S:
New prognostic biomarkers and therapeutic effect of bevacizumab for
patients with non-small-cell lung cancer. Lung Cancer (Auckl).
8:91–99. 2017.PubMed/NCBI
|
|
30
|
Tamassia N, Le Moigne V, Calzetti F,
Donini M, Gasperini S, Ear T, Cloutier A, Martinez FO, Fabbri M,
Locati M, et al: The MyD88-independent pathway is not mobilized in
human neutrophils stimulated via TLR4. J Immunol. 178:7344–7356.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang QW, Lu FL, Zhou Y, Wang L, Zhong Q,
Lin S, Xiang J, Li JC, Fang CQ and Wang JZ: HMBG1 mediates
ischemia-reperfusion injury by TRIF-adaptor independent Toll-like
receptor 4 signaling. J Cereb Blood Flow Metab. 31:593–605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carestia A, Kaufman T, Rivadeneyra L,
Landoni VI, Pozner RG, Negrotto S, D'Atri LP, Gómez RM and
Schattner M: Mediators and molecular pathways involved in the
regulation of neutrophil extracellular trap formation mediated by
activated platelets. J Leukoc Biol. 99:153–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Awasthi D, Nagarkoti S, Kumar A, Dubey M,
Singh AK, Pathak P, Chandra T, Barthwal MK and Dikshit M: Oxidized
LDL induced extracellular trap formation in human neutrophils via
TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic Biol
Med. 93:190–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu M, Wang H, Ding A, Golenbock DT, Latz
E, Czura CJ, Fenton MJ, Tracey KJ and Yang H: HMGB1 signals through
toll-like receptor (TLR) 4 and TLR2. Shock. 26:174–179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Plein LM and Rittner HL: Opioids and the
immune system-friend or foe. Br J Pharmacol. 175:2717–2725. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glattard E, Welters ID, Lavaux T, Muller
AH, Laux A, Zhang D, Schmidt AR, Delalande F, Laventie BJ,
Dirrig-Grosch S, et al: Endogenous morphine levels are increased in
sepsis: A partial implication of neutrophils. PLoS One.
5:e87912010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gregory AD and Houghton AM:
Tumor-associated neutrophils: new targets for cancer therapy.
Cancer Res. 71:2411–2416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berger-Achituv S, Brinkmann V, Abed UA,
Kuhn LI, Ben-Ezra J, Elhasid R and Zychlinsky A: A proposed role
for neutrophil extracellular traps in cancer immunoediting. Front
Immunol. 4:482013. View Article : Google Scholar : PubMed/NCBI
|