Introduction
Cervical cancer is the third leading cause of
cancer-associated mortalities in women in low-and middle-income
countries (1). The 5-year survival
rate of International Federation of Gynecology and Obstetrics
(FIGO) stage I cervical cancer reaches 90% (2–4).
However, <16% of patients survive FIGO stage IV cancer (4,5). Women
who never participated in a cervical cancer screening program are
associated with an incidence rate of cervical cancer ≥2-fold higher
than those previously screened (6).
Therefore, increasing primary screening and early detection of
cervical cancer are high priorities for improving survival
rates.
The Papanicolaou (Pap) test is the most widely used
test for primary screening of cervical cancer (7). However, accumulating data indicate that
the test alone can miss cervical lesions due to its low sensitivity
(8,9). In certain developed countries, the
human papillomavirus (HPV) DNA test is performed in combination
with the Pap test to increase the accuracy of detection (10). The Pap test and the HPV DNA test
require cells to be collected from the uterine cervix by a
physician. This procedure can cause embarrassment and anxiety, and
may lead to reluctance in being screened (11). In addition, cell-based tests are not
suitable for high-throughput screening. Instead, serum offers
advantages in the collection, storage and transportation of
specimens. Therefore, serum-based strategies are considered
advantageous for high-throughput screening.
Recent data suggest that 10–20% of cancer cases are
associated with viral infections (12,13).
Therefore, serum antibodies acquired from virus infection are
potential biomarkers for diagnosing cancer types, such as cervical
cancer (14), nasopharyngeal
carcinoma (15) and adult T-cell
leukemia (16). HPV is detected in
almost all patients with cervical cancer (17). Persistent HPV infection evokes
humoral immune responses and there have been many attempts to use
elevated levels of anti-HPV L1, E6 or E7 antibodies as markers of
cervical lesions (14,18). However, none of these biomarkers have
proved to be sufficiently sensitive. Furthermore, ≥12 types of HPV
can be involved in the development of cervical cancer (19,20) and
there are technical barriers to preparing numerous different HPV
antigens for assays. Therefore, discovering a serum biomarker for
primary screening of cervical cancer remains a priority.
Autoantibodies serve important homeostatic roles by
eliminating apoptotic and malignant cells and misfolded proteins
(21–23), and changes in autoantibody levels are
thought to occur in disease states (24–26).
Autoantibody levels may be elevated in patients with autoimmune
disease; elevated anti-nuclear antibody levels are revealed in 78%
of patients with systemic lupus erythematosus and can be detected
several years prior to disease onset (27). Elevated levels of autoantibodies have
also been investigated in several types of cancer (28–30).
However, these autoantibodies have drawbacks as independent cancer
markers because they do not provide sufficient accuracy. Only
10–30% of patients with a given cancer have been demonstrated to be
seropositive when single autoantibodies were used for screening
(31,32). Furthermore, not all autoantibodies
exhibit an increase in patients with cancer (33–35).
Changes in protein expression and post-translational
modifications occur during transitions to malignancy (36–39). In
cancer cells, overexpression, unusual expression and misfolding of
autoantigens, as well as aberrant post-translational modifications
of autoantigens, can affect their recognition by the immune system
in cancer (40), although the exact
mechanism involved is unclear. Therefore, the use of cancer cell
lines may be useful for identifying suitable autoantigens as cancer
markers. HeLa cells, the most widely used cells in cancer research,
have an altered pattern of protein expression when compared with
normal cervical cell lines (41,42). In
the present study, HeLa cells were used as a source of autoantigens
and for the first time a HeLa cell autoantibody was identified,
whose level reflects the severity of cervical lesions.
Materials and methods
Specimens
The present study was carried out with the approval
of the Institutional Review Board of the Ewha Woman's University
Mokdong Hospital, Seoul, South Korea (approval no. EUMC
2016-07-067-002). The study was conducted in accordance with the
Declaration of Helsinki. All serum samples were collected in a
prospective and consecutive manner following the collection of
written informed consent from the subjects.
A total of 130 serum samples were collected from
female patients diagnosed with no cervical abnormalities (n=29),
cervical intraepithelial neoplasia (CIN) I (n=18), CIN II (n=23),
CIN III (n=30) and cervical cancer (n=30), and stored at −80°C. All
participants underwent a liquid-based cytology examination and
those who tested positive for any abnormality in the cervix were
designated for biopsy. Each cervical lesion was graded on the basis
of hematoxylin and eosin staining of sections of formalin-fixed and
paraffin-embedded tissue samples. Hysterectomy specimens with
negative results in the hematoxylin and eosin staining examination
were classified as normal. Cervical cancer was graded according to
the FIGO staging system. All women >20 years old with
abnormalities detected in the cervical cytology examinations, and
who were designated for biopsy or surgery under suspicion of
cervical CINs or cervical cancer, were included. Immunosuppressed
individuals (those with human immunodeficiency virus infection,
having undergone a transplant operation or on any immunosuppressive
medication) or individuals with histories of other types of cancer,
were excluded. The sera from the normal group were collected
following the examination of the hysterectomy specimens. The sera
from the CIN I group were collected immediately following punch
biopsy, and those from the CIN II and CIN III groups were collected
prior to large loop excision of transformation zones. The sera from
the patients with cervical cancer were collected prior to
surgery.
Extraction of cytosolic proteins from
HeLa cells
The HeLa cell line, obtained from Korean Cell Line
Bank, were maintained in Dulbecco's Modified Eagle's medium
supplemented with 10% fetal bovine serum (InvivoGen, San Diego, CA,
USA), 100 U/ml penicillin-streptomycin (GenDepot, Katy, TX, USA)
and 25 µg/ml normocin (InvivoGen). The HeLa cells were detached
from dishes with trypsin-EDTA (GenDepot), and cell pellets were
collected by centrifugation (400 × g for 5 min). The pellets were
washed once with PBS and recovered by centrifugation (400 × g for 5
min). To obtain the cytosolic proteins, the cell pellet was lysed
with lysis buffer (20 mM Tris, 20 mM NaCl, 1 mM EDTA, 5% glycerol,
0.1% Triton X-100 and 0.1% β-mercaptoethanol; pH 5.7; 4°C; 12 h)
and the cell debris was removed by centrifugation (400 × g for 5
min).
Cation-exchange chromatography for the
isolation of HeLa-GAPDH
The loading sample was prepared by adding 10 volumes
of binding buffer (20 mM Tris, 20 mM NaCl, 1 mM EDTA, 5% glycerol,
0.02 % Triton X-100 and 0.1% β-mercaptoethanol; pH 5.7) to the HeLa
cell cytosolic proteins. A total of 1 ml POROS XS resin (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was equilibrated with
binding buffer and the sample was loaded onto the resin. Unbound
proteins were collected, the column was washed with 10 resin
volumes of binding buffer, and the bound proteins were eluted by
successive additions of elution buffer consisting of binding buffer
containing 25–375 mM NaCl at 25 mM increments. The loading sample,
flow-through, column wash and eluates were fractionated on a 12.5%
polyacrylamide gel and the proteins were visualized by silver
staining.
Western blotting
The proteins were fractionated by SDS-PAGE on 12.5%
gels and transferred to polyvinylidene difluoride membranes (Merck
KGaA, Darmstadt, Germany). Concentration of HeLa cytosolic proteins
were determined by Bradford protein assay and 9 or 18 µg of the
proteins were loaded in each lane. Equal volume of samples eluted
from cation-exchange chromatography were loaded to perform SDS-PAGE
and Western blotting analysis. Mixtures of sera from the group of
control females (n=20) and rabbit anti-human GAPDH polyclonal
antibody (cat. no.10094-T52, Sino Biological, Beijing, China) were
diluted 1:200 in TBS containing 0.1% Tween-20 (TBST) and 2.5%
skimmed milk, and 1:1000 in TBST containing 0.5% skimmed milk,
respectively, and reacted with the membranes for 2 h at room
temperature. Following washing with TBS with 1% Tween-20,
horseradish peroxidase (HRP)-conjugated goat anti-human IgG
(Sigma-Aldrich; Merck KGaA) or HRP-conjugated goat anti-rabbit IgG
(Bethyl Laboratories, Inc., Montgomery, TX, USA) was incubated with
the membranes for 1 h at room temperature. The signals were
developed using an AB Signal Chemiluminescence kit (AbClon, Inc.,
Seoul, South Korea) and photo activated on AGFA film (AGFA
Healthcare, Ghent, Belgium). Rabbit anti-beta actin polyclonal
antibody (cat.no.sc-1615-R, Santa Cruz Biotechnology, Texas, USA),
diluted 1:1,000 in TBST containing 0.5% skimmed milk, was used as a
positive internal loading control.
Identification of the major protein
purified by cation-exchange chromatography
A total of 1 µg of the protein fraction extracted
with 275 mM NaCl in the cation-exchange chromatography was
fractionated on a polyacrylamide gel and visualized by Coomassie
blue staining. The protein band of molecular mass 37 kDa was cut
out and treated with trypsin. The tryptic peptides were separated
and analyzed by nano ACQUITY ultra performance liquid
chromatography coupled directly to a LTQ-orbitrap-mass spectrometer
(LC-MS/MS). The spectra from the LC-MS/MS were processed using the
SEQUEST (Thermo Quest, San Jose, CA, USA) software and the peak
list files generated were used to query the National Center for
Biotechnology Information using the MASCOT search program (Matrix
Science Ltd., London, UK, http://www.matrixscience.com/).
ELISAs for measuring serum antibody
against HeLa cell whole cytosolic protein, purified HeLa-GAPDH or
recombinant GAPDH in the normal, CIN I, CIN II, CIN III and
cervical cancer groups
A 96-well microplate (SPL Life Sciences, Pocheon,
South Korea) was coated overnight at 4°C with 2,000, 1,000, 500,
250 or 125 ng/well HeLa cell cytosolic protein, 50 ng/well purified
HeLa-GAPDH or 50 ng/well recombinant GAPDH (rGAPDH; Sino
Biological, China), and blocked with 5% skimmed milk in PBS
containing 0.05% Tween-20 (PBST). To measure the serum antibody
against HeLa cell cytosolic protein, a mixture of normal group sera
(n=5) and a mixture of cervical cancer group sera (n=5) were
diluted 1:400 and incubated in the plate for 90 min at 37°C. To
measure the serum anti-HeLa-GAPDH antibody (Total, n=130; normal,
n=29; CIN I, n=18; CIN II, n=23; CIN III, n=30; and cervical
cancer, n=30) or anti-rGAPDH antibody levels (Total, n=129; normal,
n=29; CIN I, n=17; CIN II, n=23; CIN III, n=30; and cervical
cancer, n=30), individual sera were diluted 1:100 and incubated in
a plate coated with HeLa-GAPDH or rGAPDH. The serum samples were
diluted with 1% skimmed milk in PBST. HRP-conjugated goat
anti-human IgG polyclonal antibody or HRP-conjugated goat
anti-human IgM polyclonal antibody (both Sigma-Aldrich; Merck KGaA)
was diluted 1:5000 in 0.5% skim milk in PBST and incubated in the
wells for 1 h at 37°C. The reactions were developed with
o-phenylenediamine (Sigma-Aldrich; Merck KGaA) and measured at 492
nm using a FlexStation 3 Multi-Mode microplate reader (Molecular
Devices, LLC, Sunnyvale, CA, USA). The plates were washed 3 or 5
times with PBST between reactions.
To exclude interference by non-specific signals due
to direct binding of the serum to the polyethylene plate, a mirror
plate without coating antigen was prepared, and serum and secondary
antibody were incubated as described above. The final optical
density (OD) data were obtained using the following equation: OD
from ELISA coated with antigen-OD from ELISA without antigen.
Statistical analysis
The difference in antibody levels was analyzed using
the Kruskal-Wallis test with Dunn's multiple comparison test
(GraphPad Prism version 5.01; GraphPad Software, Inc., La Jolla,
CA, USA). The difference in ages between the groups was analyzed
using analysis of variance with Bonferroni correction (ANOVA;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate statistically significant differences.
Statistical power values (1-β error) were calculated using the
G*Power v.3.1 program. Receiver operating characteristic (ROC)
curves were plotted, and areas under the curves (AUCs) were
obtained, using GraphPad Prism version 5.01. The sensitivity,
specificity, negative predictive value (NPV), positive predictive
value (PPV) and accuracy of each assay were determined from the ROC
curves. Optimal cut-off values were determined from Youden's index,
which yields maximum values of sensitivity plus specificity based
on the ROC curves. The following parameters were calculated:
Sensitivity=number of true positives/(number of true positives +
number of false negatives); specificity=number of true
negatives/(number of true negatives + number of false positives);
NPV=number of true negatives/(number of true negatives + number of
false negatives); PPV=number of true positives/(number of true
positives + number of false positives); and accuracy=(number of
true positives + number of true negatives)/(number of true
positives + number of true negatives + number of false positives +
number of false negatives).
Results
Serum IgG against total HeLa cell
cytosolic proteins in the normal and cervical cancer groups
ELISAs were performed to investigate the overall
levels of antibodies against HeLa cell cytosolic proteins in the
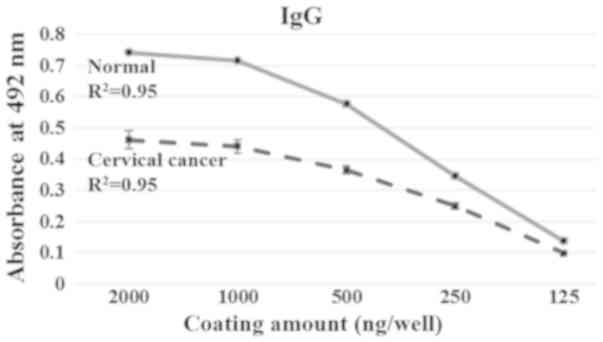
normal and cervical cancer group. As observed in Fig. 1, the IgG level was significantly
lower in the cervical cancer group than the controls, and the level
decreased as the amount of cytosolic protein used for coating
decreased. This result indicates that the overall level of serum
IgG against the whole cytosolic protein fraction in the cervical
cancer group is lower than in the normal group.
Identification of a HeLa cell
cytosolic protein with strong reactivity with sera from healthy
females
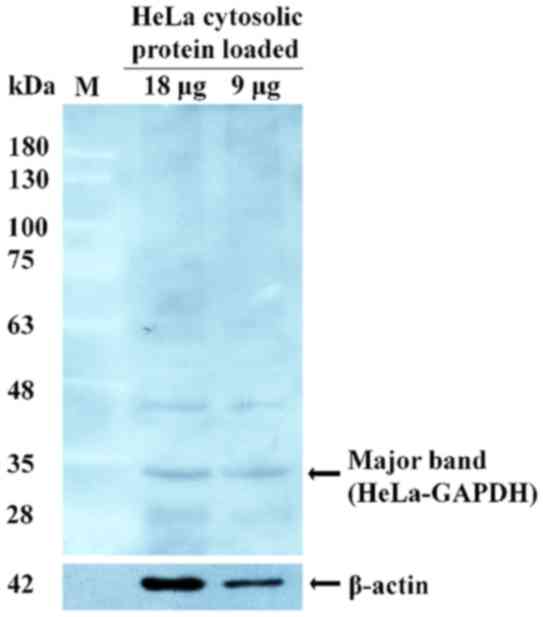
Western blot analysis was performed to detect any
major protein among the HeLa cell cytosolic proteins that reacted
strongly with the sera from healthy females. A 37-kDa protein with
strong reactivity was revealed (Fig.
2) and was identified by LC-MS/MS. The protein was termed
HeLa-GAPDH for the purposes of the present study and was identified
as GAPDH isoform-1.
Purification of HeLa-GAPDH by
cation-exchange chromatography
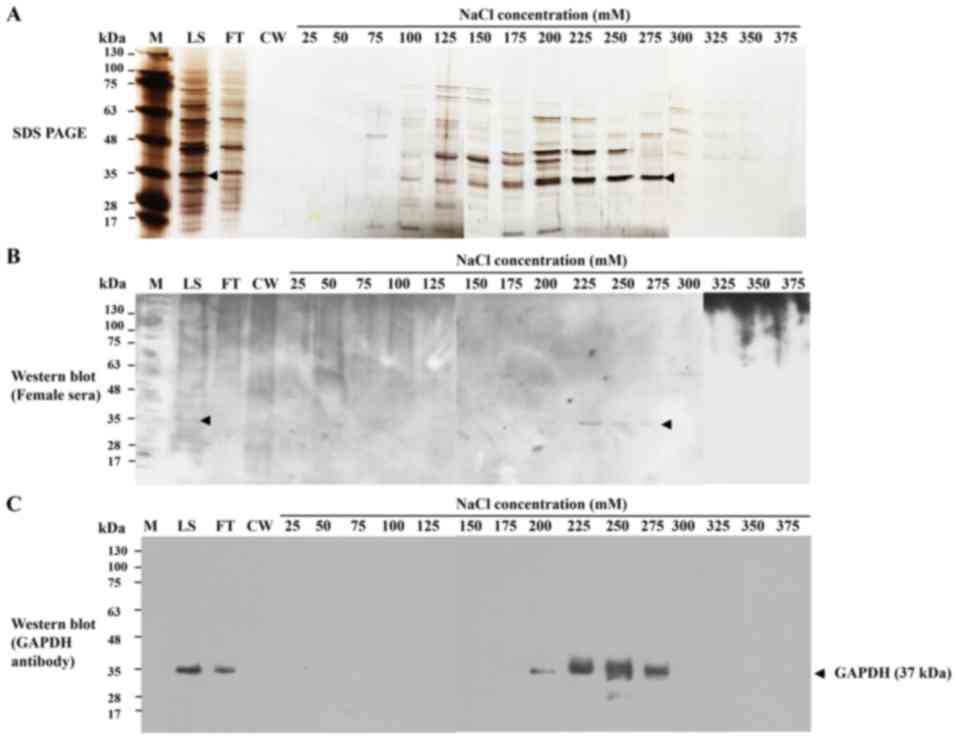
Cation-exchange chromatography was used to purify
HeLa-GAPDH from the total cytosolic proteins. The protein was
isolated with satisfactory purity in the 275-mM NaCl elution
fraction (Fig. 3A). Western blotting
confirmed that the isolated fraction reacted with control sera from
healthy females and with anti-human GAPDH antibody (Fig. 3B and C). The purified protein was
demonstrated to give a linear response with human sera and
anti-human GAPDH antibody in ELISAs (data not shown).
Serum anti-HeLa-GAPDH antibody levels
in normal, CIN I, CIN II, CIN III and cervical cancer samples
Levels of serum anti-HeLa-GAPDH IgG and IgM were
investigated in the normal, CIN (I, II and III) and cervical cancer
groups by ELISA. The clinicopathological characteristics of each
group are as follows: A total of 130 serum samples were used and
the average ages of the normal (n=29), CIN I (n=18), CIN II (n=23),
CIN III (n=30) and cervical cancer (n=30) groups were 43.6, 43.5,
45.7, 40.7 and 51.8 years, respectively. A significant difference
of ages was observed between CIN III and cervical cancer groups
(ANOVA with Bonferroni correction; P<0.05). In the cervical
cancer group, the proportions of squamous cell carcinoma,
adenocarcinoma and adenosquamous carcinoma were 60.0, 36.7 and
3.3%, respectively.
When compared with the anti-HeLa-GAPDH antibody
levels in the normal group, the anti-HeLa-GAPDH IgG level of the
CINs and cervical cancer groups generally declined as the severity
of the cervical lesions increased (CIN II, P<0.01 versus normal;
CIN III, P<0.001 versus normal; and cervical cancer, P<0.001
versus normal; Fig. 4A). Similarly,
anti-HeLa-GAPDH IgM was lower in the CIN and cervical cancer
samples than in the controls (CIN II, P<0.05 versus normal; and
cervical cancer, P<0.001 versus normal; Fig. 4B). When rGAPDH was used as the
coating antigen for the ELISAs, reduced number of serum sample
(Total, n=129; normal, n=29; CIN I, n=17; CIN II, n=23; CIN III,
n=30; and cervical cancer, n=30) was used in measuring anti-rGAPDH
antibody levels. However, the trends in levels of anti-rGAPDH IgG
and anti-rGAPDH IgM in the groups were similar to when HeLa-GAPDH
was used (Fig. 5).
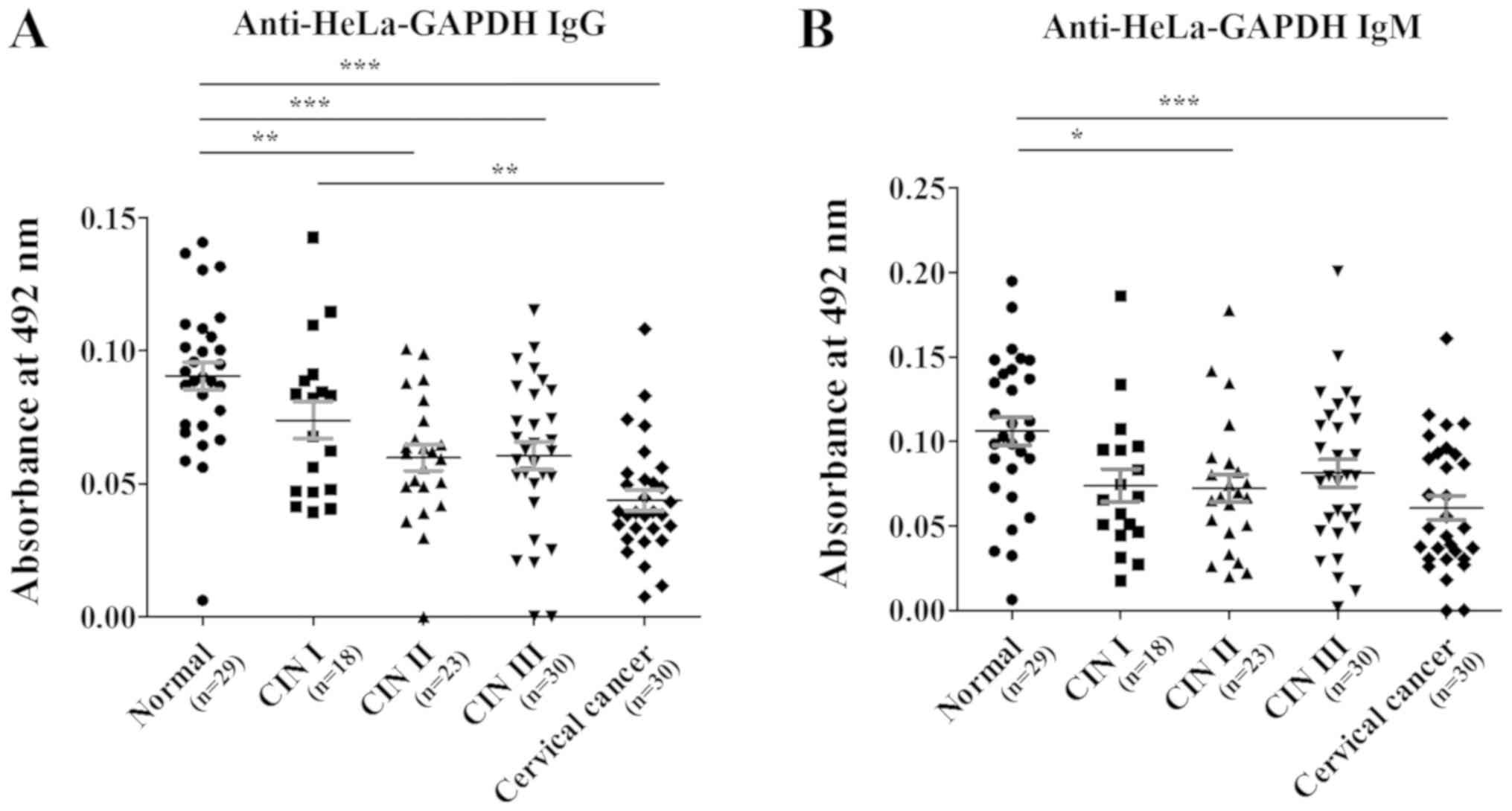 | Figure 4.Anti-HeLa-GAPDH antibody levels in
the normal, CIN I, CIN II, CIN III and cervical cancer groups. (A)
Levels of anti-HeLa-GAPDH IgG. (B) Levels of anti-HeLa-GAPDH IgM.
The central lines represent the mean values and the error bars
indicate the ranges of the standard error of the mean. Total,
n=130; normal, n=29; CIN I, n=18; CIN II, n=23; CIN III, n=30; and
cervical cancer, n=30. Statistical analysis was performed using the
Kruskal-Wallis test with Dunn's multiple comparison test.
*P<0.05, **P<0.01 and ***P<0.001, as indicated. CIN,
cervical intraepithelial neoplasia; IgG/IgM, immunoglobulin
G/M. |
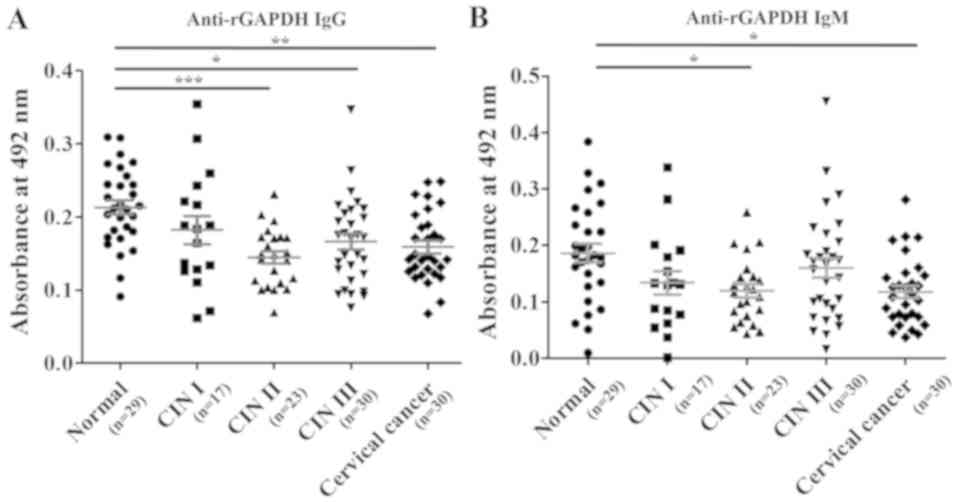 | Figure 5.Comparison of anti-rGAPDH IgG and
anti-rGAPDH IgM levels in the normal, CIN I, CIN II, CIN III and
cervical cancer groups in ELISAs using rGAPDH as the coating
antigen. (A) Levels of anti-rGAPDH IgG. (B) Levels of anti-rGAPDH
IgM. The central lines represent the mean values, and the error
bars indicate the ranges of the standard error of the mean. Total,
n=129; normal, n=29; CIN I, n=17; CIN II, n=23; CIN III, n=30; and
cervical cancer, n=30. Statistical analysis was performed using the
Kruskal-Wallis test with Dunn's multiple comparison test.
*P<0.05, **P<0.01 and ***P<0.001, as indicated. rGAPDH,
recombinant GAPDH; CIN, cervical intraepithelial neoplasia;
IgG/IgM, immunoglobulin G/M. |
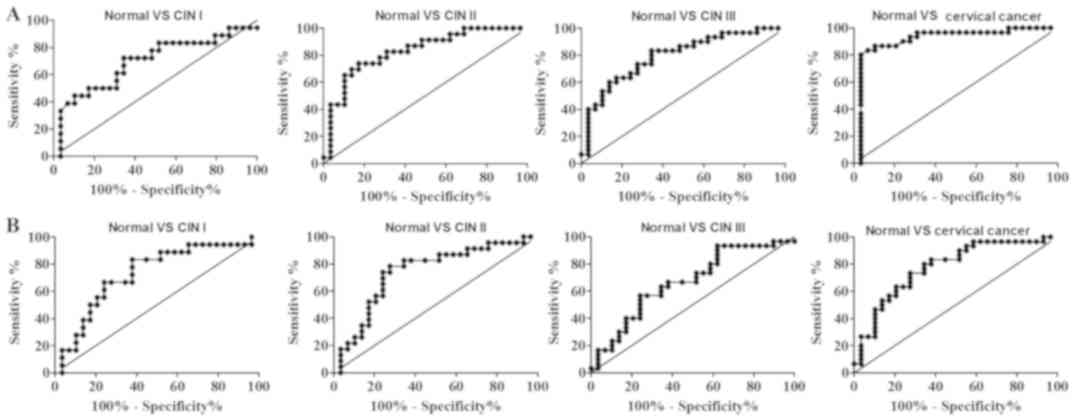
The statistical power (1-β) and diagnostic
performances (sensitivity, specificity, NPV, PPV, accuracy and AUC)
were evaluated for the ability to discriminate the various cervical
lesions from normal samples (Table
I). It was demonstrated that the anti-HeLa-GAPDH IgG levels
discriminated cervical cancer from healthy samples with high
sensitivity (80.0%) and specificity (96.6%), whereas the
anti-HeLa-GAPDH IgM levels discriminated cervical cancer from
normal with 73.3% sensitivity and 72.4% specificity. Overall,
higher AUCs for discriminating cervical cancer from normal were
obtained with anti-HeLa-GAPDH IgG than with anti-HeLa-GAPDH IgM
(AUC, 0.91 vs. 0.78, respectively; Fig.
6 and Table I). Therefore,
anti-HeLa-GAPDH IgG may be a more reliable biomarker for detecting
cervical cancer than anti-HeLa-GAPDH IgM.
 | Table I.Statistical parameters based on
anti-HeLa-GAPDH IgG or IgM levels for discriminating CINs or
cervical cancer from normal cytology, and CIN II+ from
Normal + CIN I. |
Table I.
Statistical parameters based on
anti-HeLa-GAPDH IgG or IgM levels for discriminating CINs or
cervical cancer from normal cytology, and CIN II+ from
Normal + CIN I.
| A, IgG |
|---|
|
|---|
| Comparison | Power (1-β
error)a |
Sensitivityb, % |
Specificityc, % | NPV, % | PPV, % | Accuracy, % | AUC |
|---|
| Normal vs. CIN
I | 0.48 | 72.2 | 65.5 | 79.2 | 56.5 | 68.1 | 0.69 |
| Normal vs. CIN
II | 0.99 | 73.9 | 82.8 | 80.0 | 77.3 | 78.8 | 0.83 |
| Normal vs. CIN
III | 0.98 | 83.3 | 65.5 | 79.2 | 71.4 | 74.6 | 0.79 |
| Normal vs. Cervical
cancer | 1.00 | 80.0 | 96.6 | 82.4 | 96.0 | 88.1 | 0.91 |
| Normal + CIN
Id vs. CIN
II+e | 1.00 | 74.7 | 72.3 | 61.8 | 82.7 | 73.8 | 0.78 |
|
| B, IgM |
|
|
Comparison | Power (1-β
error)a |
Sensitivityb, % |
Specificityc, % | NPV, % | PPV, % | Accuracy,
% | AUC |
|
| Normal vs. CIN
I | 0.68 | 83.3 | 62.1 | 85.7 | 57.7 | 70.2 | 0.73 |
| Normal vs. CIN
II | 0.80 | 78.3 | 72.4 | 80.8 | 69.2 | 75.0 | 0.74 |
| Normal vs. CIN
III | 0.55 | 56.7 | 75.9 | 62.9 | 70.8 | 66.1 | 0.67 |
| Normal vs. Cervical
cancer | 0.98 | 73.3 | 72.4 | 72.4 | 73.3 | 72.9 | 0.78 |
| Normal + CIN
Id vs. CIN
II+e | 0.80 | 74.7 | 53.2 | 73.8 | 54.3 | 66.9 | 0.65 |
CIN II is a clinically important end-point because
CIN II and III can progress to cervical cancer if left untreated
(43). Anti-HeLa-GAPDH IgM was
revealed to discriminate CIN II+ lesions (CIN II, CIN
III and cervical cancer) from the normal plus CIN I group with
74.7% sensitivity and 53.2% specificity, and higher sensitivity
(74.7%) and specificity (72.3%) were obtained with anti-HeLa-GAPDH
IgG (Table I; normal + CIN I vs. CIN
II+). In addition, the overall AUC values for
anti-HeLa-GAPDH IgG were higher than those for anti-HeLa-GAPDH IgM
for discriminating CIN II+ from the normal plus CIN I
group (Table I; normal + CIN I vs.
CIN II+). Therefore, the results indicate that
anti-GAPDH IgG reflects the severity of cervical lesions more
accurately than anti-GAPDH IgM. It can be concluded that anti-GAPDH
autoantibody is a potential serum biomarker for high-grade CINs and
cervical cancer.
Discussion
High-grade CINs (II and III) have a high probability
of progressing to cervical cancer (44–46).
Therefore, discriminating CIN II and more severe cervical lesions
from normal sample is important in primary screening for cervical
lesions. The present study aimed to discover a new
autoantibody-based marker for detecting cervical lesions and
revealed that serum anti-GAPDH IgG or IgM may be a useful marker
for that purpose. Anti-GAPDH IgG was identified to decrease with
increasing severity of cervical lesions, and cervical cancer was
detected with high sensitivity (80.0%) and specificity (96.6%) with
anti-HeLa-GAPDH IgG as marker. In addition, a lower anti-HeLa-GAPDH
IgG level discriminated CIN II+ from normal and CIN I
samples with 74.7% sensitivity and 72.3% specificity. The present
findings suggest that a decreased anti-GAPDH IgG level is a
promising biomarker for cervical cancer.
Autoantibodies have received much attention as serum
biomarkers for cancer screening over the past five decades
(24,47–49).
Misfolding, overexpression and aberrant glycosylation of
autoantigens are recognized to be general features of cancer
development (36,38,39,50), and
it was considered likely that alterations in the properties of
autoantigens that can elicit humoral immune responses would be
encountered (40). Therefore,
previous attempts to discover autoantibody-based cancer markers
have focused on autoantibodies whose levels increase in the
presence of cancer. However, recent studies have demonstrated that
an increase in the level of an autoantibody is only one of a
variety of changes associated with autoantibodies in cancer.
Autoantibodies not only eliminate the breakdown products of
antigens resulting from apoptosis but also protect healthy cells
from attack by the immune system of the individual (21,23,51,52). In
addition, autoantibodies can serve anti-oncogenic roles by
promoting the apoptosis of malignant cells and reducing their
invasiveness (53,54), as well as by maintaining homeostasis
(55). Decreased levels of
anti-glucose-regulated protein 78 and anti-α-enolase 1 antibodies
have been demonstrated in ovarian, breast and lung cancer (34,35,53), and
decreased levels of anti-α-enolase 1 were revealed in late stage
(stage IV) lung cancer but not in earlier stages (stage I/II)
(34). The present observations on
anti-GAPDH autoantibody levels during cervical cancer development
together with these other findings suggest that maintaining a
certain level of various autoantibodies is critical for the balance
of the immune system, and that decreased levels can be associated
with a malignant state.
GAPDH is a glycolytic enzyme that promotes the 6th
step of glycolysis (56). Increased
levels of GAPDH mRNA are detected in cervical cancer tissues and
cell lines (57,58). Malignant cells preferentially employ
aerobic glycolysis rather than oxidative phosphorylation to
generate adenosine triphosphate (59), and overexpression of glycolytic
enzymes has been suggested to be a hallmark of cancer cell
metabolism (60). Furthermore, GAPDH
is associated with an increased migratory behavior and
proliferation of cancer cells (61–63), and
its downregulation by GAPDH segregator, a triazine-based small
molecule, decreases the viability of colon carcinoma cells
(64). In addition, treatment with
antisense oligonucleotides against GAPDH, or glycolysis-targeting
anticancer agents, including 3-bromopyruvate, was demonstrated to
prevent the proliferation of human colon cancer cells and to induce
apoptosis of human and mouse cervical carcinoma cells (65,66).
Therefore, it appears that GAPDH has anti-apoptotic or
pro-proliferative activity for malignant cells, and that
maintaining GAPDH levels may be critical for preventing malignancy.
In light of the results of the present study and previous findings,
it may be that a decrease in anti-GAPDH autoantibody levels in
cervical cancer is a consequence of providing a favorable
microenvironment for the survival and proliferation of cancer
cells.
Panels of autoantibodies, generally ones that are
elevated in cancer, have been used as cancer biomarkers to overcome
the insufficient sensitivity or specificity of single
autoantibodies (29,30,32). The
EarlyCDT®-Lung test, which uses a combination of 6
autoantibodies (anti-cellular tumor antigen p53, autoimmunogenic
cancer/testis antigen NY-ESO-1, cancer-associated gene protein,
GBU4-5, Annexin I and transcription factor SOX2), provides 39%
sensitivity and 89% specificity for identifying individuals at high
risk of lung cancer (67).
Similarly, the present research group had demonstrated that a
combination of 3 autoantibodies [cancer antigen (CA)15-3,
carcinoembryonic antigen and CA19-9 autoantibodies] discriminate
cervical cancer from normal samples with 90.3% sensitivity and
82.1% specificity (68). In contrast
with previous findings, a decrease in the level of a single
autoantibody (IgG against GAPDH) was identified in the present
study to discriminate cervical cancer from normal samples with
80.0% sensitivity and 96.6% specificity. This finding indicates
that the reduced IgG response against the autoantigen may be useful
as a biomarker.
Overexpression of the GAPDH gene has been observed
in breast, ovarian, colon, lung, liver and cervical cancer
(60,69–71). In
addition, several types of non-cancerous cells, such as
inflammatory cells, naïve T lymphocytes and endothelial cells also
undergo rapid proliferation and obtain energy by aerobic glycolysis
during their activation or proliferation which are involved in
GAPDH gene expression (72).
Therefore, it will be of the interest to examine the distinctive
role of GAPDH in such non-cancerous cells as well as anti-GAPDH
antibody levels in the non-cancerous status. Understanding the
functions of anti-GAPDH autoantibody in cancer may provide new
insights for the treatment of cancer.
The limitations of this study include a limited
number of serum samples (n=130) used, although the resulting power
(0.8–1.0) satisfied the criterion for reliability. In addition, the
anti-GAPDH antibody levels were only investigated in a single
ethnic group (Korean women). Therefore, further studies involving
larger sample sizes and diversity are required to confirm whether
the decreased anti-GAPDH level in patients with cervical lesions is
a general feature.
In conclusion, the present study presents the first
evidence that decreased levels of anti-GAPDH autoantibody (IgG and
IgM), particularly anti-GAPDH IgG, is a potential serum biomarker
for detecting CINs and cervical cancer. Furthermore, it was
revealed that decreased levels of serum anti-GAPDH autoantibodies
reflect the severity of cervical lesions. Serum anti-GAPDH IgG
level, as a single indicator, was able to discriminate CIN
II+ from normal plus CIN I with high sensitivity and
specificity. It is expected that the present findings can provide a
new paradigm to the discovery of serum biomarker for cervical
lesions screening.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
NRF-2015R1D1A1A01057370).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SCK, WJ and YHK collected the serum samples and
cervical biopsies, and graded the cervical lesions. MLX, HyJK and
HoJK conceived and designed the experiments. MLX and KC performed
the experiments. MLX, HyJK, KC and HoJK analyzed the data. MLX and
HyJK wrote the paper. HoK is responsible for the integrity of the
work as a whole. All the authors have accepted responsibility for
the entire consent of this submitted article and approved
submission.
Ethics approval and consent to
participate
The present study was carried out with the approval
of the Institutional Review Board of the Ewha Woman's University
Mokdong Hospital, Seoul, South Korea (approval no. EUMC
2016-07-067-002). The study was conducted in accordance with the
Declaration of Helsinki. All serum samples were collected in a
prospective and consecutive manner following the collection of
written informed consent from the subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim SM, Choi HS and Byun JS: Overall
5-year survival rate and prognostic factors in patients with stage
IB and IIA cervical cancer treated by radical hysterectomy and
pelvic lymph node dissection. Int J Gynecol Cancer. 10:305–312.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kasamatsu T, Onda T, Sawada M, Kato T,
Ikeda S, Sasajima Y and Tsuda H: Radical hysterectomy for FIGO
stage I–IIB adenocarcinoma of the uterine cervix. Br J Cancer.
100:1400–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Survival rates for cervical cancer by
stage, . https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/survival.html
|
|
5
|
Bulk S, Visser O, Rozendaal L, Verheijen
RH and Meijer CJ: Incidence and survival rate of women with
cervical cancer in the Greater Amsterdam area. Br J Cancer.
89:834–839. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ibáñez R, Alejo M, Combalia N, Tarroch X,
Autonell J, Codina L, Culubret M, Bosch FX and de Sanjosé S:
Underscreened women remain overrepresented in the pool of cervical
cancer cases in spain: A need to rethink the screening
interventions. Biomed Res Int. 2015:6053752015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Safaeian M, Solomon D and Castle PE:
Cervical cancer prevention-cervical screening: Science in
evolution. Obstet Gynecol Clin North Am. 34739–760. (ix)2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karimi-Zarchi M, Peighmbari F, Karimi N,
Rohi M and Chiti Z: A Comparison of 3 Ways of Conventional Pap
Smear, Liquid-Based Cytology and Colposcopy vs Cervical Biopsy for
Early Diagnosis of Premalignant Lesions or Cervical Cancer in Women
with Abnormal Conventional Pap Test. Int J Biomed Sci. 9:205–210.
2013.PubMed/NCBI
|
|
9
|
Franco EL: Chapter 13: Primary screening
of cervical cancer with human papillomavirus tests. J Natl Cancer
Inst Monogr. 89–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kulasingam SL, Havrilesky LJ, Ghebre R and
Myers ER: Screening for cervical cancer: A modeling study for the
US preventive services task force. J Low Genit Tract Dis.
17:193–202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marlow LA, Waller J and Wardle J: Barriers
to cervical cancer screening among ethnic minority women: A
qualitative study. J Fam Plann Reprod Health Care. 41:248–254.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McLaughlin-Drubin ME and Munger K: Viruses
associated with human cancer. Biochim Biophys Acta. 1782:127–150.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parkin DM: The global health burden of
infection-associated cancers in the year 2002. Int J Cancer.
118:3030–3044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Combes JD, Pawlita M, Waterboer T,
Hammouda D, Rajkumar T, Vanhems P, Snijders P, Herrero R,
Franceschi S and Clifford G: Antibodies against high-risk human
papillomavirus proteins as markers for invasive cervical cancer.
Int J Cancer. 135:2453–2461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coghill AE and Hildesheim A: Epstein-Barr
virus antibodies and the risk of associated malignancies: Review of
the literature. Am J Epidemiol. 180:687–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mahieux R and Gessain A: Adult T-cell
leukemia/lymphoma and HTLV-1. Curr Hematol Malig Rep. 2:257–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luevano M, Bernard HU, Barrera-Saldaña HA,
Trevino V, Garcia-Carranca A, Villa LL, Monk BJ, Tan X, Davies DH,
Felgner PL and Kalantari M: High-throughput profiling of the
humoral immune responses against thirteen human papillomavirus
types by proteome microarrays. Virology. 405:31–40. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joura EA, Ault KA, Bosch FX, Brown D,
Cuzick J, Ferris D, Garland SM, Giuliano AR, Hernandez-Avila M, Huh
W, et al: Attribution of 12 high-risk human papillomavirus
genotypes to infection and cervical disease. Cancer Epidemiol
Biomarkers Prev. 23:1997–2008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lutz HU: Homeostatic roles of naturally
occurring antibodies: An overview. J Autoimmun. 29:287–294. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwartz-Albiez R, Monteiro RC, Rodriguez
M, Binder CJ and Shoenfeld Y: Natural antibodies, intravenous
immunoglobulin and their role in autoimmunity, cancer and
inflammation. Clin Exp Immunol. 158 (Suppl 1):S43–S50. 2009.
View Article : Google Scholar
|
|
23
|
Peng Y, Kowalewski R, Kim S and Elkon KB:
The role of IgM antibodies in the recognition and clearance of
apoptotic cells. Mol Immunol. 42:781–787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Desmetz C, Mange A, Maudelonde T and
Solassol J: Autoantibody signatures: Progress and perspectives for
early cancer detection. J Cell Mol Med. 15:2013–2024. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knight V, Merkel PA and O'Sullivan MD:
Anticytokine autoantibodies: Association with infection and immune
dysregulation. Antibodies. 5:2016.doi: 10.3390/antib5010003.
View Article : Google Scholar
|
|
26
|
Wu J and Li L: Autoantibodies in
Alzheimer's disease: Potential biomarkers, pathogenic roles, and
therapeutic implications. J Biomed Res. 30:361–372. 2016.PubMed/NCBI
|
|
27
|
Arbuckle MR, McClain MT, Rubertone MV,
Scofield RH, Dennis GJ, James JA and Harley JB: Development of
autoantibodies before the clinical onset of systemic lupus
erythematosus. N Engl J Med. 349:1526–1533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lacombe J, Mange A and Solassol J: Use of
Autoantibodies to Detect the Onset of Breast Cancer. J Immunol Res.
2014:5749812014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Liao Y, Xiang L, Jiang K, Li S,
Huangfu M and Sun S: A panel of autoantibodies as potential early
diagnostic serum biomarkers in patients with breast cancer. Int J
Clin Oncol. 22:291–296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huangfu M, Xu S, Li S, Sun B, Lee KH, Liu
L and Sun S: A panel of autoantibodies as potential early
diagnostic serum biomarkers in patients with cervical cancer.
Tumour Biol. 37:8709–8714. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tan EM and Zhang J: Autoantibodies to
tumor-associated antigens: Reporters from the immune system.
Immunol Rev. 222:328–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang JY, Casiano CA, Peng XX, Koziol JA,
Chan EK and Tan EM: Enhancement of antibody detection in cancer
using panel of recombinant tumor-associated antigens. Cancer
Epidemiol Biomarkers Prev. 12:136–143. 2003.PubMed/NCBI
|
|
33
|
Tabuchi Y, Shimoda M, Kagara N, Naoi Y,
Tanei T, Shimomura A, Shimazu K, Kim SJ and Noguchi S: Protective
effect of naturally occurring anti-HER2 autoantibodies on breast
cancer. Breast Cancer Res Treat. 157:55–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shih NY, Lai HL, Chang GC, Lin HC, Wu YC,
Liu JM, Liu KJ and Tseng SW: Anti-alpha-enolase autoantibodies are
down-regulated in advanced cancer patients. Jpn J Clin Oncol.
40:663–669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Van Hoesen K, Meynier S, Ribaux P,
Petignat P, Delie F and Cohen M: Circulating GRP78 antibodies from
ovarian cancer patients: A promising tool for cancer cell targeting
drug delivery system? Oncotarget. 8:107176–107187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pinho SS and Reis CA: Glycosylation in
cancer: Mechanisms and clinical implications. Nat Rev Cancer.
15:540–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krueger KE and Srivastava S:
Posttranslational protein modifications: Current implications for
cancer detection, prevention, and therapeutics. Mol Cell
Proteomics. 5:1799–1810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He Y, Zhou Z, Hofstetter WL, Zhou Y, Hu W,
Guo C, Wang L, Guo W, Pataer A, Correa AM, et al: Aberrant
expression of proteins involved in signal transduction and DNA
repair pathways in lung cancer and their association with clinical
parameters. PLoS One. 7:e310872012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Geradts J and Ingram CD: Abnormal
expression of cell cycle regulatory proteins in ductal and lobular
carcinomas of the breast. Mod Pathol. 13:945–953. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zaenker P, Gray ES and Ziman MR:
Autoantibody Production in Cancer-The Humoral Immune Response
toward Autologous Antigens in Cancer Patients. Autoimmun Rev.
15:477–483. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pappa KI, Lygirou V, Kontostathi G,
Zoidakis J, Makridakis M, Vougas K, Daskalakis G, Polyzos A and
Anagnou NP: Proteomic analysis of normal and cancer cervical cell
lines reveals deregulation of cytoskeleton-associated proteins.
Cancer Genomics Proteomics. 14:253–266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kontostathi G, Zoidakis J, Makridakis M,
Lygirou V, Mermelekas G, Papadopoulos T, Vougas K, Vlamis-Gardikas
A, Drakakis P, Loutradis D, et al: Cervical cancer cell line
secretome highlights the roles of transforming growth
factor-Beta-induced protein ig-h3, peroxiredoxin-2, and NRF2 on
cervical carcinogenesis. Biomed Res Int. 2017:41807032017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Demarco M, Lorey TS, Fetterman B, Cheung
LC, Guido RS, Wentzensen N, Kinney WK, Poitras NE, Befano B, Castle
PE, et al: Risks of CIN 2+, CIN 3+, and cancer by cytology and
human papillomavirus status: The foundation of risk-based cervical
screening guidelines. J Low Genit Tract Dis. 21:261–267. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Holowaty P, Miller AB, Rohan T and To T:
Natural history of dysplasia of the uterine cervix. J Natl Cancer
Inst. 91:252–258. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ostör AG: Natural history of cervical
intraepithelial neoplasia: A critical review. Int J Gynecol Pathol.
12:186–192. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McCredie MR, Sharples KJ, Paul C, Baranyai
J, Medley G, Jones RW and Skegg DC: Natural history of cervical
neoplasia and risk of invasive cancer in women with cervical
intraepithelial neoplasia 3: A retrospective cohort study. Lancet
Oncol. 9:425–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Macdonald IK, Parsy-Kowalska CB and
Chapman CJ: Autoantibodies: Opportunities for early cancer
detection. Trends Cancer. 3:198–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zaenker P and Ziman MR: Serologic
autoantibodies as diagnostic cancer biomarkers-a review. Cancer
Epidemiol Biomarkers Prev. 22:2161–2181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pedersen JW and Wandall HH: Autoantibodies
as Biomarkers in Cancer. Lab Medicine. 42:623–628. 2011. View Article : Google Scholar
|
|
50
|
de Oliveira GA, Rangel LP, Costa DC and
Silva JL: Misfolding, aggregation, and disordered segments in c-Abl
and p53 in human cancer. Front Oncol. 5:972015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shoenfeld Y and Toubi E: Protective
autoantibodies: Role in homeostasis, clinical importance, and
therapeutic potential. Arthritis Rheum. 52:2599–2606. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Siloşi I, Siloşi CA, Boldeanu MV, Cojocaru
M, Biciuşcă V, Avrămescu CS, Cojocaru IM, Bogdan M and FolcuŢi RM:
The role of autoantibodies in health and disease. Rom J Morphol
Embryol. 57 (Suppl):633–638. 2016.PubMed/NCBI
|
|
53
|
Cohen M and Petignat P: Purified
autoantibodies against glucose-regulated protein 78 (GRP78) promote
apoptosis and decrease invasiveness of ovarian cancer cells. Cancer
Lett. 309:104–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Díaz-Zaragoza M, Hernández-Ávila R,
Viedma-Rodríguez R, Arenas-Aranda D and Ostoa-Saloma P: Natural and
adaptive IgM antibodies in the recognition of tumor-associated
antigens of breast cancer (Review). Oncol Rep. 34:1106–1114. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nagele EP, Han M, Acharya NK, DeMarshall
C, Kosciuk MC and Nagele RG: Natural IgG autoantibodies are
abundant and ubiquitous in human sera, and their number is
influenced by age, gender, and disease. PLoS One. 8:e607262013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Krasnov GS, Dmitriev AA, Snezhkina AV and
Kudryavtseva AV: Deregulation of glycolysis in cancer:
Glyceraldehyde-3-phosphate dehydrogenase as a therapeutic target.
Expert Opin Ther Targets. 17:681–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hansen CN, Ketabi Z, Rosenstierne MW,
Palle C, Boesen HC and Norrild B: Expression of CPEB, GAPDH and
U6snRNA in cervical and ovarian tissue during cancer development.
APMIS. 117:53–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim JW, Kim SJ, Han SM, Paik SY, Hur SY,
Kim YW, Lee JM and Namkoong SE: Increased
glyceraldehyde-3-phosphate dehydrogenase gene expression in human
cervical cancers. Gynecol Oncol. 71:266–269. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zheng J: Energy metabolism of cancer:
Glycolysis versus oxidative phosphorylation (Review). Oncol Lett.
4:1151–1157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Altenberg B and Greulich KO: Genes of
glycolysis are ubiquitously overexpressed in 24 cancer classes.
Genomics. 84:1014–1020. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu K, Tang Z, Huang A, Chen P, Liu P,
Yang J, Lu W, Liao J, Sun Y, Wen S, et al:
Glyceraldehyde-3-phosphate dehydrogenase promotes cancer growth and
metastasis through upregulation of SNAIL expression. Int J Oncol.
50:252–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hao L, Zhou X, Liu S, Sun M, Song Y, Du S,
Sun B, Guo C, Gong L, Hu J, et al: Elevated GAPDH expression is
associated with the proliferation and invasion of lung and
esophageal squamous cell carcinomas. Proteomics. 15:3087–3100.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nicholls C, Pinto AR, Li H, Li L, Wang LH,
Simpson R and Liu JP: Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) induces cancer cell senescence by interacting with
telomerase RNA component. Proc Natl Acad Sci USA. 109:13308–13313.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jung DW, Kim WH, Seo S, Oh E, Yim SH, Ha
HH, Chang YT and Williams DR: Chemical targeting of GAPDH
moonlighting function in cancer cells reveals its role in tubulin
regulation. Chem Biol. 21:1533–1545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lea MA, Qureshi MS, Buxhoeveden M, Gengel
N, Kleinschmit J and Desbordes C: Regulation of the proliferation
of colon cancer cells by compounds that affect glycolysis,
including 3-bromopyruvate, 2-deoxyglucose and biguanides.
Anticancer Res. 33:401–407. 2013.PubMed/NCBI
|
|
66
|
Kim JW, Kim TE, Kim YK, Kim YW, Kim SJ,
Lee JM, Kim IK and Namkoong SE: Antisense oligodeoxynucleotide of
glyceraldehyde-3-phosphate dehydrogenase gene inhibits cell
proliferation and induces apoptosis in human cervical carcinoma
cell lines. Antisense Nucleic Acid Drug Dev. 9:507–513. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chapman CJ, Healey GF, Murray A, Boyle P,
Robertson C, Peek LJ, Allen J, Thorpe AJ, Hamilton-Fairley G,
Parsy-Kowalska CB, et al: EarlyCDT®-Lung test: Improved
clinical utility through additional autoantibody assays. Tumour
Biol. 33:1319–1326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jin Y, Kim SC and Kim HJ, Ju W, Kim YH and
Kim HJ: Use of autoantibodies against tumor-associated antigens as
serum biomarkers for primary screening of cervical cancer.
Oncotarget. 8:105425–105439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Révillion F, Pawlowski V, Hornez L and
Peyrat JP: Glyceraldehyde-3-phosphate dehydrogenase gene expression
in human breast cancer. Eur J Cancer. 36:1038–1042. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tokunaga K, Nakamura Y, Sakata K, Fujimori
K, Ohkubo M, Sawada K and Sakiyama S: Enhanced expression of a
glyceraldehyde-3-phosphate dehydrogenase gene in human lung
cancers. Cancer Res. 47:5616–5619. 1987.PubMed/NCBI
|
|
71
|
Hjerpe E, Egyhazi Brage S, Carlson J,
Frostvik Stolt M, Schedvins K, Johansson H, Shoshan M and
Avall-Lundqvist E: Metabolic markers GAPDH, PKM2, ATP5B and
BEC-index in advanced serous ovarian cancer. BMC Clin Pathol.
13:302013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Abdel-Haleem AM, Lewis NE, Jamshidi N,
Mineta K, Gao X and Gojobori T: The emerging facets of
non-cancerous warburg effect. Front Endocrinol (Lausanne).
8:2792017. View Article : Google Scholar : PubMed/NCBI
|