Introduction
According to the American Cancer Society estimates
in 2014, pancreatic carcinoma is the fourth most common cause of
cancer-associated mortality in the USA (1). Pancreatic carcinoma is characterized by
insidious onset, rapid progression, a high degree of malignancy,
early metastasis and poor prognosis (1). The disease has a poor prognosis due to
a lack of early diagnostic symptoms. As a result, the majority of
patients are diagnosed with mid- and terminal-staged pancreatic
cancer, which cannot be surgically removed (2). Although 15–20% of patients are
diagnosed at an early stage, only 20–25% of these patients may
survive >5 years after tumor resection (3). Despite the introduction of new
therapeutic techniques, including external beam radiotherapy,
intraoperative radiotherapy, radioactive seed implantation and
chemotherapy, the prognosis for patients with pancreatic carcinoma
remains unsatisfactory with a 5-year survival rate of <6%
(4).
Autopsy studies have suggested that 8–15% of
patients succumb to the disease due to the local destructive power
of pancreatic carcinoma rather than systemic metastatic spread,
according to different pattern of genetic mutations (5–7). Thus,
there is a limited response to chemotherapy in patients with
pancreatic carcinoma. This has led to the development and
application of an ablative technique in pancreatic cancer termed
radiofrequency ablation (RFA) (7).
The most common worldwide application of RFA for pancreatic cancer
is for the treatment of patients with stage III disease, either as
an initial strategy at the time of diagnosis or when there is no
response to standard systemic treatments (8–10).
However, RFA can also be used in patients with stage IV metastatic
disease to induce positive modulation of the immune system
(11–13). Recently, the application of RFA as an
initial treatment method has been proposed on the basis of a
presumed immunological antitumor stimulation induced by RFA; a
randomized control trial is currently being performed to evaluate
this (14). In a systemic review by
Rombouts et al (15), the
RFA-associated morbidity rate has been reported to range between
4.0 and 17.8%, and the RFA-associated mortality rate ranges between
0.0 and 3.0% when a maximal ablation temperature of 90°C is used.
Furthermore, the median survival time reported following RFA was
25.6 months (15).
In addition, different new intervention techniques,
including transarterial chemotherapy, computed tomography
(CT)-guided iodine-125 (125I) seed implantation and
radio-immunotherapy, have been investigated (16–19).
CT-guided percutaneous implantation of 125I seeds
provides positional precision with minimal invasion and maintains
the slow and continuous release of 125I seeds, which has
been suggested to be radio-biologically advantageous, allowing the
repair of non-lethal damage and re-oxygenation of hypoxic areas in
normal tissues (20,21). A number of studies have confirmed the
safety of this technique with a mortality rate of 0.006–0.031% and
a complication rate of 0.5–3.0% (22,23).
Furthermore, in a study by Yu et al (18), the incidence rates of complications
were not statistically different between the normal visceral organs
group and the visceral organs punctured group. Additionally,
patients with 3–4 punctures and patients with 5–4 punctures did not
exhibit a significant difference in adverse events, which suggests
that 125I seed implantation may be a comparatively safer
technique.
Trans-arterial chemoembolization (TACE) is another
interventional technique that has been has been demonstrated to be
efficient for the control of both symptoms and tumor masses in
patients with pancreatic cancer (24). TACE is a locoregional procedure that
provides a highly concentrated dose of chemotherapeutic drug to
tumor cells, prolonging drug-cell contact time and minimizing
systemic toxicity (25). Despite the
emergence of these aforementioned interventional techniques, to the
best of our knowledge, there is currently no consensus regarding
the best therapeutic modality for unresectable pancreatic
carcinoma. Therefore, it is necessary to investigate novel
techniques that may improve patient outcome. The present study
analyzed the effectiveness of combined interventional therapy (CIT;
TACE combined with 125I seed implantation and/or RFA)
compared with TACE alone for the treatment of unresectable
pancreatic carcinoma.
Materials and methods
Patients
Patients with unresectable pancreatic carcinoma, who
were treated either with TACE alone or with TACE combined with
CT-guided percutaneous implantation of 125I seeds and/or
radiofrequency ablation at the Interventional Radiology Department
of the Affiliated Hospital of North Sichuan Medical College
(Nanchong, China) and Pingdingshan Fifth People's Hospital
(Pingdingshan, China) between July 2012 and November 2015, were
included in the present retrospective study. Patients who did not
receive any interventional therapy and were treated with systemic
chemotherapy were considered as the control group. The criteria for
enrollment were as follows: i) Ductal adenocarcinomas of the
pancreas that were pathologically diagnosed by CT-guided fine
needle aspiration prior to interventional procedures; ii)
contraindication to curative resection because of locally advanced
primary tumor or the presence of liver metastasis; iii) presence of
measurable lesions in the liver or pancreas that corresponded to
Response Evaluation Criteria in Solid Tumors (26) target lesions; iv) Karnofsky physical
score (KPS) ≥60; and v) age ≥18 years (27). The exclusion criteria were: i)
patients with extra hepatic metastasis; ii) coagulation dysfunction
and platelet count <50×109 per liter; iii) local
infection or uncontrollable systemic infection; and iv)
contraindication for interventional therapy due to severe liver,
kidney or cardiac dysfunction. A total of 266 patients with an
average age (mean ± standard deviation) of 67.24±12.46 years met
the inclusion criteria and were enrolled in the present study. Of
these patients, 162 were male and 104 female. The current study was
approved by the Institutional Review Board of Affiliated Hospital
of North Sichuan Medical College (Nanchong, China) as well as
Pingdingshan Fifth People's Hospital (Pingdingshan, China). Written
informed consent was obtained from all the patients.
Procedure for TACE
Gemcitabine-based trans-arterial chemotherapy is a
type of therapeutic strategy recognized by the Chinese Society of
Clinical Oncology-Pancreatic Cancer Professional Committee
(28,29). Trans-arterial chemotherapy is a
routine treatment for patients with advanced pancreatic cancer in
China (28).
In the present study, the Seldinger technique
(30) was used under local
anesthesia to access the femoral artery with a 5-Fr vascular
sheath. Subsequently, a digital subtraction angiography examination
[Infinix-i core+ (INFX-800V) Canon Medical Systems,
Tustin, CA, USA] was performed following catheterization of the
celiac and superior mesenteric arteries with a 5.0-Fr
(Radiofocus® Angiographic catheter; Terumo, Tokyo,
Japan) or (Torcon® NB Advantage; Catheter Cook Medical,
Bloomington, IN, USA) catheter. Segmental or sub-segmental
tumor-feeding artery was selected using either 2.0-Fr
(Progreat®; Terumo) or a 3.0-Fr (Renegade™;
Boston Scientific Corporation, Marlborough, MA, USA) micro-catheter
depending upon the diameter of the selected vessel. Emulsions of
iodized oil (Lipiodol Ultra-Fluid; André Guerbet Laboratories,
Aulnay Sous-Bois, France), 100 mg/m2 Gemcitabine and 100
mg/m2 Oxaliplatin (both from Jiangsu Hengrui Medicine
Co., Ltd, Lianyungang, China) were infused. The amount of emulsion
was decided based on tumor size and vascularity.
The body and tail of the pancreas are supplied by
the dorsal pancreatic artery, the great pancreatic artery and the
caudal pancreatic artery; all of which generally originate from the
splenic artery (31,32). Therefore, in the current study, in
case of a lesion in the pancreatic body or tail, a full dose of
chemotherapeutic drug was infused via the splenic artery if the
aforementioned arteries originated from the splenic artery. In
patients with lesions in the head of the pancreas, one-third of the
drug was infused via the super-mesenteric artery and the other
two-thirds were infused via the gastroduodenal artery. In cases
where the super-mesenteric artery contributed to the tumor blood
supply, one-third of the drug was administered via the superior
mesenteric artery and two-thirds via the splenic artery.
Chemotherapeutic drug was infused via the celiac artery if the
tumor blood-supplying arteries originated from the common hepatic
artery or the celiac artery. Furthermore, if the tumor
blood-supplying arteries were directly super-selected, the drugs
were infused via the blood-supplying arteries using 3-Fr
catheters.
For liver metastases, TACE was performed during the
same session as pancreatic primary tumor TACE. Depending on the
tumor arterial supply, the tip of the catheter was advanced into
the right or left hepatic artery to perform selective arterial
embolization.
Procedure for 125I seed
implantation
CT scans (Siemens AG, Munich, Germany) were
performed to calculate the total volume of each tumor using a
treatment planning system, which was then used to calculate the
expected seed number to be implanted. Under CT guidance, the number
and angle of the needle directions (18-gauge; length, 150–200 mm;
Dr Japan Co., Ltd, Tokyo, Japan) were calculated according to the
puncture approach and tumor size. Subsequently, 125I
seeds (Model-6711; Beijing Atom and High Technique Industries Inc.,
Beijing, China), with a half-life of 59.4 days, a low energy level
of 27.4 KeV and a half-value layer of 0.025 mm of lead, were
implanted within the tumor maintaining a spacing of 1.0 cm
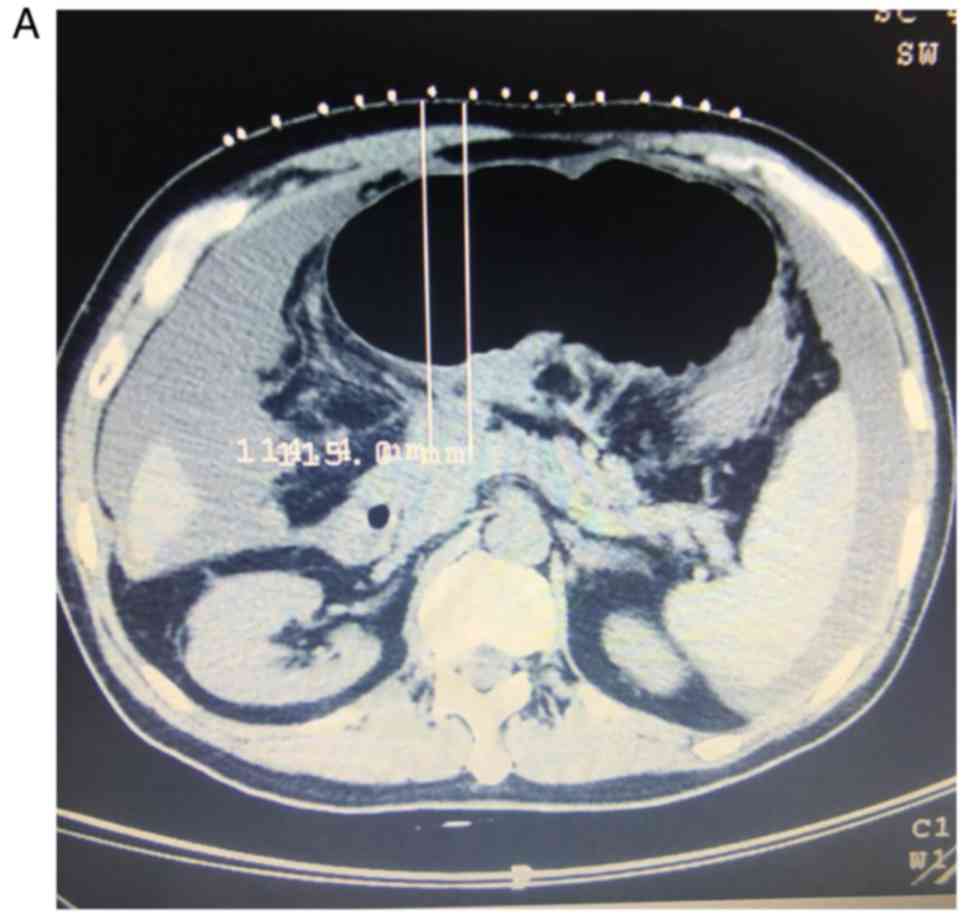
intervals. Fig. 1 presents
representative images from a patient undergoing 125I
seed implantation combined with TACE for unresectable pancreatic
carcinoma with liver metastases.
Procedure for RFA
Percutaneous RFA was performed with the patient
under conscious sedation under CT guidance. Histological
confirmation of the diagnosis was obtained in all the patients
prior to RFA. RFA was performed with a RF generator (Elektrotom HF
106®; Berchtold, Tuttlingen, Germany), generating 40–60
W of power. Prior to needle insertion, the point of entry was
planned to ensure a safe trajectory and end position. CT scan image
guidance was used to precisely place the ablation probes
percutaneously within the tumor. The tumor size, location and
geometry were considered when selecting whether a single 17-gauge
or triple cluster 17-gauge needle electrode would be applied in the
RFA procedure. In total, ~4,200 W of energy was delivered using a
saline perfused needle with the aim of ablating the entire tumor
along with a 1.0 cm ablative margin.
Systemic chemotherapy
Gemcitabine (1,000 mg/m2) diluted in
normal saline was intravenously administered for 30 min/week for
the first seven weeks (i.e. on day 1, 8, 15, 22, 29, 36 and 43)
followed by one-week rest. In subsequent cycles, all patients were
administered treatment on days 1, 8 and 15 of every four weeks
(4-week cycle) until disease progression, unacceptable toxic
effects, or withdrawal of consent. Dose reductions to 750, 550 and
425 mg/m2 were allowed for management of adverse
events.
Assessment of tumor response and pain
relief
The treatment response was evaluated 6 months after
the interventional procedure using the modified Response Evaluation
Criteria in Solid Tumors (26). A
complete response (CR) was defined as disappearance of any
intra-humoral arterial enhancement in all lesions; a partial
response (PR) was defined as a ≥30% decrease in the sum of the
diameters of viable (contrast enhancement in the arterial phase)
lesions; progressive disease (PD) was defined as a ≥20% increase in
the sum of diameters of viable lesions; and stable disease (SD) was
defined as any cases that did not qualify as either PR or PD
(26). The response rate was the sum
of CR and PR.
Pain intensity was evaluated and graded by the
Numerical Rating Scale (NRS) (33).
NRS score of 1–3 was defined as mild pain, 4–6 was defined as
moderate pain and 7–10 was defined as severe pain. A good response
was defined as severe or moderate pain decreasing to no pain
post-treatment (NRS score 6–10 reducing to NRS score 0). A medium
response was regarded as severe pain reducing to mild pain (NRS
score 7–10 reducing to NRS score 1–3) with pain-free sleep. A mild
response was regarded as severe pain reducing to moderate (NRS
score 7–10 reducing to NRS score 4–6) or a moderate pain reducing
to mild (NRS score 4–6 reducing to NRS score 1–3) following
treatment. A poor response indicated that there was no change in
the severity of pain compared with pre-treatment status (34).
Follow-up and survival rate
Patients were followed up at 3 month intervals
during the first year and at 6 month intervals thereafter until the
end of the study (36 months) or until death. Follow-up tests
included routine hematological, biochemical and serological tests,
as well as abdominal CT scans. Survival was calculated from the
date of diagnosis to the date of mortality or last follow-up. Local
recurrence was defined as tumor progression within the implanted
area or surrounding regions according to CT images. Local
recurrence and distant metastases were scored until patient
mortality and censored thereafter.
Toxicity and complications
Safety was defined according to the frequency of
procedural and procedure-associated post-procedural complications.
These were evaluated based on common terminology criteria for
adverse events (version 4.0) (35).
Statistical analysis
All statistical analyses were performed using
Statistical Package for the Social Science (SPSS) version 23.0 (IBM
Corp., Armonk, NY, USA) and GraphPad Prism version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Continuous variables are
presented as the mean ± standard deviation and categorical
variables are presented as percentages. For comparisons of clinical
characteristics of the patients between the three groups, one-way
analysis of variance (ANOVA) was used to compare continuous
variables and a χ2 test was used to compare categorical
variables. A χ2 test was also used to compare treatment
response outcomes between the groups. Overall survival curves were
produced using the Kaplan-Meier method and compared using the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics of the
patients
The clinical characteristics of all patients
involved in the present study are presented in Table I. According to the Union for
International Cancer Control 2002 staging criteria (36), 84 cases were at stage III and 182
cases presented with stage IV pancreatic cancer. A total of 143
patients exhibited a tumor in the pancreatic head and 123 presented
with a tumor in the pancreatic body or tail. The average diameter
of the tumors was 99.2±1.23 mm. The main clinical manifestations
were epigastric discomfort, abdominal pain and jaundice. The KPS
values of 21 patients were between 60 and 69, 84 patients had
values of 70–79 and 161 patients presented with values ≥80.
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
| Variable | Control (n=123), n
(%) | TACE (n=59), n
(%) | CIT (n=84), n
(%) | P-value |
|---|
| Age, years (mean ±
standard deviation) | 66.41±12.53 | 68.13±13.08 | 66.74±15.14 | 0.17 |
| Sex |
|
|
| 0.07 |
|
Male | 71 (57.72) | 38 (64.40) | 53 (63.10) |
|
|
Female | 52 (42.28) | 21(35.60) | 31(36.90) |
|
| Tumor location |
|
|
| 0.65 |
|
Head | 69 (56.10) | 27 (45.76) | 47 (55.90) |
|
|
Body/tail | 54 (43.90) | 22 (44.24) | 37 (54.10) |
|
| UICC
stagea |
|
|
| 0.45 |
| Stage
III | 55 (44.71) | 11 (18.64) | 18 (21.42) |
|
| Stage
IV | 68 (55.29) | 48 (81.36) | 66 (78.58) |
|
| Size, cm |
|
|
| 0.24 |
|
<5.0 | 37 (30.00) | 10 (16.94) | 17 (20.23) |
|
|
≥5.0 | 86 (70.00) | 49 (83.06) | 67 (79.77) |
|
| Ascites |
|
|
| 0.10 |
|
Yes | 19 (15.44) | 4 (6.78) | 5 (6.00) |
|
| No | 104 (84.56) | 55 (93.22) | 79 (94.00) |
|
| Jaundice |
|
|
| 0.14 |
|
Yes | 30 (24.39) | 22 (37.28) | 29 (34.52) |
|
| No | 93 (75.61) | 37 (62.72) | 55 (65.48) |
|
| Child-Pugh
classificationb |
|
|
| 0.12 |
| A | 0 (0.00) | 0 (0.00) | 0 (0.00) |
|
| B | 34 (27.64) | 17 (28.81) | 33 (39.28) |
|
| C | 89 (72.36) | 42 (71.19) | 51 (60.72) |
|
| Karnofsky physical
scoresc |
|
|
| 0.08 |
|
60–69 | 12 (9.75) | 4 (6.78) | 5 (5.95) |
|
|
70–79 | 22 (17.89) | 28 (47.46) | 34 (40.48) |
|
|
≥80 | 89 (72.36) | 27 (45.76) | 45 (53.57) |
|
|
Elevated CA 19-9 level | 93 (75.60) | 48 (81.35) | 64 (76.19) | 0.43 |
Treatment protocol
Primary tumor
Of the 266 patients, 143 underwent interventional
therapy whereas 123 underwent systemic therapy. The patients who
underwent systemic therapy were labeled as the control group in the
present study. Patients undergoing interventional therapy were
further divided into the TACE group (n=59), who underwent TACE
alone, and the CIT group (n=84), who underwent TACE + RFA and/or
125I seed implantation. In the CIT group, 28 patients
had undergone TACE + RFA and 47 had undergone TACE +
125I. The remaining 9 patients were treated with TACE +
RFA + 125I.
All patients who underwent TACE had a mean of three
TACE sessions per patient (range, 2–6), in four-week or eight-week
intervals. The mean ablation session per patient was two (range,
1–3). 125I seed implantation was performed only once per
tumor.
Metastases
There was a total of 68 unresectable metastatic
(UR-M) and 55 unresectable locally advance tumor(UR-L) patients in
the control group. In the TACE group, there were 48 UR-M and 11
UR-L patients, whereas, in the CIT group, 66 were UR-M and 18 UR-L.
Out of the 68 UR-M patients in the control group, 30 had oligo
nodular liver metastases (≤3 liver lesions), while 9 had multi
nodular liver metastases (>3 liver lesions). No local regional
therapy was performed for the liver metastatic tumor in the control
group other than systemic chemotherapy. Out of the 48 UR-M patients
in the TACE group, 31 had oligo nodular liver metastases, while 17
had multinodular liver metastases. All UR-M patients in the TACE
group received TACE for liver metastasis. In the CIT group, of the
66 UR-M patients, 29 had oligo nodular and 37 had multinodular
liver metastasis. Of the 66 UR-M patients in the CIT group, 49
patients underwent TACE for liver metastases. A total of 9 patients
received 125I seed implantation and 8 had RFA in
addition to TACE for their liver metastases.
Tumor response to treatment
The tumor response to treatment was evaluated at 6
months after the intervention. The patients deceased at the time of
the tumor response evaluation (i.e., at six months from the
initiation of the treatment), in whom CT could not be performed to
carry out tumor response evaluation, were not included in this
analysis. The results are presented in Table II. In the control group 17.20% of
patients demonstrated CR or PR, 36.56% had PD and 46.24% had SD. In
the TACE group, 30.61% of patients demonstrated CR or PR, 24.48%
had PD and 44.89% exhibited SD. By contrast, 51.89% of patients
experienced CR or PR in the CIT group. Furthermore, while only
20.25% had PD, 29.11% exhibited SD in the CIT group. The CR, PR,
PD, and SD values of the CIT group were significantly different
compared with control group (P<0.05 for each comparison).
Furthermore, the overall response rate in the CIT group was
revealed to be significantly higher compared with the control group
(P<0.001). Although the individual comparisons of CR, PR, PD and
SD between CIT and TACE groups exhibited no significant
differences, the overall response rate between the two groups was
significantly different (P=0.028).
 | Table II.Tumor response to treatment. |
Table II.
Tumor response to treatment.
| Variable | Control
(n=93a) | TACE
(n=49a) | CIT
(n=79a) | P-value (CIT vs.
control) | P-value (CIT vs.
TACE) |
|---|
| Treatment response,
n (%) |
| CR | 7 (7.53) | 9 (18.37) | 22 (27.84) |
<0.001b | 0.291 |
| PR | 9 (9.67) | 6 (12.24) | 19 (24.05) | 0.014b | 0.168 |
| PD | 34 (36.55) | 12 (24.48) | 16 (20.25) | 0.019b | 0.661 |
| SD | 43 (46.23) | 22 (44.89) | 23 (29.11) | 0.020b | 0.086 |
| ORR
(%) | 17.20 | 30.61 | 51.89 |
<0.001b | 0.028a |
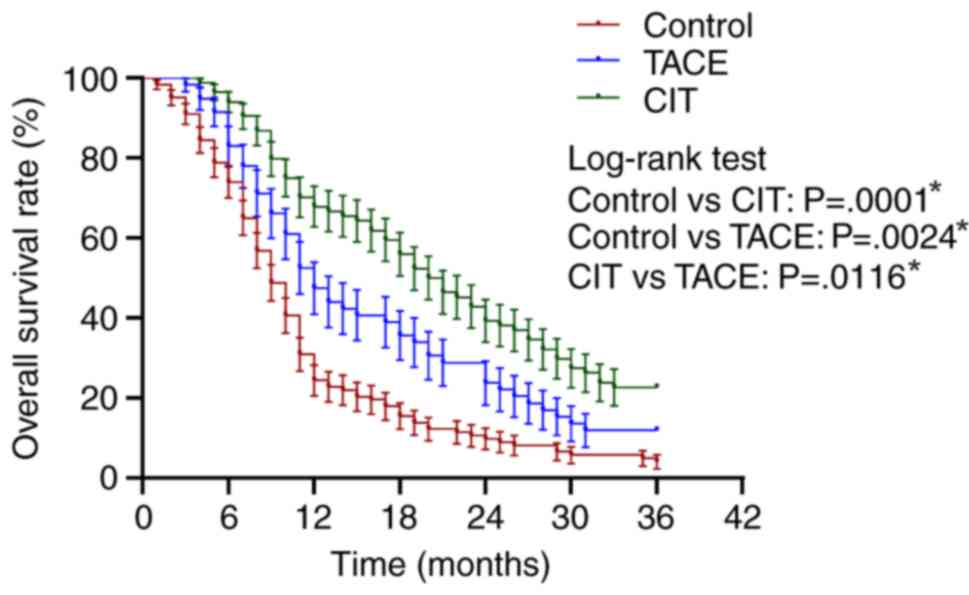
Overall survival
The results for overall survival are presented in
Table III. The overall survival
rates at 12, 24 and 36 months were 23.57, 9.75 and 4.06% for the
control group; 47.46, 25.42 and 11.86% for the TACE group; and and
67.85, 39.28 and 22.62% for the CIT group, respectively, as
presented in Fig. 2 and Table II. The overall survival rate at 36
months for the CIT group (22.62%) was significantly higher compared
with the control group (4.06%; P=0.0001) and the TACE group
(11.86%; P=0.0116). The median survival rate was 9.0, 12.0 and
20.5% for the control, TACE and CIT groups, respectively.
 | Table III.Survival analysis. |
Table III.
Survival analysis.
| Variable | Control
(n=123) | TACE (n=59) | CIT (n=84) | P-value (CIT vs.
control) | P-value (CIT vs.
TACE) |
|---|
| OS rate, months
(%) |
| 12 | 23.57 | 47.46 | 67.85 |
<0.001a | 0.011a |
| 24 | 9.57 | 25.42 | 39.28 |
<0.001a | 0.013a |
| 36 | 4.06 | 11.86 | 22.62 |
<0.001a | 0.012a |
| MST,
months | 9.00 | 12.00 | 20.50 |
<0.001a | 0.017a |
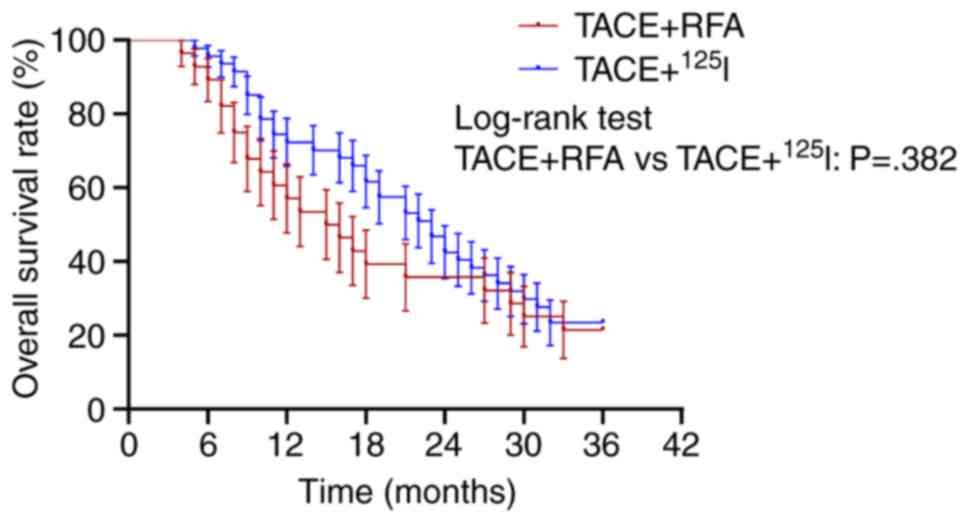
The present study further analyzed the differences
in the overall survival rates for different combinations of
interventional procedures for primary tumors. The difference in
their overall survival rates at 36 months was insignificant
(Fig. 3; TACE + RFA vs. TACE +
125I; P=0.382). However, the median survival rate for
TACE + 125I was 23.0 months, whereas that of TACE + RFA
was only 15.5 months. As only 9 patients underwent TACE +
125I + RFA, statistical analysis was not performed for
this subgroup.
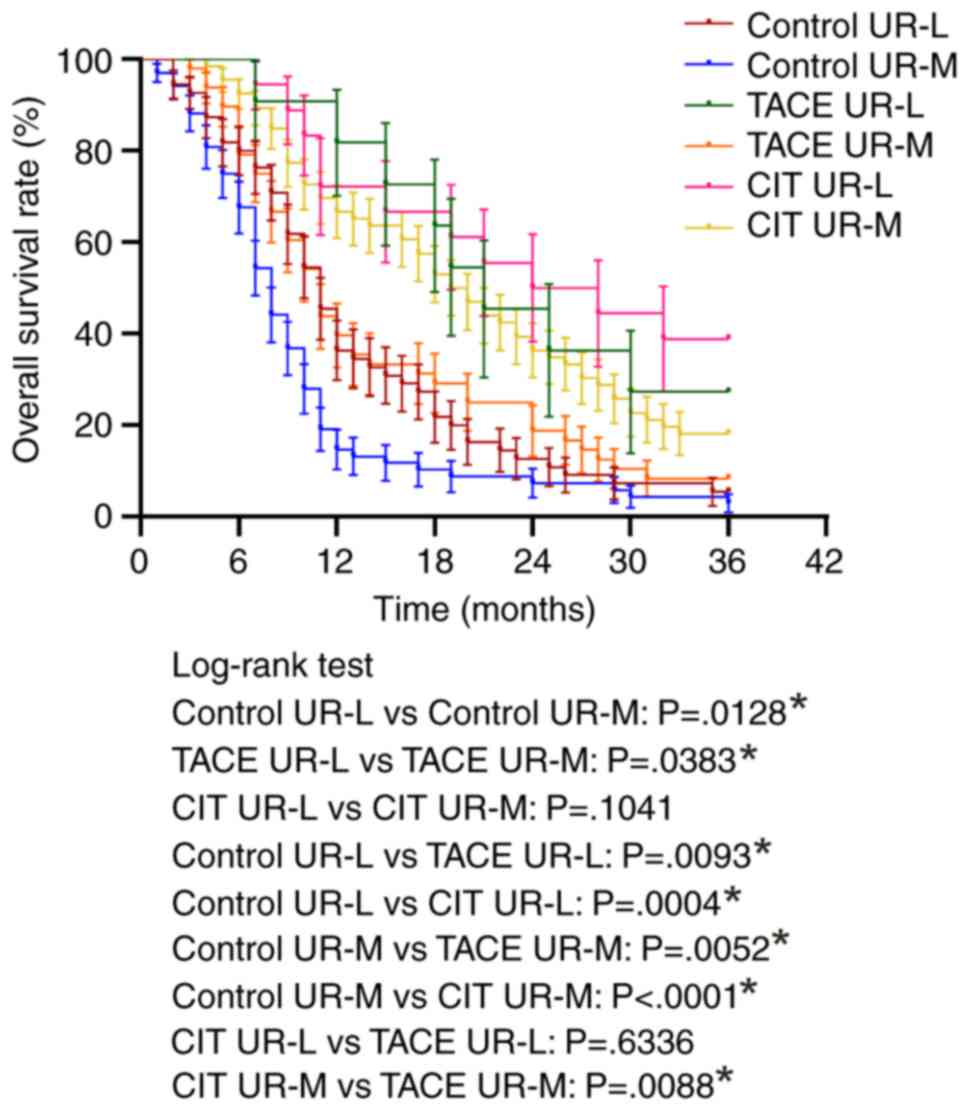
Subgroup analysis was also performed to evaluate the
overall survival rate in patients with UR-L and UR-M. The median
survival rates for all the treatment groups were significantly
higher for UR-L patients compared with UR-M patients, which
indicated that all treatments exhibited improved results for
locally advanced tumor compared with metastatic tumor (Fig. 4).
Among UR-L patients, the median survival times were
11, 21 and 26 months for patients treated with systemic
chemotherapy, TACE and CIT respectively. Among UR-M patients, the
median survival times were 8.0, 11.0 and 19.5 months for patients
treated with systemic chemotherapy, TACE and CIT, respectively.
TACE and CIT exhibited significantly improved overall survival
rates compared with systemic chemotherapy for treating both UR-M
and UR-L patients (Fig. 4). Notably,
CIT demonstrated a significantly improved overall survival rate for
UR-M patients compared with TACE (P=0.0088).
In addition, the UR-M patients were analyzed to
evaluate if the number of liver metastases affects the overall
survival rate in each treatment group. The number of liver
metastases (oligo nodular versus multi nodular metastases) did not
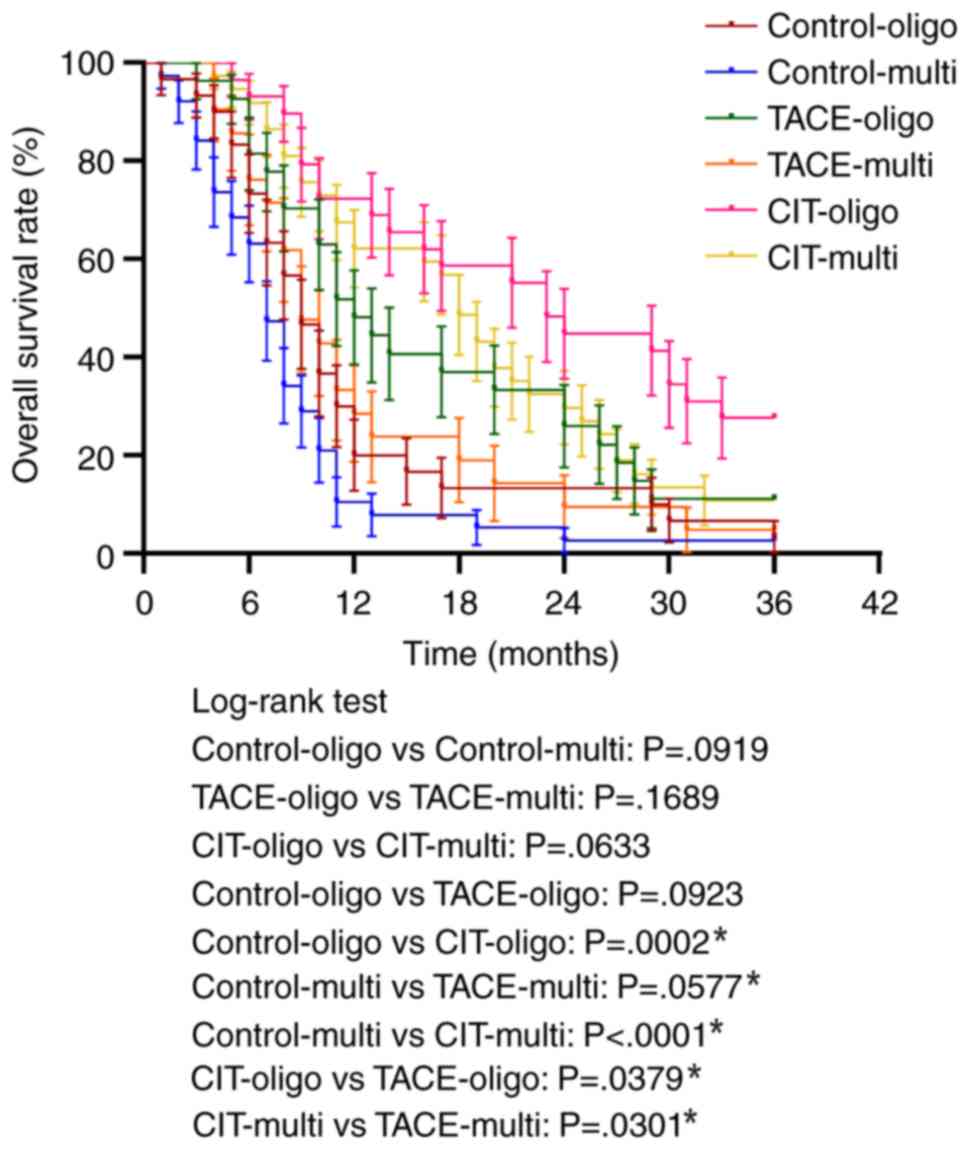
affect the overall survival rate in either group. However, the CIT
group exhibited significantly improved survival rates for treating
both oligo nodular and multi nodular metastases compared with the
control group and TACE group (Fig.
5).
Pain relief
Among all patients, 209 (78.57%) were experiencing
pain prior to treatment, of which 166 (79.42%) demonstrated a good
to medium response, while 43 (20.57%) demonstrated a mild or no
response. Among the control group, 61 patients (65.59%) achieved a
good or medium response, while 32 (34.41%) exhibited a mild or no
response. In the TACE group, 41 patients (91.66%) achieved a good
or medium response, and 7 (14.58%) achieved a mild or no response
following treatment. In the CIT group, 64 patients (94.12%)
achieved a good or medium response, and 4 patients (5.88%) achieved
a mild or no response following treatment. In summary, pain relief
was more pronounced in the CIT group compared with the TACE group
or control group.
Toxicity and complications
Few toxicity complications were observed in the
present study and no patients died during the perioperative period
in any of the groups. The drug and intervention-associated adverse
effects reported with each treatment are presented in Table IV. In the control group, the most
frequently reported non-hematological adverse events associated
with treatment were fatigue (78.04%), alopecia (54.10%) and nausea
(in 41.46%). Reported treatment-associated adverse events of grade
3 or higher were neutropenia, fatigue and peripheral neuropathy. No
patients in the control group discontinued treatment. A total of 14
patients (11.38%) had at least one dose reduction of the
chemotherapeutic agent in the control group.
 | Table IV.Adverse events associated with the
procedures. |
Table IV.
Adverse events associated with the
procedures.
| Adverse
eventsa | Systemic
chemotherapy (n=123), n (%) | TACEb (n=143), n (%) | 125I
seedsc (n=47), n
(%) | RFAd (n=28, n (%) |
|---|
| Neutropenia |
| Grade
1 | 26 (21.13) | 23 (16.08) | 6 (12.76) | 5 (17.85) |
|
Moderate-grade 2 | 10 (8.13) | 9 (6.29) | 4 (8.51) | 2 (7.14) |
| Grade
3 | 8 (6.50) | 4 (2.79) | 0 (0.00) | 0 (0.00) |
| Anemia |
| Grade
1 | 18 (14.63) | 21 (14.68) | 7 (14.89) | 6 (21.42) |
| Grade
2 | 7 (5.69) | 11 (7.69) | 5 (10.63) | 0 (0.00) |
|
Thrombocytopenia |
|
Mild-grade 1 | 27 (21.95) | 19 (13.28) | 6 (12.76) | 5 (17.85) |
|
Moderate-grade 2 | 11 (8.95) | 7 (4.89) | 2 (4.25) | 1 (3.57) |
| Elevated AST |
|
Mild-grade 1 | 25 (20.32) | 29 (20.27) | 5 (10.63) | 5 (17.85) |
|
Moderate-grade 2 | 9 (7.31) | 14 (9.79) | 3 (6.38) | 2 (7.14) |
| Grade
3 | 0 (0.00) | 3 (2.09) | 1 (2.12) | 1 (3.57) |
| Elevated ALT |
|
Mild-grade 1 | 27 (21.95) | 31 (21.68) | 6 (12.76) | 3 (10.71) |
|
Moderate-grade 2 | 8 (6.50) | 13 (9.09) | 3 (6.38) | 4 (14.28) |
| Grade
3 | 0 (0.00) | 5 (3.49) | 1 (2.12) | 0 (0.00) |
| Increased serum
bilirubin |
|
Mild-grade 1 | 15 (12.19) | 36 (25.17) | 5 (10.63) | 4 (14.28) |
| Grade
2 | 9 (7.31) | 11 (7.69) | 1 (2.12) | 2 (7.14) |
| Pulmonary
infection |
|
Mild-grade 1 | 13 (10.56) | 14 (9.79) | 2 (4.25) | 1 (3.57) |
| GI hemorrhage |
|
Mild-grade 1 | 7 (5.69) | 3 (2.09) | 5 (10.63) | 1 (3.57) |
| Pancreatic
hemorrhage |
|
Mild-grade 1 | 0 (0.00) | 0 (0.00) | 2 (4.25%) | 0 (0.00) |
| Hepatic
hemorrhage |
|
Mild-grade 1 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (7.14) |
| Fever |
| Grade
1 | 22 (17.88) | 33 (23.07) | 11 (23.40) | 8 (28.57) |
| Grade
2 | 12 (9.75) | 20 (13.98) | 3 (6.38) | 4 (14.28) |
| Fatigue |
| Grade
1 | 56 (45.52) | 39 (27.27) | 12 (25.55) | 7 (25.00) |
| Grade
2 | 29 (23.57) | 18 (12.58) | 10 (21.27) | 5 (17.85) |
| Grade
3 | 11 (8.94) | 11 (7.69) | 3 (6.38) | 3 (10.71) |
|
Nausea/vomiting |
| Grade
1 | 34 (27.64) | 35 (24.47) | 5 (10.63) | 3 (10.71) |
| Grade
2 | 17 (13.82) | 12 (8.39) | 5 (10.63) | 3 (10.71) |
| Grade
3 | 0 (0.00) | 1 (0.69) | 0 (0.00) | 0 (0.00) |
| Pain |
| Grade
1 | 50 (40.65) | 37 (25.87) | 11 (23.40) | 9 (32.14) |
| Grade
2 | 58 (47.15) | 15 (10.48) | 10 (21.27) | 6 (21.42) |
| Grade
3 | 11 (8.94) | 4 (2.79) | 4 (8.51) | 3 (10.71) |
| Peripheral
neuropathy |
| Grade
1 | 35 (28.45) | 6 (4.19) | 2 (4.25) | 1 (3.57) |
| Grade
2 | 13 (10.56) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Grade
3 | 6 (4.87) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
Among the patients receiving TACE (either alone or
in combination with other interventional treatment), the most
frequently reported non-hematological adverse event associated with
treatment was post-embolization syndrome, which is characterized by
fever, nausea, vomiting, fatigue and elevation of liver enzymes.
These patients were treated accordingly to manage their symptoms.
Treatment-associated adverse events of grade 3 or higher were
neutropenia, elevated liver enzymes and vomiting. Hospitalization
with nasogastric tube feeding performed for patients with grade 3
vomiting.
Among patients treated with RFA (either alone or in
combination with other interventional treatment), the most frequent
non-hematological adverse events were associated with
post-ablation/embolization syndrome, including pain, fever, nausea,
vomiting and elevated liver enzymes. A total of 5 patients
undergoing RFA developed hypertensive crisis, which includes a
blood pressure >220/115 mmHg and a heart rate >117 beats/min.
In each case, ablation was immediately discontinued, a selective
α-1 blocker was administered, blood pressure and heart rate
normalized after 20 min, and ablation was then continued. In total,
1 patient experienced intra-abdominal bleeding and 2 patients
experienced hepatic bleeding, which improved upon management during
the same hospitalization period.
Among patients treated with 125I seeds
(either alone or in combination with other interventional
treatment) implantation, pain, fever, nausea, vomiting and elevated
liver enzymes were the most frequently reported adverse events.
Reported treatment-associated adverse events of grade 3 or higher
were neutropenia, pain, fatigue and elevated liver enzymes. During
125I seed implantation, transgression of the bowel
during puncture did not result in substantial complications in the
present study. However, a safer approach was achieved by traversing
the stomach and avoiding the intestine and colon, particularly when
using large-bore needles. A total of 17 patients exhibited a
punctured visceral organ, including the liver (5), stomach (9) and intestine (3), during the implantation. Of these 17
patients, 0 had major adverse events; however, 5 of them
experiences minor events. The minor events were slight
gastrointestinal bleeding (<20 ml) during implantation. In
addition, 2 patients experienced intra-pancreatic bleeding, which
was managed during the same hospitalization period. Furthermore, 2
patients had seeds that migrated to nearby organs; however, no side
effects were observed in the 18 months post-treatment. Pain was the
most common adverse effect in all the groups.
Discussion
The 5-year survival rate for patients with locally
advanced pancreatic cancer is ~4% and the overall survival time is
9–15 months, which may be reduced by 3–6 months in cases with
metastases (37). Pancreatic cancer
is as a major therapeutic challenge, which is largely characterized
as a chemotherapy-refractory disease that exhibits a poor response
to currently available treatments. The median overall survival time
has been reported to be only 6.0–7.1 months for patients with
advanced pancreatic cancer undergoing chemotherapy (38). Multimodal treatment protocols,
including TACE, RFA and 125I radioactive seed
implantation, have been established for the treatment of metastatic
tumors. A continuous low-dose irradiation with 125I
seeds causes Panc-1 cell-cycle arrest in the G2/M phase and induces
apoptosis (39). Furthermore,
implantation of 125I seeds as irradiation promotes the
permeability of the surrounding vasculature and increases the
efficacy of chemotherapy (40). In
addition, thermal energy provided by RFA can cause cell death by
coagulative necrosis and protein denaturation (41). It is also thought that RFA may have
an immunogenic effect on tumors via an upregulation of heat shock
protein 70, which causes activation of dendritic cells, attracting
infiltrating immune cells and eliciting a T cell response (42,43). A
systematic review of five studies that included 158 patients
treated with RFA reported a positive impact on survival (44). Furthermore, image-guided radiotherapy
is associated with an overall and progression-free survival time of
12.1 months and 7.6 months, respectively, for patients with
pancreatic cancer (45). In summary,
brachytherapy with 125I seed implantation and RFA
provides an option for patients with advanced pancreatic cancer who
may benefit from combinational therapies. Therefore, the present
study compared the efficacy of TACE with TACE + RFA and/or
125I radioactive seed implantation for unresectable
pancreatic cancer. It was identified that TACE combined with either
125I seed implantation and/or RFA, improved the tumor
treatment response and overall survival rate compared with TACE
alone. This result suggested that RFA and radioactive seed
implantation may be used to improve loco-regional disease control
and improve survival compared with either systemic or
intra-arterial chemotherapy alone for patients with pancreatic
cancer.
In the present study, the CIT group demonstrated a
significantly higher overall response rate compared with the TACE
group (51.89 vs. 30.61; P=0.028). The overall survival rate of CIT
group was also higher compared with the TACE alone group at 3
years. This suggested that a combination of different
interventional techniques may be effective for increasing the
survival of patients with pancreatic cancer. Furthermore, all
treatment groups demonstrated a higher overall survival rate for
patients with locally advanced tumors compared with patients with
metastatic tumors. Notably, CIT yielded a significantly improved
overall survival rate for UR-M patients compared with TACE alone.
However, CIT did not demonstrate any significant difference in the
overall survival rate for treating oligo nodular or multi nodular
metastatic patients. Overall, the present study revealed that
combination therapy may be an effective option for the treatment of
patients with unresectable pancreatic cancer. However, which
specific combination achieves the best result could not be
determined in the current study. The overall survival rate obtained
with TACE + 125I was similar to that obtained with TACE
+ RFA. We hypothesize that the choice of TACE + RFA or TACE +
125I should depend on the condition of the patient, the
location of the tumor and potential complications associated with
the interventions. For example, tumors in close proximity with
adjacent organs are at higher risk if treated with radioactive
seeds. The present study could not conclude which combination is
associated with more complications. Both the TACE + 125I
and TACE + RFA groups experienced various complications, which were
managed accordingly in the present study. No life-threatening
peri-procedural complications were associated with either of the
groups. In the current study, only 9 patients received TACE +
125I + RFA; therefore, the efficacy or the safety of
this combination of treatment could not be analyzed due to the
small number of patients.
There were some limitations of the present study.
The retrospective nature and limited sample size may have reduced
the generalizability of the results. Furthermore, there may have
been a selection bias caused by non-randomization. In addition, the
treatment protocol and the nature of health care delivery differs
between different cancer institutions; therefore, the results based
on a single center study may not be generalized to other
institutions.
In conclusion, combined TACE + 125I
and/or RFA treatment may prolong the survival time compared with
TACE alone for patients with unresectable pancreatic carcinoma.
Notably, the use of concomitant RFA and 125I seed
implantation may increase post-operative complications and the rate
of adverse effects. However, the complications and adverse effects
were demonstrated to be resolved following active management of the
symptoms. Therefore, CIT may be a more optimal approach compared
with a single interventional technique to achieve an improved tumor
response and survival.
Acknowledgements
The authors wish to thank Professor Morgan A.
McClure (Affiliated Hospital of North Sichuan Medical College,
Nanchong, China) for his assistance with English grammar correction
and revision.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SKD, JLW and HFY conceived and designed the study.
SKD, JLW, BL, CZ and HFY analyzed and interpreted the data. SKD,
JLW, BL and CZ collected the data. BL and CZ performed statistical
analysis. SKD wrote the manuscript. SKD, JLW and HFY critically
revised the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The current study was approved by the Institutional
Review Board of Affiliated Hospital of North Sichuan Medical
College (Nanchong, China) and Pingdingshan Fifth People's Hospital
(Pingdingshan, China), and written informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang L, Jin H, Guo X, Yang Z, Zhao L,
Tang S, Mo P, Wu K, Nie Y Pan Y and Fan D: Distinguishing
pancreatic cancer from chronic pancreatitis and healthy individuals
by (1) H nuclear magnetic resonance-based metabonomic profiles.
Clin Biochem. 45:1064–1069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao C, Domenico DR, Kim K, Hanson DJ and
Howar JM: Observations on the developmental patterns and the
consequences of pancreatic exocrine adenocarcinoma: Findings of 154
autopsies. Arch Surg. 130:125–134. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamisawa T, Isawa T, Koike M, Tsuruta K
and Okamoto A: Hematogenous metastases of pancreatic ductal
carcinoma. Pancreas. 11:345–349. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iacobuzio-Donahue CA, Fu B, Yachida S, Luo
M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P,
et al: DPC4 gene status of the primary carcinoma correlates with
patterns of failure in patients with pancreatic cancer. J Clin
Oncol. 27:1806–1813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frigerio I, Girelli R, Giardino A, Regi P,
Salvia R and Bassi C: Short term chemotherapy followed by
radiofrequency ablation in stage III pancreatic cancer: Results
from a single center. J Hepatobiliary Pancreat Sci. 20:574–577.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Girelli R, Frigerio I, Salvia R, Barbi E,
Tinazzi Martini P and Bassi C: Feasibility and safety of
radiofrequency ablation for locally advanced pancreatic cancer. Br
J Surg. 97:220–225. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keane MG, Bramis K, Pereira SP and Fusai
GK: Systematic review of novel ablative methods in locally advanced
pancreatic cancer. World J Gastroenterol. 20:2267–2278. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spiliotis JD, Datsis AC, Michalopoulos NV,
Kekelos SP, Vaxevanidou A, Rogdakis AG and Christopoulou AN:
Radiofrequency ablation combined with palliative surgery may
prolong survival of patients with advanced cancer of the pancreas.
Langenbecks Arch Surg. 392:55–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto
K, Kubo N and Nakase Y: Selective thermocoagulation of unresectable
pancreatic cancers by using radiofrequency capacitive heating.
Pancreas. 20:14–20. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Date RS and Siriwardena AK: Radiofrequency
ablation of the pancreas. II: Intra-operative ablation of
non-resectable pancreatic cancer. A description of technique and
initial outcome. JOP. 6:588–592. 2005.PubMed/NCBI
|
|
14
|
Landman J: Differences in immunologic
response to cryoablation versus radiofrequency ablation in the
treatment of renal cell carcinoma. ClinicalTrial, RCT No.
NCT03409224. 2018.
|
|
15
|
Rombouts SJ, Vogel JA, van Santvoort HC,
van Lienden KP, van Hillegersberg R, Busch OR, Besselink MG and
Molenaar IQ: Systematic review of innovative ablative therapies for
the treatment of locally advanced pancreatic cancer. Br J Surg.
102:182–193. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu PH, Pan CC, Huang ZL, Li W, Zhao M and
Zhou ZW: Percutaneous radiofrequency ablation approach through the
spleen: Initial case report for pancreatic tail gastrinoma. Chin J
Cancer. 29:836–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paiella S, Salvia R, Girelli R, Frigerio
I, Giardino A, D'Onofrio M, De Marchi G and Bassi C: Role of local
ablative techniques (radiofrequency ablation and irreversible
electroporation) in the treatment of pancreatic cancer. Updates
Surg. 68:307–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu YP, Yu Q, Guo JM Jiang HT, Di XY and
Zhu Y: Effectiveness and security of CT-guided percutaneous
implantation of 125I seeds in pancreatic carcinoma. Br J Radiol.
87:201306422014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vogl TJ, Mohamed SA, Albrecht MH,
Gruber-Roh T, Lin H, Nour Eldin NEA, Bednarova I, Naguib NN and
Panahi B: Transarterial chemoembolization in pancreatic
adenocarcinoma with liver metastases: MR-based tumor response
evaluation, apparent diffusion coefficient (ADC) patterns, and
survival rates. Pancreatology. 18:94–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ceha HM, van Tienhoven G, Gouma DJ,
Veenhof CH, Schneider CJ, Rauws EA, Phoa SS and González González
D: Feasibility and efficacy of high dose conformal radiotherapy for
patients with locally advanced pancreatic carcinoma. Cancer.
89:2222–2229. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du YQ, Li ZS and Jin ZD:
Endoscope-assisted brachytherapy for pancreatic cancer: From tumor
killing to pain relief and drainage. J Interv Gastroenterol.
1:23–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paulsen SD, Nghiem HV, Negussie E, Higgins
EJ, Caoili EM and Francis IR: Evaluation of imaging-guided core
biopsy of pancreatic masses. AJR Am J Roentgenol. 187:769–772.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith EH: Complications of percutaneous
abdominal fine-needle biopsy. Review. Radiology. 178:253–258. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vogl TJ, Zangos S, Heller M, Hammerstingl
RM, Böcher E, Jacob U and Bauer RW: Transarterial chemoperfusion
with gemcitabine and mitomycin c in pancreatic carcinoma: Results
in locally recurrent tumors and advanced tumor stages. Rofo.
179:1181–1188. 2007.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang ZM, Pan CC, Wu PH, Zhao M, Li W,
Huang ZL and Yi RY: Efficacy of minimally invasive therapies on
unresectable pancreatic cancer. Chin J Cancer. 32:334–341. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karnofsky DA and Burchenal JH: Evaluation
of chemotherapeutic agents. The Clinical Evaluation of
Chemotherapeutic Agents in Cancer. Macleod CM: Columbia University
Press. (New York, NY). p2051949.
|
|
28
|
Liu X, Yang X, Zhou G, Chen Y, Li C and
Wang X: Gemcitabine-based regional intra-arterial infusion
chemotherapy in patients with advanced pancreatic adenocarcinoma.
Medicine (Baltimore). 95:e30982016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M,
Liu L, Liu C, Xu J, Gao YT, Zheng Y, et al: Cancer statistics:
Current diagnosis and treatment of pancreatic cancer in Shanghai,
China. Cancer Lett. 346:273–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seldinger SI: Catheter replacement of the
needle in percutaneous arteriography; A new technique. Acta Radiol.
39:368–376. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu ZX, Yang XZ, Cai JQ, Liao LM, Yang L,
Lin YN and Tan JM: Digital subtraction angiography and computed
tomography angiography of predominant artery feeding pancreatic
body and tail. Diabetes Technol Ther. 13:537–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Donatini B: A systematic study of the
vascularisation of the pancreas. Surg Radiol Anat. 12:173–180.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
‘Pain Intensity Instruments’, . National
Institutes of Health - Warren Grant Magnuson Clinical Center.
July;2003.http://mvltca.net/Presentations/mvltca.pdfAugust
5–2018
|
|
34
|
Wang H, Wang J, Jiang Y, Li J, Tian S, Ran
W, Xiu D and Gao Y: The investigation of 125I seed implantation as
a salvage modality for unresectable pancreatic carcinoma. J Exp
Clin Cancer Res. 32:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Common terminology criteria for adverse
events (version 4.0). https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5×11.pdfAugust
19–2018
|
|
36
|
Morin E, Cheng S, Mete O, Serra S, Araujo
PB, Temple S, Cleary S, Gallinger S, Greig PD, McGilvray I, et al:
Hormone profiling, WHO 2010 grading, and AJCC/UICC staging in
pancreatic neuroendocrine tumor behavior. Cancer Med. 2:701–711.
2013.PubMed/NCBI
|
|
37
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saif MW: New developments in the treatment
of pancreatic cancer. highlights from the ‘44th ASCO annual
meeting’. Chicago, IL, USA. May 30-June 3, 2008. JOP. 9:391–397.
2008.PubMed/NCBI
|
|
39
|
Wang J, Wang J, Liao A, Zhuang H and Zhao
Y: The direct biologic effects of radioactive 125I seeds on
pancreatic cancer cells PANC-1, at continuous low-dose rates.
Cancer Biother Radiopharm. 24:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cron GO, Beghein N, Crokart N, Chavée E,
Bernard S, Vynckier S, Scalliet P and Gallez B: Changes in the
tumor microenvironment during low-dose-rate permanent seed
implantation iodine-125 brachytherapy. Int J Radiat Oncol Biol
Phys. 63:1245–1251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nikfarjam M, Muralidharan V and Christophi
C: Mechanisms of focal heat destruction of liver tumors. J Surg.
Res. 127:208–223. 2005.
|
|
42
|
Teng LS, Jin KT, Han N and Cao J:
Radiofrequency ablation, heat shock protein 70 and potential
anti-tumor immunity in hepatic and pancreatic cancers: A
minireview. Hepatobiliary Pancreat Dis Int. 9:361–365.
2010.PubMed/NCBI
|
|
43
|
Dromi SA, Walsh MP, Herby S, Traughber B,
Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR, et al:
Radiofrequency ablation induces antigen-presenting cell
infiltration and amplification of weak tumor-induced immunity.
Radiology. 251:58–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fegrachi S, Besselink MG, van Santvoort
HC, van Hillegersberg R and Molenaar IQ: Radiofrequency ablation
for unresectable locally advanced pancreatic cancer: A systematic
review. HPB (Oxford). 16:119–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Son SH, Song JH, Choi BO, Kang YN, Lee MA,
Kang KM and Jang HS: The technical feasibility of an image-guided
intensity-modulated radiotherapy (IG-IMRT) to perform a
hypofractionated schedule in terms of toxicity and local control
for patients with locally advanced or recurrent pancreatic cancer.
Radiat Oncol. 7:2032012. View Article : Google Scholar : PubMed/NCBI
|