Introduction
A gynecological tumor is a type of malignant tumor
that occurs in the female reproductive system and seriously
threatens the life of the patient. Among the types of gynecological
tumor, cervical cancer (CC), endometrial carcinoma (EC) and vulvar
carcinoma (VC) are the top three most common tumors of the female
genital system, besides ovarian cancer (1). Despite an overall decline in the
incidence and mortality rates due to increased understanding of the
disease, gynecological cancer remains a significant health care
burden worldwide (1). Early
detection and treatment are essential for improving patient
outcomes; however, these require improved understanding of the
molecular pathology of the disease, in addition to identification
of appropriate biomarkers and drug targets. Previous studies have
demonstrated that the occurrence of CC is closely associated with
human papillomavirus (HPV) infection (2–4). VC can
be separated into two types, including one type that more
frequently occurs in young females. This type involves the
progression of a vulvar intraepithelial neoplasia caused by HPV
infection, particularly HPV 16 and 18 (5). Based on pathogenetic perspectives, EC
is also classified into two groups according to estrogen dependence
(6). Although there have been a
number of previous etiology studies, the exact pathogenesis of
these three types of cancer remains unclear.
There are certain pathological and etiological
associations between CC and VC, as both are squamous cell cancers
and both are associated with HPV infection (2,5). Unlike
CC and VC, EC is associated with sex hormones, which is similar to
common invasive tumors in females, including breast and ovarian
cancer (6). In addition, clinical
diagnoses of these three cancer types rely predominantly on
pathology (7). Precise biomarkers in
early stages of CC, EC and VC remain unknown.
It is understood that cervical, endometrial and
vulvar tissues all originate from the same embryological origin,
the paramesonephric ducts, which give rise to the whole female
reproductive tract and develop into different organs, following
complex regulatory process (8). For
this reason, although there a number of differences between CC, EC
and VC, it has been hypothesized that these three types of
gynecological tumor share a similar mechanism and certain specific
marker molecules may be common to their tumorigenesis and
development. Therefore, a comprehensive analysis may improve
understanding of these three types of tumor.
Advancements in biotechnology have improved the
availability of high-throughput data, including genomic, proteomic
and metabolomics data, which supports in-depth scientific research.
High-throughput data can assist with effective early diagnosis,
prognosis prediction and investigations of molecular mechanisms for
numerous types of disease. The present study used GSE63678
microarray data downloaded from the Gene Expression Omnibus (GEO)
to determine the differentially expressed genes (DEGs, which were
identified between cancerous samples and non-cancerous samples) of
CC, EC and VC (9). Subsequently,
functional enrichment analyses were performed, including gene
ontology (GO) and pathway analysis, and a protein-protein
interaction (PPI) network was generated to identify the significant
biological terms associated with the DEGs. The genes that were
screened out by the PPI network were considered as the hub genes,
which may serve important roles in the mechanism of CC, EC and VC.
In addition, a gene-pathway network was constructed and further
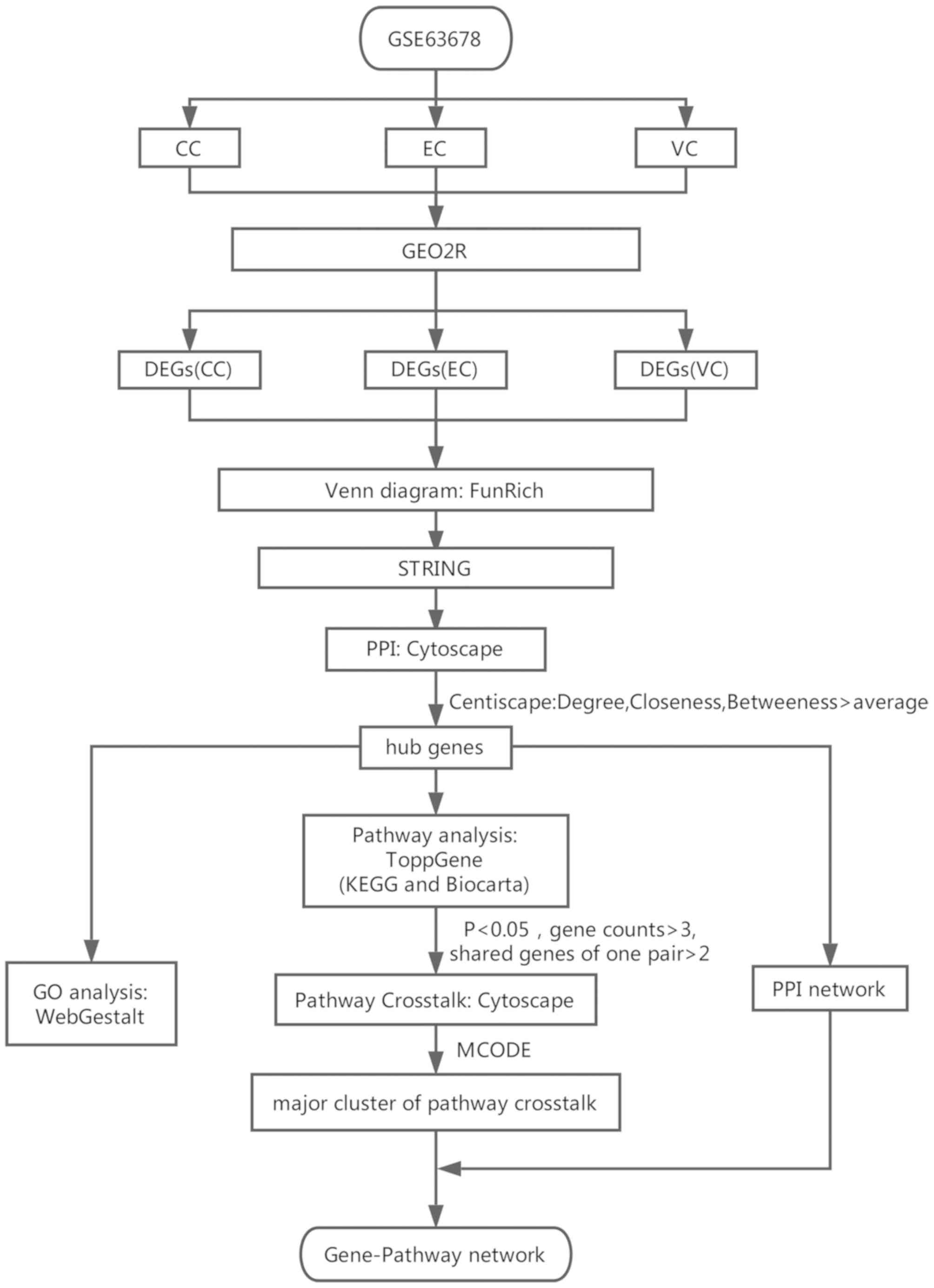
analysis was performed. The complete flowchart of the present study
is presented in Fig. 1. In summary,
the current study may provide a new perspective for elucidating the
biological significance of three types of gynecological cancer, and
assist with the identification of potential candidate biomarkers
for diagnosis, prognosis and therapy.
Materials and methods
Microarray data
The gene expression profile GSE63678 on the platform
of the GPL571 Affymetrix Human Genome U133A 2.0 Array was
downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). GSE63678 is a
dataset submitted by Pappa et al (9), containing 18 cancer samples, including
five cervical, seven endometrial and six vulvar samples, and 17
normal samples, including, five cervical, five endometrial and
seven vulvar samples.
Identification of DEGs
GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r) is an
interactive network analysis tool of the GEO database based on R,
in which two sets of samples can be compared under the same
experimental conditions (10).
Associated gene data were divided into CC, EC and VC groups.
Subsequently, the GEO2R (10) tool
and limma package (11) available
through Bioconductor (version 3.8) of R Studio (version 3.5) were
used to compare the gene expression of the CC, EC and VC groups.
P<0.05 and a fold-change >2 were considered to indicate a
DEG. The distribution of the DEGs in the three tumor types was
presented as a Venn diagram using FunRich software (version 3.0)
(12).
Construction of the PPI network and
identification of hub genes
Search Tool for the Retrieval of Interacting Genes
(http://string-db.org/; version 10.5) is a
software system that is commonly used to search for known proteins
and predict interactions (13). The
experimentally validated interactions with a combined score >0.7
were selected as significant and DEGs with a connection number
<2 were removed. The PPI network was visualized using Cytoscape
(https://cytoscape.org/; version 3.6.0). The nodes
with degree, closeness and betweenness scores higher than the mean,
as calculated by the Cytoscape plugin Centiscape, were considered
hub nodes.
GO analysis of the hub genes
WEB-based Gene SeT AnaLysis Toolkit (http://www.webgestalt.org/; revision 2017) is a
popular software tool for functional enrichment analysis, which
covers seven biological contexts, including GO (14). Therefore, this software was used in
the present study for GO enrichment analysis. The false discovery
rate (FDR) was set at <0.05 to conduct the GO analysis of the
DEGs.
Pathway enrichment analysis of the hub
genes
The hub genes were uploaded to ToppGene (https://toppgene.cchmc.org/,) for pathway enrichment
analysis. The two frequently used databases, Kyoto Encyclopedia of
Gene and Genomes (KEGG; www.genome.jp/kegg) and Biocarta (www.biocarta.com), were used to perform this analysis
(15). The FDR was set at
<0.05.
Pathway crosstalk analysis
The enriched pathways were recruited for further
crosstalk analysis to investigate the associations between them. As
described previously (15), to
measure the association between two pathways, Jaccard coefficient
(JC)=A∩B/A∪B and overlap coefficient (OC)=A∩B/min(|A|,|B|) were
adopted, where A and B are the gene items contained in the two
pathways, min is the minimum, ∩ is the intersection of A and B, and
∪ is the union of A and B. Since limited biological information was
available, pathways containing <3 genes were excluded.
Similarly, the pathway pairs with <2 overlapping genes were
removed. Subsequently, the pathway network was presented with
Cytoscape according to the JC and OC value of each selected pair
(16), and the MCODE plug-in
(17) (version 1.4.2; apps.cytoscape.org/apps/MCODE) for
Cytoscape was used to find clusters and highly interconnected
regions in any network was used to analyze the clusters.
Gene-pathway network analysis
To further investigate the developmental mechanisms
of CC, EC and VC, the hub genes were mapped into a crosstalk
network. By analyzing the interactions between the genes and
pathways with KEGG and Biocarta, the connected nodes were linked
with arrows. The gene-pathway network was constructed and
visualized in Cytoscape. The degree was calculated and nodes with a
degree greater than the mean degree of all nodes were selected to
constitute a sub network.
Results
Identification of DEGs
Following screening with the criteria of P<0.05
and fold-change >2, a total of 1,219 DEGs were identified. In
the CC group 138 DEGs were revealed, including 87 upregulated
genes. In addition, 479 DEGs were identified in the EC group,
including 272 upregulated genes. Finally, 734 DEGs, including 172
upregulated genes, were revealed in the VC group. As demonstrated
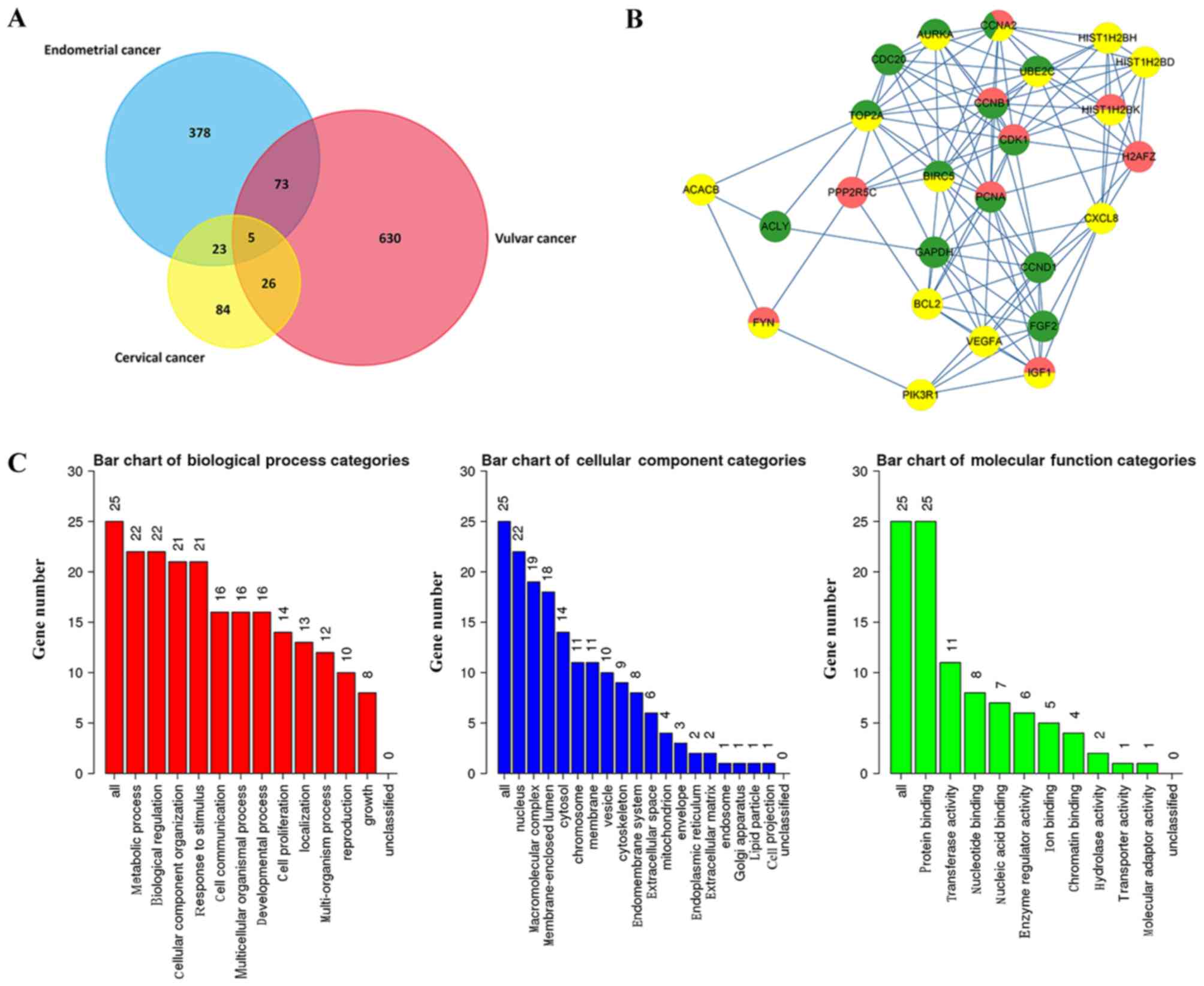
in Fig. 2A, 84, 378 and 630 DEGs
were exclusively identified in CC, EC and VC groups, respectively.
However, 23 DEGs were present in both the CC and EC group, 73 DEGs
were identified in both the EC and VC groups, and 26 DEGs were
revealed in both the CC and VC groups. Furthermore, five mutual
genes, including signal sequence receptor subunit 1 (SSR1), flap
structure-specific endonuclease 1 (FEN1), cyclin A2 (CCNA2), signal
transducer and activator of transcription 1 (STAT1) and C-X-C motif
chemokine ligand 12 (CXCL12), were identified in all three
groups.
Hub genes and PPI network
Following calculation by Centiscape, the mean values
of degree, closeness and betweenness were 12.64080, 3.73×10-4 and
2081.81034, respectively. Additionally, 25 hub genes were
identified, including six downregulated genes and 19 upregulated
genes (Table I). Three histone
cluster family members were revealed as hub genes, including
histone H2B type 1-H (HIST1H2BH), histone cluster 1 H2B family
member D (HIST1H2BD) and histone cluster 1 H2B family member K
(HIST1H2BK), and the five hub genes were cell cycle regulatory
proteins, including CCNA2, cyclin B1 (CCNB1), cyclin D1 (CCND1),
aurora kinase A (AURKA) and cell division cycle 20 (CDC20).
Furthermore certain genes associated with tumor progression were
identified, including vascular endothelial growth factor A (VEGFA),
FYN proto-oncogene, Src family tyrosine kinase (FYN), baculoviral
IAP repeat containing 5 (BIRC5) and the apoptosis regulator B-cell
lymphoma 2 (BCL2).
 | Table I.Topological parameters of the hub
genes. |
Table I.
Topological parameters of the hub
genes.
| Gene | Degree | Betweeness | Closeness | Group | Regulation |
|---|
| Mean | 13 | 2081.81034 |
3.73×10−4 | – | – |
| CDK1 | 115 | 20448.4838 |
5.21×10−4 | CC/EC | Up |
| CCNB1 | 100 | 16335.9142 |
5.23×10−4 | CC/EC | Up |
| CDC20 | 92 | 8148.54809 |
4.76×10−4 | EC | Up |
| CCNA2 | 91 | 8126.90458 |
4.89×10−4 | CC/EC/VC | Up |
| AURKA | 90 | 9181.94344 |
4.76×10−4 | EC/VC | Up |
| TOP2A | 87 | 21409.6376 |
5.15×10−4 | EC/VC | Up |
| UBE2C | 82 | 14916.6491 |
4.82×10−4 | EC/VC | Up |
| BIRC5 | 80 | 16279.0157 |
5.13×10−4 | EC/VC | Up |
| PCNA | 62 | 26080.2036 |
5.25×10−4 | CC/EC | Up |
| VEGFA | 56 | 32712.0335 |
5.43×10−4 | VC | Up |
| PIK3R1 | 46 | 24576.8505 |
4.91×10−4 | VC | Down |
| HIST1H2BK | 43 | 8689.20717 |
4.70×10−4 | CC/VC | Up |
| HIST1H2BD | 43 | 8689.20717 |
4.70×10−4 | VC | Up |
| HIST1H2BH | 42 | 8291.98846 |
4.70×10−4 | VC | Up |
| ACACB | 41 | 40853.547 |
4.98×10−4 | VC | Down |
| CXCL8 | 40 | 28927.396 |
5.16×10−4 | VC | Up |
| H2AFZ | 39 | 8793.56814 |
4.67×10−4 | CC | Up |
| IGF1 | 38 | 16278.2011 |
5.07×10−4 | CC/VC | Down |
| ACLY | 37 | 34157.4527 |
4.81×10−4 | EC | Up |
| CCND1 | 36 | 28548.907 |
5.39×10−4 | EC | Up |
| GAPDH | 33 | 41598.1258 |
5.39×10−4 | EC | Up |
| PPP2R5C | 32 | 10238.8057 |
4.75×10−4 | CC | UP |
| FYN | 32 | 17254.3572 |
5.22×10−4 | CC/VC | Down |
| FGF2 | 32 | 19465.3169 |
5.20×10−4 | EC | Down |
| BCL2 | 32 | 17923.6004 |
4.86×10−4 | VC | Down |
The PPI network of the 25 hub genes with 25 nodes
and 114 edges is presented in Fig.
2B. The top five genes with the highest degrees were CDK1,
CCNB1, CDC20, CCNA2 and AURKA. All five of these genes are
associated with cell cycle regulation, which indicates that cell
cycle dysfunction serves an important role in the development of
gynecological tumors.
GO enrichment analysis
A total of 25 DEGs were used to perform GO
enrichment analysis (Fig. 2C). For
cellular component terms, 22 out of the 25 genes were revealed to
be located in the ‘nucleus’ and approximately 80% were identified
to participate in the ‘macromolecular complex’ (19 genes) and
‘membrane-enclosed lumen’ (18 genes). In the biological process
category, the DEGs were associated with ‘biological regulation’ (22
genes), ‘metabolic process’ (22 genes), ‘cellular component
organization’ (21 genes) and ‘response to stimulus’ (21 genes). In
the molecular function category all 25 DEGs were associated with
‘protein binding’ (25 genes).
Pathway enrichment analysis of the hub
genes
By uploading the 25 genes into ToppGene, 86
significant pathways were identified. The biological processes
involved in these pathways can be divided into the following five
main categories: i) viral infections and cancer formation,
including ‘viral carcinogenesis’ and ‘hepatitis B’, ii)
tumorigenesis and development, including ‘colorectal cancer’ and
‘proteoglycans in cancer’, iii) signal transduction, including
‘PI3K-Akt signaling pathway’ and ‘AMPK signaling pathway’, iv)
endocrinology and metabolism, including ‘AGE-RAGE signaling pathway
in diabetic complications’ and ‘endocrine resistance’, and v)
others, including ‘genes encoding secreted soluble factors’ and
‘NFAT and hypertrophy of the heart (transcription in the broken
heart)’. In addition, 19 pathways were identified to be
downregulated and 17 pathways were revealed to be upregulated
(Table II).
 | Table II.Pathways enriched in three types of
gynecological cancer. |
Table II.
Pathways enriched in three types of
gynecological cancer.
| Pathway | Regulation | P-value | Genes in the
pathway |
|---|
| Viral
carcinogenesis | – |
3.43×10−9 | HIST1H2BD, CCND1,
CDK1, HIST1H2BH, CDC20, PIK3R1, HIST1H2BK, CCNA2 |
| Hepatitis B | – |
9.66×10−9 | BIRC5, CCND1, BCL2,
PIK3R1, PCNA, CXCL8, CCNA2 |
| AMPK signaling
pathway | – |
1.13×10−7 | CCND1, PPP2R5C,
IGF1, ACACB, PIK3R1, CCNA2 |
| Oocyte meiosis | – |
1.31×10−7 | AURKA, PPP2R5C,
IGF1, CDK1, CDC20, CCNB1 |
| Cell cycle | Up |
1.31×10−7 | CCND1, CDK1, CDC20,
PCNA, CCNA2, CCNB1 |
| EGFR tyrosine
kinase inhibitor resistance | – |
4.35×10−7 | FGF2, BCL2, IGF1,
PIK3R1, VEGFA |
| Pathways in
cancer | – |
6.44×10−7 | FGF2, BIRC5, CCND1,
BCL2, IGF1, PIK3R1, CXCL8, VEGFA |
|
Progesterone-mediated oocyte
maturation | – |
1.15×10−6 | IGF1, CDK1, PIK3R1,
CCNA2, CCNB1 |
| AGE-RAGE signaling
pathway in diabetic complications | – |
1.35×10−6 | CCND1, BCL2,
PIK3R1, CXCL8, VEGFA |
| HIF-1 signaling
pathway | – |
1.49×10−6 | BCL2, IGF1, GAPDH,
PIK3R1, VEGFA |
| Focal adhesion | – |
2.13×10−6 | CCND1, BCL2, IGF1,
FYN, PIK3R1, VEGFA |
| PI3K-Akt signaling
pathway | – |
3.48×10−6 | FGF2, CCND1, BCL2,
PPP2R5C, IGF1, PIK3R1, VEGFA |
| p53 Signaling
Pathway | – |
3.94×10−6 | CCND1, BCL2,
PCNA |
| IL-7 Signal
Transduction | Down |
4.77×10−6 | BCL2, FYN,
PIK3R1 |
| Colorectal
cancer | – |
5.72×10−6 | BIRC5, CCND1, BCL2,
PIK3R1 |
| p53 signaling
pathway | – |
1.00×10−5 | CCND1, IGF1, CDK1,
CCNB1 |
| Melanoma | – |
1.00×10−5 | FGF2, CCND1, IGF1,
PIK3R1 |
| Cyclins and Cell
Cycle Regulation | Up |
1.23×10−5 | CCND1, CDK1,
CCNB1 |
| Platinum drug
resistance | – |
1.25×10−5 | BIRC5, BCL2,
PIK3R1, TOP2A |
| Regulation of BAD
phosphorylation | Down |
1.80×10−5 | BCL2, IGF1,
PIK3R1 |
| Prostate
cancer | – |
2.52×10−5 | CCND1, BCL2, IGF1,
PIK3R1 |
| Endocrine
resistance | – |
3.71×10−5 | CCND1, BCL2, IGF1,
PIK3R1 |
| Proteoglycans in
cancer | – |
4.47×10−5 | FGF2, CCND1, IGF1,
PIK3R1, VEGFA |
| Bladder cancer | Up |
7.25×10−5 | CCND1, CXCL8,
VEGFA |
| Sphingolipid
signaling pathway | – |
8.32×10−5 | BCL2, PPP2R5C, FYN,
PIK3R1 |
| FoxO signaling
pathway | – |
1.29×10−4 | CCND1, IGF1,
PIK3R1, CCNB1 |
| Systemic lupus
erythematosus | Up |
1.32×10−4 | H2AFZ, HIST1H2BD,
HIST1H2BH, HIST1H2BK |
| NFAT and
Hypertrophy of the heart (Transcription in the broken heart) | Down |
1.66×10−4 | FGF2, IGF1,
PIK3R1 |
| Breast cancer | – |
1.80×10−4 | FGF2, CCND1, IGF1,
PIK3R1 |
| Activation of Src
by Protein-tyrosine phosphatase alpha | Up |
2.11×10−4 | CDK1, CCNB1 |
| Sonic Hedgehog
(SHH) Receptor Ptc1 Regulates cell cycle | Up |
2.11×10−4 | CDK1, CCNB1 |
| AKAP95 role in
mitosis and chromosome dynamics | Up |
2.52×10−4 | CDK1, CCNB1 |
| Glioma | – |
2.75×10−4 | CCND1, IGF1,
PIK3R1 |
| Pancreatic
cancer | – |
2.75×10−4 | CCND1, PIK3R1,
VEGFA |
| Expression of
cyclins regulates progression through the cell cycle by activating
cyclin-dependent kinases. | Up |
2.98×10−4 | CCND1, CCNA2 |
| The IGF-1 Receptor
and Longevity | Down |
4.00×10−4 | IGF1, PIK3R1 |
| Alcoholism | Up |
4.22×10−4 | H2AFZ, HIST1H2BD,
HIST1H2BH, HIST1H2BK |
| B Cell Survival
Pathway | – |
4.57×10−4 | BIRC5, PIK3R1 |
| Small cell lung
cancer | – |
6.12×10−4 | CCND1, BCL2,
PIK3R1 |
| Stathmin and breast
cancer resistance to antimicrotubule agents | Up |
6.48×10−4 | CDK1, CCNB1 |
| Epstein-Barr virus
infection | – |
6.64×10−4 | BCL2, CDK1, PIK3R1,
CCNA2 |
| Skeletal muscle
hypertrophy is regulated via AKT/mTOR pathway | Down |
7.19×10−4 | IGF1, PIK3R1 |
| Rap1 signaling
pathway | – |
7.54×10−4 | FGF2, IGF1, PIK3R1,
VEGFA |
| IGF-1 Signaling
Pathway | Down |
7.94×10−4 | IGF1, PIK3R1 |
| Ras signaling
pathway | – |
1.01×10−3 | FGF2, IGF1, PIK3R1,
VEGFA |
| Erk and PI-3 Kinase
Are Necessary for Collagen Binding in Corneal Epithelia | Down |
1.04×10−3 | FYN, PIK3R1 |
| Cell Cycle: G2/M
Checkpoint | Up |
1.04×10−3 | CDK1, CCNB1 |
| Influence of Ras
and Rho proteins on G1 to S Transition | – |
1.22×10−3 | CCND1, PIK3R1 |
| Genes related to
IL4 rceptor signaling in B lymphocytes | Down |
1.32×10−3 | BCL2, PIK3R1 |
| Inactivation of
Gsk3 by AKT causes accumulation of b-catenin in Alveolar
Macrophages | Up |
1.32×10−3 | CCND1, PIK3R1 |
| Cholinergic
synapse | Down |
1.41×10−3 | BCL2, FYN,
PIK3R1 |
| Cell Cycle: G1/S
Check Point | Up |
1.42×10−3 | CCND1, CDK1 |
| VEGF, Hypoxia, and
Angiogenesis | – |
1.52×10−3 | PIK3R1, VEGFA |
| HTLV–I
infection | – |
1.57×10−3 | CCND1, CDC20,
PIK3R1, PCNA |
| Control of skeletal
myogenesis by HDAC and calcium/calmodulin-dependent kinase
(CaMK) | Down |
1.63×10−3 | IGF1, PIK3R1 |
| Apoptosis-multiple
species | – |
1.97×10−3 | BIRC5, BCL2 |
| How Progesterone
Initiates Oocyte Membrane | Up |
2.09×10−3 | CDK1, CCNB1 |
| Measles | – |
2.36×10−3 | CCND1, FYN,
PIK3R1 |
|
Aldosterone-regulated sodium
reabsorption | Down |
2.47×10−3 | IGF1, PIK3R1 |
| Apoptosis | – |
2.56×10−3 | BIRC5, BCL2,
PIK3R1 |
| IL-2 Receptor Beta
Chain in T cell Activation | Down |
2.60×10−3 | BCL2, PIK3R1 |
| Signaling pathways
regulating pluripotency of stem cells | Down |
2.62×10−3 | FGF2, IGF1,
PIK3R1 |
| Fluid shear stress
and atherosclerosis | – |
2.78×10−3 | BCL2, PIK3R1,
VEGFA |
| Phospholipase D
signaling pathway | – |
3.01×10−3 | FYN, PIK3R1,
CXCL8 |
| Jak-STAT signaling
pathway | – |
3.63×10−3 | CCND1, BCL2,
PIK3R1 |
| Members of the BCR
signaling pathway | Down |
3.80×10−3 | BCL2, PIK3R1 |
| Hedgehog signaling
pathway | – |
3.96×10−3 | CCND1, BCL2 |
| T Cell Receptor
Signaling Pathway | Down |
3.96×10−3 | FYN, PIK3R1 |
| Endometrial
cancer | – |
4.47×10−3 | CCND1, PIK3R1 |
| Genes encoding
secreted soluble factors | – |
4.58×10−3 | FGF2, IGF1, CXCL8,
VEGFA |
| Acute myeloid
leukemia | – |
5.39×10−3 | CCND1, PIK3R1 |
| Non-small cell lung
cancer | – |
5.97×10−3 | CCND1, PIK3R1 |
| VEGF signaling
pathway | – |
6.18×10−3 | PIK3R1, VEGFA |
| Viral
myocarditis | – |
6.18×10−3 | CCND1, FYN |
| Longevity
regulating pathway-multiple species | Down |
6.80×10−3 | IGF1, PIK3R1 |
| Renal cell
carcinoma | – |
7.45×10−3 | PIK3R1, VEGFA |
| Fc epsilon RI
signaling pathway | – |
8.13×10−3 | FYN, PIK3R1 |
| Prolactin signaling
pathway | – |
8.60×10−3 | CCND1, PIK3R1 |
| Chronic myeloid
leukemia | – |
8.84×10−3 | CCND1, PIK3R1 |
| Longevity
regulating pathway | – |
1.36×10−2 | IGF1, PIK3R1 |
| Genes related to
Wnt-mediated signal transduction | Up |
1.36×10−2 | CCND1, GAPDH |
| Rheumatoid
arthritis | Up |
1.39×10−2 | CXCL8, VEGFA |
| NF-kappa B
signaling pathway | – |
1.54×10−2 | BCL2, CXCL8 |
| Amoebiasis | – |
1.57×10−2 | PIK3R1, CXCL8 |
| Cdc25 activates the
cdc2/cyclin B complex to induce the G2/M transition. | Up |
1.60×10−2 | CDK1 |
| Inflammatory
mediator regulation of TRP channels | Down |
1.60×10−2 | IGF1, PIK3R1 |
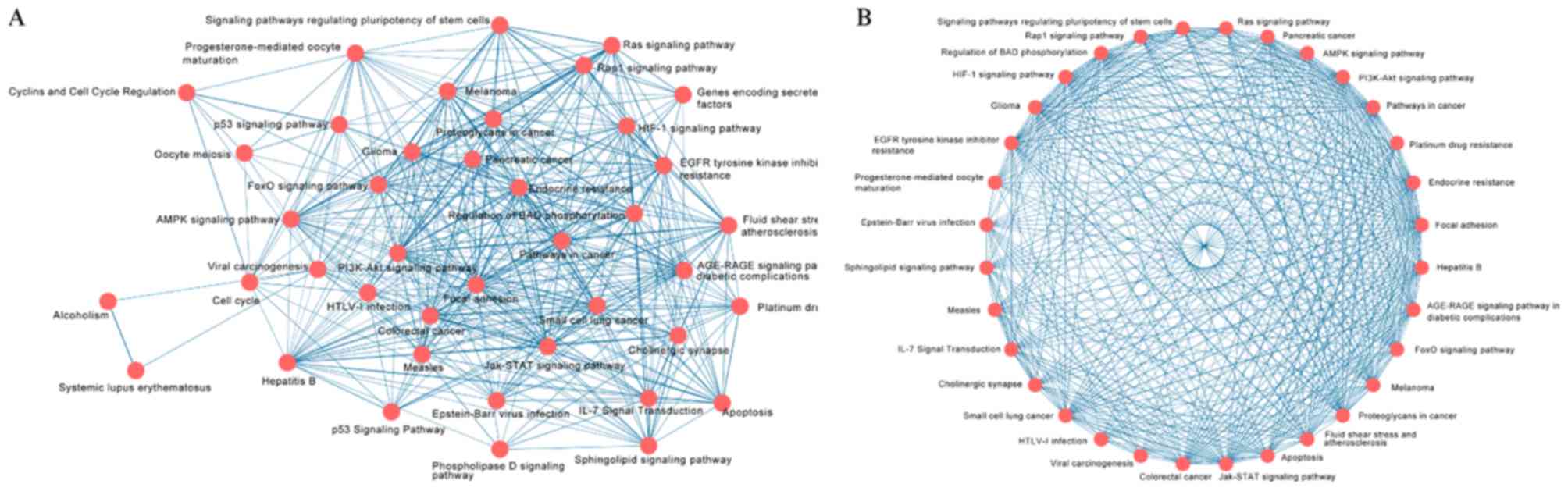
Pathway crosstalk analysis
To further investigate how the identified pathways
interact with each other, a pathway crosstalk analysis was
conducted among the pathways that met the criteria. The approach
was based on the assumption that two pathways can be considered to
be associated if they share a proportion of genes (18). A total of 45 pathways contained more
than two hub genes, of which 41 pathways met the criterion for
crosstalk analysis.
The network of crosstalk, which includes these 41
pathways, is presented in Fig. 3A.
The thickness of edge connecting two nodes represents the strength
of the association between them, which was measured by the mean
value of OC and JC. Using MCODE, two major clusters were identified
from the whole network. The simple cluster involves three pathways
associated with cell cycle, including ‘Cell cycle’, ‘Oocyte
meiosis’ and ‘Cyclins and Cell Cycle Regulation’. The complicated
cluster containing a total of 32 nodes and 376 edges is presented
in Fig. 3B. The five aforementioned
types of pathways were interconnected to form the complex network,
which indicates the complexity of the pathogenesis of CC, EC and
VC.
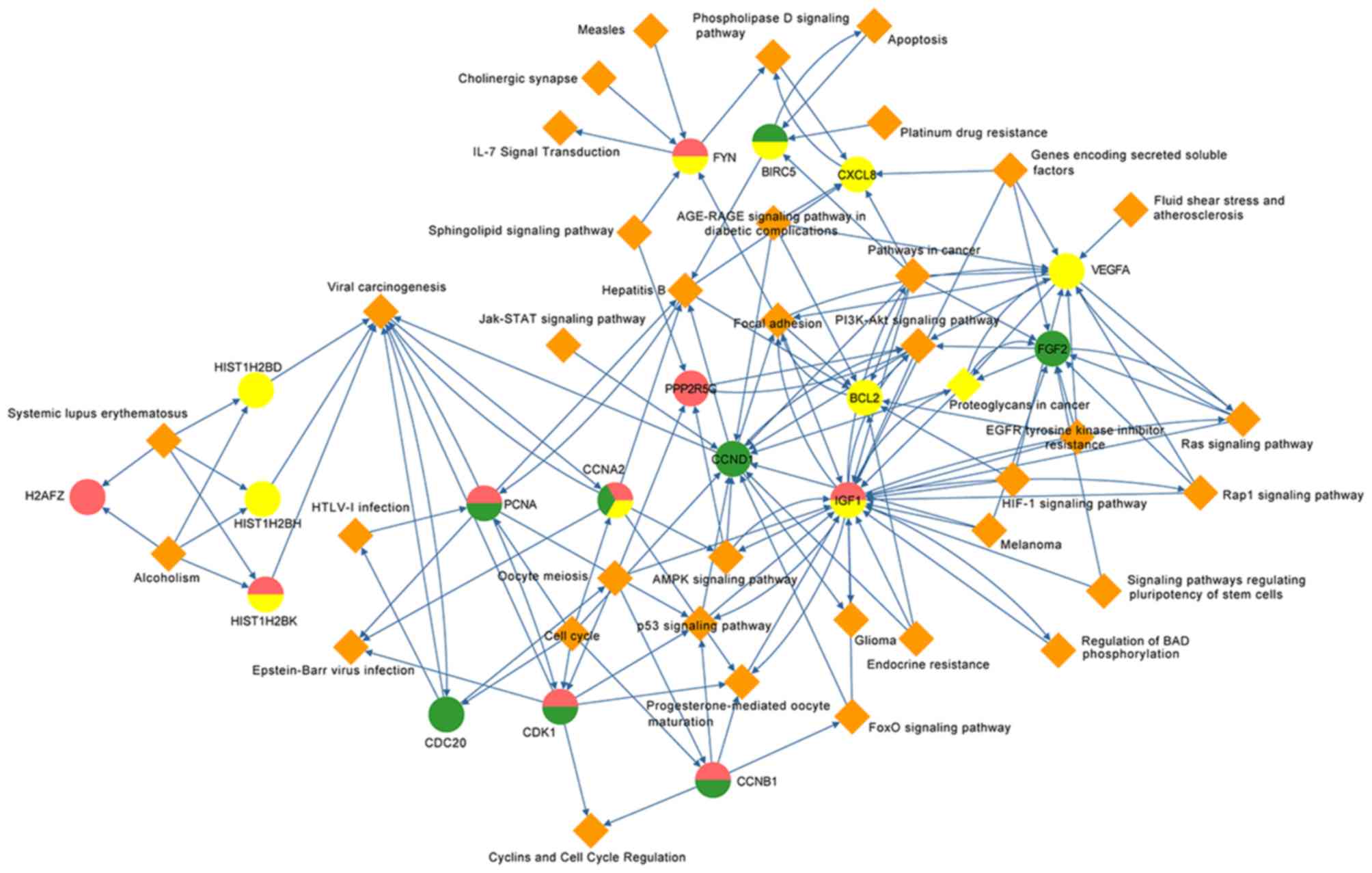
Gene-pathway network construction of
DEGs
By mapping the hub genes into the complicated
sub-network according to the KEGG and Biocarta databases, a
potential gene-pathway network was constructed to verify the
associations between the candidate pathways and genes (Fig. 4). This network included 37 important
pathways and 18 hub genes, including CCND1 presented in the middle
with direct or indirect associations with all other genes. As the
only overlapping gene of all three groups, CCNA2 possessed
complicated connections with ‘viral carcinogenesis’, ‘hepatitis B’,
‘cell cycle’ and six other pathways. In addition, insulin-like
growth factor-1 (IGF1), fibroblast growth factor 2 (FGF2) and CCND1
were located close to the middle of the gene-pathway network.
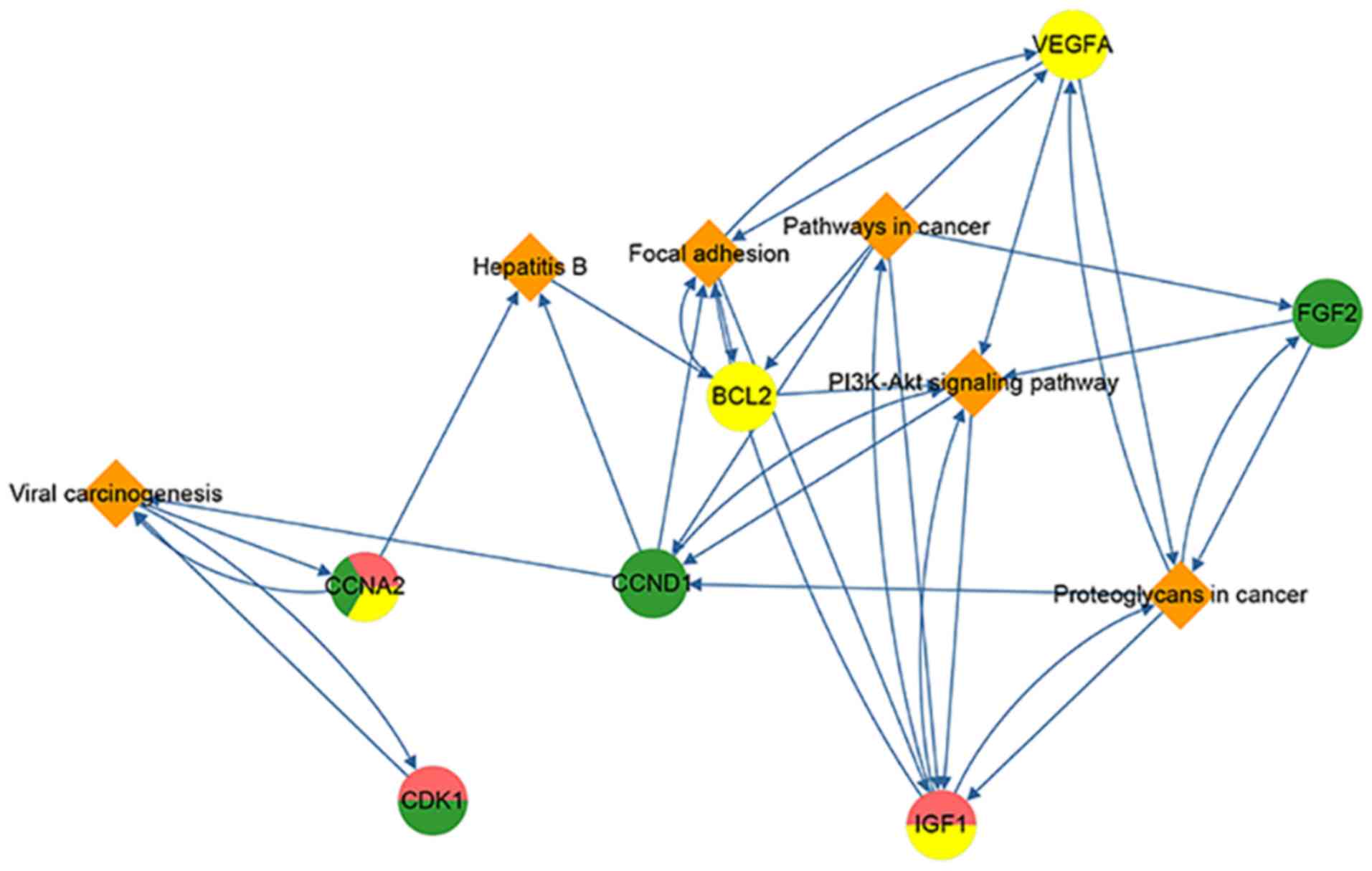
Sub gene-pathway network of DEGs
To screen the key factors, including genes and
pathways, in the gene-pathway network, the degrees of all of nodes
were calculated and nodes with a degree greater than the mean
degree of all nodes were selected (Fig.
5). Seven genes (CCNA2, CDK1, CCND1, BCL2, IGF1, FGF2 and
VEGFA) and six pathways (‘Viral carcinogenesis’, ‘Hepatitis B’,
‘Focal adhesion’, ‘Pathways in cancer’, ‘PI3K-Akt signaling
pathway’ and ‘Proteoglycans in cancer’) were selected. Since these
gene and pathways had more connections with other nodes, they were
considered to more likely serve a role in CC, EC and VS.
Discussion
In the past few decades, gynecological cancer
research has developed rapidly, particularly regarding the
recognition of etiological factors. Although a number of studies
have investigated CC, EC and VC separately, few studies have
focused on these three types of cancer in combination. The aim of
the present study was to perform a systematic and comprehensive
analysis to investigate the pathogenesis of CC, EC and VC and make
a preliminary assessment of the associations between these three
cancer types.
By performing an analysis of microarray data, 1,219
DEGs were identified, including 138 in CC, 479 in EC and 734 in VC.
Five DEGs were revealed in all three cancer types, which suggests
these genes may participate in a number of important biological
processes in gynecological cancer and may serve as crucial
biomarkers following further research. Together with the 25 hub
genes identified, these data may provide a direction for future
research on gynecological cancer and assist with the identification
of clinical biological targets.
Pathway enrichment analysis indicated that 86
pathways are closely associated with the 25 hub genes. In
particular, it was identified that viral infection and
carcinogenesis pathways were significantly enriched, including
‘viral carcinogenesis’, ‘HTLV-1 infection’ and ‘hepatitis B’, which
supports the association of virus with the three gynecological
cancer types, particularly CC. Furthermore, cancer-association
pathways, including ‘pathways in cancer’ and ‘proteoglycans in
cancer’ were revealed to be associated with the biological process
of the three malignant tumor types. Notably, multiple different
types of human cancer, including melanoma, prostate cancer, bladder
cancer, breast cancer and glioma, were also identified to be
associated with the 25 hub genes. This indicates that gynecological
cancer types may exhibit homologous mechanisms with tumor types of
other systems.
With the gene-pathway sub-network model, seven
critical hub genes and six important pathways of the three
gynecological cancer types were identified. The hub genes with the
highest degrees included CDK1, which was enriched in CC and VC. As
reported, CDK1 is a member of the Ser/Thr protein kinase family and
is encoded by cell division cycle gene 2 (cdc2) (19). In addition, CDK1 has been revealed to
serve a role in numerous types of cancer, including EC (20), breast cancer (21) and ovarian cancer (22). Consistent with the present
bioinformatics results, CDK1 has been demonstrated to serve a
comprehensive role in mediating genetic networks involved in the
progression of CC; therefore, it may be an important therapeutic
target for improving prognosis (23). A study regarding ovarian cancer
identified that CDK1 is associated with proliferation and can serve
as a prognostic factor in epithelial ovarian cancer (22). In EC, the overexpression of CDK1 in
endometrial carcinoma cells is closely associated with the
occurrence of tumors, indicating a role in tumor prognosis
(24). The CDK1 gene can contribute
to the carcinogenesis of HPV (25),
and CC and VC are associated with HPV infection; therefore, CDK1
may be an important molecule in the pathogenesis of gynecological
tumors.
Another cell cycle regulatory gene, CCNA2, was
revealed as a shared DEG of CC, EC and VC, and complicated
connections were identified between it and other nodes. According
to recent studies, CCNA2 belongs to the highly conversed cyclin
family and is expressed in multiple tissues in the human body,
including numerous types of cancer, which indicates it may serve a
role in cancer transformation and progression (26,27). Gao
et al (28) revealed that
CCNA2 is a prognostic biomarker for estrogen receptor-positive
breast cancer and is associated with Tamoxifen resistance. Combined
with another biological analysis of EC that demonstrated CCNA2 is
one of the top two upregulated nodes (29), the present study hypothesizes that
CCNA2 serves a role as a biomarker in gynecological tumors
(29). In addition, a study
associated with ovarian cancer revealed a similar result, in which
CCNA2 was upregulated in the chemo-resistant epithelial ovarian
cancer (30). Therefore, it can be
suggested that CCNA2 is a potential biomarker in gynecological
cancer; however, this requires in vivo or in vitro
experimental verification. CCND1 is an important positive regulator
of the G1/S phase of the cell cycle and has been identified as a
co-factor of HPV in the initiation of cervical carcinogenesis
(31). Similar studies regarding EC
and VC have also widely been reported (32–34).
BCL2 and IGF1 were revealed as the only two
downregulated genes in the sub-network. BCL2 is an intracellular
membrane protein that prevents apoptotic cell death and
overexpression of BCL2 can block p53-mediated G1 arrest (35). Kamaraddi et al (36) demonstrated that BCL2 expression is
higher in malignant lesions compared with premalignant lesions,
which differs from the current findings. It has been suggested that
alterations of BCL2 expression are associated with early events in
cervical tumorigenesis and a lower BCL2 expression level has also
been demonstrated to be associated with an improved 5-year survival
rate and prognosis (37). The
significance of BCL2 in gynecological tumors requires further
investigation. Furthermore, IGF1 is closely associated with the
occurrence of numerous tumor types; however, its exact mechanism
remains unclear. Iyer et al (38) identified that IGF-1 expression levels
in advanced CC increase with chemo-radiotherapy and decline during
follow-up (38). With a limited
specificity in gynecologic tumors, IGF1 is of limited value in the
early prediction of gynecological tumors; however it may serve a
role in targeted treatment strategies, and the assessment and
improvement of prognosis (39,40).
Angiogenesis serves an important role in tumor
growth, development, progression and metastasis (41). As a pro-angiogenesis factor, VEGFA is
involved in the proliferation, differentiation and migration of
endothelial cells, and participates in the invasion and metastasis
of numerous types of cancer (42).
Chen et al (43) demonstrated
that VEGFA may be a target for inhibiting angiogenesis in EC 42).
Similarly, Hua and Tian (44)
revealed that CCL4 can promote cell proliferation, invasion and
migration of EC by targeting the VEGFA signal pathway (44). Combined with the present results,
this indicates that VEGFA serves an important role in gynecological
tumor invasion and metastasis.
FGF2 is a typical fibroblast growth factor that
stimulates the growth of various cell types, from fibroblasts to
tumor cells (45). In addition, FGF2
is a fundamental signaling molecule in tumor-induced angiogenesis
(46). It has been demonstrated that
FGF2 is mitogenic in various cell types and is associated with the
regulation of tumor angiogenesis and metastasis (47). Certain studies regarding the receptor
family of FGF2 have revealed that it is associated with the
occurrence and development of CC, in addition to HPV16 infection
(48,49). Aberrant FGF/FGF receptor signaling
has been demonstrated in multiple types of tumor (50,51). The
expression level of FGF2 has been revealed to be higher in EC
compared with normal tissues, and the highest expression level was
observed in tumors with dedifferentiation, myometrial invasion and
advanced staging (52). Therefore,
angiogenesis has an important impact in the pathogenesis of
gynecological cancers.
‘PI3K-Akt signaling pathway’, ‘hepatitis B’,
‘pathways in cancer’, ‘focal adhesion’, ‘viral carcinogenesis’ and
‘proteoglycans in cancer’ were located in the sub-network, which
indicates that these processes serve an important role in the
pathogenesis of CC, EC and VC. It is understood that the PI3K/Akt
signaling pathway serves a central role in cell growth and
proliferation, and it has also been suggested that its deregulation
is associated with cancer (53).
Yung et al (54) demonstrated
that the activation of AMPK could significantly inhibit CC cell
growth. Similar studies regarding the PI3K/Akt pathway in EC have
also been reported (55,56), and it has been considered as a
therapeutic target (57). According
to previous studies, the PI3K/Akt signaling pathway can serve as a
therapeutic target in EC (57) and
ovarian cancer (58,59), and can be mediated by molecules,
including VEGFA (40). FGF2 has also
been reported to serve an angiogenic role by the PI3K/Akt pathway
(60). Furthermore, BCL2 is a major
downstream mediator of the PI3K/Akt pathway and serves a pivotal
role in tumor response (61,62). It has been reported that CCNA2
expression promotes the migration, invasion and metastasis of
hepatocellular carcinoma and ovarian cancer cells via the PI3K/Akt
signaling pathway (63). Therefore,
this crucial pathway in cancer cells may be involved in the early
developmental stages of formation and invasion. As indicated by the
present results, the molecular mechanisms underlying CC, EC and VC
are complicated, and further studies are required to fully
understand their pathological mechanisms.
Similarly, Suman and Mishra (64) identified that the aurora kinase
pathway has a crucial function in the pathogenesis of five
gynecological cancer types, including breast cancer, EC, CC,
ovarian cancer and VC, by analyzing the common core genes from the
GSE63678, GSE57297 and GSE26712 datasets. Furthermore, the present
study identified seven genes (CCNA2, CDK1, CCND1, BCL2, IGF1, FGF2
and VEGFA) and six pathways (‘viral carcinogenesis’, ‘hepatitis B’,
‘focal adhesion’, ‘pathways in cancer’, ‘PI3K-Akt signaling
pathway’ and ‘proteoglycans in cancer’) that may serve an important
role in CC, EC and VC. A number of factors are involved in the
progression of cancer; the present study focused on the factors
associated with the female reproductive system. The results may
provide comprehensive evidence that promotes the understanding of
cancers of the female genital tract. While previous studies have
focused on co-expressed DEGs (23,64), the
present study aimed to establish a gene-pathway network based on
the analysis of DEGs. Additionally, previous studies investigated
genes co-expressed by the five cancer types (breast cancer, EC, CC,
ovarian cancer and VC). However, the current study not only
investigated the co-expressed DEGs of CC, EC and VC, but also
examined the DEGs co-expressed by any combination of two of the
cancer types. In summary, the current study focused of the
associations between three types of tumor, which may make it more
comprehensive compared with previous studies.
Notably, there are certain limitations to the
present study. The sample number was relatively small, which to a
certain extent reduces the credibility of gene enrichment. Subject
to conditions, long-term assessments of the patients' clinical
conditions were not available. In addition, the literature
regarding the pathways associated with CC, EC and EC, except for
the PI3K/Akt pathway is limited; therefore, the present study
lacked a solid foundation to adequately discuss the current
results. Finally, certain genes that are associated with the
pathogenesis of gynecological types of cancer may not have been
statistically analyzed, possibly due to the exclusion criteria that
was applied.
In conclusion, the pathogenesis of CC, EC and VC is
complicated. By performing a comprehensive analysis, the present
study revealed a library of DEGs in CC, EC and VC, and identified
25 hub genes. Subsequently, viral infection, tumorigenesis,
inflammation and the endocrine system were revealed to be involved
in the development of these three types of cancer. Finally, a
molecular network of CC, EC and VC was constructed. Most notably,
it was identified that the PI3K/Akt pathway serves an important
role in the three types of gynecological cancer and seven hub genes
(CCNA2, CDK1, CCND1, FGF2, IGF1, BCL2 and VEGFA) present in the
sub-network may act as therapeutic targets, and assist with early
diagnosis and prevention. The present study may support the
elucidation of the underlying mechanisms in CC, EC and VC, which
would promote early detection and the development of targeted
therapy. Further investigations that aim to improve understanding
of the mechanisms of these three cancer types will be vital for
developing highly sensitive and multifactorial strategies for the
prevention, diagnosis and treatment of CC, VC and EC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, YY and WZ conceived and designed the study. YY,
WW, and KW analyzed the data. YL wrote the manuscript with
contributions from all authors. All authors contributed to the
interpretation of the data and writing the manuscript. The final
version of the manuscript was reviewed and approved by all the
authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ronco G, Dillner J, Elfström KM, Tunesi S,
Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi
P, et al: Efficacy of HPV-based screening for prevention of
invasive cervical cancer: Follow-up of four European randomised
controlled trials. Lancet. 383:524–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Castellsagué X: Natural history and
epidemiology of HPV infection and cervical cancer. Gynecol Oncol
110 (3 Suppl 2). S4–S7. 2008. View Article : Google Scholar
|
|
5
|
Alkatout I, Schubert M, Garbrecht N,
Weigel MT, Jonat W, Mundhenke C and Günther V: Vulvar cancer:
Epidemiology, clinical presentation, and management options. Int J
Womens Health. 7:305–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DiSaia PJ, Creasman WT, Mannel RS,
McMeekin DS and Mutch DG: SPEC-clinical gynecologic oncology.
2017.Elsevier Health Sciences. PubMed/NCBI
|
|
8
|
Klattig J and Englert C: The Müllerian
duct: Recent insights into its development and regression. Sex Dev.
1:271–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pappa KI, Rodolakis A, Christodoulou I,
Gazouli M, Markaki S, Antsaklis A and Anagnou NP: Comparative
assessment of lymph node micrometastasis in cervical, endometrial
and vulvar cancer: Insights on the real time qRT-PCR approach
versus immunohistochemistry, employing dual molecular markers.
Biomed Res Int. 2014:1876842014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res 41 (Database Issue).
D991–D995. 2013.
|
|
11
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pathan M, Keerthikumar S, Ang CS, Gangoda
L, Quek CY, Williamson NA, Mouradov D, Sieber OM, Simpson RJ, Salim
A, et al: FunRich: An open access standalone functional enrichment
and interaction network analysis tool. Proteomics. 15:2597–2601.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res 45
(Database Issue). D362–D368. 2017. View Article : Google Scholar
|
|
14
|
Wang J, Duncan DT, Shi Z and Zhang B:
WEB-based GEne SeT analysis toolkit (WebGestalt): Update 2013.
Nucleic Acids Res 41 (Web Server Issue). W77–W83. 2013. View Article : Google Scholar
|
|
15
|
Hu Y, Pan Z, Hu Y, Zhang L and Wang J:
Network and pathway-based analyses of genes associated with
parkinson's disease. Mol Neurobiol. 54:4452–4465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Franz M, Lopes CT, Huck G, Dong Y, Sumer O
and Bader GD: Cytoscape.js: A graph theory library for
visualisation and analysis. Bioinformatics. 32:309–311.
2016.PubMed/NCBI
|
|
17
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia P, Kao CF, Kuo PH and Zhao Z: A
comprehensive network and pathway analysis of candidate genes in
major depressive disorder. BMC Syst Biol. 5 (Suppl 3):S122011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nigg EA: Mitotic kinases as regulators of
cell division and its checkpoints. Nat Rev Mol Cell Biol. 2:21–32.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li L, Qu YW and Li YP: Over-expression of
miR-1271 inhibits endometrial cancer cells proliferation and
induces cell apoptosis by targeting CDK1. Eur Rev Med Pharmacol
Sci. 21:2816–2822. 2017.PubMed/NCBI
|
|
21
|
Kang J, Sergio CM, Sutherland RL and
Musgrove EA: Targeting cyclin-dependent kinase 1 (CDK1) but not
CDK4/6 or CDK2 is selectively lethal to MYC-dependent human breast
cancer cells. BMC Cancer. 14:322014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xi Q, Huang M, Wang Y, Zhong J, Liu R, Xu
G, Jiang L, Wang J, Fang Z and Yang S: The expression of CDK1 is
associated with proliferation and can be a prognostic factor in
epithelial ovarian cancer. Tumour Biol. 36:4939–4948. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo Y, Wu Y, Peng Y, Liu X, Bie J and Li
S: Systematic analysis to identify a key role of CDK1 in mediating
gene interaction networks in cervical cancer development. Ir J Med
Sci. 185:231–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bidus MA, Risinger JI, Chandramouli GV,
Dainty LA, Litzi TJ, Berchuck A, Barrett JC and Maxwell GL:
Prediction of lymph node metastasis in patients with endometrioid
endometrial cancer using expression microarray. Clin Cancer Res.
12:83–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang W, Liu Y, Zhao N, Chen H, Qiao L,
Zhao W and Chen JJ: Role of Cdk1 in the p53-independent abrogation
of the postmitotic checkpoint by human papillomavirus E6. J Virol.
89:2553–2562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruan JS, Zhou H, Yang L, Wang L, Jiang ZS
and Wang SM: CCNA2 facilitates epithelial-to-mesenchymal transition
via the integrin αvβ3 signaling in NSCLC. Int J Clin Exp Pathol.
10:8324–8333. 2017.
|
|
27
|
He Y, Liu J, Zhao Z and Zhao H:
Bioinformatics analysis of gene expression profiles of esophageal
squamous cell carcinoma. Dis Esophagus. 30:1–8. 2017. View Article : Google Scholar
|
|
28
|
Gao T, Han Y, Yu L, Ao S, Li Z and Ji J:
CCNA2 is a prognostic biomarker for ER+ breast cancer and tamoxifen
resistance. PLoS One. 9:e917712014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Zhang W, Li X, Li D, Zhang X, Yin
Y, Deng X and Sheng X: Prognostic factors and genes associated with
endometrial cancer based on gene expression profiling by
bioinformatics analysis. Arch Gynecol Obstet. 293:1287–1295. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ju W, Yoo BC, Kim IJ, Kim JW, Kim SC and
Lee HP: Identification of genes with differential expression in
chemoresistant epithelial ovarian cancer using high-density
oligonucleotide microarrays. Oncol Res. 18:47–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Catarino R, Matos A, Pinto D, Pereira D,
Craveiro R, Vasconcelos A, Lopes C and Medeiros R: Increased risk
of cervical cancer associated with cyclin D1 gene A870G
polymorphism. Cancer Genet Cytogenet. 160:49–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rolfe KJ, Crow JC, Benjamin E, Reid WM,
Maclean AB and Perrett CW: Cyclin D1 and retinoblastoma protein in
vulvar cancer and adjacent lesions. Int J Gynecol Cancer.
11:381–386. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lerma E, Esteller M, Herman JG and Prat J:
Alterations of the p16/Rb/cyclin-D1 pathway in vulvar carcinoma,
vulvar intraepithelial neoplasia, and lichen sclerosus. Hum Pathol.
33:1120–1125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moreno-Bueno G, Rodríguez-Perales S,
Sánchez-Estévez C, Marcos R, Hardisson D, Cigudosa JC and Palacios
J: Molecular alterations associated with cyclin d1 overexpression
in endometrial cancer. Int J Cancer. 110:194–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kurvinen K, Syrjänen K and Syrjänen S: p53
and bcl-2 proteins as prognostic markers in human
papillomavirus-associated cervical lesions. J Clin Oncol.
14:2120–2130. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kamaraddi S, Ashwini NU, Honnappa S and
Swarup A: Expression of bcl-2 marker in premalignant lesions of
cervical cancer. Int J Reprod Contrac Obstet Gynecol. 5:965–969.
2016. View Article : Google Scholar
|
|
37
|
Aletra C, Ravazoula P, Scopa C, Kounelis
S, Sotiropoulou G, Kourounis G, Ladopoulos I and Bonikos D:
Expression of bcl-2 and bax in cervical intraepithelial neoplasia
and invasive squamous cell carcinoma of the uterine cervix. Eur J
Gynaecol Oncol. 21:494–498. 2000.PubMed/NCBI
|
|
38
|
Iyer P, Radhakrishnan V, Vyas R and
Trivedi S: Study on the effect of chemo-radiation on the serum
levels of IGF-I in patients with cancer cervix stage IIIB. Ind J
Gynecol Oncol. 15:342017. View Article : Google Scholar
|
|
39
|
Peyrat JP, Bonneterre J, Hecquet B, Vennin
P, Louchez MM, Fournier C, Lefebvre J and Demaille A: Plasma
insulin-like growth factor-1 (IGF-1) concentrations in human breast
cancer. Eur J Cancer 29A. 492–497. 1993. View Article : Google Scholar
|
|
40
|
Vanamala J, Reddivari L, Radhakrishnan S
and Tarver C: Resveratrol suppresses IGF-1 induced human colon
cancer cell proliferation and elevates apoptosis via suppression of
IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer.
10:2382010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liao Y, Lu W, Che Q, Yang T, Qiu H, Zhang
H, He X, Wang J, Qiu M, Zou Y, et al: SHARP1 suppresses
angiogenesis of endometrial cancer by decreasing hypoxia-inducible
factor-1α level. PLoS One. 9:e999072014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baeriswyl V and Christofori G: The
angiogenic switch in carcinogenesis. Semin Cancer Biol. 19:329–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen HX, Xu XX, Tan BZ, Zhang Z and Zhou
XD: MicroRNA-29b inhibits angiogenesis by targeting VEGFA through
the MAPK/ERK and PI3K/Akt signaling pathways in endometrial
carcinoma. Cell Physiol Biochem. 41:933–946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hua F and Tian Y: CCL4 promotes the cell
proliferation, invasion and migration of endometrial carcinoma by
targeting the VEGF-A signal pathway. Int J Clin Exp Pathol.
10:11288–11299. 2017.
|
|
45
|
Itoh N: The Fgf families in humans, mice,
and zebrafish: Their evolutional processes and roles in
development, metabolism, and disease. Biol Pharm Bull.
30:1819–1825. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Presta M, Dell'Era P, Mitola S, Moroni E,
Ronca R and Rusnati M: Fibroblast growth factor/fibroblast growth
factor receptor system in angiogenesis. Cytokine Growth Factor Rev.
16:159–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Folkman J and Klagsbrun M: Angiogenic
factors. Science. 235:442–447. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cheng YM, Chou CY, Hsu YC and Chen MJ:
Influence of HPV16 E6/7 on the expression of FGF2 and FGFR type B
in cervical carcinogenesis. Reprod Sci. 19:580–586. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheng YM, Chou CY, Chang FM and Wing LYC:
Fibroblast growth factor receptor 1 (FGFR1) overexpression play a
possible role in cervical carcinogenesis: 0453. Int J Gynecol
Cancer. 728:2006.
|
|
50
|
Huang JK, Ma L, Song WH, Lu BY, Huang YB,
Dong HM, Ma XK, Zhu ZZ and Zhou R: LncRNA-MALAT1 promotes
angiogenesis of thyroid cancer by modulating tumor-associated
macrophage FGF2 protein secretion. J Cell Biochem. 118:4821–4830.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee PS and Secord AA: Targeting molecular
pathways in endometrial cancer: A focus on the FGFR pathway. Cancer
Treat Rev. 40:507–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fujimoto J, Hori M, Ichigo S and Tamaya T:
Expressions of the fibroblast growth factor family (FGF-1, −2 and
−4) mRNA in endometrial cancers. Tumour Biol. 17:226–233. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yung MM, Chan DW, Liu VW, Yao KM and Ngan
HY: Activation of AMPK inhibits cervical cancer cell growth through
AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer. 13:3272013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Y, Goodfellow R, Li Y, Yang S,
Winters CJ, Thiel KW, Leslie KK and Yang B: NEDD4 ubiquitin ligase
is a putative oncogene in endometrial cancer that activates
IGF-1R/PI3K/Akt signaling. Gynecol Oncol. 139:127–133. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Slomovitz BM and Coleman RL: The
PI3K/AKT/mTOR pathway as a therapeutic target in endometrial
cancer. Clin Cancer Res. 18:5856–5864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pavlidou A and Vlahos NF: Molecular
alterations of PI3K/Akt/mTOR pathway: A therapeutic target in
endometrial cancer. ScientificWorldJournal. 2014:7097362014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cheaib B, Auguste A and Leary A: The
PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities
and challenges. Chin J Cancer. 34:4–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mabuchi S, Kuroda H, Takahashi R and
Sasano T: The PI3K/AKT/mTOR pathway as a therapeutic target in
ovarian cancer. Gynecol Oncol. 137:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Boras EA, Matou-nasri S, Kuprinski J,
Badimon L, Potempa LA and Slevin M: Abstract 181: Common angiogenic
signalling pathways induced by monomeric c-reactive protein and
FGF-2 through phosphatidylinositol 3-kinase. Stroke.
43:A1812012.
|
|
61
|
Liu Y, Liu H, Zou J, Zhang B and Yuan Z:
Dengue virus subgenomic RNA induces apoptosis through the
Bcl-2-mediated PI3k/Akt signaling pathway. Virology. 448:15–25.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Luna-López A, Triana-Martínez F,
López-Diazguerrero NE, Ventura-Gallegos JL, Gutiérrez-Ruiz MC,
Damián-Matsumura P, Zentella A, Gómez-Quiroz LE and Königsberg M:
Bcl-2 sustains hormetic response by inducing Nrf-2 nuclear
translocation in L929 mouse fibroblasts. Free Radic Biol Med.
49:1192–1204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gopinathan L, Tan SL, Padmakumar VC,
Coppola V, Tessarollo L and Kaldis P: Loss of Cdk2 and cyclin A2
impairs cell proliferation and tumorigenesis. Cancer Res.
74:3870–3879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Suman S and Mishra A: Network analysis
revealed aurora kinase dysregulation in five gynecological types of
cancer. Oncol Lett. 15:1125–1132. 2018.PubMed/NCBI
|