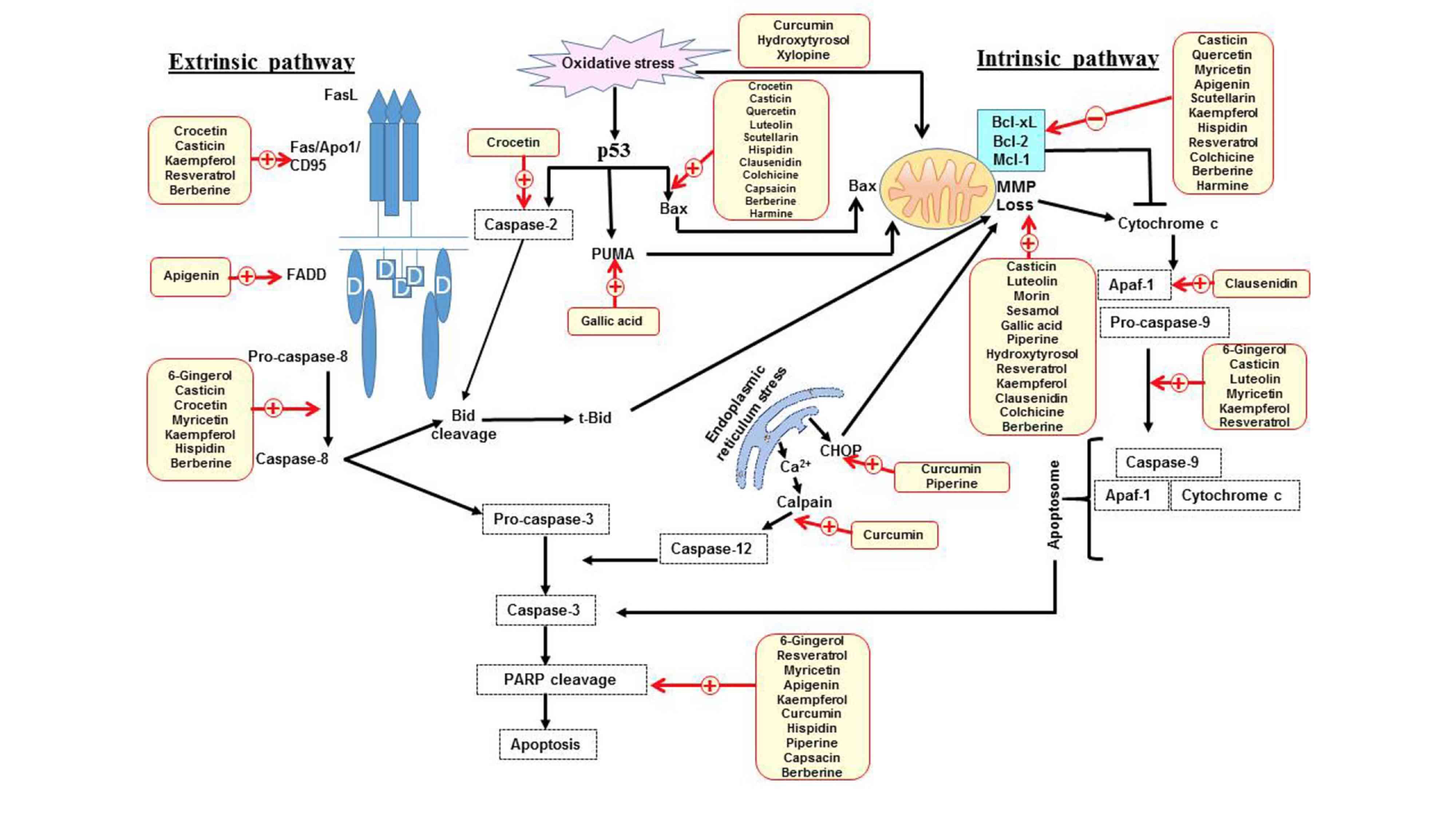
Colorectal cancer (CRC) is the third most common
cancer diagnosed in men and women and is the fourth leading cause
of cancer-associated mortality worldwide (1). In 2018, >30,000 CRC-associated
mortalities were reported in the USA (2). According to the World Health
Organization, in 2008, 1.2 million new CRC cases were reported
globally (3). Annually >0.6
million patients succumb due to CRC (4), and a family history of CRC or chronic
inflammatory bowel disease is a contributing factor for disease
progression (5). Additionally, a
sedentary lifestyle, low physical activity and unhealthy dietary
patterns, including diets with low fiber and a high content of red
meat or fat, cigarette smoking and alcohol abuse, are the major
causes of CRC development. The majority of CRC cases are diagnosed
at the advanced stages of disease, which makes curative treatment
impossible (6).
Understanding the developmental pathways in tumor
cells that promote the growth and metastasis of tumors is important
in order to identify the molecular targets of cancer therapeutics.
The majority of CRC cases occur as a result of genetic and
epigenetic modifications (7–9). Studies investigating human cancer,
including CRC, have demonstrated a central role of p53 in tumor
suppression (10). Almost 50% of CRC
cases are reported to have a mutation in p53 (9), which promotes cell proliferation,
invasion, metastasis and resistance to a variety of anticancer
drugs, such as 5 fluorouracil (11,12).
Mutations in the KRAS, BRAF and neuroblastoma RAS viral oncogene
genes have also been reported in CRC (13). Additionally, mutations of the
adenomatous polyposis coli gene in CRC promotes the dysfunction of
β-catenin and activates the Wnt pathway, which is an activator of
the key cell cycle regulatory genes cyclin D1 and c-Myc, which
provide suitable conditions for cellular proliferation (13). In CRC, the activation of NF-κB
upregulates a number of genes responsible for the generation of
pro-inflammatory mediators and cytokines, which are essential for
CRC cell propagation (14).
Additionally, the PI3K/Akt pathway promotes tumor proliferation via
inhibition of apoptosis and stimulation of the cell cycle (15).
High levels of reactive oxygen species (ROS) have
been detected in almost all types of cancer and been demonstrated
to potentiate numerous aspects of tumor development and
progression. Under physiological conditions, the intracellular ROS
levels are not high enough to induce cell damage. However, any
imbalance in the redox status of the cell results in oxidative
stress, which exerts an important function in the initiation,
promotion and progression of carcinogenesis (16). Physical agents, chemical agents,
inflammation and infection potentiate oxidative stress, which
directly damages DNA and promotes tumorigenesis (17). Under mild oxidative stress, wild-type
p53 is reported to induce the expression of antioxidant enzymes,
and stimulates cell repair and survival mechanisms (18,19).
However, upon acute oxidative stress, p53 reduces the expression of
detoxifying enzymes and stimulates apoptosis (20). By contrast, mutated p53 exhibited in
cancer cells cannot induce the expression of antioxidant enzymes to
detoxify higher levels of ROS, whereas it upregulates cell
proliferative gene expression, which promotes the propagation of
DNA damage (21).
Apoptosis is a tightly regulated mechanism of cell
death and a stress response to toxic stimuli that is required to
maintain intestinal epithelial cell homeostasis. Spontaneous
apoptosis continuously occurs in the normal, unstressed intestine
and stress-induced apoptosis occurs following genotoxic insult,
including exposure to DNA-damaging agents. In cancer cells,
dysregulation of the apoptotic process results in disturbance of
tissue homeostasis, which then results in uncontrolled
proliferation of cells (22).
Caspases function as initiators and executors of apoptosis.
Initiator caspases, including caspase-8 and −9, which are involved
in the extrinsic and intrinsic apoptotic pathways, activate
effector caspases, including caspase-3 and −7, which cleave several
proteins, including poly(ADP ribose) polymerase-1 (PARP-1) in cells
(23,24). PARP-1 is a nuclear enzyme involved in
DNA repair, DNA stability and transcriptional regulation. PARP-1
cleavage prevents recruitment of enzymes to the site of DNA damage
and is considered to be a hallmark of apoptosis (Fig. 1) (25).
The endoplasmic reticulum (ER) is responsible for
the synthesis, folding and maturation of proteins (26). Conditions that result in ER stress
can activate cell protective mechanisms; however, if the stress is
excessive or prolonged then it will eliminate cells via the
intrinsic apoptosis pathway (26).
This switch between pro-survival and pro-apoptotic pathways is due
to the induction of a transcriptional factor C/EBP homologous
protein (CHOP) (27), which has been
reported to downregulate the anti-apoptotic protein Bcl-2 (28). Additionally, ER stress increases
intracellular calcium (Ca2+) levels, which activates
calpain-induced cleavage of anti-apoptotic B-cell lymphoma-extra
large (Bcl-xl) and increases caspase-12 activity, which then
activates caspase-9 independent of apoptotic protease activating
factor 1 (Apaf-1), followed by the activation of caspase-3
(Fig. 1) (29,30).
The activation of apoptosis is an important
mechanism for CRC prevention and treatment (31–33).
Dietary phytochemicals are known to prevent the initiation of
carcinogenesis via the induction of antioxidant enzymes and block
the progression of carcinogenic cells via the induction of
apoptosis and cell cycle arrest (34,35).
Almost 50% the approved anticancer drugs are derived from natural
products or their derivatives (36,37). To
establish novel compounds that can be utilized in combined therapy
to potentiate the effect of chemotherapeutic drugs, a number of
studies have been performed to identify agents present in diet or
herbs that interfere with proliferative cell signaling pathways
(34–38). The aim of the present study was to
compile available literature published between 2008–2018 regarding
the mechanisms of apoptosis induced by phytochemicals in CRC cells
in preclinical and clinical settings. This may assist in providing
a solid foundation for future research options in this field.
Phytochemicals are a non-nutritive group of
compounds naturally present in fruits, vegetables, spices, grains
and herbs, which have health promoting and disease preventing
characteristics (39). In
preclinical and clinical studies of different types of tumor, the
consumption of fruits and vegetables has been reported to exert
beneficial health effects (39,40). CRC
is strongly associated with dietary factors and the association of
phytochemicals with CRC prevention has been reported in several
studies (41–43). Numerous phytochemicals exhibit
chemo-preventive effects in CRC due to their antioxidative and ROS
scavenging activities. However, various phytochemicals are also
known to induce apoptosis by promoting ROS generation (44–62).
Table I details a list of
ROS-inducing phytochemicals in CRC.
The induction of apoptosis and inhibition of tumor
cell proliferation by cell cycle arrest are markers for the
evaluation of phytochemical anticancer activities. Table II details a list of phytochemicals
that can induce CRC cell cycle arrest at different phases of the
cell cycle (45,48,51,54,55,57,59,60,63–84).
Phytochemicals are classified according to their chemical
structure, for example, carotenoids, alkaloids and phenolic
compounds such as flavonoids, phenolic acid, stilbenes
(resveratrol), curcuminoids, tannins and cumarins (85). The following sections discuss the
apoptotic mechanisms of different classes in detail.
Carotenoids are colored lipid soluble pigments
present in plants, fungi, bacteria and algae, and also have been
identified in numerous foods, including fruit, vegetables and fish.
Carotenoids are responsible for providing bright coloration to
plants and animal. There are >600 carotenoids with natural
structural variations, which are divided into lycopenes,
β-carotenes, luteins and zeaxanthins (86).
Carotenoids are the most characteristic and
important components present in saffron (Crocus sativus L.)
stigmas. In ancient times, the Arabs, Indians and Chinese used
carotenoids for the treatment of various diseases, including
cancer. Crocetin is the most potent carotenoid in saffron (86,87).
Crocetin can induce different apoptotic mechanisms in colon cancer
cells with varying p53 statuses. The presence of wild-type p53 in
HCT 116 cells trans-activates Bax along with upregulation of
p53-induced death domain protein, which cleaves and activates Bid
via caspase-2 (88). However, in
functional p53-impaired cells (HCT 116 p53-/-), augmentation of the
p53-paralogue p73 was observed, which upregulates Fas to cleave Bid
through the Fas-associated death domain (FADD)-caspase-8-pathway
(88).
Ginger contains numerous phenolic compounds,
including 6-gingerol, 6-shagol, 6-paradol and zingerone (89). Among these compounds, 6-gingerol has
been extensively investigated for its cytotoxic effects in various
types of cancer, including colon cancer (83,84).
6-gingerol inhibits the proliferation of SW480 colon cancer cells
by arresting them at the G2/M phase and induces
apoptosis via activation of caspase-8, −9, −3 and −7 and PARP
cleavage (75).
Flavonoids are one of the largest groups of
naturally-occurring phenols, including flavones, flavanols,
isoflavones, flavonols, flavanones and flavanonols (90). Flavonoids are present in fruits,
vegetables, grains, bark, roots, stems, flowers, tea and wine.
Along with carotenoids, they are responsible for the vivid colors
in fruits and vegetables (91).
Flavonoids are known for their anti-oxidative, anti-inflammatory,
anti-mutagenic and anti-carcinogenic properties (91).
Quercetin is a major dietary flavonoid that has been
identified in a wide range of fruits, vegetables and beverages,
including tea and wine. Quercetin is known for its antioxidant,
anti-inflammatory and anti-proliferative properties (50). In HT-29 colon cancer cells quercetin
treatment decreases cell viability, arrests the cell cycle at the
G1 phase and induces apoptosis (76,92).
Quercetin inhibits the PI3K-mediated cell survival signaling
pathway via phosphorylation of its downstream target Akt (92). Additionally, quercetin decreases the
expression of COP9 signalosome subunit 6 (CSN6), a subunit of the
constitutive photomorphogenesis 9 multiprotein complex (76). Akt is a known regulator of CSN6,
which promotes carcinogenesis by stabilizing the viral oncogene
homolog Myc (76). Furthermore,
quercetin-treatment targets CSN6 genes to induce apoptosis, as it
downregulates Myc and Bcl-2 expression, and increases the
expression of p53 and Bax (76,92).
Quercetin has also been demonstrated to suppress the Wnt/β-catenin
and NF-κB pathways in CRC cells (93,94).
Additionally, treatment of HT-29 cells with quercetin upregulates
AMP-activated protein kinase, a physiological cellular energy
sensor, which markedly suppresses cell proliferation (92).
Luteolin (3,4,5,7-tetrahydroxyflavone) is a common
flavonoid that exists in numerous types of plants including fruits,
vegetables and medicinal herbs (95). Plants rich in luteolin have been used
in Chinese traditional medicine for the treatment of various
diseases, including hypertension, inflammatory disorders and cancer
(95). Luteolin decreases the cell
viability of HT-29 cells without affecting normal colon cells and
it has been observed to induce apoptosis of HT-29 cells by
activating the mitochondria-mediated caspase pathway (96). Treatment of HT-29 cells with luteolin
results in a loss of the mitochondrial membrane potential, an
increase in mitochondrial Ca2+ level, upregulation of
Bax, downregulation of Bcl-2, release of cytochrome c from
the mitochondria to the cytosol and an increase in the levels of
the active forms of caspase-9 and caspase-3 (96).
Morin is a flavonoid primarily identified in the
leaves of common guava, onion and almonds, and in members of the
Moraceae family, including mulberry, figs and Chinese herbs. The
pro-oxidative effect of morin in SW480 cells results in a
disturbance of the mitochondrial function, which results in the
activation of the intrinsic and the extrinsic apoptosis pathways
(56). Additionally, morin induces a
significant reduction in glucose transporter-1 expression, which
results in a decline in cellular glucose uptake, resulting in an
impaired mitochondrial function, which further sensitizes cells to
undergo apoptosis via the intrinsic apoptosis pathway (56).
Myricetin is a flavonoid present in fruits,
vegetables, tea, berries and medicinal plants (98). Myricetin has been reported to exhibit
anticancer activity in the colon cancer HCT-115 cells via
activation of nucleoside diphosphate kinase, which has been
reported to induce apoptosis and inhibit metastasis in various
types of cancer, such as hepatocellular carcinoma and pancreatic
cancer (99,100). Additionally, myricetin activates
caspase-3,- 8 and −9, and PARP, and downregulates the
anti-apoptotic Bcl-2 protein to induce apoptosis in colon cancer
cells (98).
Apigenin is a common flavonoid present in numerous
plants, fruits and vegetables. The primary source of its
consumption is chamomile tea, which is prepared from dried flowers
of Matricaria chamomilla (101). Apigenin has been reported to exert
anti-proliferative and anti-metastatic effects in a variety of CRC
cell lines, such as those for lung cancer and osteosarcoma
(102,103). Additionally, apigenin has been
demonstarted to inhibit the Wnt/β-catenin signaling pathway
(101,103) and suppress the phosphorylation of
STAT3 (104), which in turn results
in the downregulation of the anti-apoptotic proteins Bcl-xl and
myeloid cell leukemia sequence-1 (Mcl-1), which stimulates the
cleavage of PARP and the apoptosis of colon cancer cells (104). Furthermore, an in vivo study
revealed that apigenin increases the apoptotic index of SW480 colon
cancer cells via an upregulation of FADD (101).
Kaempferol is a flavonol present in fruits and
vegetables, including apples, onions, and green and black tea
(105). A high intake of kaempferol
has been reported to reduce the risk of colon cancer (105). Kaempferol induces apoptosis of
HT-29 colon cancer cells via activation of the extrinsic and
intrinsic apoptosis pathways. Kaempferol initiates the extrinsic
apoptosis pathway by increasing the level of FasL, which binds with
the Fas receptor and activates caspase-8. Caspase-8 then cleaves
Bid and interacts with the intrinsic apoptosis pathway via
translocation of t-Bid to the mitochondria, as evident by the
release of cytochrome c, activation of caspase-9, −7 and −3,
and PARP cleavage (106).
Additionally, kaempferol decreases the expression of the
anti-apoptotic protein Bcl-xl and reduces Akt activity (106).
Sesamol is a phenolic compound present in sesame
seeds that has been extensively investigated in different types of
cancer, such as hepatocellular carcinoma and skin tumors (111,112).
However, to the best of our knowledge, only a single study has been
performed with CRC cells (57).
Sesamol acts as an antioxidant at lower concentrations, while at
higher concentrations it exhibits pro-oxidant effects, which
decrease the viability of colon cancer HCT116 cells via
interruption of the cell cycle at the S-phase and induces apoptosis
via mitochondrial dysfunction (57).
Gallic acid is a type of phenolic acid that is
present in dietary substances, including blackberries, chocolate,
walnuts, raspberries, clove, vinegar, wine, green tea and herbs,
including oak, drosera, golden root, stinging nettle and Chinese
mahogany (58,113). Gallic acid is associated with
oxidative stress and arrests HCT-15 cells at the sub G1
phase (58). Additionally, gallic
acid has been reported to activate p53 upregulated modulator of
apoptosis, which is a pro-apoptotic protein that potentiates the
release of cytochrome c from the mitochondria via disruption
of the mitochondrial membrane potential (MMP), which demonstrates
the involvement of the intrinsic apoptosis pathway (114).
Trans-resveratrol, a natural stilbene present in
wine and grapes, has been extensively investigated for its
anti-inflammatory and anticancer activities (119). It has been reported to inhibit cell
proliferation signaling pathways in a number of studies (120–123).
In SW-620 and LoVo cells it has been reported to induce apoptosis
via the mitochondria-dependent and -independent pathways via an
upregulation of pro-apoptotic proteins and downregulation of
anti-apoptotic proteins (121,122).
Resveratrol has also been investigated in
combination with etoposide in CRC cell lines (120–122).
Synergistic effects have been observed on cell growth inhibition
via downregulation of mitogen-activated protein kinase signaling
pathways and an increase in apoptosis via activation of p53
(124–126). Additionally, a combination of
resveratrol and grape seed extract has been reported to suppress
Wnt/β-catenin signaling and increase mitochondria-dependent
apoptosis in in vitro and in vivo models (126).
Piperine is an amide alkaloid extracted from the
fruits of black and long pepper plants (Piper nigrum Linn.
and Piper longum Linn.) (45). Piperine arrests the cell cycle and
inhibits CRC cell proliferation independent of p53 status (45,65).
Piperine induces apoptosis by inhibiting the cell survival PI3K/Akt
signaling pathway and upregulating ER stress response proteins,
including inositol-requiring enzyme-1α, CHOP and binding
immunoglobulin protein, which results in MMP loss, cytochrome
c release and PARP cleavage, which indicates a role of the
intrinsic pathway in piperine-induced apoptosis (65).
As aforementioned, a number of phytochemical groups
have been demonstrated to induce apoptosis of CRC cells via
multiple pathways in in vitro studies. However, clinical
studies have been only performed with a limited number of
phytochemicals. A phase I pilot study with resveratrol reported
that it downregulates Wnt target gene expression in the normal
colonic mucosa of patients with CRC, while it upregulates Wnt
target genes, including myc and cyclin D1, in colon cancer
(138). The mechanism of this
increase remains to be completely elucidated and requires further
investigation. The resveratrol-induced downregulation of the
Wnt-associated genes in normal colonoic mucosa may exert a
protective effect as Wnt and its downstream effectors are known to
regulate processes involved in tumor initiation, tumor growth and
metastasis (139). Another study
that investigated the efficacy of resveratrol in patients with
colorectal adenocarcinoma demonstrated a 5% reduction in tumor cell
proliferation, which indicates that daily oral doses of resveratrol
at 0.5 or 1.0 g are sufficient to induce anti-carcinogenic effects
(140). However, further clinical
evaluation is required before it may be used as an alternative to
non-steroidal anti-inflammatory agents and selective cyclooxygenase
inhibitors in CRC chemoprevention (140).
Flavonols are understood to inhibit colorectal
carcinogenesis via multiple mechanisms, including attenuation of
inflammation (141–144). Elevated blood levels of IL-6 have
also been observed in colorectal adenoma (145). In a 4-year, randomized,
multi-center, nutritional intervention trial study it was
demonstrated that a high flavonol intake results in a reduction of
serum IL-6 level, which decreases the risk of colorectal adenoma
recurrence (146).
An open non-randomized clinical study was performed
with curcumin at dose of 4 or 2 g in patients with ≥8 aberrant
crypt foci (ACF) in a colonoscopic examination. Curcumin at a 4 g
daily dose for 30 days reduced ACF by 40%, while a 2 g dose
exhibited no effect (147). Another
clinical study demonstrated that administration of curcumin
increased the body weight of patients with CRC, reduced serum TNF-α
levels, upregulated p53 and increased DNA fragmentation in CRC
cells (148).
Despite significant progress in the diagnosis and
treatment of cancer, the incidence of CRC is increasing worldwide
and is expected to rise by 60% by 2030 (1). Mutations or alterations in cancer are a
major challenge for effective management. Due to the high incidence
of resistance and adverse effects-associated with chemotherapeutic
drugs, there is an urgent requirement to develop more effective
therapeutics (149). Phytochemicals
are known sources of various compounds that are currently used as
chemotherapeutic drugs (36,37). The current review summarized
previously published studies regarding the effect of phytochemicals
in CRC. To date, an extensive number of studies have been performed
to identify molecular pathways involved in CRC and the effects of
specific phytochemicals have been examined, primarily in
preclinical trials. However, only a limited number have been
performed in clinical settings. Therefore, for the majority of
phytochemicals it is too early to conclude their anticancer
properties. Further extensive research on phytochemicals is
required to promote understanding and elucidate their molecular
targets, drug interactions, ideal dosages, long-term safety and
adverse effects.
Not applicable.
The present study was supported by JSPS KAKENHI
(grant no. 17K09154).
Not applicable.
KA and SFZ contributed to the planning and design
of the study; KA drafted the manuscript; KA, SFZ, ZC, DZ, SAS and
HI performed the critical revisions of the manuscript and reviewed
the intellectual content.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Wang TY and Niu XC: Increased
plasma levels of pentraxin 3 are associated with poor prognosis of
colorectal carcinoma patients. Tohoku J Exp Med. 240:39–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology.
138:2101–2114.e5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hafström L, Johansson H and Ahlberg J:
Does diagnostic delay of colorectal cancer result in malpractice
claims? A retrospective analysis of the Swedish board of
malpractice from 1995–2008. Patient Saf Surg. 6:132012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boland CR and Goel A: Microsatellite
instability in colorectal cancer. Gastroenterology.
138:2073–2087.e3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lao VV and Grady WM: Epigenetics and
colorectal cancer. Nat Rev Gastroenterol Hepatol. 8:686–700. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pino MS and Chung DC: The chromosomal
instability pathway in colon cancer. Gastroenterology.
138:2059–2072. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naccarati A, Polakova V, Pardini B,
Vodickova L, Hemminki K, Kumar R and Vodicka P: Mutations and
polymorphisms in TP53 gene--an overview on the role in colorectal
cancer. Mutagenesis. 27:211–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie Q, Wu MY, Zhang DX, Yang YM, Wang BS,
Zhang J, Xu J, Zhong WD and Hu JN: Synergistic anticancer effect of
exogenous wild-type p53 gene combined with 5-FU in human colon
cancer resistant to 5-FU in vivo. World J Gastroenterol.
22:7342–7352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bykov VJN, Eriksson SE, Bianchi J and
Wiman KG: Targeting mutant p53 for efficient cancer therapy. Nat
Rev Cancer. 18:89–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Testa U, Pelosi E and Castelli G:
Colorectal cancer: Genetic abnormalities, tumor progression, tumor
heterogeneity, clonal evolution and tumor-initiating cells. Med Sci
(Basel). 6:1–113. 2018.
|
|
14
|
Wang S, Liu Z, Wang L and Zhang X:
NF-kappaB signaling pathway, inflammation and colorectal cancer.
Cell Mol Immunol. 6:327–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
PerŠe M: Oxidative Stress in the
Pathogenesis of Colorectal Cancer: Cause or Consequence? BioMed Res
Int. 7257102013.PubMed/NCBI
|
|
17
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative Stress: Harms and Benefits for Human Health. Oxid Med
Cell Longev. 2017:84167632017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cano CE, Gommeaux J, Pietri S, Culcasi M,
Garcia S, Seux M, Barelier S, Vasseur S, Spoto RP, Pébusque MJ, et
al: Tumor protein 53-induced nuclear protein 1 is a major mediator
of p53 antioxidant function. Cancer Res. 69:219–226. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Flöter J, Kaymak I and Schulze A:
Regulation of Metabolic Activity by p53. Metabolites. 7:1–18. 2017.
View Article : Google Scholar
|
|
20
|
Kalo E, Kogan-Sakin I, Solomon H,
Bar-Nathan E, Shay M, Shetzer Y, Dekel E, Goldfinger N, Buganim Y,
Stambolsky P, et al: Mutant p53R273H attenuates the expression of
phase 2 detoxifying enzymes and promotes the survival of cells with
high levels of reactive oxygen species. J Cell Sci. 125:5578–5586.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Zhang C and Feng Z: Tumor
suppressor p53 and its gain-of-function mutants in cancer. Acta
Biochim Biophys Sin (Shanghai). 46:170–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmed K, Tabuchi Y and Kondo T:
Hyperthermia: An effective strategy to induce apoptosis in cancer
cells. Apoptosis. 20:1411–1419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao K and Tait SWG: Apoptosis and Cancer:
Force Awakens, Phantom Menace, or Both? Int Rev Cell Mol Biol.
337:135–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Khattouti A, Selimovic D, Haikel Y and
Hassan M: Crosstalk between apoptosis and autophagy: Molecular
mechanisms and therapeutic strategies in cancer. J Cell Death.
6:37–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chaitanya GV, Steven AJ and Babu PP:
PARP-1 cleavage fragments: Signatures of cell-death proteases in
neurodegeneration. Cell Commun Signal. 8:312010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shiraishi H, Okamoto H, Yoshimura A and
Yoshida H: ER stress-induced apoptosis and caspase-12 activation
occurs downstream of mitochondrial apoptosis involving Apaf-1. J
Cell Sci. 119:3958–3966. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harding HP, Novoa I, Zhang Y, Zeng H, Wek
R, Schapira M and Ron D: Regulated translation initiation controls
stress-induced gene expression in mammalian cells. Mol Cell.
6:1099–1108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rao RV, Castro-Obregon S, Frankowski H,
Schuler M, Stoka V, del Rio G, Bredesen DE and Ellerby HM: Coupling
endoplasmic reticulum stress to the cell death program. An
Apaf-1-independent intrinsic pathway. J Biol Chem. 277:21836–21842.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abraha AM and Ketema EB: Apoptotic
pathways as a therapeutic target for colorectal cancer treatment.
World J Gastrointest Onco. 8:583–591. 2016. View Article : Google Scholar
|
|
32
|
Stoian M, State N, Stoica V and Radulian
G: Apoptosis in colorectal cancer. J Med Life. 7:160–164.
2014.PubMed/NCBI
|
|
33
|
Zhang B, Fang C, Deng D and Xia L:
Research progress on common adverse events caused by targeted
therapy for colorectal cancer. Oncol Lett. 16:27–33. 2018.(review).
PubMed/NCBI
|
|
34
|
Lee JH, Khor TO, Shu L, Su ZY, Fuentes F
and Kong AN: Dietary phytochemicals and cancer prevention: Nrf2
signaling, epigenetics, and cell death mechanisms in blocking
cancer initiation and progression. Pharmacol Ther. 137:153–171.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zaidi SF, Ahmed K, Saeed SA, Khan U and
Sugiyama T: Can diet modulate helicobacter pylori associated
gastric pathogenesis? An evidence-based analysis. Nutr Cancer.
69:979–989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nobili S, Lippi D, Witort E, Donnini M,
Bausi L, Mini E and Capaccioli S: Natural compounds for cancer
treatment and prevention. Pharmacol Res. 59:365–378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rejhová A, Opattová A, Čumová A, Slíva D
and Vodička P: Natural compounds and combination therapy in
colorectal cancer treatment. Eur J Med Chem. 144:582–594. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
González-Vallinas M, González-Castejón M,
Rodríguez-Casado A and Ramírez de Molina A: Dietary phytochemicals
in cancer prevention and therapy: A complementary approach with
promising perspectives. Nutr Rev. 71:585–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee KW, Bode AM and Dong Z: Molecular
targets of phytochemicals for cancer prevention. Nat Rev Cancer.
11:211–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS
and Giovannucci E: The mediterranean and dietary approaches to stop
hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr.
92:1429–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nomura AMY, Wilkens LR, Murphy SP, Hankin
JH, Henderson BE, Pike MC and Kolonel LN: Association of vegetable,
fruit, and grain intakes with colorectal cancer: The Multiethnic
Cohort Study. Am J Clin Nutr. 88:730–737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Duijnhoven FJ, Bueno-De-Mesquita HB,
Ferrari P, Jenab M, Boshuizen HC, Ros MM, Casagrande C, Tjønneland
A, Olsen A, Overvad K, et al: Fruit, vegetables, and colorectal
cancer risk: The European Prospective Investigation into Cancer and
Nutrition. Am J Clin Nutr. 89:1441–1452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gunasekaran S, Venkatachalam K and
Namasivayam N: p-Methoxycinnamic acid, an active phenylpropanoid
induces mitochondrial mediated apoptosis in HCT-116 human colon
adenocarcinoma cell line. Environ Toxicol Pharmacol. 40:966–974.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yaffe PB, Doucette CD, Walsh M and Hoskin
DW: Piperine impairs cell cycle progression and causes reactive
oxygen species-dependent apoptosis in rectal cancer cells. Exp Mol
Pathol. 94:109–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Banerjee K and Mandal M: Oxidative stress
triggered by naturally occurring flavone apigenin results in
senescence and chemotherapeutic effect in human colorectal cancer
cells. Redox Biol. 5:153–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Watson JL, Hill R, Yaffe PB, Greenshields
A, Walsh M, Lee PW, Giacomantonio CA and Hoskin DW: Curcumin causes
superoxide anion production and p53-independent apoptosis in human
colon cancer cells. Cancer Lett. 297:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Singh N, Shrivastav A and Sharma RK:
Curcumin induces caspase and calpain-dependent apoptosis in HT29
human colon cancer cells. Mol Med Rep. 2:627–631. 2009.PubMed/NCBI
|
|
49
|
Liu B, Yuan B, Zhang L, Mu W and Wang C:
ROS/p38/p53/Puma signaling pathway is involved in emodin-induced
apoptosis of human colorectal cancer cells. Int J Clin Exp Med.
8:15413–15422. 2015.PubMed/NCBI
|
|
50
|
Raja SB, Rajendiran V, Kasinathan NK, P A,
Venkatabalasubramanian S, Murali MR, Devaraj H and Devaraj SN:
Differential cytotoxic activity of Quercetin on colonic cancer
cells depends on ROS generation through COX-2 expression. Food Chem
Toxicol 106 (Pt A). 92–106. 2017. View Article : Google Scholar
|
|
51
|
Kwon O, Soung NK, Thimmegowda NR, Jeong
SJ, Jang JH, Moon DO, Chung JK, Lee KS, Kwon YT, Erikson RL, et al:
Patulin induces colorectal cancer cells apoptosis through EGR-1
dependent ATF3 up-regulation. Cell Signal. 24:943–950. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miki H, Uehara N, Kimura A, Sasaki T, Yuri
T, Yoshizawa K and Tsubura A: Resveratrol induces apoptosis via
ROS-triggered autophagy in human colon cancer cells. Int J Oncol.
40:1020–1028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han MH, Kim GY, Yoo YH and Choi YH:
Sanguinarine induces apoptosis in human colorectal cancer HCT-116
cells through ROS-mediated Egr-1 activation and mitochondrial
dysfunction. Toxicol Lett. 220:157–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li M, Song LH, Yue GG, Lee JKM, Zhao LM,
Li L, Zhou X, Tsui SK, Ng SS, Fung KP, et al: Bigelovin triggered
apoptosis in colorectal cancer in vitro and in vivo via
upregulating death receptor 5 and reactive oxidative species. Sci
Rep. 7:42176–42188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shang HS, Liu JY, Lu HF, Chiang HS, Lin
CH, Chen A, Lin YF, Chung JG, Ng SS, et al: Casticin induced
apoptotic cell death and altered associated gene expression in
human colon cancer colo 205 cells. Environ Toxicol. 32:2041–2052.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sithara T, Arun KB, Syama HP, Reshmitha TR
and Nisha P: Morin inhibits proliferation of sw480 colorectal
cancer cells by inducing apoptosis mediated by reactive oxygen
species formation and uncoupling of Warburg effect. Front
Pharmacol. 8:6402017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Khamphio M, Barusrux S and Weerapreeyakul
N: Sesamol induces mitochondrial apoptosis pathway in HCT116 human
colon cancer cells via pro-oxidant effect. Life Sci. 158:46–56.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Subramanian AP, Jaganathan SK, Mandal M,
Supriyanto E and Muhamad II: Gallic acid induced apoptotic events
in HCT-15 colon cancer cells. World J Gastroenterol. 22:3952–3961.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lim JH, Lee YM, Park SR, Kim DH and Lim
BO: Anticancer activity of hispidin via reactive oxygen
species-mediated apoptosis in colon cancer cells. Anticancer Res.
34:4087–4093. 2014.PubMed/NCBI
|
|
60
|
Waziri PM, Abdullah R, Yeap SK, Omar AR,
Kassim NK, Malami I, How CW, Etti IC and Abu ML: Clausenidin
induces caspase-dependent apoptosis in colon cancer. BMC Complement
Altern Med. 16:2562016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huang Z, Xu Y and Peng W: Colchicine
induces apoptosis in HT-29 human colon cancer cells via the AKT and
c-Jun N-terminal kinase signaling pathways. Mol Med Rep.
12:5939–5944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Santos LS, Silva VR, Menezes LRA, Soares
MBP, Costa EV and Bezerra DP: Xylopine induces oxidative stress and
causes G2/M phase arrest, triggering caspase-mediated apoptosis by
p53-independent pathway in HCT116 cells. Oxid Med Cell Longev.
2017:71268722017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sun G, Zheng Z, Lee MH, Xu Y, Kang S, Dong
Z, Wang M, Gu Z, Li H and Chen W: Chemoprevention of Colorectal
Cancer by Artocarpin, a Dietary Phytochemical from Artocarpus
heterophyllus. J Agric Food Chem. 65:3474–3480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kaur M, Velmurugan B, Tyagi A, Deep G,
Katiyar S, Agarwal C and Agarwal R: Silibinin suppresses growth and
induces apoptotic death of human colorectal carcinoma LoVo cells in
culture and tumor xenograft. Mol Cancer Ther. 8:2366–2374. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yaffe PB, Power Coombs MR, Doucette CD,
Walsh M and Hoskin DW: Piperine, an alkaloid from black pepper,
inhibits growth of human colon cancer cells via G1 arrest and
apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog.
54:1070–1085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang D, Zhang X, Zhang W and Rengarajan T:
Vicenin-2 inhibits Wnt/β-catenin signaling and induces apoptosis in
HT-29 human colon cancer cell line. Drug Des Devel Ther.
12:1303–1310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dasiram JD, Ganesan R, Kannan J,
Kotteeswaran V and Sivalingam N: Curcumin inhibits growth potential
by G1 cell cycle arrest and induces apoptosis in p53-mutated COLO
320DM human colon adenocarcinoma cells. Biomed Pharmacother.
86:373–380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Agarwal A, Kasinathan A, Ganesan R,
Balasubramanian A, Bhaskaran J, Suresh S, Srinivasan R, Aravind KB
and Sivalingam N: Curcumin induces apoptosis and cell cycle arrest
via the activation of reactive oxygen species-independent
mitochondrial apoptotic pathway in Smad4 and p53 mutated colon
adenocarcinoma HT29 cells. Nutr Res. 51:67–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu B, Zhou Z, Zhou W, Liu J, Zhang Q, Xia
J, Liu J, Chen N, Li M and Zhu R: Resveratrol inhibits
proliferation in human colorectal carcinoma cells by inducing
G1/S-phase cell cycle arrest and apoptosis through
caspase/cyclin-CDK pathways. Mol Med Rep. 10:1697–1702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Eldhose B, Gunawan M, Rahman M, Latha MS
and Notario V: Plumbagin reduces human colon cancer cell survival
by inducing cell cycle arrest and mitochondria-mediated apoptosis.
Int J Oncol. 45:1913–1920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kim HJ, Park JH and Kim JK:
Cucurbitacin-I, a natural cell-permeable triterpenoid isolated from
Cucurbitaceae, exerts potent anticancer effect in colon cancer.
Chem Biol Interact. 219:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Amin A, Bajbouj K, Koch A, Gandesiri M and
Schneider-Stock R: Defective autophagosome formation in p53-null
colorectal cancer reinforces crocin-induced apoptosis. Int J Mol
Sci. 16:1544–1561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li CY, Huang WF, Wang QL, Wang F, Cai E,
Hu B, Du JC, Wang J, Chen R, Cai XJ, et al: Crocetin induces
cytotoxicity in colon cancer cells via p53-independent mechanisms.
Asian Pac J Cancer Prev. 13:3757–3761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
74. Lee YJ, Kang YR, Lee SY, Jin Y, Han DC
and Kwon BM: Ginkgetin induces G2-phase arrest in HCT116 colon
cancer cells through the modulation of b-Myb and miRNA34a
expression. Int J Oncol. 51:1331–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Radhakrishnan EK, Bava SV, Narayanan SS,
Nath LR, Thulasidasan AKT, Soniya EV and Anto RJ: [6]-Gingerol
induces caspase-dependent apoptosis and prevents PMA-induced
proliferation in colon cancer cells by inhibiting MAPK/AP-1
signaling. PLoS One. 9:e1044012014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang L, Liu Y, Wang M, Qian Y, Dong X, Gu
H, Wang H, Guo S and Hisamitsu T: Quercetin-induced apoptosis of
HT-29 colon cancer cells via inhibition of the Akt-CSN6-Myc
signaling axis. Mol Med Rep. 14:4559–4566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cho HJ and Park JHY: Kaempferol induces
cell cycle arrest in HT-29 human colon cancer cells. J Cancer Prev.
18:257–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
López de Las Hazas MC, Piñol C, Macià A
and Motilva MJ: Hydroxytyrosol and the colonic metabolites derived
from virgin olive oil intake induce cell cycle arrest and apoptosis
in colon cancer cells. J Agric Food Chem. 65:6467–6476. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lee SH and Clark R: Anti-Tumorigenic
Effects of Capsaicin in Colon Cancer. J Food Chem Nanotechnol.
2:162–167. 2016. View Article : Google Scholar
|
|
80
|
Jin J, Lin G, Huang H, Xu D, Yu H, Ma X,
Zhu L, Ma D and Jiang H: Capsaicin mediates cell cycle arrest and
apoptosis in human colon cancer cells via stabilizing and
activating p53. Int J Biol Sci. 10:285–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chidambara Murthy KN, Jayaprakasha GK and
Patil BS: The natural alkaloid berberine targets multiple pathways
to induce cell death in cultured human colon cancer cells. Eur J
Pharmacol. 688:14–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xu LN, Lu BN, Hu MM, Xu YW, Han X, Qi Y
and Peng JY: Mechanisms involved in the cytotoxic effects of
berberine on human colon cancer HCT-8 cells. Biocell. 36:113–120.
2012.PubMed/NCBI
|
|
83
|
Cai Y, Xia Q, Luo R, Huang P, Sun Y, Shi Y
and Jiang W: Berberine inhibits the growth of human colorectal
adenocarcinoma in vitro and in vivo. J Nat Med. 68:53–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu J, Li Q, Liu Z, Lin L, Zhang X, Cao M
and Jiang J: Harmine induces cell cycle arrest and mitochondrial
pathway-mediated cellular apoptosis in SW620 cells via inhibition
of the Akt and ERK signaling pathways. Oncol Rep. 35:3363–3370.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tailor D and Singh RP: Dietary and
non-dietary phytochemicals in cancer control. Nutrition, Diet and
Cancer. Shankar S and Shrivastava RK: Springer. (New York).
585–622. 2012. View Article : Google Scholar
|
|
86
|
Milani A, Basirnejad M, Shahbazi S and
Bolhassani A: Carotenoids: Biochemistry, pharmacology and
treatment. Br J Pharmacol. 174:1290–1324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Gutheil WG, Reed G, Ray A, Anant S and
Dhar A: Crocetin: An agent derived from saffron for prevention and
therapy for cancer. Curr Pharm Biotechnol. 13:173–179. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ray P, Guha D, Chakraborty J, Banerjee S,
Adhikary A, Chakraborty S, Das T and Sa G: Crocetin exploits
p53-induced death domain (PIDD) and FAS-associated death domain
(FADD) proteins to induce apoptosis in colorectal cancer. Sci Rep.
6:32979–32989. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chakraborty D, Bishayee K, Ghosh S, Biswas
R, Mandal SK and Khuda-Bukhsh AR: [6]-Gingerol induces caspase 3
dependent apoptosis and autophagy in cancer cells: drug-DNA
interaction and expression of certain signal genes in HeLa cells.
Eur J Pharmacol. 694:20–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ju SA, Park SM, Lee YS, Bae JH, Yu R, An
WG, Suh JH and Kim BS: Administration of 6-gingerol greatly
enhances the number of tumor-infiltrating lymphocytes in murine
tumors. Int J Cancer. 130:2618–2628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5:e472016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kim HJ, Kim SK, Kim BS, Lee SH, Park YS,
Park BK, Kim SJ, Kim J, Choi C, Kim JS, et al: Apoptotic effect of
quercetin on HT-29 colon cancer cells via the AMPK signaling
pathway. J Agric Food Chem. 58:8643–8650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Refolo MG, DAlessandro R, Malerba N,
Laezza C, Bifulco M, Messa C, Caruso MG, Notarnicola M and Tutino
V: Anti-proliferative and pro apoptotic effects of flavonoid
quercetin are mediated by CB1 receptor in human colon cancer cell
lines. J Cell Physiol. 230:2973–2980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhang XA, Zhang S, Yin Q and Zhang J:
Quercetin induces human colon cancer cells apoptosis by inhibiting
the nuclear factor-kappa B Pathway. Pharmacogn Mag. 11:404–409.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Liu Y, Lang T, Jin B, Chen F, Zhang Y,
Beuerman RW, Zhou L and Zhang Z: Luteolin inhibits colorectal
cancer cell epithelial-to-mesenchymal transition by suppressing
CREB1 expression revealed by comparative proteomics study. J
Proteomics. 161:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kang KA, Piao MJ, Ryu YS, Hyun YJ, Park
JE, Shilnikova K, Zhen AX, Kang HK, Koh YS, Jeong YJ, et al:
Luteolin induces apoptotic cell death via antioxidant activity in
human colon cancer cells. Int J Oncol. 51:1169–1178. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang N, Zhao Y, Wang Z, Liu Y and Zhang Y:
Scutellarin suppresses growth and causes apoptosis of human
colorectal cancer cells by regulating the p53 pathway. Mol Med Rep.
15:929–935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lee JH, Choi YJ, Park SH and Nam MJ:
Potential role of nucleoside diphosphate kinase in
myricetin-induced selective apoptosis in colon cancer HCT-15 cells.
Food Chem Toxicol 116 (Pt B). 315–322. 2018. View Article : Google Scholar
|
|
99
|
Seydi E, Rasekh HR, Salimi A, Mohsenifar Z
and Pourahmad J: Myricetin selectively induces apoptosis on
cancerous hepatocytes by directly targeting their mitochondria.
Basic Clin Pharmacol Toxicol. 119:249–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Phillips PA, Sangwan V, Borja-Cacho D,
Dudeja V, Vickers SM and Saluja AK: Myricetin induces pancreatic
cancer cell death via the induction of apoptosis and inhibition of
the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer
Lett. 308:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xu M, Wang S, Song YU, Yao J, Huang K and
Zhu X: Apigenin suppresses colorectal cancer cell proliferation,
migration and invasion via inhibition of the Wnt/β-catenin
signaling pathway. Oncol Lett. 11:3075–3080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhou Z, Tang M, Liu Y, Zhang Z, Lu R and
Lu J: Apigenin inhibits cell proliferation, migration, and invasion
by targeting Akt in the A549 human lung cancer cell line.
Anticancer Drugs. 28:446–456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Liu X, Li L, Lv L, Chen D, Shen L and Xie
Z: Apigenin inhibits the proliferation and invasion of osteosarcoma
cells by suppressing the Wnt/β-catenin signaling pathway. Oncol
Rep. 34:1035–1041. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Maeda Y, Takahashi H, Nakai N, Yanagita T,
Ando N, Okubo T, Saito K, Shiga K, Hirokawa T, Hara M, et al:
Apigenin induces apoptosis by suppressing Bcl-xl and Mcl-1
simultaneously via signal transducer and activator of transcription
3 signaling in colon cancer. Int J Oncol. 52:1661–1673. 2018.
|
|
105
|
Bobe G, Sansbury LB, Albert PS, Cross AJ,
Kahle L, Ashby J, Slattery ML, Caan B, Paskett E, Iber F, et al:
Dietary flavonoids and colorectal adenoma recurrence in the Polyp
Prevention Trial. Cancer Epidemiol Biomarkers Prev. 17:1344–1353.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lee HS, Cho HJ, Yu R, Lee KW, Chun HS and
Park JHY: Mechanisms underlying apoptosis-inducing effects of
Kaempferol in HT-29 human colon cancer cells. Int J Mol Sci.
15:2722–2737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tong W, Wang Q, Sun D and Suo J: Curcumin
suppresses colon cancer cell invasion via AMPK-induced inhibition
of NF-κB, uPA activator and MMP9. Oncol Lett. 12:4139–4146. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yang X, Li Z, Wu Q, Chen S, Yi C and Gong
C: TRAIL and curcumin codelivery nanoparticles enhance
TRAIL-induced apoptosis through upregulation of death receptors.
Drug Deliv. 24:1526–1536. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Shakibaei M, Kraehe P, Popper B, Shayan P,
Goel A and Buhrmann C: Curcumin potentiates antitumor activity of
5-fluorouracil in a 3D alginate tumor microenvironment of
colorectal cancer. BMC Cancer. 15:2502015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Shakibaei M, Mobasheri A, Lueders C, Busch
F, Shayan P and Goel A: Curcumin enhances the effect of
chemotherapy against colorectal cancer cells by inhibition of NF-κB
and Src protein kinase signaling pathways. PLoS One. 8:e572182013.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Liu Z, Ren B, Wang Y, Zou C, Qiao Q, Diao
Z, Mi Y, Zhu D and Liu X: Sesamol induces human hepatocellular
carcinoma cells apoptosis by impairing mitochondrial function and
suppressing autophagy. Sci Rep. 7:457282017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Bhardwaj R, Sanyal SN, Vaiphei K, Kakkar
V, Deol PK, Kaur IP and Kaur T: Sesamol induces apoptosis by
altering expression of bcl-2 and bax proteins and modifies skin
tumor development in Balb/c mice. Anticancer Agents Med Chem.
17:726–733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Daglia M, Di Lorenzo A, Nabavi SF, Talas
ZS and Nabavi SM: Polyphenols: well beyond the antioxidant
capacity: gallic acid and related compounds as neuroprotective
agents: you are what you eat! Curr Pharm Biotechnol. 15:362–372.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yang C, Xie X, Tang H, Dong X, Zhang X and
Huang F: Transcriptome analysis reveals GA induced apoptosis in
HCT116 human colon cancer cells through calcium and p53 signal
pathways. RSC Advances. 8:12449–12458. 2018. View Article : Google Scholar
|
|
115
|
Rubió L, Macià A, Valls RM, Pedret A,
Romero MP, Solà R and Motilva MJ: A new hydroxytyrosol metabolite
identified in human plasma: Hydroxytyrosol acetate sulphate. Food
Chem. 134:1132–1136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
de Las Hazas MCL, Motilva MJ, Piñol C and
Macià A: Application of dried blood spot cards to determine olive
oil phenols (hydroxytyrosol metabolites) in human blood. Talanta.
159:189–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Mosele JI, Martín-Peláez S, Macià A,
Farràs M, Valls RM, Catalán Ú and Motilva MJ: Faecal microbial
metabolism of olive oil phenolic compounds: In vitro and in vivo
approaches. Mol Nutr Food Res. 58:1809–1819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sun L, Luo C and Liu J: Hydroxytyrosol
induces apoptosis in human colon cancer cells through ROS
generation. Food Funct. 5:1909–1914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bertelli AA, Ferrara F, Diana G, Fulgenzi
A, Corsi M, Ponti W, Ferrero ME and Bertelli A: Resveratrol, a
natural stilbene in grapes and wine, enhances intraphagocytosis in
human promonocytes: A co-factor in antiinflammatory and anticancer
chemopreventive activity. Int J Tissue React. 21:93–104.
1999.PubMed/NCBI
|
|
120
|
Buhrmann C, Shayan P, Popper B, Goel A and
Shakibaei M: Sirt1 is required for resveratrolmediated
chemopreventive effects in colorectal cancer cells. Nutrients.
8:1452016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chen H, Jin ZL and Xu H: MEK/ERK signaling
pathway in apoptosis of SW620 cell line and inhibition effect of
resveratrol. Asian Pac J Trop Med. 9:49–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yuan SX, Wang DX, Wu QX, Ren CM, Li Y,
Chen QZ, Zeng YH, Shao Y, Yang JQ, Bai Y, et al: BMP9/p38 MAPK is
essential for the antiproliferative effect of resveratrol on human
colon cancer. Oncol Rep. 35:939–947. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Saud SM, Li W, Morris NL, Matter MS,
Colburn NH, Kim YS and Young MR: Resveratrol prevents tumorigenesis
in mouse model of Kras activated sporadic colorectal cancer by
suppressing oncogenic Kras expression. Carcinogenesis.
35:2778–2786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
De Maria S, Scognamiglio I, Lombardi A,
Amodio N, Caraglia M, Cartenì M, Ravagnan G and Stiuso P:
Polydatin, a natural precursor of resveratrol, induces cell cycle
arrest and differentiation of human colorectal Caco-2 cell. J
Transl Med. 11:2642013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kumazaki M, Noguchi S, Yasui Y, Iwasaki J,
Shinohara H, Yamada N and Akao Y: Anti-cancer effects of naturally
occurring compounds through modulation of signal transduction and
miRNA expression in human colon cancer cells. J Nutr Biochem.
24:1849–1858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Reddivari L, Charepalli V, Radhakrishnan
S, Vadde R, Elias RJ, Lambert JD and Vanamala JKP: Grape compounds
suppress colon cancer stem cells in vitro and in a rodent model of
colon carcinogenesis. BMC Complement Altern Med. 16:2782016.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sivakumar G: Colchicine semisynthetics:
Chemotherapeutics for cancer? Curr Med Chem. 20:892–898. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Risinger AL, Giles FJ and Mooberry SL:
Microtubule dynamics as a target in oncology. Cancer Treat Rev.
35:255–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lee SH, Richardson RL, Dashwood RH and
Baek SJ: Capsaicin represses transcriptional activity of β-catenin
in human colorectal cancer cells. J Nutr Biochem. 23:646–655. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Guamán Ortiz LM, Tillhon M, Parks M, Dutto
I, Prosperi E, Savio M, Arcamone AG, Buzzetti F, Lombardi P and
Scovassi AI: Multiple effects of berberine derivatives on colon
cancer cells. BioMed Res Int. 2014:9245852014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wang Y, Liu Q, Liu Z, Li B, Sun Z, Zhou H,
Zhang X, Gong Y and Shao C: Berberine, a genotoxic alkaloid,
induces ATM-Chk1 mediated G2 arrest in prostate cancer cells. Mutat
Res. 734:20–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Li J, Gu L, Zhang H, Liu T, Tian D, Zhou M
and Zhou S: Berberine represses DAXX gene transcription and induces
cancer cell apoptosis. Lab Invest. 93:354–364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Yan K, Zhang C, Feng J, Hou L, Yan L, Zhou
Z, Liu Z, Liu C, Fan Y, Zheng B, et al: Induction of G1 cell cycle
arrest and apoptosis by berberine in bladder cancer cells. Eur J
Pharmacol. 661:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Tillhon M, Guamán Ortiz LM, Lombardi P and
Scovassi AI: Berberine: New perspectives for old remedies. Biochem
Pharmacol. 84:1260–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wang L, Liu L, Shi Y, Cao H, Chaturvedi R,
Calcutt MW, Hu T, Ren X, Wilson KT, Polk DB, et al: Berberine
induces caspase-independent cell death in colon tumor cells through
activation of apoptosis-inducing factor. PLoS One. 7:e364182012.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Xu LN, Lu BN, Hu MM, Xu YW, Han X, Qi Y
and Peng JY: Mechanisms involved in the cytotoxic effects of
berberine on human colon cancer HCT-8 cells. Biocell. 36:113–20.
2012.PubMed/NCBI
|
|
137
|
Patel K, Gadewar M, Tripathi R, Prasad SK
and Patel DK: A review on medicinal importance, pharmacological
activity and bioanalytical aspects of beta-carboline alkaloid
‘Harmine’. Asian Pac J Trop Biomed. 2:660–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Nguyen AV, Martinez M, Stamos MJ, Moyer
MP, Planutis K, Hope C and Holcombe RF: Results of a phase I pilot
clinical trial examining the effect of plant-derived resveratrol
and grape powder on Wnt pathway target gene expression in colonic
mucosa and colon cancer. Cancer Manag Res. 1:25–37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Patel KR, Brown VA, Jones DJ, Britton RG,
Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA,
et al: Clinical pharmacology of resveratrol and its metabolites in
colorectal cancer patients. Cancer Res. 70:7392–7399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Ferguson LR and Philpott M: Cancer
prevention by dietary bioactive components that target the immune
response. Curr Cancer Drug Targets. 7:459–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Camuesco D, Comalada M, Rodríguez-Cabezas
ME, Nieto A, Lorente MD, Concha A, Zarzuelo A and Gálvez J: The
intestinal anti-inflammatory effect of quercitrin is associated
with an inhibition in iNOS expression. Br J Pharmacol. 143:908–918.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Kwon KH, Murakami A, Tanaka T and Ohigashi
H: Dietary rutin, but not its aglycone quercetin, ameliorates
dextran sulfate sodium-induced experimental colitis in mice:
Attenuation of pro-inflammatory gene expression. Biochem Pharmacol.
69:395–406. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Camuesco D, Comalada M, Concha A, Nieto A,
Sierra S, Xaus J, Zarzuelo A and Gálvez J: Intestinal
anti-inflammatory activity of combined quercitrin and dietary olive
oil supplemented with fish oil, rich in EPA and DHA (n-3)
polyunsaturated fatty acids, in rats with DSS-induced colitis. Clin
Nutr. 25:466–476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Kim S, Keku TO, Martin C, Galanko J,
Woosley JT, Schroeder JC, Satia JA, Halabi S and Sandler RS:
Circulating levels of inflammatory cytokines and risk of colorectal
adenomas. Cancer Res. 68:323–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Bobe G, Albert PS, Sansbury LB, Lanza E,
Schatzkin A, Colburn NH and Cross AJ: Interleukin-6 as a potential
indicator for prevention of high-risk adenoma recurrence by dietary
flavonols in the polyp prevention trial. Cancer Prev Res (Phila).
3:764–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Carroll RE, Benya RV, Turgeon DK, Vareed
S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C,
Meyskens FL Jr, et al: Phase IIa clinical trial of curcumin for the
prevention of colorectal neoplasia. Cancer Prev Res (Phila).
4:354–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
He ZY, Shi CB, Wen H, Li FL, Wang BL and
Wang J: Upregulation of p53 expression in patients with colorectal
cancer by administration of curcumin. Cancer Invest. 29:208–213.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Chakraborty S and Rahman T: The
difficulties in cancer treatment. Ecancermedicalscience.
6:ed162012.PubMed/NCBI
|