Introduction
Psammoma bodies (PBs) are basophilic structures with
laminated concretions, with a diameter generally ranging from
20–100 µm (1). PBs are composed of
calcium apatite and are most commonly observed in papillary thyroid
carcinoma (PTC), meningioma, and papillary serous
cystadenocarcinoma of the ovary (2).
Studies on serous cystadenocarcinoma of the ovary and meningioma
demonstrated that collagen production by neoplastic cells and
subsequent calcification led to the formation of PBs (2,3).
However, an ultrastructural study of PTC revealed that tumor cell
necrosis was responsible for the formation of PBs (2,4).
Although various theories have been published to explain the origin
of PBs, the underlying biochemical mechanism is poorly understood
(2–4).
PBs have been reported in ~50% of PTC cases, which
makes it an important marker for the pathological diagnosis of PTC
(5). In addition, PBs are of great
significance in the diagnosis of meningiomas (6) and serous cystadenocarcinomas of ovary
(2), with incidence rates of 45 and
33%, respectively. Although PBs from various types of neoplasms
share identical light-microscopic characteristics, their
significance in diverse types of tumors should be further
investigated. Cai et al (7)
found PBs to be useful predictors of aggressive tumor behavior and
patients with PTC with PBs had a poorer prognosis compared with
those without PBs (7). However, Das
(2) reported that PBs not only lead
to degeneration or death of tumor cells, but serve as a barrier
against the spread of the neoplasm.
In renal cell carcinoma (RCC), PBs are most commonly
observed in Xp11.2 translocation RCC (Xp11.2 tRCC) (8) and papillary (P)RCC (9). Xp11.2 tRCC, a rare and aggressive type
of cancer, harbors a chromosomal translocation involving the Xp11.2
breakpoint and transcription factor binding to IGHM enhancer 3
(TFE3) gene fusion (10,11).
Microscopically, Xp11.2 tRCC mimics clear cell and PRCC (10,11). The
typical morphology of Xp11.2 tRCC includes PBs, irregular
calcifications, hyaline globules and nested architecture,
particularly for the subtype with the UBX domain containing tether
for SLC2A4 (ASPL)-TFE3 gene fusion (10,12).
Furthermore, hyaline globules are considered as precursors of PBs
and irregular calcifications in PCT (13). However, to the best of our knowledge,
the incidence and significance of PBs, irregular calcifications,
hyaline globules and nested architecture in Xp11.2 tRCC have only
been described in reports with low patient numbers. Therefore, the
aim of the present study was to investigate the association between
the presence of these specific morphologies and clinicopathological
characteristics. A group of PRCC cases were further enrolled due to
the high incidence of PBs (9).
Materials and methods
Ethics approval
All procedures were approved by the Medical Ethics
Committee for Human Experiments of Nanjing Drum Tower Hospital
(Nanjing, China).
Diagnostic methods
A total of 47 patients with Xp11.2 tRCC were
selected between January 2007 and September 2017 at the Nanjing
Drum Tower Hospital. Patients were diagnosed by applying
self-designed break-apart TFE3 fluorescence in situ
hybridization (FISH) probes. Briefly, ASPL-TFE3 dual-fusion
FISH probes were used for diagnosing ASPL-TFE3 tRCC. The
break-apart and the dual-fusion FISH probe results were validated
by reverse transcription-PCR analysis (13). The inclusion criteria comprised
patients with RCC who were positive for TFE3 by
immunohistochemistry and FISH. Exclusion criteria comprise patients
without integrated clinical and pathological data.
Clinicopathological
characteristics
According to the 2016 World Health Organization
classification of renal cell carcinoma, patients with PRCC were
divided into type 1 (31 cases) and type 2 (64 cases) (14). Of the 95 cases of PRCC, 31 were type
1 and 64 were type 2. Immediately after excision, tumor samples
were fixed in buffered 10% formalin for at least 24 h, embedded in
paraffin, cut into 4-µm thick sections and stained by hematoxylin
and eosin (0.5–1%) for 5–20 min at room temperature. The
hematoxylin and eosin-stained sections of all 142 cases were
retrospectively reviewed by two experienced pathologists. To
further investigate the role of PBs in Xp11.2 tRCC and PRCC, each
group was divided into two subgroups according to the presence of
PBs. Besides, sensitivity, specificity and Youden's index of PB in
the diagnosis of Xp11.2 RCC was measured by FISH. Clinical data
including patient age at onset, sex, maximum tumor diameter,
American Joint Committee on Cancer (AJCC) stage (15) and surgical procedure were obtained
from reviewing medical records.
Survival data
All patients were followed every 3 months during the
first year, every 6 months during the following 4 years and
annually after 5 years until the time of death or the loss of
follow-up. The survival data were obtained from latest follow-up
information. Progression-free survival (PFS) was defined as the
time interval between the date of surgery and the date of
disease-progression or censoring at the time of the last follow-up.
Overall survival (OS) was defined from the date of surgery to the
mortality date or the last follow-up.
Statistical analysis
Continuous variables are presented as mean ±
standard deviation and were compared using Student's t-test or one
way analysis of variance followed by Student-Newman-Keuls post-hoc
test in case of multiple group comparisons. Categorical variables
were expressed as counts and percentages, and χ2 or
Fisher exact test were used for comparisons. Due to selection bias
between subgroups, propensity score matching (PSM) was preformed to
balance the distribution of covariates. A non-parsimonious logistic
regression model encompassing patients' age, sex, tumor diameter,
AJCC stage, surgical procedure and tumor subtype was used to
calculate a propensity score for each patient. A 1:1 matching ratio
was used in the propensity analyses. Following PSM, matched
covariates were re-compared between the two groups with statistical
tests. Subsequently, PFS and OS curves were obtained by
Kaplan-Meier analysis and statistic comparisons were undertaken
using the log-rank test.
In all tests, a two-sided P<0.05 was considered
to indicate statistically significant differences. Statistical
analysis was performed with SPSS software version 23.0 (IBM Corp.).
GraphPad Prism software version 5.0 (GraphPad Software, Inc.) was
used for graphics of survival curves.
Results
Frequency of PBs in different tumor
types
Among the 47 cases of Xp11.2 tRCC, 13 (27.7%) were
identified as ASPL-TFE3 tRCC. The incidence rates of PBs in
ASPL-TFE3 tRCC and type 1 PRCC were 69.2 and 41.9%,
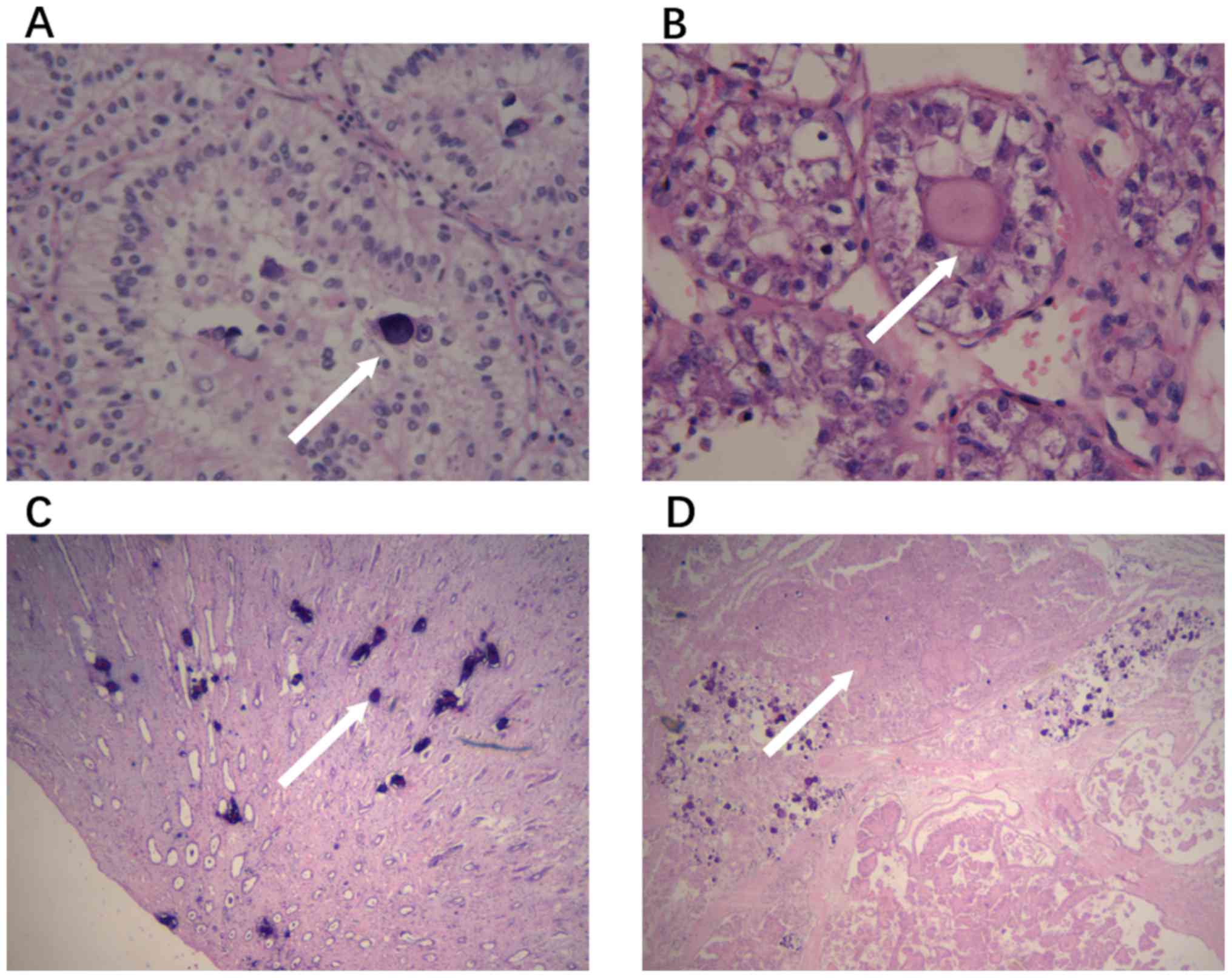
respectively. The presence of PBs, irregular calcifications,
hyaline globules and nested architecture was determined and
recorded (Fig. 1). Table I presents the baseline clinical
characteristics and frequency of PBs, irregular calcifications,
hyaline globules and nested architecture in all the patients. In
the Xp11.2 tRCC group, the incidence rate of PBs, hyaline globules
and nested architecture was significantly higher compared with that
in PRCCs (all P<0.05).
 | Table I.Clinicopathological characteristics
of Xp11.2 tRCC and PRCC. |
Table I.
Clinicopathological characteristics
of Xp11.2 tRCC and PRCC.
| Characteristic | Xp11.2 tRCC
(n=47) | PRCC (n=95) | P-value |
|---|
| Age (years) | 31.32±15.66 | 54.47±14.02 | <0.001 |
| Sex |
|
| 0.050 |
|
Male | 20 (42.6) | 25 (26.3) |
|
|
Female | 27 (57.4) | 70 (73.7) |
|
| Tumor size
(cm) | 5.43±2.39 | 5.01±2.60 | 0.360 |
| AJCC stage |
|
| 0.608 |
| 1 | 29 (61.7) | 63 (66.3) |
|
| 2 | 4 (8.5) | 8 (8.4) |
|
| 3 | 11 (23.4) | 18 (18.9) |
|
| 4 | 3 (6.4) | 6 (6.3) |
|
| Psammoma body | 25 (53.2) | 29 (30.5) | 0.009 |
| Irregular
calcification | 29 (61.7) | 42 (44.2) | 0.050 |
| Hyaline
globule | 35 (74.5) | 53 (55.8) | 0.031 |
| Nested
architecture | 16 (34.0) | 14 (14.7) | 0.008 |
Association of other morphological
characteristics with PBs
Hyaline globules were present in 22 cases (88.0%) of
Xp11.2 tRCC with PBs, as opposed to 13 (59.1%) cases without PBs
(P=0.053). In addition, 91.7% (33/36) cases of Xp11.2 tRCC
displayed PBs or irregular calcifications, as opposed to 18.2%
(2/11) cases without PBs (P<0.001). Similarly, a significantly
higher number of PRCC cases with PBs or irregular calcifications
contained hyaline globules, as opposed to cases without PBs or
irregular calcifications [45/54 (83.3%) vs. 8/41 (19.5%),
respectively; P<0.001]. In addition, the sensitivity,
specificity and Youden's index of PBs in diagnosing Xp11.2 tRCC
were 53.2, 69.5 and 22.7% respectively. For nested architecture,
another histopathological characteristic of Xp11.2 tRCC, the
sensitivity, specificity and Youden's index were 34.0, 85.3 and
19.3%, respectively. When PBs were combined with nested
architecture, the specificity in diagnosing Xp11.2 tRCC increased
to 93.7%; however, sensitivity dropped to 17.0% (Table II).
 | Table II.The role of psammoma bodies and
nested architecture in diagnosing Xp11.2 tRCC. |
Table II.
The role of psammoma bodies and
nested architecture in diagnosing Xp11.2 tRCC.
| Characteristic | Sensitivity
(%) | Specificity
(%) | Youden's index
(%) |
|---|
| Psammoma body | 53.2 | 69.5 | 22.7 |
| Nested
architecture | 34.0 | 85.3 | 19.3 |
| Psammoma body +
Nested architecture | 93.7 | 17.0 | 10.7 |
The clinicopathological characteristics of patients
with Xp11.2 tRCC and PRCC before and after PSM are summarized in
Tables III and IV, respectively. Prior to PSM, only the
AJCC stages in the PRCC group were proven to differ significantly
between the groups with and without PB (P=0.015). After PSM, 44
patients with Xp11.2 tRCC and 58 with PRCC were selected and no
significant differences in clinicopathological characteristics,
including patient age, sex distribution, tumor size, AJCC stage and
subtype was associated with the presence of PBs.
 | Table III.Comparisons of clinicopathological
characteristics of Xp11.2 tRCC based on the presence of PB. |
Table III.
Comparisons of clinicopathological
characteristics of Xp11.2 tRCC based on the presence of PB.
|
|
| PB |
|---|
|
|
|
|
|---|
| Characteristic | No PB (n=22) | All (n=25) |
P-valuea | PSM-adjusted
(n=22) |
P-valuea |
|---|
| Age
(years)b | 29.32±18.41 | 32.84±13.51 | 0.465 | 32.68±13.64 | 0.500 |
| Sexc |
|
| 0.831 |
| 1 |
|
Female | 13 (59.09) | 14 (56.00) |
| 13 (59.09) |
|
|
Male | 9 (40.91) | 11 (44.00) |
| 9 (40.91) |
|
| Tumor size
(cm)b | 6.00±2.55 | 4.92±2.17 | 0.166 | 5.03±2.28 | 0.173 |
| AJCC
stagec |
|
| 0.458 |
| 0.666 |
| 1 | 12 (54.55) | 17 (86.00) |
| 14 (63.64) |
|
| 2 | 3 (13.64) | 1 (4.00) |
| 1 (4.55) |
|
| 3 | 5 (22.72) | 6 (24.00) |
| 6 (27.26) |
|
| 4 | 2 (9.09) | 1 (4.00) |
| 1 (4.55) |
|
|
Subtypec |
|
| 0.173 |
| 0.173 |
|
ASPL | 4 (18.18) | 9 (36.00) |
| 8 (36.36) |
|
|
Non-ASPL | 18 (81.82) | 16 (64.00) |
| 14 (63.64) |
|
 | Table IV.Comparisons of clinicopathological
characteristics of PRCC based on the presence of PB. |
Table IV.
Comparisons of clinicopathological
characteristics of PRCC based on the presence of PB.
|
|
| No PB |
|---|
|
|
|
|
|---|
| Characteristic | PB (n=29) | Original
(n=66) |
P-valuea | PSM-adjusted
(n=29) |
P-valuea |
|---|
| Age
(years)b | 52.07±15.67 | 55.53±13.22 | 0.265 | 59.17±13.42 | 0.053 |
| Sexc |
|
| 0.409 |
| 1 |
|
Male | 6 (20.69) | 19 (28.79) |
| 6 (20.69) |
|
|
Female | 23 (79.31) | 47 (71.21) |
| 23 (79.31) |
|
| Tumor size
(cm)b | 4.36±2.24 | 5.30±2.72 | 0.115 | 5.21±2.96 | 0.224 |
| AJCC
stagec |
|
| 0.015 |
| 0.058 |
| 1 | 24 (82.76) | 39 (59.09) |
| 18 (62.07) |
|
| 2 | 3 (10.34) | 5 (7.57) |
| 4 (13.79) |
|
| 3 | 1 (3.45) | 17 (25.76) |
| 6 (20.69) |
|
| 4 | 1 (3.45) | 5 (7.57) |
| 1 (3.45) |
|
|
Subtypec |
|
| 0.093 |
| 0.097 |
| 1 | 13 (44.83) | 18 (27.27) |
| 7 (24.14) |
|
| 2 | 16 (55.17) | 48 (72.73) |
| 22 (75.86) |
|
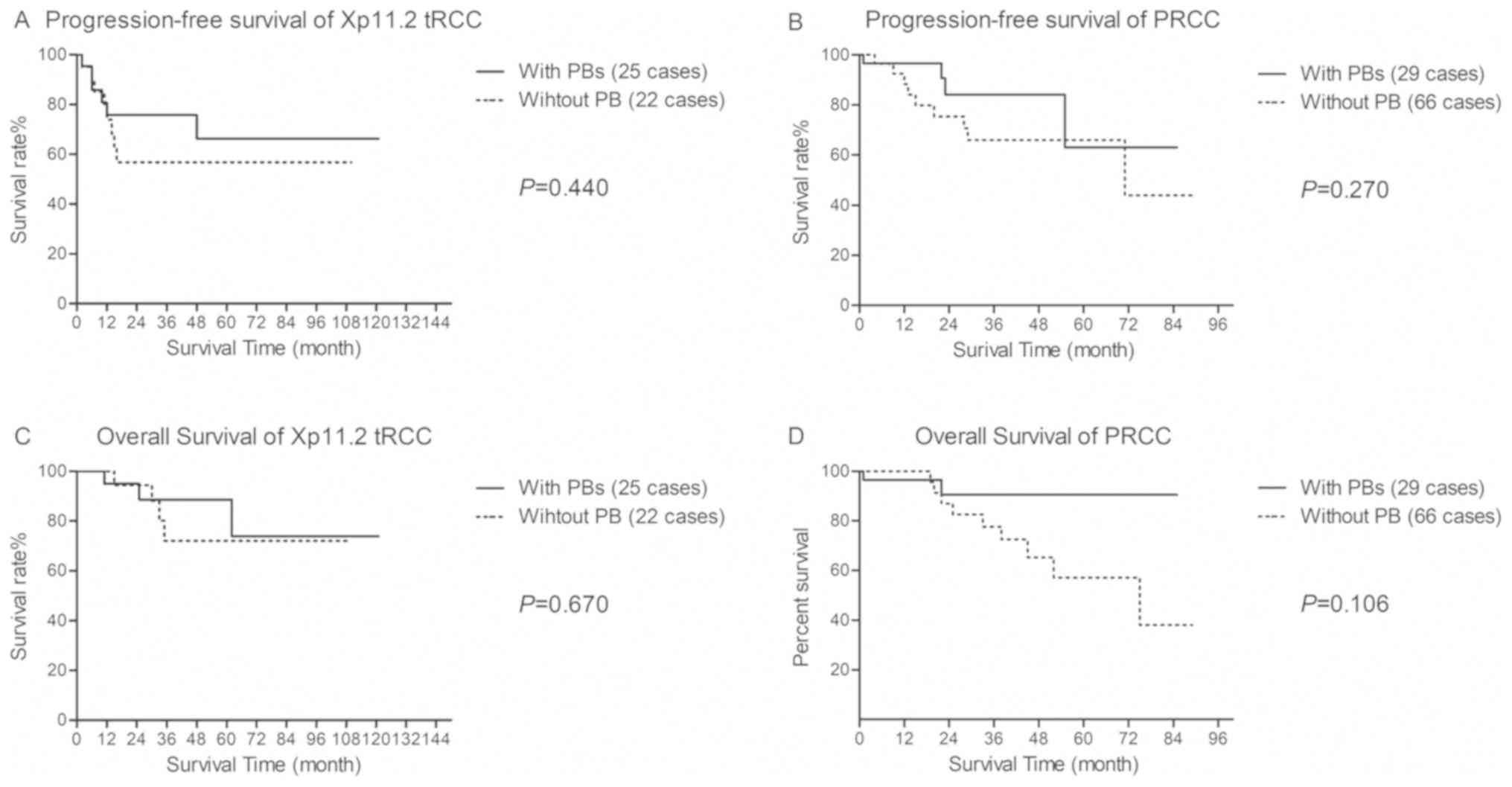
Follow-up and survival data
Except for two patients, no patients were lost to
follow-up. The mean follow-up duration was 44.69±31.56 months
(range 5–121 months) in the Xp11.2 tRCC group and 27.23±21.40
months (range 1–89 months) in the PRCC group. During follow-up, 14
patients with Xp11.2 tRCC (29.8%) and 22 patients with PRCC (23.2%)
showed disease progression. The 5-year OS rate for patients with
Xp11.2 tRCC and PRCC was 81.8 and 67.1%, respectively (P=0.196).
After adjusting the covariates using PSM, there were no statistical
differences in the survival curves for PFS and OS based on PB
occurrence (Fig. 2).
Discussion
Since PBs were first described, various mechanisms
underlying PB morphogenesis have been suggested and the hypothesis
of dystrophic calcification appears to be the most likely
explanation. During the process of dystrophic calcification, events
such as thickening of the basal lamina, vascular thrombosis,
calcification and tumor cell necrosis occur sequentially (4). The formation of papillae, where the
precursors of PBs usually originate, is an important morphological
characteristic associated with PBs (2). After analyzing the IgG distribution of
blood vessels, whorls and PBs of meningiomas, Tabuchi et al
(16) hypothesized that humoral
immunity participates in the formation of whorls and PBs. Another
important contributor of the formation of PBs is type IV collagen
(3). Described by a nested formation
around the basement membrane, type IV collagen may be involved in
anchoring PBs to the site of development of meningiomas (3). Additionally, type IV collagen was found
to be selectively expressed in PB-forming ovarian cancer cell
lines, which suggested that type IV collagen slows down the
formation of PBs during the growth of ovarian cancer cells
(17).
In RCC, PBs have mainly been reported for Xp11.2
tRCC and PRCC (8,9), and occasionally for chromophobe RCC
(18) and renal oncocytoma (19). Unlike the aforementioned studies,
which were largely supported by immunohistochemistry and molecular
techniques, the present study was a morphological study to
summarize the incidence of PBs in PRCC and Xp11.2 tRCC. Published
reports of Xp11.2 tRCC with identified PBs are summarized in
Table V, to reflect that the
incidence rate of PB in Xp11.2 tRCC varies greatly among different
reports (10,20–25). To
the best of our knowledge, there has been no previous report about
the clinical significance of PBs in Xp11.2 tRCC.
 | Table V.Literature described cases of Xp11.2
tRCC involving psammoma bodies. |
Table V.
Literature described cases of Xp11.2
tRCC involving psammoma bodies.
| Author, year | Patients (n) | Diagnosis
methods | Incident rate of PB
(%) | (Refs.) |
|---|
| Qu et al,
2016 | 30 | IHC+FISH | 26.7 | (20) |
| Rao et al,
2011 | 19 | IHC+CK | 94.7 | (21) |
| Zou et al,
2014 | 9 | IHC | 77.8 | (22) |
| He et al,
2014 | 6 | IHC | 83.3 | (23) |
| Komai et al,
2009 | 7 | IHC+CK | 42.9 | (24) |
| Argani et
al, 2007 | 28 | IHC+PCR | 50.0 | (10) |
| Camparo et
al, 2008 | 31 | IHC+CK | 62.0 | (25) |
Compared with PRCC, Xp11.2 tRCC shares a similar
histopathological structure, but exhibits a more aggressive course
(10,11). In the diagnosis of Xp11.2 tRCC,
TFE3 immunohistochemical staining serves as the basic
method, with high false-positive rates and low predictive values
(26). Therefore, FISH, PCR or
karyotype analysis is applied for a definitive diagnosis (12). Although frequently present in Xp11.2
tRCC and PRCC, the diagnostic and predictive role of PBs has not
yet been fully elucidated (8,9). The
present study included 47 cases of Xp11.2 tRCC and 95 cases of
PRCC, and demonstrated that the incidence of PBs in Xp11.2 tRCC was
significantly higher compared with that in PRCC. The specificity of
PBs combined with nested architecture in diagnosing Xp11.2 tRCC
reached 93.7%, suggesting a potential diagnostic role in Xp11.2
tRCC screening.
Numerous studies have reported the association
between hyaline globules and PBs/irregular calcifications (3,13,27). In
a PTC case diagnosed by fine-needle aspiration, Haji et al
(27) found only few PBs, but
numerous laminated hyaline globules. Immunohistochemical staining
using paraffin-embedded sections revealed that these globules were
positive for von Kossa staining, but negative for thyroglobulin,
suggesting an early or precursor form of PBs. In the study from Das
et al (13), the presence of
hyaline degeneration among cases with and without PBs/irregular
calcifications was 80.0% vs. 22.7%, respectively. Similarly, the
statistical analysis of the present study favored the hypothesis
that hyaline globules may be precursors of PBs in Xp11.2 tRCC and
PRCC.
The predictive role of PBs has been associated with
tumor types (2,7). In the PRCC group, type 1 tumors
contained more PBs and displayed a more favorable behavior
(9). In the Xp11.2 tRCC group,
ASPL-TFE3 tRCC exhibited a higher incidence of PBs compared
with other fusion types. However, ASPL-TFE3 tRCC has been
reported to be more likely associated with lymph node metastasis
and disease progression (10,11,28).
Based on this, it is crucial to investigate whether PBs exert
opposite effects in different tumor types. However, the PSM data
obtained in the present study suggested that the presence of PBs
was not associated with any of the observed clinicopathological
characteristics, including tumor size, AJCC stage or survival, in
PRCC and Xp11.2 tRCC.
To the best of our knowledge, the present study was
the first to systematically investigate the incidence and
significance of PBs, irregular calcifications, hyaline globules and
nested architecture in Xp11.2 tRCC and PRCC. However, there were
certain limitations. This was a retrospective study and the sample
size of Xp11.2 tRCC was low due to its scarcity; however, it is
believed to be one of the largest single-center clinical reports on
Xp11.2 tRCC. Additionally, as patients received various adjuvant
therapies following surgery, selection bias persisted even after
PSM was performed. Furthermore, other subtypes of RCC, including
chromophobe RCC and renal oncocytoma, were not included in the
present study due to the small number of cases and their favorable
prognosis. Further studies are required to elucidate the
biochemical mechanisms underlying the development of PBs in
RCC.
The incidence rate of PBs in Xp11.2 tRCC was found
to be significantly higher compared with that in PRCC, and the
presence of hyaline globules was associated with PBs and irregular
calcifications. However, the presence of PBs was not found to be
associated with tumor subtype or prognosis in Xp11.2 tRCC or PRCC.
When PBs and nested architecture were present simultaneously in
RCC, additional methods may be used to investigate the potential of
Xp11.2 tRCC occurrence.
Acknowledgements
The authors would like to thank Professor Xiaogong
Li, Professor Gutian Zhang, Dr Linfang Yao and Dr Xiaozhi Zhao
(Department of Urology, Nanjing Drum Tower Hospital) for providing
patient information.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572512).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WG, DL and HG provided the idea and acquired funding
for the studies. NL and FQ made substantial contributions to the
conception or design of the work. NL, KW, WZ, ZW, SA and WM
analyzed and interpreted the patient data regarding psammoma bodies
in Xp11.2 translocation renal cell carcinoma and papillary renal
cell carcinoma. FQ, LX, JY and MC performed the histological
examinations of the renal cell carcinomas and were major
contributors to the writing of the manuscript. WG, LX and HG were
involved in drafting and revising the manuscript. All the authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Medical Ethics
Committee for Human Experiments of Nanjing Drum Tower Hospital
(Nanjing, China). At time of hospitalization, patients provided
written informed consent for the collection and use of their
tissues.
Patient consent for publication
The patients, or parents, guardians or next of kin
provided written informed consent for the publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AJCC
|
American Joint Committee on Cancer
|
|
FISH
|
fluorescence in situ
hybridization
|
|
OS
|
overall survival
|
|
PBs
|
psammoma bodies
|
|
PRCC
|
papillary renal cell carcinoma
|
|
PSF
|
progression-free survival
|
|
PSM
|
propensity score matching
|
|
PTC
|
papillary thyroid carcinoma
|
|
RCC
|
renal cell carcinoma
|
|
Xp11.2 tRCC
|
Xp11.2 translocation renal cell
carcinoma
|
References
|
1
|
Fadare O, Chacho MS and Parkash V:
Psammoma bodies in cervicovaginal smears: Significance and
practical implications for diagnostic cytopathology. Adv Anat
Pathol. 11:250–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Das DK: Psammoma body: A product of
dystrophic calcification or of a biologically active process that
aims at limiting the growth and spread of tumor? Diagn Cytopathol.
37:534–541. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han J, Daniel JC and Pappas GD: Expression
of type VI collagen in psammoma bodies: Immunofluorescence studies
on two fresh human meningiomas. Acta Cytol. 40:177–181. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johannessen JV and Sobrinho-Simoes M: The
origin and significance of thyroid psammoma bodies. Lab Invest.
43:287–296. 1980.PubMed/NCBI
|
|
5
|
Pyo JS, Kang G, Kim DH, Park C, Kim JH and
Sohn JH: The prognostic relevance of psammoma bodies and
ultrasonographic intratumoral calcifications in papillary thyroid
carcinoma. World J Surg. 37:2330–2335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carneiro SS, Scheithauer BW, Nascimento
AG, Hirose T and Davis DH: Solitary fibrous tumor of the meninges:
A lesion distinct from fibrous meningioma. A clinicopathologic and
immunohistochemical study. Am J Clin Pathol. 106:217–224. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai YF, Wang QX, Ni CJ, Guo GL, Li Q, Wang
OC, Wu L, Du HY, You J and Zhang XH: The clinical relevance of
psammoma body and hashimoto thyroiditis in papillary thyroid
carcinoma: A large case-control study. Medicine (Baltimore).
94:e18812015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Armah HB and Parwani AV: Xp11.2
translocation renal cell carcinoma. Arch Pathol Lab Med.
134:124–129. 2010.PubMed/NCBI
|
|
9
|
Delahunt B, Eble JN, McCredie MR,
Bethwaite PB, Stewart JH and Bilous AM: Morphologic typing of
papillary renal cell carcinoma: Comparison of growth kinetics and
patient survival in 66 cases. Hum Pathol. 32:590–595. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Argani P, Olgac S, Tickoo SK, Goldfischer
M, Moch H, Chan DY, Eble JN, Bonsib SM, Jimeno M, Lloreta J, et al:
Xp11 translocation renal cell carcinoma in adults: Expanded
clinical, pathologic, and genetic spectrum. Am J Surg Pathol.
31:1149–1160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Yang Y, Gan W, Xu L, Ye Q and Guo
H: Newly designed break-apart and ASPL-TFE3 dual-fusion FISH assay
are useful in diagnosing Xp11.2 translocation renal cell carcinoma
and ASPL-TFE3 renal cell carcinoma: A STARD-compliant article.
Medicine (Baltimore). 94:e8732015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu N, Wang Z, Gan W, Xiong L, Miao B,
Chen X, Guo H and Li D: Renal cell carcinoma associated with Xp11.2
translocation/TFE3 gene fusions: Clinical features, treatments and
prognosis. PLoS One. 11:e01668972016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Das DK, Mallik MK, Haji BE, Ahmed MS,
Al-Shama'a M, Al-Ayadhy B, George SS, Sathar SA and Junaid TA:
Psammoma body and its precursors in papillary thyroid carcinoma: A
study by fine-needle aspiration cytology. Diagn Cytopathol.
31:380–386. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-Part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tabuchi K, Kawakami Y and Nishimoto A:
Immunohistochemical demonstration of IgG in meningioma. Acta
Neurochir (Wien). 55:201–211. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kiyozuka Y, Nakagawa H, Senzaki H, Uemura
Y, Adachi S, Teramoto Y, Matsuyama T, Bessho K and Tsubura A: Bone
morphogenetic protein-2 and type IV collagen expression in psammoma
body forming ovarian cancer. Anticancer Res. 21:1723–1730.
2001.PubMed/NCBI
|
|
18
|
Cohen RJ, Weinstein S, Robertson T,
Sellner LN, Dawkins HJ and McNeal JE: Variant chromophobe renal
cell carcinoma. Arch Pathol Lab Med. 124:904–906. 2000.PubMed/NCBI
|
|
19
|
Amin MB, Crotty TB, Tickoo SK and Farrow
GM: Renal oncocytoma: A reappraisal of morphologic features with
clinicopathologic findings in 80 cases. Am J Surg Pathol. 21:1–12.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu Y, Gu C, Wang H, Chang K, Yang X, Zhou
X, Dai B, Zhu Y, Shi G, Zhang H and Ye D: Diagnosis of adults
Xp11.2 translocation renal cell carcinoma by immunohistochemistry
and FISH assays: Clinicopathological data from ethnic Chinese
population. Sci Rep. 6:216772016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rao Q, Chen JY, Wang JD, Ma HH, Zhou HB,
Lu ZF and Zhou XJ: Renal cell carcinoma in children and young
adults: Clinicopathological, immunohistochemical, and VHL gene
analysis of 46 cases with follow-up. Int J Surg Pathol. 19:170–179.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou H, Kang X, Pang LJ, Hu W, Zhao J, Qi
Y, Hu J, Liu C, Li H, Liang W, et al: Xp11 translocation renal cell
carcinoma in adults: A clinicopathological and comparative genomic
hybridization study. Int J Clin Exp Pathol. 7:236–245.
2014.PubMed/NCBI
|
|
23
|
He J, Huan Y, Qiao Q, Zhang J and Zhang
JS: Renal carcinomas associated with Xp11.2 translocations: Are CT
findings suggestive of the diagnosis? Clin Radiol. 69:45–51. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Komai Y, Fujiwara M, Fujii Y, Mukai H,
Yonese J, Kawakami S, Yamamoto S, Migita T, Ishikawa Y, Kurata M,
et al: Adult Xp11 translocation renal cell carcinoma diagnosed by
cytogenetics and immunohistochemistry. Clin Cancer Res.
15:1170–1176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Camparo P, Vasiliu V, Molinie V, Couturier
J, Dykema KJ, Petillo D, Furge KA, Comperat EM, Lae M, Bouvier R,
et al: Renal translocation carcinomas: Clinicopathologic,
immunohistochemical, and gene expression profiling analysis of 31
cases with a review of the literature. Am J Surg Pathol.
32:656–670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klatte T, Streubel B, Wrba F, Remzi M,
Krammer B, de Martino M, Waldert M, Marberger M, Susani M and
Haitel A: Renal cell carcinoma associated with transcription factor
E3 expression and Xp11.2 translocation: Incidence, characteristics,
and prognosis. Am J Clin Pathol. 137:761–768. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haji BE, Ahmed MS, Prasad A, Omar MS and
Das DK: Papillary thyroid carcinoma with an adenoid cystic pattern:
Report of a case with fine-needle aspiration cytology and
immunocytochemistry. Diagn Cytopathol. 30:418–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ellis CL, Eble JN, Subhawong AP,
Martignoni G, Zhong M, Ladanyi M, Epstein JI, Netto GJ and Argani
P: Clinical heterogeneity of Xp11 translocation renal cell
carcinoma: Impact of fusion subtype, age, and stage. Mod Pathol.
27:875–886. 2014. View Article : Google Scholar : PubMed/NCBI
|