Introduction
Acute leukemia (AL) is a malignant clonogenic
disease that results from the uncontrolled proliferation of
abnormal hematopoietic stem cells. Based on the types of cells
affected, AL can be divided into two categories: Acute myelocytic
leukemia and acute lymphoblastic leukemia (ALL) (1). Treatments for AL include single-drug
chemotherapy, molecular targeting therapy, hematopoietic stem cell
transplantation and cellular immunotherapy. Chemotherapy is
currently the main treatment strategy due to limited sources of
donor bone marrow, high cost and severe complications associated
with transplantation; however, a considerable number of patients
exhibit no remission or early recurrence after chemotherapy, which
may be caused by drug resistance, minimal residual disease after
remission and persistence of leukemic stem cells (2,3).
Anthracycline-type drugs, such as Adriamycin (also
termed doxorubicin), are frequently used for AL chemotherapy.
Adriamycin kills leukemic cells by inhibiting DNA replication and
RNA transcription. The dose of anthracyclines correlates with its
efficiency in the clearance of leukemic cells, but increases in the
dose are also associated with elevated toxic side effects (4,5). The
heart is a major organ affected by the side effects of
anthracycline drugs, including acute heart failure, congestive
cardiomyopathy and occult ventricular dysfunction (6,7). In
addition, the efficacy of the drug is also restricted by the
development of drug resistance in leukemic cells (8). Therefore, it is crucial to develop a
novel strategy for treating AL to bypass the resistance of leukemic
cells to anthracycline-type drugs and limit the side effects.
Quercetin is a plant flavonol derived from the
flavonoid group of polyphenols; it is found in a number of fruits,
vegetables, leaves and grains (9).
It is commonly used as an ingredient of dietary supplements,
beverages and foods. It is a potential anti-oxidant capable of
modulating the activity of various cellular enzymes (10,11).
Quercetin has an anti-tumor effect on a variety of tumor cells,
including leukemia, lymphoma, colon, ovarian, cervical, prostate
and breast cancer cells (12–16).
However, whether quercetin is able to modify the therapeutic
efficacy of anthracycline-type drugs (such as Adriamycin) for
leukemia is yet to be determined.
The present study explored the combined AL treatment
with quercetin and Adriamycin, and the results suggested that
quercetin and Adriamycin may be used in combination to treat
malignant hematopathy.
Materials and methods
Clinical samples
The study recruited 20 patients with refractory,
Adriamycin-resistant acute leukemia (17) (excluding acute promyelocyte leukemia)
treated at the Department of Hematology of the Affiliated Hospital
of Inner Mongolia Medical University (Hohhot, China) between
November 2015 and December 2016 (Table
I). The inclusion criteria were: i) Patients treated with two
courses of induction chemotherapy; ii) patients with recurrence
6–12 months following complete response (CR) or patients with
ineffective re-treatment using a standard chemotherapy regimen; and
iii) patients with two or more recurrences. Patients who met any
one of the aforementioned criteria were determined to be cases of
refractory AL. A 5-ml vacuum heparin sodium tube was used to
collect peripheral blood from the patients. This study was approved
by the Ethics Committee of the Affiliated Hospital of Inner
Mongolia Medical University. All patients signed the informed
consent form.
 | Table I.Clinical characteristics of 20 cases
of refractory drug-resistant acute leukemia. |
Table I.
Clinical characteristics of 20 cases
of refractory drug-resistant acute leukemia.
| No. | Sex | Age (years) | Diagnosis |
Immunophenotyping | Chromosome | Fusion gene and gene
mutation | Initial leukocyte
number (×109cells/l) | Chemotherapy
history | Remarks |
|---|
| 1 | Female | 82 | M5 |
CD14/CD13/CD33/CD64/CD117+ | 48,XX,+8,+m | WT1+ | 103.17 | DA,AA,FA, low dose
Ara-C | Non-remission |
| 2 | Female | 61 | M2 |
CD7CD13/CD33/CD117+ |
44,XX,dup(12)(q12q21),- 15,-18 | WT1+ | 96.28 | CAG,DA, HA | Non-remission |
| 3 | Female | 75 | M4 |
CD11b/CD13/CD33/CD34+ |
49,XX,+8,+13,+22,inv(16) (p13;q22) |
MLL-AF6− | 30.89 | FA,IA,AA,CAG | Non-remission |
| 4 | Female | 39 | M2 |
CD13/CD33/MPO+ |
46,XX,t(8;21)(q22;q22) |
AML1/ETO− | 108.45 | DA,AA,MA,FA | Recurrence |
| 5 | Female | 52 | M4 |
CD13/CD33/CD34+ |
46,XX,6p-,t(8:11)(q23;q14) |
MLL-SEPT6+ | 50.22 |
DA,AA,HAD,CAG,IA | Recurrence |
| 6 | Female | 68 | M2 |
CD13/CD33/CD34+ |
46,XX,i(1q),7q- |
AML1/ETO− | 348.13 | DA,FM, CAG,IA,
DCAG | Recurrence |
| 7 | Female | 52 | M2 |
CD13/CD33/CD7/MPO+ | 47,XX,+19 |
AML1/ETO− | 84.04 | DA,MA, AA | Non-remission |
| 8 | Female | 27 | M5 |
CD14/CD13/CD33/CD64/CD117+ |
46,XX,add(1)(p36),t(8;16) (p11;p13) |
WT1+ | 30.27 |
HAD,FA,DCAG,FIA,MAC | Non-remission |
| 9 | Female | 31 | M2 |
CD13/CD33/CD34/MPO+ |
46,XX,t(8;21)(q22q22) |
AML1/ETO− | 20.09 | DA,AA,IA,MA | Recurrence |
| 10 | Male | 60 | M5 |
CD13/CD33/CD64/CD117+ |
46,XY,del(11)(q23),del(12) (p13) |
WT1− | 129.33 | DA,FA, IA,CAG | Non-remission |
| 11 | Male | 58 | M5 |
CD11b/CD13/CD33/CD34/CD117+ |
46,XY,del(11)(q23) |
WT1+ | 46.32 | MA,CAG, HA,AA | Non-remission |
| 12 | Male | 40 | M5 |
CD14/CD11b/CD13/CD33/CD117+ |
46,XY,del(7)(q22),t(s;21)
(q22;q22),del(9)(q11q22) |
WT1+ | 110.16 | DA,AA,
FA,IA,MA | Recurrence |
| 13 | Male | 56 | M2 |
CD13/CD33/CD7/MPO+ |
46,XY,t(8;21)(q22;q22) |
AML1/ETO+ | 29.74 | MA,HA, DA,CAG | Non-remission |
| 14 | Male | 68 | M2 |
CD117/CD34/CD13/CD123/CD33+ |
46,XY,inv(16)(p13;q22) |
FLT3-ITD+ | 19.55 | DA,MA, CAG | Non-remission |
| 15 | Male | 76 | M2 |
CD13/CD33/CD34/MPO+ |
45,X,-Y,t(8;21)(q22;q22) |
AML1/ETO+ | 44.02 | IA,CAG,low dose
Ara-C | Non-remission |
| 16 | Female | 58 | B-ALL |
CD19/CyCD22/CD34/HLA-DR+ | Normal
karyotype |
BCR/ABL− | 10.18 | CAG,HA, VDLP,V
DCLP,HD-MTX | Recurrence |
| 17 | Female | 27 | B-ALL |
CD10/CD19/CyCD22/CD20/CD34/HLA-DR+ |
46,XX,t(9;22)(q34;q11) |
BCR/ABL+ | 94.29 | VDLP,VDCLP,
Hyper-CVAD combined with Imatinib, Dasatinib | Recurrence |
| 18 | Female | 58 | B-ALL |
CD19/CyCD22/CD34/CyCD79a/HLA-DR+ | 46,XX,t(11;12)
(p10;p10),t(4;6)(q26;p2) |
BCR/ABL− | 26.01 | VDLP,FLAG,MA | Recurrence |
| 19 | Female | 55 | B-ALL |
HLA-DR/CD38/CD123/CD19/CD9/CD79a+ |
46,XX,2q-t(9;22) |
BCR/ABL− | 118.34 |
VDCLP,HD-MTX,CAG | Non-remission |
| 20 | Male | 34 | B-ALL |
HLA-DR/CD19/CD10/TDT/CD79a+ |
46,XY,t(9;22)(q34;q11) |
BCR/ABL− | 209.58 | VDLP,FLAG,MA* | Non-remission |
Cell Counting Kit-8 assay (CCK-8)
Ficoll lymphocyte separation solution (Tianjin Hao
Yang Biological Products Technology Co., Ltd, Tianjin, China) was
used to separate lymphocytes in the peripheral blood of patients,
cell density was adjusted and 5×104 cells/well were
placed in a 96-well cell culture plate. The maximum drug
concentrations of Adriamycin (Shenzhen Wanle Pharmaceutical Co.,
Ltd., Shenzhen, China) and quercetin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) were set as 6 and 75.5 µg/ml, respectively,
based on a preliminary experiment (data not shown). The samples
were separated into one negative control group (Con) and six
experimental groups (6 wells/group). Three experimental groups were
treated with 0.06, 0.6 or 6 µg/ml Adriamycin, and three groups were
treated with 0.03, 0.3 or 3 µg/ml Adriamycin combined with 75.5
µg/ml quercetin, based on a preliminary experiment (data not
shown). The cells were incubated at 37°C with saturated humidity
and 5% CO2. At 24, 48 and 72 h, 20 µl of CCK-8 solution
(lot no. JM754; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was added to each well. Cells were incubated for an
additional 4 h at 37°C, and the optical density (OD) of each well
at 450 nm was detected using a BioTek ELX800 Automatic Enzyme
Labeling Instrument microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). Cell proliferation inhibition rate was
calculated as follows: Proliferation inhibition rate=[(OD value of
the Con group-OD value of the experimental group)/OD value of the
Con group] × 100.
Mouse model of T-ALL
C57BL/6J (CD45.2+) and
B6.SJL-PtprcaPepcb/BoyJ (B6.SJL;CD45.1+; donor mice for
leukemia cells) mice (all female; age, 6–8 weeks; weight, 15–18 g;
n=28 mice/group) were purchased from the Academy of Military
Medical Sciences (Beijing, China) and maintained in the Central
Laboratory of the Affiliated Hospital of Inner Mongolia Medical
University. The mice received food and water ad libitum.
They were kept under specific pathogen-free conditions at 20±2°C
and 40–60% relative humidity under a 12:12 h light/dark cycle. All
animal experiments were approved by the Ethics Committee of the
Affiliated Hospital of Inner Mongolia Medical University. A
non-irradiated, Notch1-induced T-ALL model was established as
previously described (18). Briefly,
the mice were sacrificed, disinfected with ethanol and the spleens
and the femurs of the mice were removed. The bone marrow cells were
repeatedly flushed out using a 1-ml syringe, and the spleen cells
were sieved by grinding. Finally, the cells were collected by
centrifugation and stored frozen. Green fluorescent protein
(GFP)+CD45.1+ leukemic cells were isolated
from either the spleen or the bone marrow of mice with B6.SJL
leukemia and transplanted by tail vein injection into C57BL/6 J
female mice (1×106 cells/mouse) without irradiation.
Successful transplantation was confirmed by flow cytometry, as
previously described (19). The mice
were randomly divided into four groups (n=7 mice/group): Untreated,
quercetin, Adriamycin or Adriamycin combined with quercetin. For
the low-dose Adriamycin experiments, the mice were
intraperitoneally (i.p.) administered 0.9% saline, 50 mg/kg
quercetin, 1 mg/kg Adriamycin, or 1 mg/kg Adriamycin + 50 mg/kg
quercetin, respectively, daily for 10 days, starting at day 4
post-transplantation. For the high-dose Adriamycin experiments, the
mice were i.p. administered 0.9% saline, 50 mg/kg quercetin, 2
mg/kg Adriamycin or 2 mg/kg Adriamycin + 50 mg/kg quercetin,
respectively, daily for 10 days, starting at 4 days after
transplantation. The survival duration of each group was recorded.
Additionally, peripheral blood was collected from the tail in
EDTA-modified tubes on days 4 (first day of dosing), 7 (starting
point of leukemic accumulation in untreated mice), 11 (first death
in untreated mice), 16 (end of dosing), 23 (negligible drug levels,
based on the half-life of Adriamycin) and 31 (end of study)
following transplantation, and blood cells and platelets were
counted using an XN2000 blood cell analyzer (Sysmex Corporation,
Kobe, Japan).
Determination of superoxide dismutase
(SOD) activity and malondialdehyde (MDA)content in the mouse
heart
Non-irradiated T-ALL mice were administered the
allocated treatment daily for 10 days, starting 4 days following
transplantation. On day 31 post-transplantation, the mice were
sacrificed and heart tissue was collected. Following homogenization
and dilution with nine times the volume of saline, the tissue
samples were centrifuged at 800 × g for 5 min and supernatant was
collected. SOD activity and MDA content in the homogenate were
measured using a SOD Activity Detection kit (lot no. A064; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) and an MDA
Detection kit (lot no. A003-2; Nanjing Jiancheng Bioengineering
Institute), respectively.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ±
standard deviation of at least three independent tests. For cell
culture data, the comparison among groups was performed using the
Wilcoxon rank-sum test, and α was adjusted according to the number
of comparisons (α‣=α/number of comparisons). Analysis of variance
with the Student-Newman-Keuls test was used to analyze the
differences among the groups in mouse experiments. Survival curves
were compared using Kaplan-Meier and log-rank (Mantel-Cox) test,
and α was adjusted according to the number of comparisons
(α′=α/number of comparisons). P<0.05 was considered to indicate
a statistically significant difference.
Results
Quercetin enhances the cytotoxicity of
Adriamycin to leukemic cells from patients
A number of available leukemic cell lines have been
selected based on pre-existing intrinsic features that are
favorable to the establishment of in vitro culture, which
may influence drug resistance and survival (20). Therefore, primary leukemic cells
directly isolated from the peripheral blood of patients with drug
resistance were used in the present study to represent the actual
conditions observed in the clinical setting.
Primary leukemic cells were treated with a series of
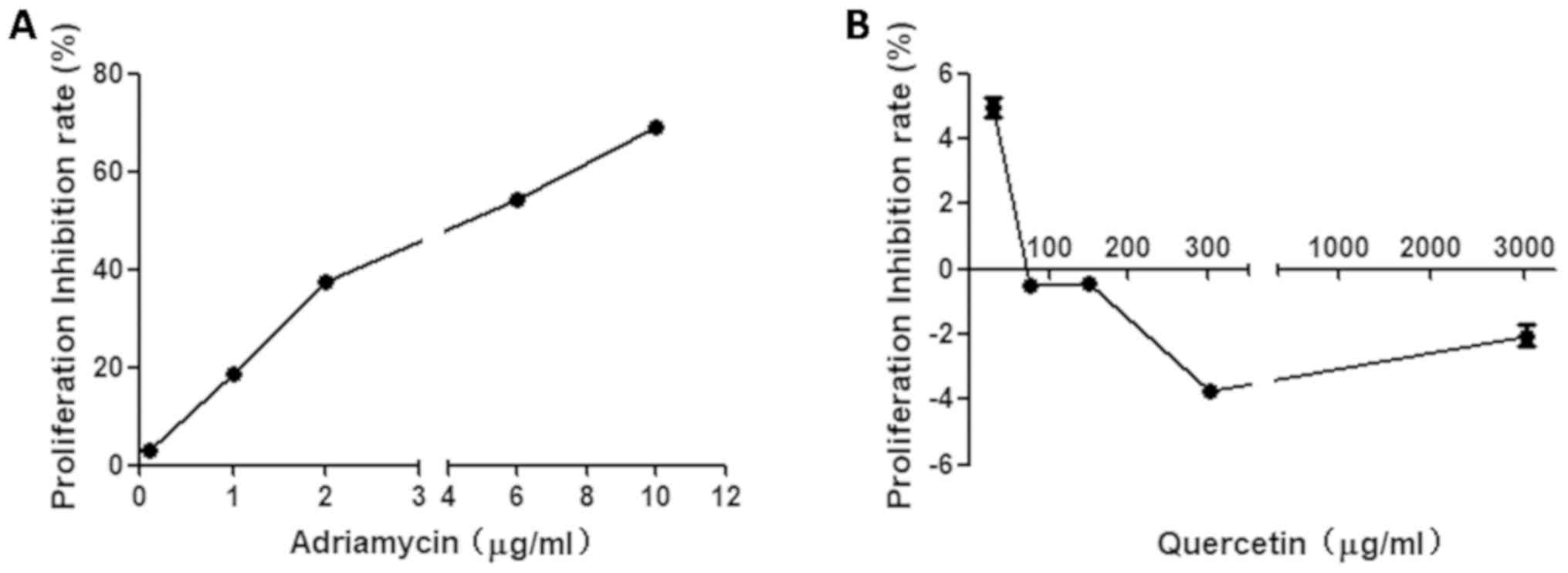
concentrations of Adriamycin or quercetin. The results demonstrated
that the inhibition rate of Adriamycin at 6 µg/ml was close to 50%
(IC50, 5.6 µg/ml; Fig.
1A). Treatment with 75.5 µg/ml or 151 µg/ml quercetin did not
affect cell proliferation (Fig. 1B).
Regarding the concentration of the quercetin solution that could
interfere with the measurement of cell viability, 75.5 µg/ml was
chosen for the following experiments, as derived from Fig. 1.
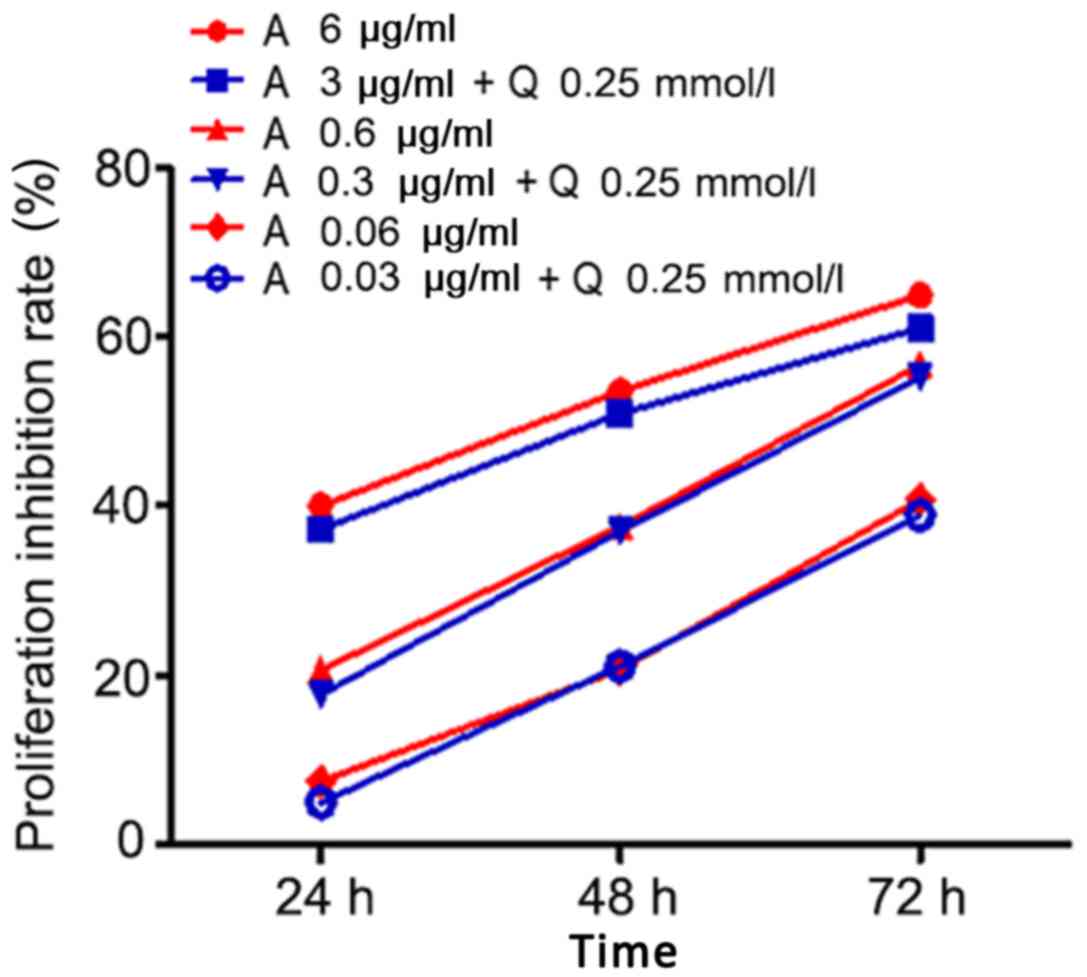
A gradient dose of Adriamycin was combined with
quercetin to investigate whether quercetin enhanced the cytotoxic
effect of Adriamycin. The results revealed that Adriamycin
efficiently suppressed the proliferation of primary leukemic cells
(Fig. 2); this effect depended on
treatment duration (24, 48 or 72 h) and dose (0.06, 0.6 or 6
µg/ml). Longer treatment and higher drug dose suppressed cell
growth more efficiently. When half doses of Adriamycin (0.03, 0.3
or 3 µg/ml) were co-administered with quercetin (75.5 µg/ml), the
treatment resulted in similar suppression of cell growth (Fig. 2), which indicated that quercetin may
increase the sensitivity of leukemic cells to Adriamycin.
Quercetin and high-dose Adriamycin
co-treatment enhances the survival of mice with leukemia
The mouse model of non-irradiated T-ALL was
established to further explore the potential of quercetin to
enhance Adriamycin efficiency in treating AL. Following the
transplantation of leukemic cells into recipient mice, the survival
duration was recorded. The analysis demonstrated that administering
low-dose Adriamycin alone, quercetin alone or low-dose Adriamycin
combined with quercetin did not significantly alter the survival
duration of mice with T-ALL (Fig.
3A). Administration of high-dose Adriamycin alone reduced the
median survival time to 14 days, although administering high-dose
Adriamycin combined with quercetin (median survival time 51 days)
markedly extended the survival of mice with T-ALL compared with
untreated mice (P<0.05; Fig. 3B).
The mice in the low-dose Adriamycin group exhibited a longer
survival time compared with the Adriamycin + quercetin group, but
the difference was not significant (Fig.
3A).
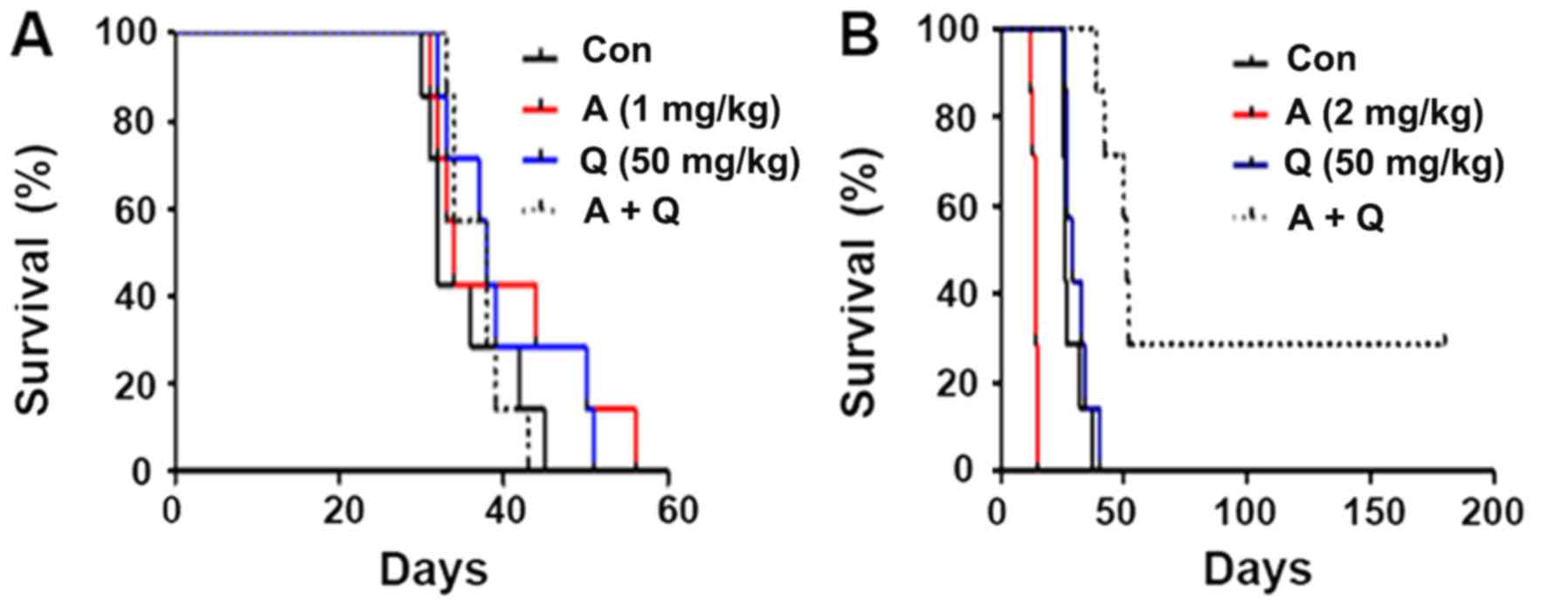 | Figure 3.Survival time of mice with T-ALL is
enhanced by co-treatment with high-dose Adriamycin and quercetin.
(A) Mice with T-ALL leukemia were treated with low-dose Adriamycin
alone, quercetin alone or their combination, and the median
survival duration was calculated from the survival record. Median
survival: Con, 32 days; A, 34 days; Q, 38 days; A+Q, 38 days. (B)
Mice with T-ALL leukemia were treated with high-dose Adriamycin
alone, quercetin alone or their combination, and the median
survival duration was calculated. Median survival: Con, 26 days; A,
14 days; Q, 29 days; A+Q, 51 days. A, Adriamycin; Con, untreated
control; Q, quercetin; T-ALL, T cell acute lymphoblastic
leukemia. |
Peripheral blood from T-ALL model mice was analyzed
to determine the mechanism of enhanced survival with high-dose
Adriamycin + quercetin combination treatment. The analysis revealed
that the numbers of white blood cells (WBCs) and red blood cells
(RBCs) were altered by drug treatment following transplantation, as
determined by flow cytometry. There were no differences in the
changes in blood cells at 4 days among the groups, with the
exception of an increase in RBCs in the Adriamycin + quercetin
group compared with the untreated group. The number of WBCs in
peripheral blood was significantly reduced in the Adriamycin and
Adriamycin + quercetin groups compared with the untreated group on
days 7, 11, and 16 following transplantation (P<0.01 and
P<0.001); however, no differences were observed at 23 and 31
days (Fig. 4A). Quercetin alone did
not significantly change the number of WBCs compared with the
untreated group (Fig. 4A). The RBC
count indicated that Adriamycin significantly (P<0.01) reduced
the number of RBCs at an earlier stage (before day 23) following
transplantation compared with the untreated group (Fig. 4B). Platelets (PLTs) began to decrease
significantly in the untreated and quercetin-alone groups from day
11 after transplantation, whereas Adriamycin and Adriamycin +
quercetin co-treatment restored this reduction to the level
comparable to healthy C57BL/6 mice (normal group) (Fig. 4C), which indicated that Adriamycin
may alleviate leukemia by preventing the dramatic decrease in the
number of PLTs.
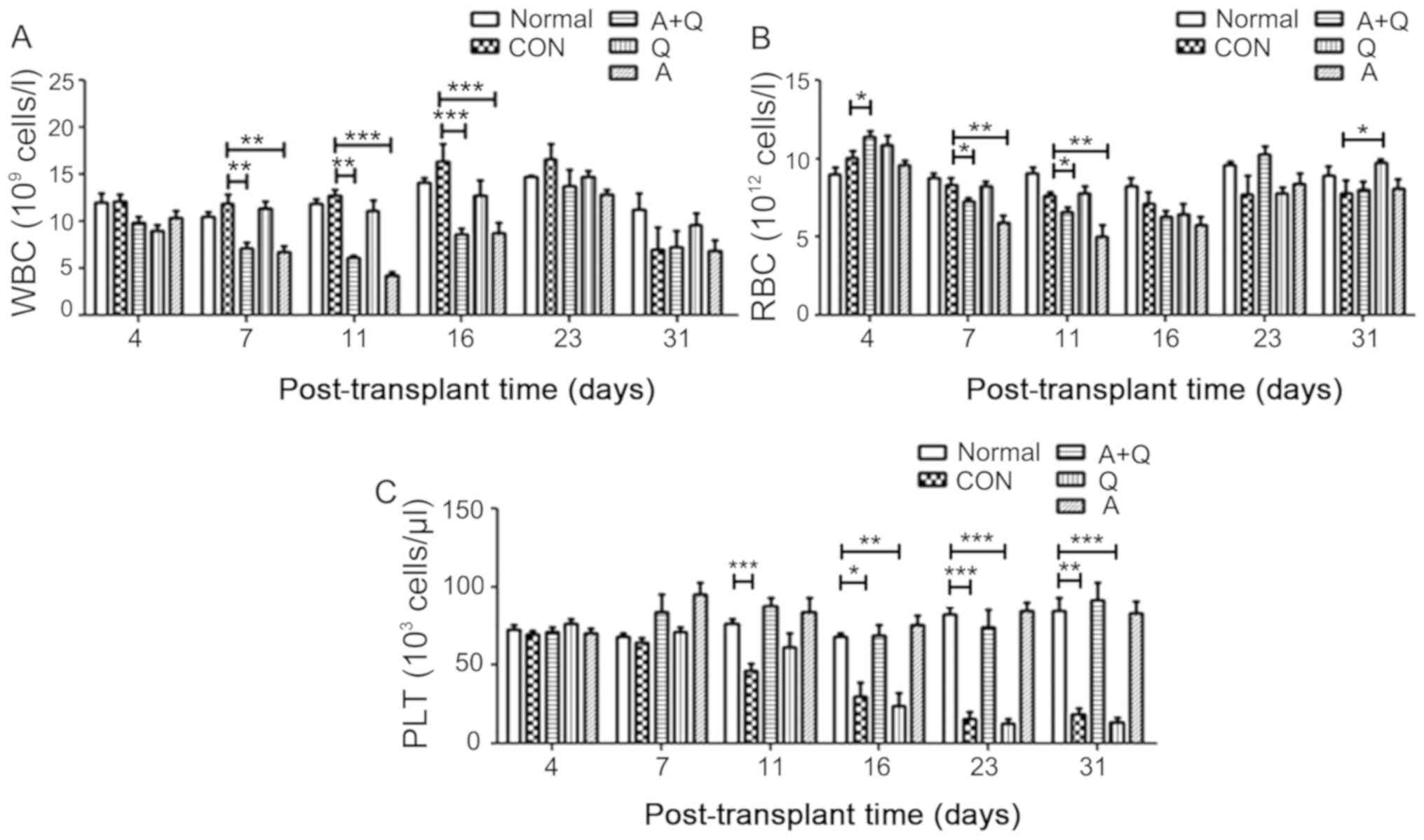 | Figure 4.Peripheral blood cells and components
are altered by Adriamycin and quercetin in mice with T-ALL. (A)
WBCs, (B) RBCs and (C) PLTs were counted in peripheral blood from
normal healthy C57BL/6 mice, untreated CON mice with T-ALL or mice
with T-ALL treated with A (2 mg/kg), Q (50 mg/kg) or their
combination. n=7 for each group. *P<0.05,**P<0.01 and
***P<0.001. A, Adriamycin; CON, untreated control; PLT,
platelets; Q, quercetin; RBC, red blood cells; T-ALL, T cell acute
lymphoblastic leukemia; WBC, white blood cells. |
Quercetin attenuates
Adriamycin-induced oxidative stress in the heart
Several toxic side effects of Adriamycin have been
reported, including the induction of oxidative injury in the heart,
which is monitored based on SOD activity and MDA content (4,21). In
the present study, quercetin was demonstrated to be beneficial in
reducing the side effects of Adriamycin; in cardiac tissues of
mice, SOD activity in the Adriamycin (low dose or high dose) +
quercetin combination treatment group was higher compared with the
respective Adriamycin-alone (low dose or high dose) group (Fig. 5A). MDA content in the Adriamycin (low
dose or high dose) + quercetin combination treatment group was
lower compared with the Adriamycin-alone (low dose or high dose)
group (Fig. 5B). Higher SOD activity
and lower MDA content indicated enhanced anti-oxidant capacity and
reduced oxidative damage, respectively. Thus, the results suggested
that quercetin may reduce the cardiac toxicity of Adriamycin by
reducing oxidative injury to cardiac tissue.
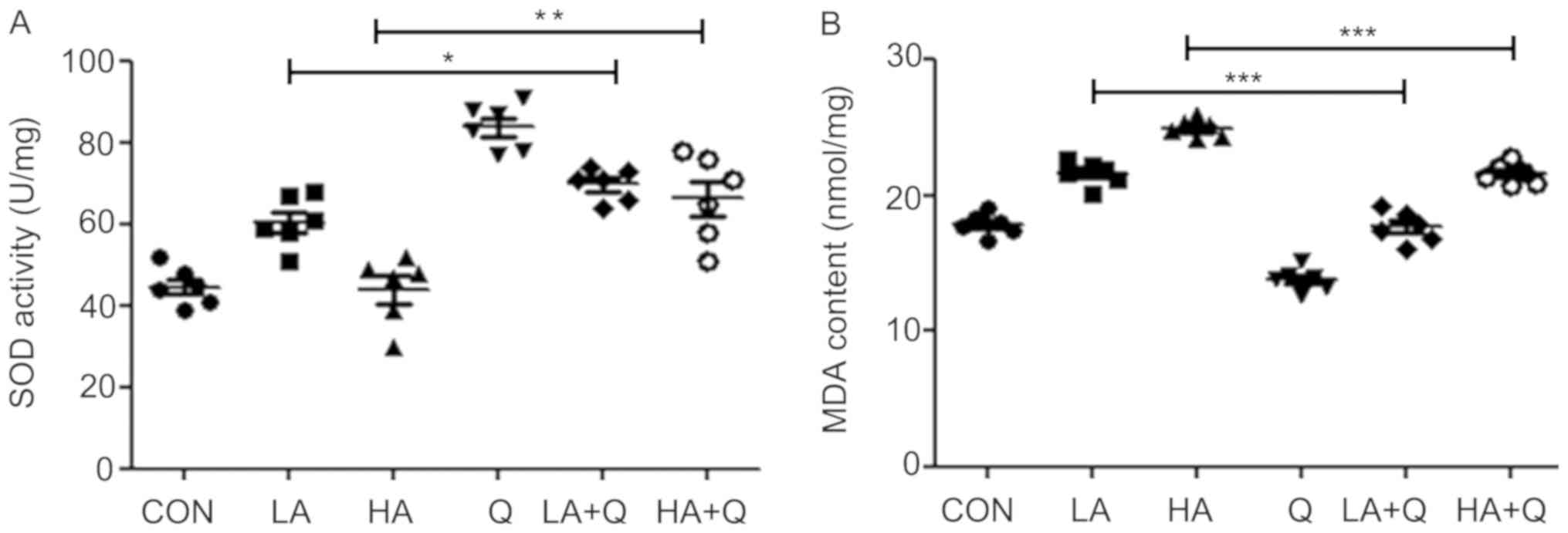 | Figure 5.Quercetin attenuates
Adriamycin-induced oxidative stress in the heart. (A) SOD activity
and (B) MDA content in the heart were monitored on day 31
post-transplantation in CON mice with T-ALL without treatment or
treated with LA (1 mg/kg), HA (2 mg/kg), Q (50 mg/kg), or their
combination (LA+Q or HA+Q). *P<0.05, **P<0.01 and
***P<0.001. CON, untreated; HA, high-dose Adriamycin; LA,
low-dose Adriamycin; MDA, malondialdehyde; Q, quercetin; SOD,
superoxide dismutase (SOD); T-ALL, T cell acute lymphoblastic
leukemia. |
Discussion
Leukemia is a severe malignancy of the hematopoietic
system. AL displays rapid progression and high mortality (1,5).
Chemotherapy is currently the main strategy for treating AL
(22). Adriamycin is a commonly used
anthracycline-type chemotherapy drug that has toxic side effects
when a high dose is administered (4). In addition, drug resistance to
Adriamycin in cancer cells is widely reported, limiting the
effectiveness of chemotherapy (6,7).
Originally developed as a food supplement, quercetin has been
reported to exert an anti-tumor effect on a variety of cancers,
including leukemia, lymphoma, colon, ovarian, cervical, prostate
and breast cancer (23,24). In addition, quercetin protects the
cardiovascular system from damage during aging or disease (25). A previous study has demonstrated that
quercetin inhibits the proliferation of P388 leukemic cells
(12). The aim of the present study
was to investigate the anti-leukemic effect of quercetin on
cultured leukemic cells and a mouse model of leukemia, with a
particular focus on enhancing the therapeutic effect of Adriamycin
and reducing its side effects.
The present study used primary leukemic cells
isolated from patients with AL exhibiting drug resistance to
determine the effect of drug treatment. The results demonstrated
that quercetin, Adriamycin and their combination inhibited the
proliferation of primary leukemic cells; the suppression depended
on the concentration and duration of drug treatment. Notably, when
quercetin was co-administered, a two-fold lower dose of Adriamycin
was required to achieve a similar growth-inhibitory effect on
leukemic cells to that of Adriamycin alone. This result indicated
that quercetin may enhance the inhibitory activity of
Adriamycin.
The enhancing effect of quercetin on Adriamycin was
also indicated in a mouse model of leukemia. Most animal models of
leukemia are generated in immunodeficient mice; for example, mice
in which the immune system has been destroyed by irradiation
(26,27). However, these models do not reflect
immune alteration, which is crucial in patients during
tumorigenesis or drug response. Therefore, the present study was
conducted in a non-irradiated, immunocompetent mouse model.
NOTCH1 is a type I transmembrane receptor involved
in signal transduction; activation of the NOTCH1 signaling pathway
is present in >50% of patients with T-ALL (18). In agreement with this clinical
observation, the transplantation of hematopoietic leukemic cells
harboring mutant Notch1 effectively induces T-ALL in recipient mice
(18). Taking advantage of this
model, the present study explored the therapeutic effect of
Adriamycin and quercetin. Of note, based on the Kaplan-Meier
survival curves, low-dose Adriamycin treatment was more effective
in extending mouse survival time compared with high-dose Adriamycin
treatment, which was contrary to the results obtained from primary
leukemic cells and indicated that in vitro experiments
cannot replace the biological complexity of a whole organism for
the study of diseases. In addition, high-dose Adriamycin combined
with quercetin effectively inhibited leukemia development,
reflected by the extended survival. This was consistent with the
result observed in primary leukemic cells that quercetin enhanced
the treatment effect of Adriamycin.
The anti-leukemic effect of Adriamycin was
dose-dependent. However, with the increase in the dose of
Adriamycin, its toxic side effects became more severe. The most
severe side effect is cardiac injury (7,28). A
previous study has found that administering Adriamycin to BALB/c
nude mice transplanted with the leukemic cell line P388 causes
cardiac injury, which is alleviated following co-treatment with
Adriamycin and quercetin (12). The
mechanism underlying the cardiac toxicity of Adriamycin may be
oxidative damage to cardiomyocytes, which is associated with
various types of heart injury (24,28). SOD
scavenges superoxide radicals in cells to protect them from damage.
MDA is the product of lipid peroxidation in the cell membrane,
which is a marker for oxidative damage in cells (12). The present study demonstrated that
quercetin increased SOD activity and decreased MDA content in the
hearts of non-irradiated mice with T-ALL, which indicated that
quercetin may reduce the cardiac toxicity of Adriamycin by
attenuating oxidative stress, thus promoting the survival of mice
with leukemia under high-dose Adriamycin treatment.
In conclusion, high-dose Adriamycin effectively
inhibited the proliferation of leukemic cells, but the toxic side
effects of high-dose Adriamycin limited the efficacy of the drug in
mice with T-ALL. Quercetin was beneficial for both cultured primary
leukemic cells and mice with T-ALL treated with Adriamycin by
enhancing the pharmacological effect of Adriamycin and/or
restricting oxidative damage to the heart. These results shed light
on the development of a novel strategy for treating AL. To further
clarify the molecular mechanism of quercetin and Adriamycin
co-treatment in the suppression of growth in refractory acute
leukemia, transcriptome sequencing is planned for future
experimental studies.
Acknowledgements
Not applicable.
Funding
This study was funded by The National Natural
Science Foundation of China (grant no. 81560022); The Natural
Science Foundation of Inner Mongolia (grant nos. 2014MS0843, 2015MS
(LH) 0815); The Science and Technology Innovation Fund of Inner
Mongolia (grant no. 02039003); the Major Scientific Research
Project of the Affiliated Hospital of Inner Mongolia Medical
University (grant no. NYFY ZD2014003); the Science and Technology
Million Projects of Inner Mongolia Medical University (grant no.
YKD2012KJBW006); and the Science and Technology Planning Project of
Inner Mongolia Autonomous Region in 2016.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and HD conceived and supervised the study. YS
designed the experiments. YS, XS, HC, LY and HD performed the
experiments. YS, HD and YH analyzed the data. YS wrote the
manuscript. YS, HD and YH revised the manuscript. All authors
reviewed the results and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional research committee and with the 1964 Declaration of
Helsinki and its later amendments. Ethical approval was received
from the Ethics Committee of the Affiliated Hospital of Inner
Mongolia Medical University All patients signed informed consent.
All animal experiments were approved by the Ethics Committee of the
Affiliated Hospital of Inner Mongolia Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chan KW: Acute lymphoblastic leukemia.
Curr Probl Pediatr Adolesc Health Care. 32:40–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma JJ, Chen Y and Yu L: Research progress
on drug-resistance of acute lymphoblastic leukemia-review. Zhongguo
Shi Yan Xue Ye Xue Za Zhi. 24:261–265. 2016.(In Chinese).
PubMed/NCBI
|
|
3
|
Jing H and Feng R: Research progress on
minimal residual disease in acute leukemia detected by
multiparametric flow cytometry. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
22:847–851. 2014.(In Chinese). PubMed/NCBI
|
|
4
|
Johnson-Arbor K and Dubey R: Doxorubicin.
StatPearls. (Treasure Island, FL). 2019.
|
|
5
|
Mehta-Shah N, Ratner L and Horwitz SM:
Adult T-cell leukemia/lymphoma. J Oncol Pract. 13:487–492. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ballmann C, Denney T, Beyers RJ, Quindry
T, Romero M, Selsby JT and Quindry JC: Long-term dietary quercetin
enrichment as a cardioprotective countermeasure in mdx mice. Exp
Physiol. 102:635–649. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lipshultz SE, Lipsitz SR, Sallan SE,
Dalton VM, Mone SM, Gelber RD and Colan SD: Chronic progressive
cardiac dysfunction years after doxorubicin therapy for childhood
acute lymphoblastic leukemia. J Clin Oncol. 23:2629–2636. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Padron E and Fernandez H: Anthracycline
dose intensification in young adults with acute myeloid leukemia.
Ther Adv Hematol. 3:17–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Yao J, Han C, Yang J, Chaudhry MT,
Wang S, Liu H and Yin Y: Quercetin, inflammation and immunity.
Nutrients. 8:1672016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Darband SG, Kaviani M, Yousefi B,
Sadighparvar S, Pakdel FG, Attari JA Mohebbi I, Naderi S and
Majidinia M: Quercetin: A functional dietary flavonoid with
potential chemo-preventive properties in colorectal cancer. J Cell
Physiol. 233:6544–6560. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Francaux M and Deldicque L: Using
polyphenol derivatives to prevent muscle wasting. Curr Opin Clin
Nutr Metab Care. 21:159–163. 2018.PubMed/NCBI
|
|
12
|
Han Y, Yu H, Wang J, Ren Y, Su X and Shi
Y: Quercetin alleviates myocyte toxic and sensitizes anti-leukemic
effect of Adriamycin. Hematology. 20:276–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alam RT, Zeid EH and Imam TS: Protective
role of quercetin against hematotoxic and immunotoxic effects of
furan in rats. Environ Sci Pollut Res Int. 24:3780–3789. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brito AF, Ribeiro M, Abrantes AM, Pires
AS, Teixo RJ, Tralhão JG and Botelho MF: Quercetin in cancer
treatment, alone or in combination with conventional therapeutics?
Curr Med Chem. 22:3025–3039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lamson DW and Brignall MS: Antioxidants
and cancer therapy II: Quick reference guide. Altern Med Rev.
5:152–163. 2000.PubMed/NCBI
|
|
16
|
Chen C, Zhou J and Ji C: Quercetin: A
potential drug to reverse multidrug resistance. Life Sci.
87:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Belli JA and Harris JR: Adriamycin
resistance and radiation response. Int J Radiat Oncol Biol Phys.
5:1231–1234. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu X, Shen H, Tian C, Yu H, Zheng G,
XuFeng R, Ju Z, Xu J, Wang J and Cheng T: Kinetics of normal
hematopoietic stem and progenitor cells in a Notch1-induced
leukemia model. Blood. 114:3783–3792. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma S, Shi Y, Pang Y, Dong F, Cheng H, Hao
S, Xu J, Zhu X, Yuan W, Cheng T and Zheng G: Notch1-induced T cell
leukemia can be potentiated by microenvironmental cues in the
spleen. J Hematol Oncol. 7:712014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garnett MJ and McDermott U: The evolving
role of cancer cell line-based screens to define the impact of
cancer genomes on drug response. Curr Opin Genet Dev. 24:114–119.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cappetta D, De Angelis A, Sapio L,
Prezioso L, Illiano M, Quaini F, Rossi F, Berrino L, Naviglio S and
Urbanek K: Oxidative stress and cellular response to doxorubicin: A
common factor in the complex milieu of anthracycline
cardiotoxicity. Oxidative medicine and cellular longevity.
2017:15210202017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brown PA, Shah B, Fathi A, Wieduwilt M,
Advani A, Aoun P, Barta SK, Boyer MW, Bryan T, Burke PW, et al:
NCCN Guidelines Insights: Acute Lymphoblastic Leukemia, Version
1.2017. J Natl Compr Canc Netw. 15:1091–1102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HS, Wannatung T, Lee S, Yang WK, Chung
SH, Lim JS, Choe W, Kang I, Kim SS and Ha J: Quercetin enhances
hypoxia-mediated apoptosis via direct inhibition of AMPK activity
in HCT116 colon cancer. Apoptosis. 17:938–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maso V, Calgarotto AK, Franchi GC Jr,
Nowill AE, Filho PL, Vassallo J and Saad ST: Multitarget effects of
quercetin in leukemia. Cancer Prev Res (Phila). 7:1240–1250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen Y, Croft KD, Hodgson JM, Kyle R, Lee
IL, Wang Y, Stocker R and Ward NC: Quercetin and its metabolites
improve vessel function by inducing eNOS activity via
phosphorylation of AMPK. Biochem Pharmacol. 84:1036–1044. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rivina L, Davoren M and Schiestl RH:
Radiation-induced myeloid leukemia in murine models. Hum Genomics.
8:132014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Almosailleakh M and Schwaller J: Murine
models of acute myeloid leukaemia. Int J Mol Sci. 20(pii):
E4532019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Segredo MP, Salvadori DM, Rocha NS,
Moretto FC, Correa CR, Camargo EA, de Almeida DC, Reis RA, Freire
CM, Braz MG, et al: Oxidative stress on cardiotoxicity after
treatment with single and multiple doses of doxorubicin. Hum Exp
Toxicol. 33:748–760. 2014. View Article : Google Scholar : PubMed/NCBI
|