Introduction
Gastric cancer (GC) is the fourth most common type
of cancer and the second most common cause of cancer-associated
mortality worldwide (1). In China,
the morbidity and mortality rates of GC have gradually increased,
which is a major public health concern (2). Although the causes of GC, including
environmental and genetic factors (3,4), are
commonly known, there are no effective strategies available to
prevent its development. The clinical outcome of patients with
advanced GC remains poor, despite major advances in treatment
strategies, including surgery, radiotherapy and chemotherapy
(5). Therefore, it is crucial to
identify a sensitive and specific biomarker that could predict GC
prognosis and may be used as a target for GC treatment.
Reactive oxygen species (ROS) are involved in cell
growth, differentiation, progression and death (6), and include chemically active
oxygen-containing atoms or atom groups, including superoxide
radical, hydrogen peroxide and hydroxyl radical (7). Low concentrations of ROS are crucial
for biological processes, including intracellular signaling and
defense against microorganisms (8).
In cancer cells, ROS contribute to cancer progression by amplifying
genomic instability (9). However,
ROS can also induce severe cellular damage, including to cancer
cells (10). Both ROS-elevating and
ROS-eliminating strategies can therefore be developed to treat
cancer eliminate cancer cells. ROS in excess is known to attack DNA
(11). The level of oxidative DNA
damage is increased in various types of tumor, including melanoma,
colorectal and pancreatic cancers, which strongly suggests its
involvement in cancer development (12). DNA damage serves a key role in
carcinogenesis (13). ROS may be
involved in the three stages of carcinogenesis, which are
initiation, promotion and progression (14). ROS in excess are normally eliminated
by cellular antioxidant defense systems (15), which protect cell against oxidative
damage.
Methionine sulfoxide reductase (MSR) in an enzyme
that reduces methionine sulfoxide into methionine (16). MSR is considered as an important
antioxidant enzyme for cellular ROS scavenging (17). MSRs are commonly identified in
various organisms ranging from bacteria to mammals, and are also
involved in protein repair and protein function regulation
(18). MSRs are evolutionarily
conserved. There are two isomers produced during methionine
oxidation: Methionine-S-sulfoxide and methionine-R-sulfoxide, which
are reduced by MSRA and MSRB, respectively (19). The human MSRA gene is located on
chromosome 8 and codes for a protein located in the mitochondria,
cytosol and nucleus (20–22). The human genome contains three MSRB
genes that code for the proteins, MSRB1, MSRB2 and MSRB3 (23). MSRB1, also termed selenoprotein R, is
present in the cytoplasm and nucleus; whereas, MSRB2 is present in
the mitochondria. There are two forms of human MSRB3; MSRB3A, which
is localized in the endoplasmic reticulum, and MSRB3B, which is
localized in the mitochondria. MSRB3A and MSRB3B are generated via
alternative first-exon splicing (24).
As an important member of the MSR family, MSRB3 is
involved in the response to oxidative stress-induced tissue
alteration (25). MSRB3 can prevent
oncogene-induced DNA damage (26).
This suggests that MSRB3 and cancer may be associated. However, to
the best of our knowledge, only a few studies have reported the
effects of MSRB3 in cancer cells. Morel et al (26) demonstrated that the expression of
MSRB3 promotes malignant transformation of breast stem cells. Kwak
et al (17) reported that
MSRB3 deficiency leads to breast, lung and liver cancer cell
apoptosis. In addition, the functional role of MSRB3 in the
protection against oncogene-induced DNA damage may be applicable to
a broad range of tumors including breast, lung and colorectal
cancers (26). Although MSRB3 is
highly expressed in the stomach (27), the role of MSRB3 in GC has not yet
been elucidated. Therefore, the study of MSRB3 expression in human
GC may provide some indications about its role in GC.
To the best of our knowledge, the expression of
MSRB3 in GC and its clinical relevance have not yet been
investigated. The present study aimed to investigate the expression
of MSRB3 in GC samples and to determine whether MSRB3 may be
associated with GC clinical outcomes. In addition, the Cancer
Genome Atlas (TCGA) database was used to validate the results.
Materials and methods
Patients and tissue samples
A total of 90 formalin-fixed, paraffin-embedded GC
tissues samples and paired adjacent normal tissues (at a distance
of 5 cm away from the edge of the cancerous tissue) were collected
from the Department of Pathology, Nanjing Drum Tower Hospital Group
Suqian People's Hospital (Suqian, China). All samples were
pathologically confirmed as GC and were collected from patients who
underwent surgical resection between May 2007 and April 2008. The
surgical specimens were fixed with 10% formalin at room temperature
for 24 h. Formalin-fixed, paraffin-embedded tissue blocks were
processed by pathologists in accordance with standard procedures.
The sections were stored at room temperature under dry conditions.
Patients had not received preoperative radiotherapy, chemotherapy
or biotherapy for cancer. The patients consisted of 70 (77.8%) men
and 20 (22.2%) women, and the age range was 34–83 years (median, 66
years old). A total of 62 patients succumbed to the disease during
the follow-up and the median follow-up time was 38 months. The
present study protocol was approved by the Ethics and Research
Committees of Nanjing Drum Tower Hospital Group Suqian People's
Hospital (Suqian, China) and was conducted in accordance with the
principles outlined in The Declaration of Helsinki. Written
informed consent was obtained from all patients included in the
study. The tissue microarray (TMA) was made in accordance with the
standard method (28) using a manual
microarray device (Beecher Instruments). The TMA blocks contained
complete clinical data, including age, sex, maximum tumor diameter,
histological differentiation, depth of invasion, lymph node
metastasis, distant metastasis and TNM stage (29).
Data mining
The TCGA database was used to further investigate
the association between MSRB3 mRNA expression and the overall
survival (OS) time of patients with GC. The TCGA database is a
public patient database, which includes high-throughput genome
sequencing of >11,000 tumor tissues and matched normal tissues
from patients (30). According to
parameters defined in previous studies (31,32), the
MSRB3 expression and clinical data from the TCGA database were
downloaded from the TCGA website (http://cancergenome.nih.gov/). A total of 442 GC
samples that contained detailed MSRB3 expression data were
available for this analysis. Patients had received no pretreatment
and data comprised fully characterized tumors, OS time and complete
RNAseq information. The median MSRB3 mRNA level was used as the
cut-off value in tumor tissue. According to this level [median,
478.40; interquartile range (IQR), 230.63–1039.47], patients were
subdivided into low and high MSRB3 expression groups for further
analysis as follows: For the low MSRB3 expression group, the median
was 229.36 and the IQR was 156.51–342.33. For the high MSRB3
expression group, the median was 1,035.65 and the IQR was
670.30–1,832.16.
Immunohistochemistry analysis
Some 4-µm-thick sections were cut from TMA blocks.
Staining was conducted using the streptavidin-peroxidase method,
according to the manufacturer's protocol (Thermo Fisher Scientific,
Inc.). Briefly, sections were dewaxed in xylene and rehydrated in
increasing ethanol gradients of 100, 95, 90, 85 and 70%. Then
samples were boiled at 100°C in citrate buffer for antigen
retrieval. Samples were blocked with 5% normal goat serum (cat. no.
KL-D1418; Kalang Biologicals) for 30 min at 37°C and incubated with
rabbit anti-human MSRB3 polyclonal primary antibody (1:500; cat.
no. NBP1-84259; Novus Biologicals; Littleton; CO; USA) overnight at
4°C. Subsequently, samples were washed in PBS and incubated with
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
(H+L) highly cross-adsorbed secondary antibody (1:1,000; cat. no.
A16110; Thermo Fisher Scientific, Inc.) at room temperature for 30
min. The peroxidase reaction was developed by 3,3′-diaminobenzidine
staining. Eventually, sections were lightly counterstained with
hematoxylin at room temperature for 3 min and mounted on glass
slides.
Immunostaining was evaluated by two experienced
pathologists using an inverted microscope (IX73; Olympus
Corporation; magnification, ×200) in a double-blinded manner. A
total of five microscopic fields from representative MSRB3 immune
responses were examined. MSRB3 expression was scored based on
staining intensity and percentage of positive cells, as previously
described (33). Briefly, the
percentage of positive cells was scored as 0, 1, 2, 3 or 4 for
5–25, 26–50, 51–75 and 76–100% of positive cells, respectively. The
staining intensity was scored as 0, 1, 2 or 3 for a negative, weak,
moderate on strong signal, respectively. The immunoreactivity score
(IRS) was calculated as follows: IRS=staining percentage ×
intensity. IRS <6 was considered as low expression and IRS ≥6
was considered as high expression. A total of 64 and 26 cases had
high and low expression of MSRB3 in GC tissues, respectively.
However, 48 and 42 cases cases had high and low expression of MSRB3
in GC tissues, respectively, in non-cancerous tissues. The
diagnostic value of MSRB3 for GC was therefore estimated with a
sensitivity of 71.1% [64/(64+26)×100%=71.1%] and a specificity of
46.7% [42/(42+48)×100%=46.7%].
Statistical analysis
All statistical analyses were conducted using SPSS
16.0 software (SPSS Inc.). McNemar's test was used to analyze the
difference in MSRB3 expression between different types of tissue.
The association between MSRB3 expression and clinicopathological
characteristics of patients with GC was analyzed by χ2
test. Kaplan-Meier survival curves were plotted and a log-rank test
was used to compare the curves. The Cox's proportional hazards
model was used to determine the factors that were significantly
associated with OS time. A two-sided P<0.05 was considered to
indicate a statistically significant difference.
Results
Aberrant overexpression of MSRB3 in GC
tissues and the diagnostic value of MSRB3 in patients with GC
MSRB3 expression was detected by immunohistochemical
staining in 90 cases of human GC tissues and paired adjacent normal
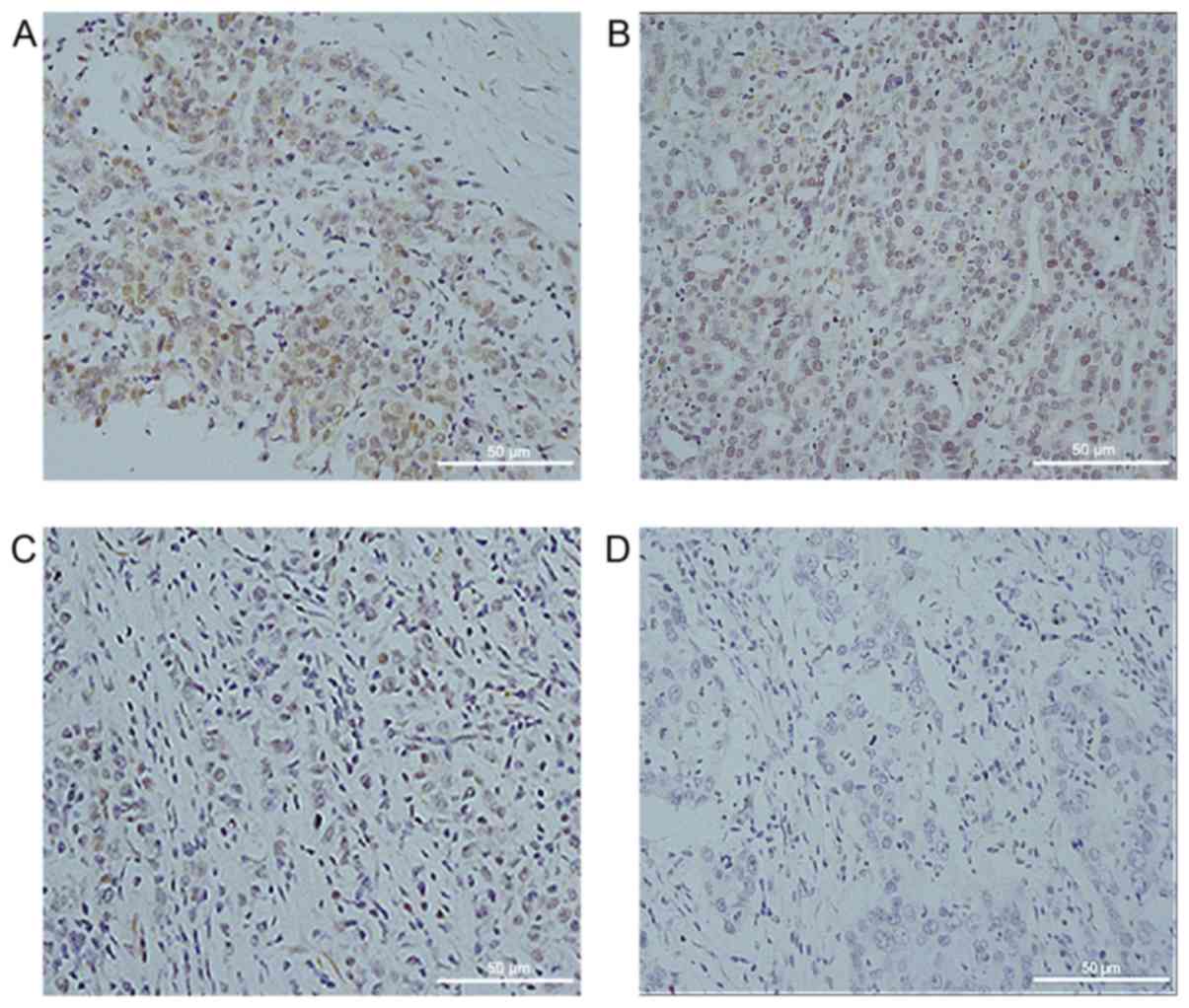
tissues. As presented in Fig. 1,
MSRB3 was localized in the cytoplasm of GC cells. Higher MSRB3
expression levels are presented in Fig.
1A and B, whereas lower expression levels are presented in
Fig. 1C and D. Among the 90 GC
samples, 64 (71.1%) exhibited a high expression of MSRB3, whereas
48 (53.3%) of the 90 paired adjacent normal tissues presented with
a high MSRB3 expression level (Table
I). In addition, MSRB3 protein expression was significantly
different in GC tissues compared with that in the paired adjacent
normal tissues (P=0.017; Table I).
The diagnostic value of MSRB3 for GC was estimated with a
sensitivity of 71.1% and a specificity of 46.7% (data not
shown).
 | Table I.Methionine sulfoxide reductases B3
expression level in different GC and adjacent normal tissues of
patients with GC. |
Table I.
Methionine sulfoxide reductases B3
expression level in different GC and adjacent normal tissues of
patients with GC.
|
| Adjacent normal
tissues |
|
|---|
|
|
|
|
|---|
| GC tissues | High
expression | Low expression | Total |
|---|
| High
expression | 36 | 28 | 64 |
| Low expression | 12 | 14 | 26 |
| Total | 48 | 42 | 90 |
Associations between MSRB3 protein
expression and clinicopathological characteristics of patients with
GC
The associations between MSRB3 expression and the
clinicopathological characteristics of patients with GC were
investigated. The retrieved data included patient age, sex, maximum
tumor diameter, histological differentiation, depth of invasion,
lymph node metastasis, distant metastasis and TNM stage. MSRB3
expression levels in the 90 GC samples were not significantly
associated with age (P=0.731), sex (P=0.901), maximum tumor
diameter (P=0.850), histological differentiation (P=0.972), depth
of invasion (P=0.403), lymph node metastasis (P=0.850), distant
metastasis (P=0.697) or TNM stage (P=0.745; Table II). The maximum tumor diameter was
determined according to the Borrmann classification of cancers
(34); this classification
identified a diffuse invasion with an unclear boundary in 3
patients. The maximum diameter of tumor for these 3 patients was
therefore difficult to determine by pathologists. Therefore, the
total number of patients that were evaluated for the maximum tumor
diameter was only 87 (Table
II).
 | Table II.MSRB3 expression and clinical
characteristics of 90 patients with gastric cancer. |
Table II.
MSRB3 expression and clinical
characteristics of 90 patients with gastric cancer.
|
|
| MSRB3
expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Cases, n | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.731 |
|
<65 | 39 | 12 | 27 |
|
|
≥65 | 51 | 14 | 37 |
|
| Sex |
|
|
| 0.901 |
|
Female | 20 | 6 | 14 |
|
|
Male | 70 | 20 | 50 |
|
| Maximum tumor
diameter, cm |
|
|
| 0.850 |
|
<5 | 30 | 9 | 21 |
|
| ≥5 | 57 | 16 | 41 |
|
| Histological
differentiation |
|
|
| 0.972 |
|
Moderate-high | 24 | 7 | 17 |
|
|
Poor | 66 | 19 | 47 |
|
| Depth of
invasion |
|
|
| 0.403 |
|
T1-2 | 11 | 2 | 9 |
|
|
T3-4 | 79 | 24 | 55 |
|
| Lymph node
metastasis |
|
|
| 0.850 |
| N0 | 23 | 7 | 16 |
|
| N+ | 67 | 19 | 48 |
|
| Distant
metastasis |
|
|
| 0.697 |
| M0 | 86 | 24 | 62 |
|
| M1 | 4 | 2 | 2 |
|
| TNM stage |
|
|
| 0.745 |
|
I/II | 37 | 10 | 27 |
|
|
III/IV | 53 | 16 | 37 |
|
High MSRB3 expression is associated
with poor clinical outcomes in patients with GC
The prognostic value of MSRB3 was evaluated by a
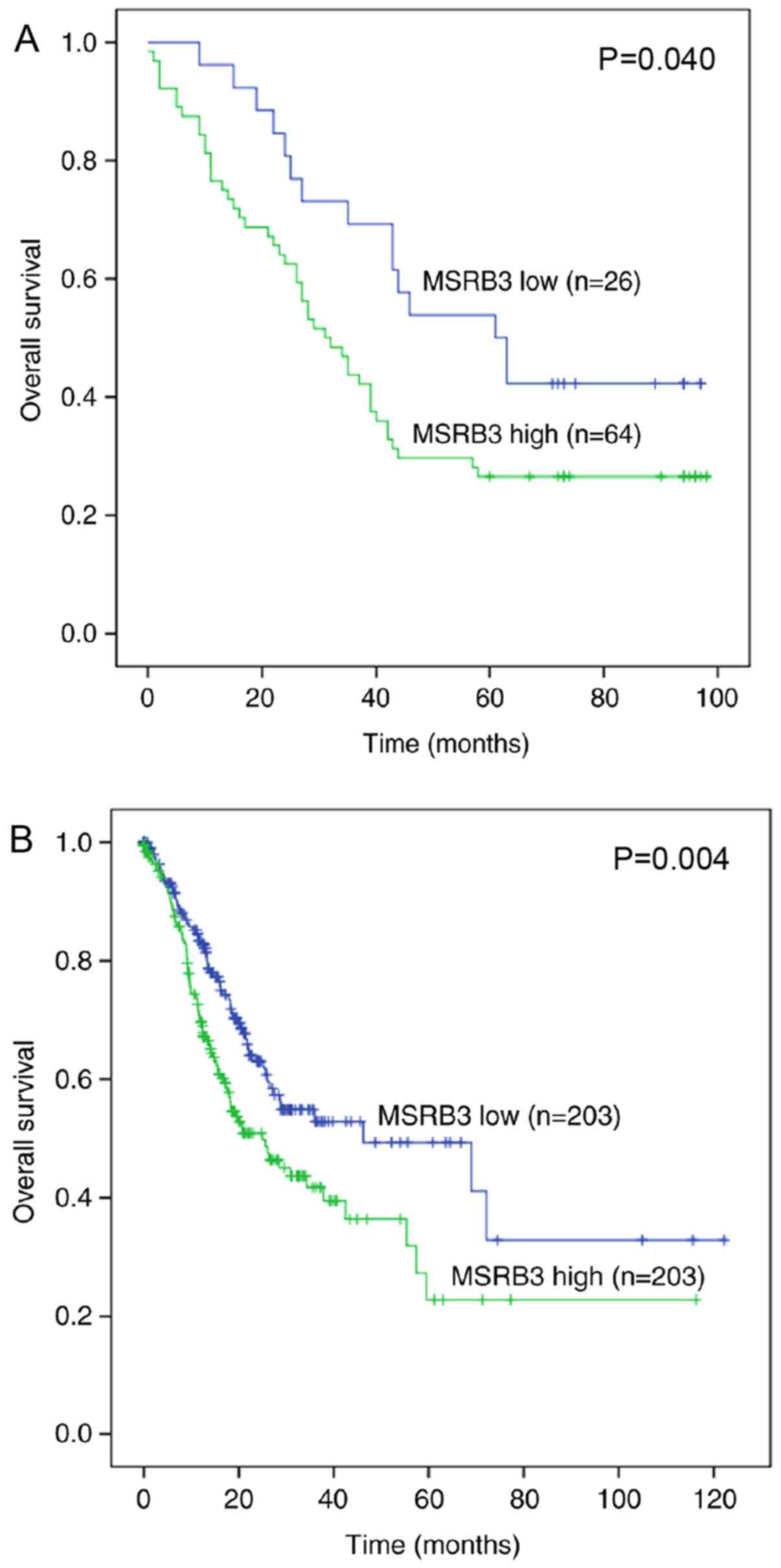
Kaplan-Meier survival curve and log-rank test. The Kaplan-Meier
analysis demonstrated that patients with higher MSRB3 expression
exhibited a poorer prognosis compared with those with lower MSRB3
expression (P=0.040; Fig. 2A).
Univariate and multivariate Cox regression analyses were used to
compare the effect of MSRB3 expression and other
clinicopathological parameters on patient outcome. The univariate
analysis suggested that maximum tumor diameter (P=0.002), depth of
invasion (P=0.008), lymph node metastasis (P=0.006), TNM stage
(P=0.001) and MSRB3 expression (P=0.045) were significantly
associated with OS time; however, OS time was not associated with
age, sex, histological differentiation or distant metastasis
(Table III). Furthermore,
multivariate analysis demonstrated that MSRB3 expression was an
independent predictor in patients with GC [Hazard ratio (HR),
1.813; 95% confidence interval (CI), 1.001–3.281; P=0.049; Table III].
 | Table III.Univariate and multivariate Cox
regression analysis of overall survival time in 90 patients with
gastric cancer. |
Table III.
Univariate and multivariate Cox
regression analysis of overall survival time in 90 patients with
gastric cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age: <65 vs. ≥65
years | 1.518 | 0.906–2.544 | 0.113 |
|
|
|
| Sex: Female vs.
male | 0.936 | 0.516–1.698 | 0.827 |
|
|
|
| Maximum tumor
diameter: <5 vs. ≥5 cm | 2.559 | 1.401–4.677 | 0.002a | 1.818 | 0.986–3.351 | 0.056 |
| Histological
differentiation: Moderate-high vs. poor | 1.480 | 0.815–2.687 | 0.198 |
|
|
|
| Depth of invasion:
T1-2 vs. T3-4 | 6.817 | 1.661–27.980 | 0.008a | 10.038 | 1.329–75.839 | 0.025a |
| Lymph node
metastasis: N0 vs. N+ | 2.595 | 1.314–5.122 | 0.006a | 2.167 | 0.793–5.922 | 0.132 |
| Distant metastasis:
M0 vs. M1 | 2.251 | 0.809–6.261 | 0.120 |
|
|
|
| TNM stage: I/II vs.
III/IV | 2.586 | 1.488–4.495 | 0.001a | 1.138 | 0.505–2.564 | 0.756 |
| MSRB3 expression:
Low vs. high | 1.817 | 1.013–3.257 | 0.045a | 1.813 | 1.001–3.281 | 0.049a |
To validate the prognostic significance of MSRB3 in
a large group of patients with GC, the TCGA database was used to
examine the association between MSRB3 mRNA expression and
prognosis. A Kaplan-Meier survival curve and log-rank test
indicated that high MSRB3 mRNA expression in 442 patients with GC
was associated with a poorer OS time (P=0.004; Fig. 2B) compared with a low MSRB3
expression level. Univariate analysis indicated that age (P=0.020),
depth of invasion (P=0.003), lymph node metastasis (P<0.001)
distant metastasis (P<0.001), TNM stage (P<0.001) and MSRB3
expression (P=0.004) were significantly associated with OS time;
however, OS time was not associated with sex or histological
differentiation (Table IV). The
results from multivariate analysis suggested that MSRB3 may be an
independent prognostic factor in patients with GC (HR, 1.755; 95%
CI, 1.248–2.466; P=0.001; Table
IV).
 | Table IV.Univariate and multivariate Cox
regression analysis of overall survival time in 442 patients with
gastric cancer from The Cancer Genome Atlas database. |
Table IV.
Univariate and multivariate Cox
regression analysis of overall survival time in 442 patients with
gastric cancer from The Cancer Genome Atlas database.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age: <65 vs. ≥65
years | 1.451 | 1.061–1.984 | 0.020a | 1.806 | 1.268–2.573 | 0.001 |
| Sex: Female vs.
male | 1.094 | 0.797–1.503 | 0.579 |
|
|
|
| Histological
differentiation: Moderate-high vs. poor | 1.308 | 0.950–1.801 | 0.100 |
|
|
|
| Depth of invasion:
T1-2 vs. T3-4 | 1.837 | 1.234–2.733 | 0.003a | 1.275 | 0.781–2.083 | 0.331 |
| Lymph node
metastasis: N0 vs. N+ | 2.159 | 1.460–3.191 |
<0.001a | 1.620 | 0.921–2.851 | 0.094 |
| Distant metastasis:
M0 vs. M1 | 2.612 | 1.578–4.323 |
<0.001a | 2.313 | 1.282–4.173 | 0.005a |
| TNM stage: I/II vs.
III/IV | 2.128 | 1.520–2.981 |
<0.001a | 1.242 | 0.731–2.111 | 0.423 |
| MSRB3 mRNA
expression: Low vs. high | 1.589 | 1.158–2.180 | 0.004a | 1.755 | 1.248–2.466 | 0.001a |
Discussion
The present study investigated the expression of
MSRB3 in GC tissues and its association with the
clinicopathological characteristics and prognosis of patients with
GC. The results revealed that MSRB3 expression level in GC samples
was significantly higher compared with in corresponding normal
gastric tissues (P=0.017). The current results also suggested that
high MSRB3 expression was associated with a poorer prognosis for
patients with GC, which was consistent with the data obtained from
the TCGA database. In addition, the expression of MSRB3 was
demonstrated to be an independent prognostic factor in patients
with GC at the mRNA and protein levels.
MSRB3 is present in the ER and mitochondria of human
cells (23), and acts as an
antioxidant and protein repair enzyme by specifically catalyzing
the reduction of methionine-R-sulfoxide residues in proteins
(35). The highest MSRB3 expression
levels are observed in the bladder, heart, skeletal muscles, aorta
and lung (27). MSRB3 is also highly
expressed in the digestive system, including the stomach and
intestines (27). Physiologically,
MSRB3 is important for mouse and human hearing (36,37), and
serves a crucial role in normal cell proliferation (38,39).
MSRB3 deficiency causes G1/S cell cycle arrest by
regulating the expression of heme oxygenase-1, p21 and p27, thereby
inhibiting normal cell proliferation (38,39). In
addition, MSRB3 deficiency increases intracellular ROS levels,
leading to redox imbalance, and also increases the Bax/Bcl-2 ratio
and cytochrome c release from the mitochondria into the cytosol,
which results in caspases activation and cell apoptosis (40,41).
The effect of MSRB3 on cancer cells has been
previously investigated. Morel et al (26) demonstrated that the expression of
MSRB3 enables mammary stem cells to bypass critical anti-tumor
barriers by preventing oncogene-induced cellular stress, thereby
promoting malignant transformation. Kwak et al (42) further reported that MSRB3 is involved
in the regulation of cancer cell proliferation and apoptosis. In
addition, MSRB3 deficiency has been demonstrated to lead to breast,
lung and liver cancer cell apoptosis (17). These previous findings were
consistent with the results from the present study, which
demonstrated that increased MSRB3 expression was associated with
poor clinical outcomes of patients with GC. Furthermore, TCGA
database analysis of MSRB3 mRNA expression validated these results.
MSRB3 is understood to serve a crucial role in cell protection
(24); notably, the results from the
current study suggest that this protective effect may also include
the protection of cancer cells.
Based on the results from the present study, low
MSRB3 expression was hypothesized to cause GC cell apoptosis, which
could lead to better clinical outcomes. A previous study
demonstrated that low expression of MSRB3 causes a negative
response (cancer cell death), whereas its high expression leads to
a positive response (cancer cell proliferation) (42). MSRB3 may also be involved in the
apoptosis of GC cells, as it is involved in lung, breast, and liver
cancer cells (17). The underlying
mechanism of high MSRB3 expression-mediated poor prognosis in
patients with GC requires further investigation. Studies on the
caspase family may help elucidate the mechanisms involved in MSRB3
downregulation-induced cell death.
MSRB3 expression level was not associated with any
clinicopathological parameters of patients with GC, but was
significantly associated with OS time. Particularly, high MSRB3
mRNA and protein expression levels were significantly associated
with poorer OS time. Since MSRB3 was not associated with
clinicopathological characteristics, it was suggested to be an
independent prognostic factor. This hypothesis was subsequently
verified by Cox regression analysis. Multivariate Cox regression
analysis confirmed that MSRB3 expression was a significant
independent prognostic factor in patients with GC. This result was
further verified by the analysis of 442 GC cases from the TCGA
database. Numerous studies have analyzed the clinicopathologic
prognostic factors for GC from various countries. Certain
controversial factors, including sex, age, tumor diameter and
differentiation, depth of invasion, lymph node metastasis, distant
metastasis and TNM stage have been considered as independent
predictors (43,44). However, no consensus on the optimum
predictors was reached. In the present study, besides MSRB3
expression, depth of invasion, age and distant metastasis were
considered as independent prognostic factors. TNM stage was
excluded from the multivariate analysis. Maximum tumor diameter,
depth of invasion, lymph node metastasis, TNM stage and MSRB3
expression were identified to be significantly associated with OS
time in the univariate analysis. Subsequently, these five factors
were further analyzed by multivariate analysis in order to screen
for independent prognostic factors. Since the factor TNM stage is
associated with maximum tumor diameter and lymph node metastasis,
it was considered not to be an independent factor; therefore, it
was excluded from the multivariate analysis. By contrast, MSRB3
expression was revealed to be independent of other factors and may
therefore be considered as an independent prognostic factor. These
results indicate that MSRB3 may serve an important role in cancer
and serve as a prognostic biomarker for GC.
Some limitations to this study should be addressed.
Firstly, the present study was a single-center study enrolling
limited number of patients. Secondly, the retrospective design
covered a period of 10 years and tissue sections from paraffin
blocks may exhibit a considerable diminution in antigenicity.
Thirdly, many cases were excluded because of neoadjuvant
chemotherapy, which would cause collection bias. Eventually, this
study only used immunohistochemistry technique, which may cause
bias in the results. Further investigation using a multi-center and
prospective study and involving additional techniques is necessary
to improve the accuracy of the results.
In conclusion, the results from the present study
demonstrated that MSRB3 expression was increased in GC tissues and
was associated with poorer prognosis in patients with GC. This
suggests that MSRB3 may be considered as a potential novel
prognostic biomarker for patients with GC and as an effective
molecular target for GC treatment. However, further investigation
is required to fully elucidate the mechanisms underlying the role
of MSRB3 in GC.
Acknowledgements
The authors would like to thank Dr Xiaohong Shi and
Dr Xiaoling Jiang (Suqian Hospital Affiliated to Xuzhou Medical
University, Xuzhou, China) for evaluating the immunostaining
signals.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JWu provided ideas, designed the study, interpreted
the data and helped writing and revising the manuscript in a
critical way. XM and HH prepared the tissue sections and analyzed
the data. JWa and MZ conducted the immunohistochemistry. XM
performed the statistical analysis. JWa participated in the
analyses of experimental data and of the online public data from
The Cancer Genome Atlas. The manuscript was written by XM, and
reviewed by JWa and JWu. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics and
Research Committees of Nanjing Drum Tower Hospital Group Suqian
People's Hospital (Suqian, China) and was conducted in accordance
with the principles outlined in The Declaration of Helsinki.
Written informed consent was provided by the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen YH, Xie ZB, Yue AM, Wei QD, Zhao HF,
Yin HD, Mai W, Zhong XG and Huang SR: Expression level of
microRNA-195 in the serum of patients with gastric cancer and its
relationship with the clinicopathological staging of the cancer.
Eur Rev Med Pharmacol Sci. 20:1283–1287. 2016.PubMed/NCBI
|
|
3
|
Miao R, Guo X, Zhi Q, Shi Y, Li L, Mao X,
Zhang L and Li C: VEZT, a novel putative tumor suppressor,
suppresses the growth and tumorigenicity of gastric cancer. PLoS
One. 8:e744092013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Y, Pan K, Li S, Xia J, Wang W, Chen
J, Zhao J, Lü L, Wang D, Pan Q, et al: Decreased expression of
V-set and immunoglobulin domain containing 1 (VSIG1) is associated
with poor prognosis in primary gastric cancer. J Surg Oncol.
106:286–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee J, Lim DH, Kim S, Park SH, Park JO,
Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, et al: Phase III trial
comparing capecitabine plus cisplatin versus capecitabine plus
cisplatin with concurrent capecitabine radiotherapy in completely
resected gastric cancer with D2 lymph node dissection: The ARTIST
trial. J Clin Oncol. 30:268–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matés JM, Pérez-Gómez C and Núñez de
Castro I: Antioxidant enzymes and human diseases. Clin Biochem.
32:595–603. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krause KH: Aging: A revisited theory based
on free radicals generated by NOX family NADPH oxidases. Exp
Gerontol. 42:256–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valko M, Izakovic M, Mazur M, Rhodes CJ
and Telser J: Role of oxygen radicals in DNA damage and cancer
incidence. Mol Cell Biochem. 266:37–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schumacker PT: Reactive oxygen species in
cancer cells: Live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pelicano H, Carney D and Huang P: ROS
stress in cancer cells and therapeutic implications. Drug Resist
Updat. 7:97–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cooke MS, Evans MD, Dizdaroglu M and Lunec
J: Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB
J. 17:1195–1214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klaunig JE and Kamendulis LM: The role of
oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol.
44:239–267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Halliwell B: Antioxidants in human health
and disease. Annu Rev Nutr. 16:33–50. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HY and Gladyshev VN: Methionine
sulfoxide reductases: Selenoprotein forms and roles in antioxidant
protein repair in mammals. Biochem J. 407:321–329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwak GH, Kim TH and Kim HY:
Down-regulation of MsrB3 induces cancer cell apoptosis through
reactive oxygen species production and intrinsic mitochondrial
pathway activation. Biochem Biophys Res Commun. 483:468–474. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HY: The methionine sulfoxide reduction
system: Selenium utilization and methionine sulfoxide reductase
enzymes and their functions. Antioxid Redox Signal. 19:958–969.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim DH, Han JY, Kim JR, Lee YS and Kim HY:
Methionine sulfoxide reductase B in the endoplasmic reticulum is
critical for stress resistance and aging in Drosophila. Biochem
Biophys Res Commun. 419:20–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HY and Gladyshev VN: Role of
structural and functional elements of mouse methionine-S-sulfoxide
reductase in its subcellular distribution. Biochemistry.
44:8059–8067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim G, Cole NB, Lim JC, Zhao H and Levine
RL: Dual sites of protein initiation control the localization and
myristoylation of methionine sulfoxide reductase A. J Biol Chem.
285:18085–18094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vougier S, Mary J and Friguet B:
Subcellular localization of methionine sulphoxide reductase A
(MsrA): Evidence for mitochondrial and cytosolic isoforms in rat
liver cells. Biochem J. 373:531–537. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HY and Gladyshev VN: Methionine
sulfoxide reduction in mammals: Characterization of
methionine-R-sulfoxide reductases. Mol Biol Cell. 15:1055–1064.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwak GH, Lim DH, Han JY, Lee YS and Kim
HY: Methionine sulfoxide reductase B3 protects from endoplasmic
reticulum stress in Drosophila and in mammalian cells. Biochem
Biophys Res Commun. 420:130–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Jia P, Jia Y, Li Y, Webster KA,
Huang X, Achary M, Lemanski SL and Lemanski LF: Anoxia, acidosis,
and intergenic interactions selectively regulate methionine
sulfoxide reductase transcriptions in mouse embryonic stem cells. J
Cell Biochem. 112:98–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morel AP, Ginestier C, Pommier RM, Cabaud
O, Ruiz E, Wicinski J, Devouassoux-Shisheboran M, Combaret V,
Finetti P, Chassot C, et al: A stemness-related ZEB1-MSRB3 axis
governs cellular pliancy and breast cancer genome stability. Nat
Med. 23:568–578. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hansel A, Jung S, Hoshi T and Heinemann
SH: A second human methionine sulfoxide reductase (hMSRB2) reducing
methionine-R-sulfoxide displays a tissue expression pattern
distinct from hMSRB1. Redox Rep. 8:384–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oakley GJ, Fuhrer K and Seethala RR:
Brachyury, SOX-9, and podoplanin, new markers in the skull base
chordoma vs chondrosarcoma differential: A tissue microarray-based
comparative analysis. Mod Pathol. 21:1461–1469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tomczak K, Czerwińska P and Wiznerowicz M:
The Cancer Genome Atlas (TCGA): An immeasurable source of
knowledge. Contemp Oncol (Pozn). 19:A68–A77. 2015.PubMed/NCBI
|
|
31
|
Jiang YZ, Yu KD, Zuo WJ, Peng WT and Shao
ZM: GATA3 mutations define a unique subtype of luminal-like breast
cancer with improved survival. Cancer. 120:1329–1337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Liang L, Dai W, Cai G, Xu Y, Li X,
Li Q and Cai S: Prognostic impact of programed cell death-1 (PD-1)
and PD-ligand 1 (PD-L1) expression in cancer cells and tumor
infiltrating lymphocytes in colorectal cancer. Mol Cancer.
15:552016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ueno H, Jones AM, Wilkinson KH, Jass JR
and Talbot IC: Histological categorisation of fibrotic cancer
stroma in advanced rectal cancer. Gut. 53:581–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Espejo Romero H and Navarrete Siancas J:
Classification of stomach adenocarcinomas. Rev Gastroenterol Peru.
23:199–212. 2003.(In Spanish). PubMed/NCBI
|
|
35
|
Kwak GH, Kim JR and Kim HY: Expression,
subcellular localization, and antioxidant role of mammalian
methionine sulfoxide reductases in Saccharomyces cerevisiae. BMB
Rep. 42:113–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwon TJ, Cho HJ, Kim UK, Lee E, Oh SK, Bok
J, Bae YC, Yi JK, Lee JW, Ryoo ZY, et al: Methionine sulfoxide
reductase B3 deficiency causes hearing loss due to stereocilia
degeneration and apoptotic cell death in cochlear hair cells. Hum
Mol Genet. 23:1591–1601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ahmed ZM, Yousaf R, Lee BC, Khan SN, Lee
S, Lee K, Husnain T, Rehman AU, Bonneux S, Ansar M, et al:
Functional null mutations of MSRB3 encoding methionine sulfoxide
reductase are associated with human deafness DFNB74. Am J Hum
Genet. 88:19–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwak GH, Kim KY and Kim HY: Methionine
sulfoxide reductase B3 deficiency stimulates heme oxygenase-1
expression via ROS-dependent and Nrf2 activation pathways. Biochem
Biophys Res Commun. 473:1033–1038. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee E, Kwak GH, Kamble K and Kim HY:
Methionine sulfoxide reductase B3 deficiency inhibits cell growth
through the activation of p53-p21 and p27 pathways. Arch Biochem
Biophys. 547:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ran Q, Liang H, Gu M, Qi W, Walter CA,
Roberts LJ II, Herman B, Richardson A and Van Remmen H: Transgenic
mice overexpressing glutathione peroxidase 4 are protected against
oxidative stress-induced apoptosis. J Biol Chem. 279:55137–55146.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Torres M: Mitogen-activated protein kinase
pathways in redox signaling. Front Biosci. 8:d369–d391. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kwak GH and Kim HY: MsrB3 deficiency
induces cancer cell apoptosis through p53-independent and ER
stress-dependent pathways. Arch Biochem Biophys. 621:1–5. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Murakami D, Tsujitani S, Osaki T, Saito H,
Katano K, Tatebe S and Ikeguchi M: Expression of phosphorylated Akt
(pAkt) in gastric carcinoma predicts prognosis and efficacy of
chemotherapy. Gastric Cancer. 10:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yokota T, Ishiyama S, Saito T, Teshima S,
Narushima Y, Murata K, Iwamoto K, Yashima R, Yamauchi H and Kikuchi
S: Lymph node metastasis as a significant prognostic factor in
gastric cancer: A multiple logistic regression analysis. Scand J
Gastroenterol. 39:380–384. 2004. View Article : Google Scholar : PubMed/NCBI
|