Introduction
Regucalcin (RGN), a calcium binding protein with a
molecular weight of 33 kDa, was first isolated by Japanese
scientists from the rat liver in the 1980s (1–4). RGN
serves a principal role in the maintenance of intracellular calcium
homeostasis and liver metabolism. It also influences the activities
of numerous enzymes including pyruvate kinase, succinate kinase,
glycogen phosphorylase and adenosine (5–8). Since
the 1990s, numerous studies have reported an association between
RGN and carcinoma, where the upregulation of RGN expression in
various cancer types inhibited tumor growth and metastasis. A
previous study demonstrated that the downregulation of RGN
expression using the RNAi technique significantly enhanced the
proliferation and migration capacities of HepG2 cells (9).
Cervical cancer (CC) is a common tumor, which has a
high rate of morbidity and mortality in developing countries
(10). According to the global
cancer statistics in 2018, incidence and mortality rates of
cervical cancer in women worldwide are 6.6 and 7.5%, respectively
(11). Cervical adenocarcinoma (CA)
is a unique type of cervical cancer with an increasing rate of
morbidity, even in young people (12,13).
Furthermore, the rate of ovarian metastasis in CA is high compared
with that of squamous carcinoma, while its sensitivity to
radiotherapy and chemotherapy is significantly lower (14–16).
Gene therapy is becoming one of the potential therapeutic
strategies for cervical cancer due to its safety and specificity
(17). In the present study,
exogenous RGN was transfected into HeLa cells, a human
papillomavirus-associated endocervical adenocarcinoma cell line, to
analyze the effect of RGN in CA, and to determine the signaling
proteins involved.
Materials and methods
Clinical association between
regucalcin (RGN) and cervical cancer
The clinical data for RGN expression in cervical and
endocervical cancers (CESC) was derived from The Cancer Genome
Atlas (TCGA) database (https://tcga-data.nci.nih.gov/tcga). RGN expression
was analyzed in six aspects: principal cancer stage, individual
cancer stage, patient's race, weight, age and histological
subtype.
Cell culture and lentivirus
infection
HeLa cells were obtained from the Chinese Academy of
Sciences (Beijing, China). A lentiviral vector encoding RGN-cDNA
was purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China).
The cDNA sequence was as follows:
ATGTCTTCCATTAAGATTGAGTGTGTTTTGCCAGAGAACTGCCGGTGTGGTGAGTCTCCAGTATGGGAGGAAGTGTCCAACTCTCTGCTCTTTGTAGACATTCCTGCAAAAAAGGTTTGCCGGTGGGATTCATTCACCAAGCAAGTACAGCGAGTGACCATGGATGCCCCAGTCAGCTCCGTGGCTCTTCGCCAGTCGGGAGGCTATGTTGCCACCATTGGAACAAAGTTCTGTGCTTTGAACTGGAAAGAACAATCAGCAGTTGTCTTGGCCACGGTGGATAACGACAAGAAAAACAATCGCTTCAATGATGGGAAGGTGGATCCCGCCGGGAGGTACTTTGCTGGCACCATGGCTGAGGAAACAGCTCCAGCAGTTCTTGAGCGGCACCAGGGGGCCCTGTACTCCCTCTTTCCTGATCACCACGTGAAAAAGTACTTTGACCAGGTGGACATTTCCAATGGTTTGGATTGGTCGCTAGACCACAAAATCTTCTATTACATTGACAGCCTGTCCTACTCCGTGGATGCCTTTGACTATGACCTGCAGACAGGACAGATCTCCAACCGCAGAAGTGTTTACAAGCTAGAAAAGGAAGAACAAATCCCAGATGGAATGTGTATTGATGCTGAGGGGAAGCTCTGGGTGGCCTGTTACAATGGAGGAAGAGTGATTCGTTTAGATCCTGTGACAGGGAAAAGACTTCAAACTGTGAAGTTGCCTGTTGATAAAACAACTTCATGCTGCTTTGGAGGGAAGAATTACTCTGAAATGTATGTGACCTGCGCCCGGGATGGGATGGACCCCGAGGGTCTTTTGAGGCAACCTGAAGCTGGTGGAATTTTCAAGATAACTGGTCTGGGGGTCAAAGGAATTGCTCCCTACTCCTATGCGGGATGA.
HeLa cells were maintained in Dulbecco's modified Eagle's medium
(DMEM; HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher
Scientific, Inc., Waltham, MA, USA). To produce a cell line that
highly expressed RGN, 5×105 cells/well were incubated
overnight in a 6-well plate, and the RGN lentiviral or empty
vectors were added (multiplicity of infection, 20). Following a 24
h infection period, the cells were treated with puromycin (2 µg/ml)
for 72 h to screen for positive clones. Successfully infected cells
were verified by the positive expression of green fluorescent
protein.
Reverse transcription-quantitative
PCR
The expression of RGN mRNA was determined using the
PrimeScript™ RT reagent kit and the SYBR Premix Ex Taq™ II kit
(Takara Bio, Inc., Otsu, Japan). All steps were performed according
to the manufacturer's protocols. Primer sequences were as follows:
RGN forward, 5′-GTGGATGCCTTTGACTATGACC-3′, and reverse,
5′-CTTCCCCTCAGCATCAATACAC-3′; GAPDH forward,
5′-CGAGATCCTCAACCAATCAA-3′, and reverse 5′-GGTGGTCCAGGGTCGTTACT-3′.
PCR reaction conditions were as follows: 20 sec at 95°C, 30 sec at
60°C and 30 sec at 72°C, repeated for 40 cycles. The
2−ΔΔCq method was used to analyze mRNA expression
(18).
Western blot analysis
RGN-transfected and empty vector control cells
cultured in 25 cm2 culture flasks were lysed using 500
µl RIPA lysate supplemented with 1 mM PMSF. The protein
concentrations were determined using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology, Haimen, China) and
30 µg total protein/lane was separated using SDS-PAGE (separation
gel, 12%; spacer gel, 5%). The separated proteins were transferred
to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), blocked with 5% non-fat milk and incubated with
the following primary antibodies overnight at 4°C: RGN (1:1,000;
cat. no. 17947-1-AP; ProteinTech Group, Inc., Chicago, IL, USA),
β-catenin (1:1,000; cat. no. 51067-2-AP; ProteinTech Group, Inc.),
p-glycogen synthase kinase (GSK)-3β (1:1,000; cat. no. ab75814;
Abcam, Cambridge, UK), GSK-3β (1:1,000; cat. no. 22104-1-AP;
ProteinTech Group, Inc.), matrix metalloproteinase (MMP)-3
(1:1,000; cat. no. 17873-1-AP; ProteinTech Group, Inc.), MMP-7
(1:800; cat. no. 10374-2-AP; ProteinTech Group, Inc.), MMP-9
(1:800; cat. no. 10375-2-AP; ProteinTech Group, Inc.), E-cadherin
(1:1,000; cat. no. 20874-1-AP; ProteinTech Group, Inc.), N-cadherin
(1:1,000; cat. no. 22018-1-AP; ProteinTech Group, Inc.), Vimentin
(1:1,000; cat. no. 10366-1-AP; ProteinTech Group, Inc.) and GAPDH
(1:1,000; cat. no. 10494-1-AP; ProteinTech Group, Inc.). GAPDH was
selected as internal reference. The membranes were washed with TBS
+ 0.1% Tween-20 (TBST) three times for 5 min and subsequently
incubated with secondary goat anti-rabbit IgG-HRP (1:5,000; cat.
no. E-AB-1003; Elabscience Biotechnology Co., Ltd., Wuhan, China)
for 1 h at 37°C. The membranes were washed with TBST three times
for 10 min. Enhanced chemiluminescence ultra-sensitive luminescence
solution (Applygen, Beijing, China) was used to develop the bands.
Densitometry was performed using ImageJ v1.48 software (National
Institutes of Health, Bethesda, MD, USA).
Migration assay
800 µl/well DMEM with 10% FBS was added to a 24-well
plate prior to the addition of Transwell inserts. HeLa cells
(3×104) were resuspended in 200 µl DMEM and loaded into
the upper chamber. Following a 24 h incubation period in 37°C, the
membranes were fixed with 4% paraformaldehyde for 15 min at room
temperature (RT) and stained with 0.1% crystal violet for 10 min at
RT. The cells on the inner surface of membrane were removed by a
cotton swab, whereas those on the outer surface were counted by an
optical microscope (magnification, ×400; Olympus Corporation,
Tokyo, Japan) in five random fields.
Invasion assay
Cellular invasive capacity was analyzed using a
Matrigel assay. Matrigel (BD Biosciences; Becton, Dickinson and
Company, Franklin, Lakes, NJ, USA) was diluted with DMEM at a ratio
of 1:9 and added to the upper chamber. Once the gel had set, 800 µl
DMEM with 10% FBS was added to 24-well plates, and 3×104
cells were inserted into the chamber. The cells were incubated at
37°C for 24 h, fixed with 4% paraformaldehyde for 15 min and
stained with 0.1% crystal violet for 10 min at RT. The cells on the
inner surface of membrane were removed, whereas those on the outer
surface were counted using an optical microscope (magnification,
×400) in five random fields.
Cell proliferation assay
HeLa cells were cultured in 96-well plates
(3×103 cells/well) for 24, 48, 72 and 96 h. The culture
medium was removed and 100 µl DMEM with 10% FBS and 10 µl Cell
Counting Kit-8 assay buffer (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added to each well. Following a 4-h incubation
period, the absorbance was determined at 450 nm. The experiment was
performed in triplicate.
Colony formation assay
A total of 500 cells/well were seeded into 6-well
plates and gently dispersed by agitation. The cells were cultured
at 37°C with 5% CO2 for 14 days with frequent
observation. The culture medium was discarded as soon as colonies
were visible, and the wells were gently washed twice with PBS. The
cells were subsequently fixed and stained with 0.1% crystal violet
for 10 min at RT. The samples were gently washed with water and
allowed to dry naturally, prior to the colonies being photographed
and counted.
Cell cycle analysis
Cell cycle analysis was conducted using a cell cycle
detection kit (Vazyme Piscataway, NJ, USA). 2×106 HeLa
cells were washed and fixed with ice-cold ethanol (70%) for 8 h at
4°C. The cells were centrifuged at 300 × g for 5 min at 4°C,
resuspended in 400 µl PBS supplemented with 20 µl RNase A solution,
and incubated in a water bath at 37°C for 1.5 h. Subsequently, 400
µl PI Staining Solution (Beyotime Institute of Biotechnology,
Shanghai, China) was added, and the cells were incubated at 4°C for
0.5 h prior to flow cytometric analysis using a flow cytometer
(FACScalibur; Becton Dickinson, Cockeysville, MD, USA) and
CellQuest software (Becton Dickinson).
Statistical analysis
The data are described as the mean ± standard
deviation. The experiments were performed in triplicate. Student's
t-test and one-way ANOVA were used to analyze the experimental
results and bioinformatics analysis. Student-Newman-Keuls post hoc
test was used for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
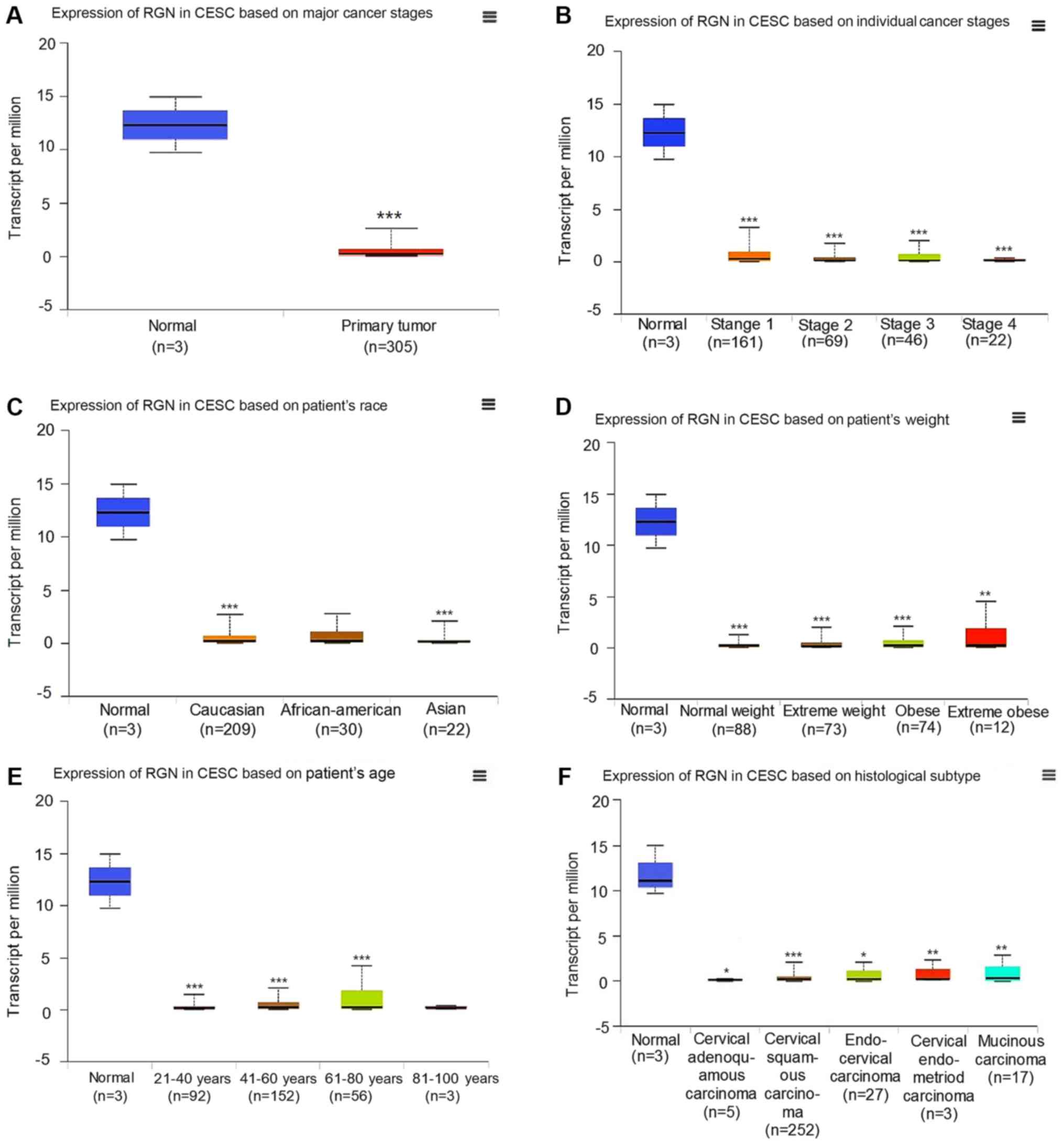
The expression of RGN is reduced in
the cancerous tissues of patients with CESC compared with those of
healthy controls
Based on clinical information in TCGA, it was
revealed that the expression of RGN in CESC tissues was reduced
compared with those of normal tissues (Fig. 1A), though this was not associated
with individual cancer stage, patient's race, patient's weight,
patient's age or histological subtype (Fig. 1B-F).
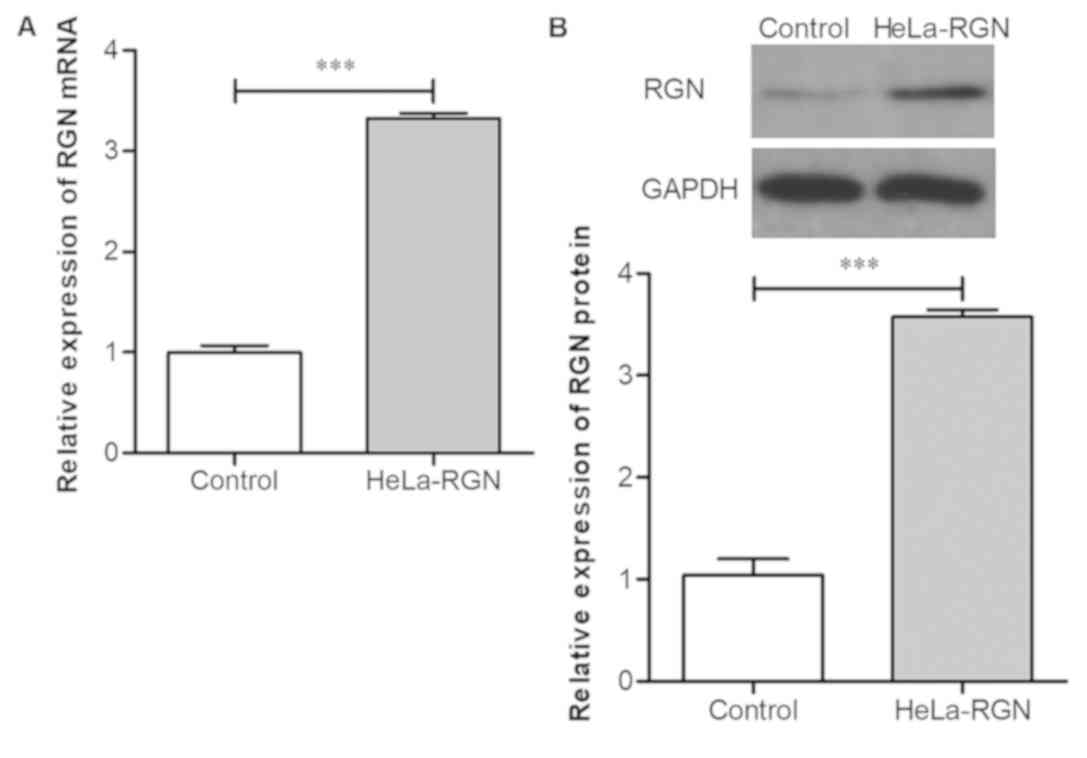
Exogenous RGN is successfully
expressed in HeLa cells following lentivirus-mediated
transfection
HeLa cells were infected with lentivirus-mediated
RGN or empty lentivirus. The expression levels of RGN mRNA in RGN
transfectants was significantly increased compared with those
transfected with the empty vector (Fig.
2A; P<0.001). RGN protein expression level in the RGN
transfectants was also increased significantly compared with the
controls (Fig. 2B; P<0.001). The
results indicated that HeLa cells that highly express RGN were
successfully produced.
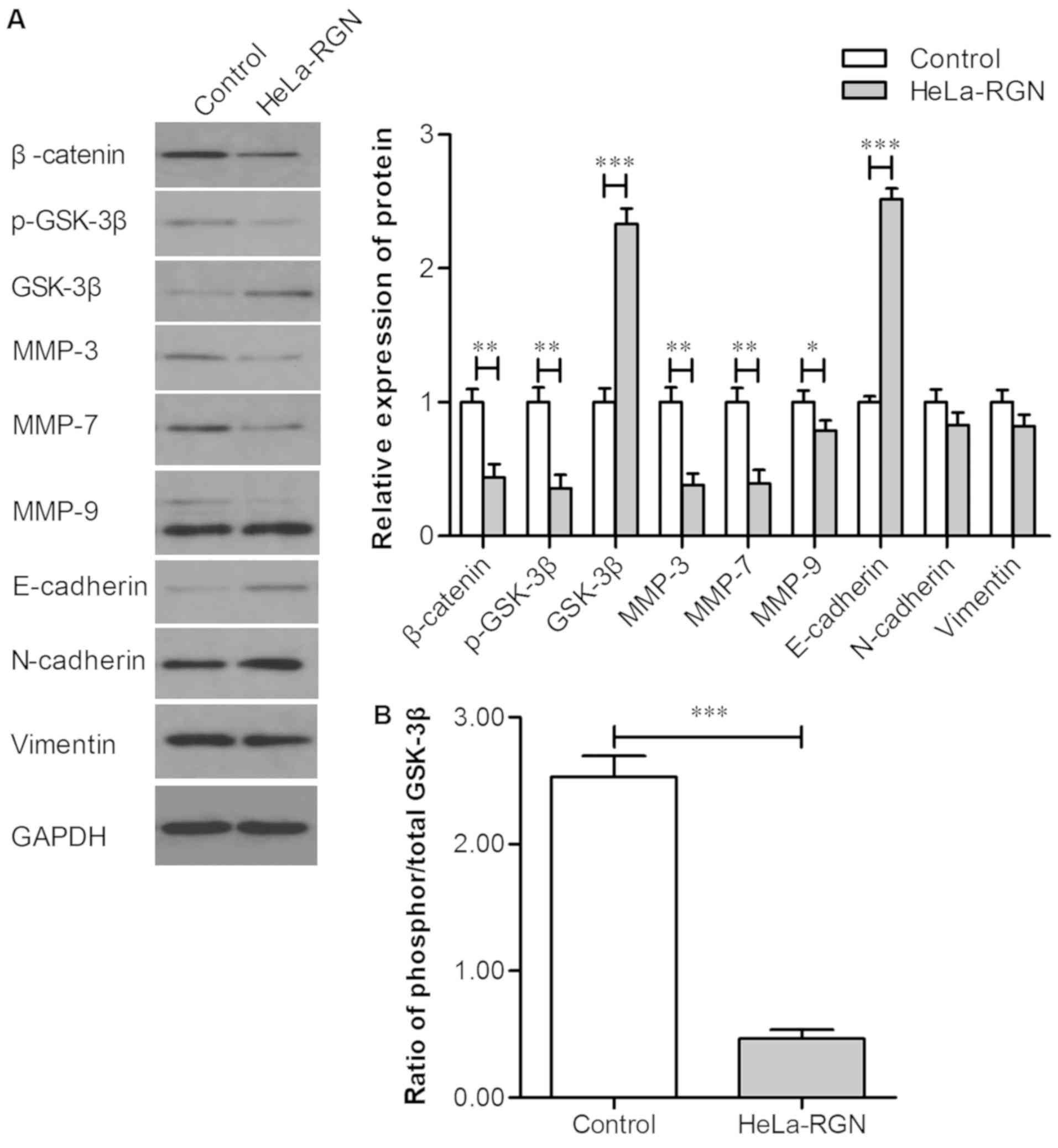
Exogenous RGN influences the
expression of β-catenin, p-GSK-3β, GSK-3β, MMP-3, MMP-7, MMP-9 and
E-cadherin
The expression levels of β-catenin, p-GSK-3β,
GSK-3β, MMP-3, MMP-7, MMP-9, E-cadherin, N-cadherin and vimentin
were measured using western blotting. The expression levels of
β-catenin, p-GSK-3β, MMP-3, MMP-7 and MMP-9 in RGN transfectants
was markedly downregulated, whilst E-cadherin and GSK-3β expression
was upregulated (Fig. 3A). There was
no significant change in the expression levels of N-cadherin and
vimentin. The ratio of phosphorylated/total GSK-3β is displayed in
Fig. 3B, and reveals that the
average ratio of phosphorylated/total GSK-3β in the controls was
higher compared with that of the HeLa-RGN cell line. The
aforementioned results indicate that exogenous RGN may effectively
inhibit the Wnt/β-catenin pathway and subsequent
epithelial-mesenchymal transition (EMT).
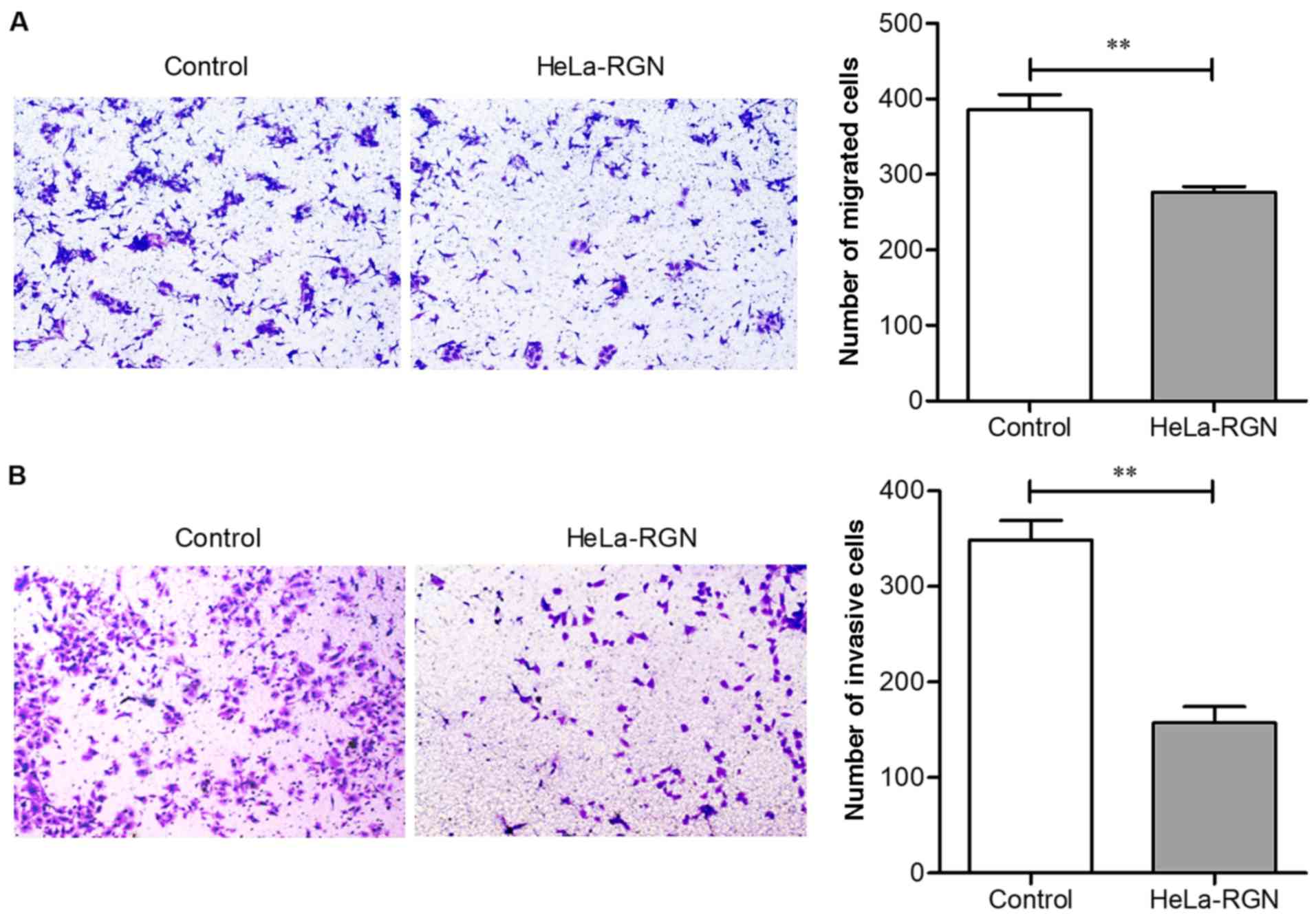
Overexpression of RGN depresses
cellular migration and invasion
Transwell and Matrigel assays were used to evaluate
the migration and invasion abilities of HeLa cells, respectively.
Fig. 4A illustrates that the
migration ability of HeLa cells was significantly depressed by RGN
(number of migrated cells: 385.67±35.02 vs. 276.33±13.32,
P<0.01), and Fig. 4B reveals that
invasiveness was also depressed in RGN transfectants (number of
invaded cells: 348.33±20.48 vs. 157.00±9.14, P<0.01). The
aforementioned results suggest that RGN overexpression may have the
ability to inhibit tumor cell metastasis.
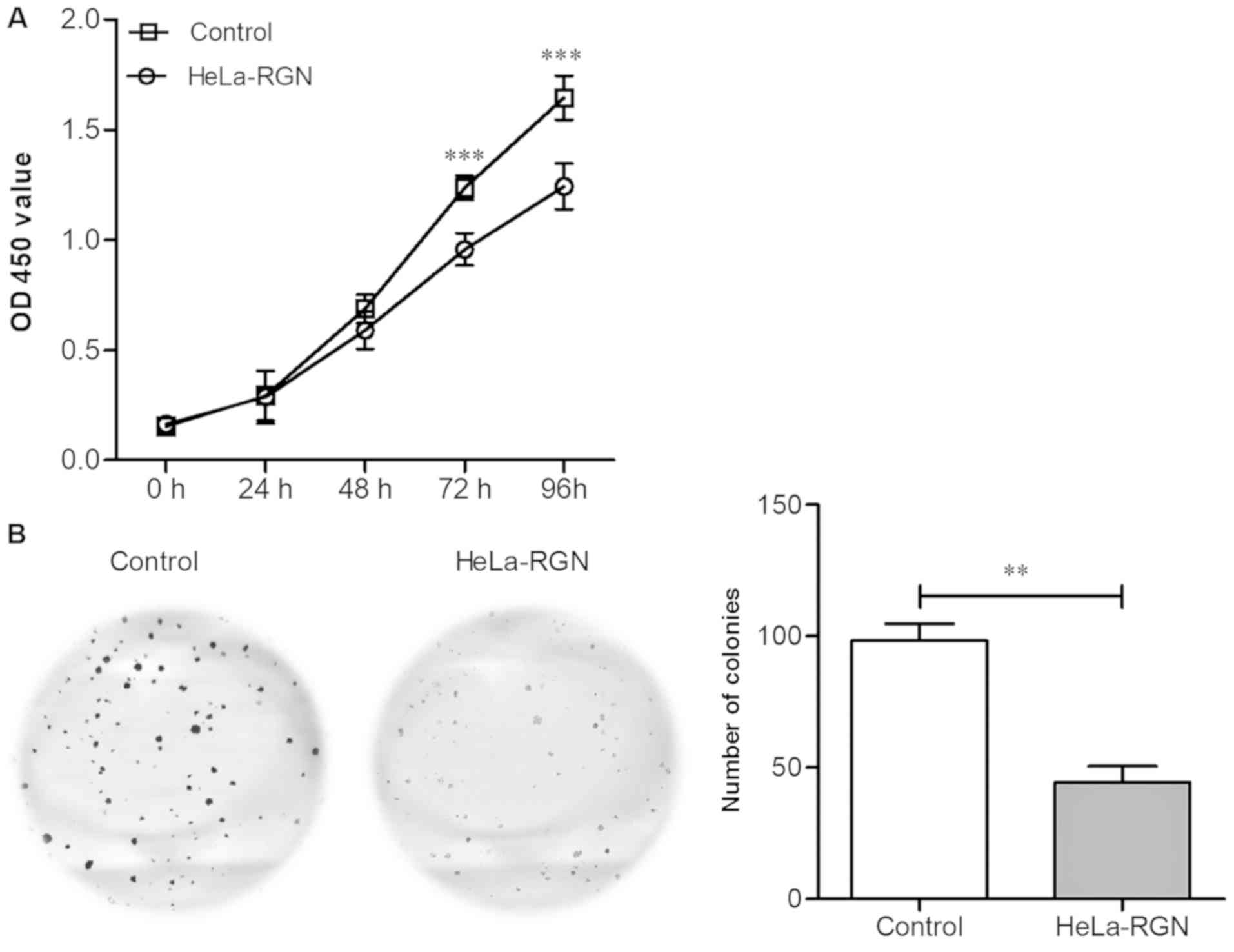
RGN overexpression inhibits cell
proliferation
Cell growth was analyzed using the CCK-8 assay.
Fig. 5A demonstrates that compared
with the control group, the proliferation rate of the RGN
transfectants was significantly lower at 72 h (P<0.001). Cell
proliferation was analyzed using a colony formation assay.
Following 14 days of incubation, cell colonies formed of RGN
transfectants were lower in number compared with those of the empty
vector controls (P<0.01; Fig.
5B). The experimental results revealed that RGN effectively
inhibits HeLa cell proliferation.
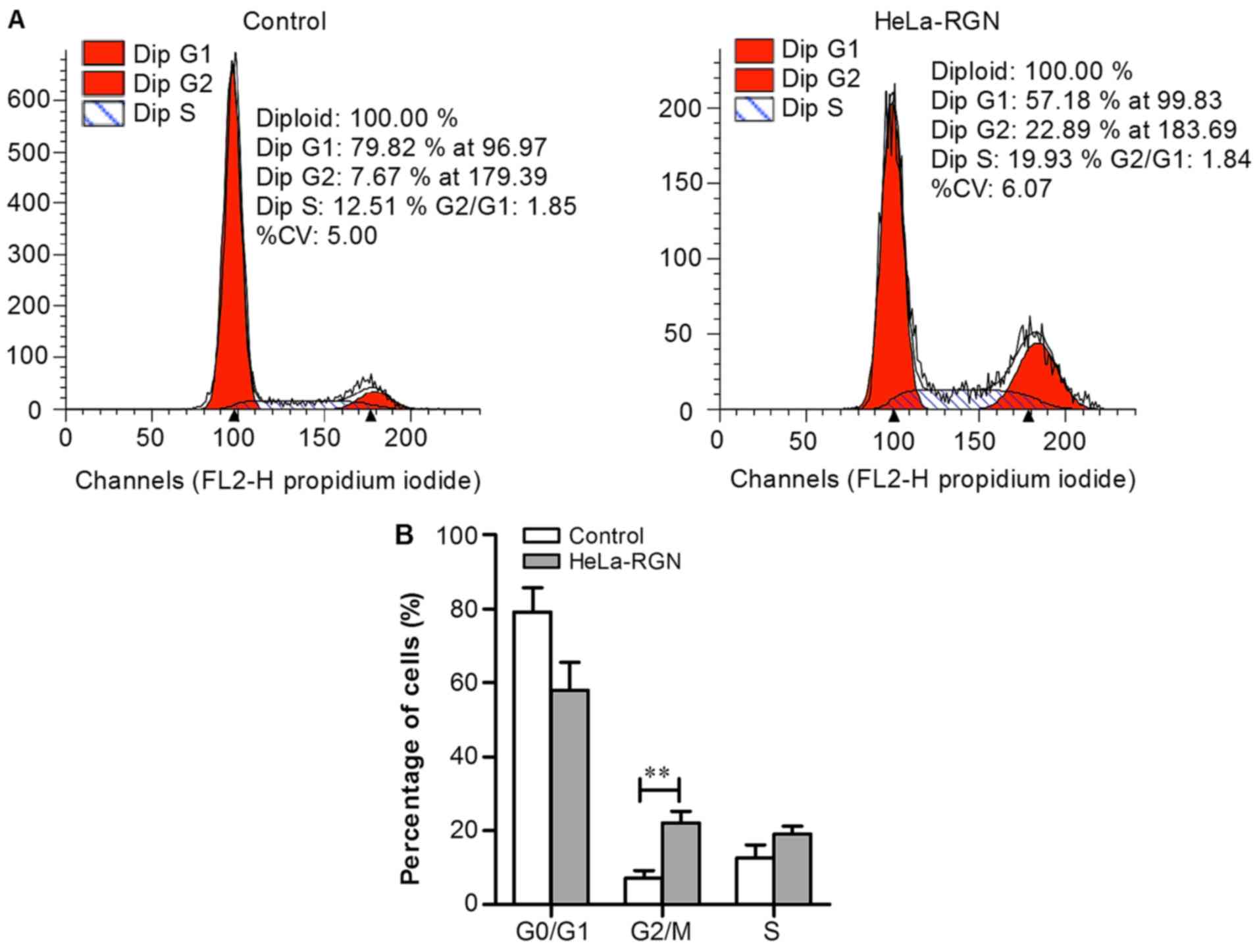
RGN upregulation halts the cell cycle
at the G2/M phase
Flow cytometry was used to compare the distribution
of RGN transfectants and control cells between the cell cycle
phases. The number of diploid HeLa-RGN cells in the G2/M
phase was greater compared with those in the control group
(Fig. 6A). The proportion of RGN
transfectants in the G2/M phase was higher compared with
that of the empty vector transfectants (P<0.01, Fig. 6B), which suggests that RGN may
depress tumor growth by retarding the cell cycle at the
G2/M phase.
Discussion
In the present study, lentivirus-mediated RGN was
successfully transfected into HeLa cells, which markedly reduced
cellular proliferation, migration and invasion. The effect of
exogenous RGN expression on specific signaling pathways was
analyzed using western blotting. The expression of β-catenin,
p-GSK-3β, MMP-3, MMP-7 and MMP-9 was downregulated, whilst
E-cadherin and GSK-3β expression was upregulated in cells
overexpressing RGN. The results suggest that RGN may regulate
proliferation and metastasis of HeLa cells by blocking the
activation of the Wnt/β-catenin signaling pathway and EMT.
The inhibitory effects of RGN on cancer cell
proliferation and metastasis have been reported in a number of
studies. It has been demonstrated that RGN depresses H4-II-E cell
proliferation by attenuating DNA synthesis, suppressing the
activity of Ca2+/calmodulin-dependent protein kinase,
protein kinase C and protein tyrosine kinase, or by altering the
mRNA expression of various intracellular signaling-associated
factors, including p21 and Insulin-like growth factor 1 (19–23).
Proliferation may also be inhibited when
transferring exogenous RGN into lung cancer cells, or by incubating
pancreatic cancer cells with RGN-supplemented media (24,25). It
was also demonstrated that RGN transgenic rats possessed a lower
propensity for breast cancer, in addition to lower cellular
proliferation and metastasis rates (26,27).
More recently, Yamaguchi et al (28) reported that the overexpression of RGN
in the colorectal adenocarcinoma cancer cell line, RKO, suppressed
proliferation and induced cell cycle arrest at the G1
and G2/M phases. The results of the present study
revealed that the proliferation, migration and invasion abilities
of RGN transfectants were significantly inhibited. Yamaguchi et
al (24,28,29) also
demonstrated that RGN promoted cell cycle arrest at the
G1 and G2/M phases in A549, HepG2 and RKO
cells. The present study on HeLa cells revealed that RGN halted the
cell cycle at the G2/M phase, but not at the
G1 phase. This suggests that the influence of RGN on the
cell cycle varies between cell types.
Numerous studies have demonstrated that RGN is
associated with various signaling pathways, including the nuclear
factor-κB, Akt, mitogen-activated protein kinase and
stress-activated protein kinases/Jun amino-terminal kinases
pathways (29,30). The overexpression of RGN suppressed
the expression of multiple oncogenes including K-ras,
Ha-ras, c-fos, c-jun, c-myc and chk,2, or
enhanced the expression of certain anti-oncogenes including
p53 and Rb (28,31,32). The
Wnt/β-catenin signaling pathway, which is associated with cell
proliferation and metastasis, is involved in cervical
carcinogenesis (33,34). Wnt signaling is activated by the
Frizzled family of transmembrane receptors; Wnt binding promotes
the binding of β-catenin and T-cell factor/lymphoid enhancer
factors in the cytosol, and their subsequent nuclear translocation;
this results in the activation of target genes, including the MMPs
(35–37). Prior to Wnt signaling activation,
β-catenin is sequestered by E-cadherin in the cytoplasm. When
β-catenin translocates to the nucleus, E-cadherin expression
decreases, a phenomenon associated with EMT. During EMT, the
expression of E-cadherin decreases significantly, while the
expression of N-cadherin and vimentin increases (38–40).
Additionally, the enhancement of GSK-3β activity results in the
ubiquitination, and subsequent phosphorylation and degradation
β-catenin (41). In the present
study, β-catenin, p-GSK-3β, MMP-3, MMP-7 and MMP-9 expression were
downregulated, while E-cadherin and GSK-3β expression were
upregulated in RGN transfectants, indicating that exogenous RGN
expression may inhibit the activation of Wnt/β-catenin signaling,
enhance cell adhesion and alter cell morphology to an epithelial
phenotype.
In conclusion, the present study may suggest that
RGN is a protective factor in CA. Though the association between
RGN, Wnt/β-catenin and EMT requires further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460432, 81572994);
Guangxi Nature Science Foundation (grant nos. 2015GXNSFDA139017,
2015GXNSFBA139155 and 2017GXNSFAA198063); Guangxi Science and
Technology Research and Technology Development Project (grant no.
15104001-7); Guangxi Nanning Qingxiu District Science and
Technology Research and Technology Development Project (grant no.
2014S03) and the Open Fund of Guangxi Key Laboratory of Biological
Targeting Diagnosis and Therapy Research (grant no.
KFJJ2014-03).
Availability of data and materials
All data generated or analyzed during the present
study are included in this publication.
Authors' contributions
SZ and XL conceived the study. XL and YH were major
contributors in writing the manuscript. SZ, XL, YH, SG, MX, XB, MS,
AC, SC, FW and QH performed the experiments and data analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamaguchi M and Sugii K: Properties of
calcium-binding protein isolated from the soluble fraction of
normal rat liver. Chem Pharm Bull (Tokyo). 29:567–570. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamaguchi M and Yoshida H: Regulatory
effect of calcium-binding protein isolated from rat liver cytosol
on activation of fructose 1,6-diphosphatase by Ca2+-calmodulin.
Chem Pharm Bull (Tokyo). 33:4489–4493. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamaguchi M and Mori S: Effect of Ca2+ and
Zn2+ on 5′-nucleotidase activity in rat liver plasma membranes:
Hepatic calcium-binding protein (regucalcin) reverses the Ca2+
effect. Chem Pharm Bull (Tokyo). 36:321–325. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamaguchi M, Mori S and Suketa Y: Effects
of Ca2+ and V5+ on glucose-6-phosphatase activity in rat liver
microsomes: The Ca2+ effect is reversed by regucalcin. Chem Pharm
Bull (Tokyo). 37:388–390. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaguchi M and Shibano H: Effect of
calcium-binding protein on the activation of phosphorylase a in rat
hepatic particulate glycogen by Ca2+. Chem Pharm Bull (Tokyo).
35:2581–2584. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamaguchi M and Shibano H: Calcium-binding
protein isolated from rat liver cytosol reverses activation of
pyruvate kinase by Ca2+. Chem Pharm Bull (Tokyo). 35:2025–2029.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaguchi M and Shibano H: Reversible
effect of calcium-binding protein on the Ca2+-induced activation of
succinate dehydrogenase in rat liver mitochondria. Chem Pharm Bull
(Tokyo). 35:3766–3700. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaguchi M, Mori S and Kato S:
Calcium-binding protein regucalcin is an activator of
(Ca2+-Mg2+)-adenosine triphosphatase in the plasma membranes of rat
liver. Chem Pharm Bull (Tokyo). 36:3532–3539. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang SC, Liang MK, Huang GL, Jiang K,
Zhou SF and Zhao S: Inhibition of SMP30 gene expression influences
the biological characteristics of human Hep G2 cells. Asian Pac J
Cancer Prev. 15:1193–1196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Denny L: Cervical cancer: Prevention and
treatment. Discov Med. 14:125–131. 2012.PubMed/NCBI
|
|
11
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith HO, Tiffany MF, Qualls CR and Key
CR: The rising incidence of adenocarcinoma relative to squamous
cell carcinoma of the uterine cervix in the United States-a 24-year
population-based study. Gynecol Oncol. 78:97–105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galic V, Herzog TJ, Lewin SN, Neugut AI,
Burke WM, Lu YS, Hershman DL and Wright JD: Prognostic significance
of adenocarcinoma histology in women with cervical cancer. Gynecol
Oncol. 125:287–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimada M, Kigawa J, Nishimura R,
Yamaguchi S, Kuzuya K, Nakanishi T, Suzuki M, Kita T, Iwasaka T and
Terakawa N: Ovarian metastasis in carcinoma of the uterine cervix.
Gynecol Oncol. 101:234–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang YT, Wang CC, Tsai CS, Lai CH, Chang
TC, Chou HH, Hsueh S, Chen CK, Lee SP and Hong JH: Long-term
outcome and prognostic factors for adenocarcinoma/adenosquamous
carcinoma of cervix after definitive radiotherapy. Int J Radiat
Oncol Biol Phy. 80:429–436. 2011. View Article : Google Scholar
|
|
16
|
Katanyoo K, Tangjitgamol S, Chongthanakorn
M, Tantivatana T, Manusirivithaya S, Rongsriyam K and Cholpaisal A:
Treatment outcomes of concurrent weekly carboplatin with radiation
therapy in locally advanced cervical cancer patients. Gynecol
Oncol. 123:571–576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yee GP, de Souza P and Khachigian LM:
Current and potential treatments for cervical cancer. Curr Cancer
Drug Targets. 13:205–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kubista M, Andrade JM, Bengtsson M,
Forootan A, Jonák J, Lind K, Sindelka R, Sjöback R, Sjögreen B,
Strömbom L, et al: The real-time polymerase chain reaction. Mol
Aspects Med. 27:95–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inagaki S and Yamaguchi M: Suppressive
role of endogenous regucalcin in the enhancement of protein kinase
activity with proliferation of cloned rat hepatoma cells (H4-II-E).
J Cell Biochem Suppl. 36 (Suppl):S12–S18. 2001. View Article : Google Scholar
|
|
20
|
Inagaki S and Yamaguchi M: Regulatory role
of endogenous regucalcin in the enhancement of nuclear
deoxyribonuleic acid synthesis with proliferation of cloned rat
hepatoma cells (H4-II-E). J Cell Biochem. 82:704–711. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Misawa H, Inagaki S and Yamaguchi M:
Suppression of cell proliferation and deoxyribonucleic acid
synthesis in the cloned rat hepatoma H4-II-E cells overexpressing
regucalcin. J Cell Biochem. 84:143–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamaguchi M and Daimon Y: Overexpression
of regucalcin suppresses cell proliferation in cloned rat hepatoma
H4-II-E cells: Involvement of intracellular signaling factors and
cell cycle-related genes. J Cell Biochem. 95:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamaguchi M: Role of regucalcin in
maintaining cell homeostasis and function (review). Int J Mol Med.
15:371–389. 2005.PubMed/NCBI
|
|
24
|
Yamaguchi M, Osuka S, Shoji M, Weitzmann
MN and Murata T: Survival of lung cancer patients is prolonged with
higher regucalcin gene expression: Suppressed proliferation of lung
adenocarcinoma A549 cells in vitro. Mol Cell Biochem. 430:37–46.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi M and Murata T: Suppressive
effects of exogenous regucalcin on the proliferation of human
pancreatic cancer MIA PaCa-2 cells in vitro. Int J Mol Med.
35:1773–1778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marques R, Vaz CV, Maia CJ, Gomes M, Gama
A, Alves G, Santos CR, Schmitt F and Socorro S: Histopathological
and in vivo evidence of regucalcin as a protective molecule in
mammary gland carcinogenesis. Exp Cell Res. 330:325–335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maia C, Santos C, Schmitt F and Socorro S:
Regucalcin is under-expressed in human breast and prostate cancers:
Effect of sex steroid hormones. J Cell Biochem. 107:667–676. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamaguchi M, Osuka S and Murata T:
Prolonged survival of patients with colorectal cancer is associated
with a higher regucalcin gene expression: Overexpression of
regucalcin suppresses the growth of human colorectal carcinoma
cells in vitro. Int J Oncol. 53:1313–1322. 2018.PubMed/NCBI
|
|
29
|
Yamaguchi M, Osuka S, Weitzmann MN,
El-Rayes BF, Shoji M and Murata T: Prolonged survival in
hepatocarcinoma patients with increased regucalcin gene expression:
HepG2 cell proliferation is suppressed by overexpression of
regucalcin in vitro. Int J Oncol. 49:1686–1694. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamaguchi M, Osuka S, Weitzmann MN,
El-Rayes BF, Shoji M and Murata T: Prolonged survival in pancreatic
cancer patients with increased regucalcin gene expression:
Overexpression of regucalcin suppresses the proliferation in human
pancreatic cancer MIA PaCa-2 cells in vitro. Int J Oncol.
48:1955–1964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsurusaki Y and Yamaguchi M: Role of
regucalcin in liver nuclear function: Binding of regucalcin to
nuclear protein or DNA and modulation of tumor-related gene
expression. Int J Mol Med. 14:277–281. 2004.PubMed/NCBI
|
|
32
|
Tsurusaki Y and Yamaguchi M:
Overexpression of regucalcin modulates tumor-related gene
expression in cloned rat hepatoma H4-II-E cells. J Cell Biochem.
90:619–626. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jacques BE, Montgomery WH IV, Uribe PM,
Yatteau A, Asuncion JD, Resendiz G, Matsui JI and Dabdoub A: The
role of Wnt/β-catenin signaling in proliferation and regeneration
of the developing basilar papilla and lateral line. Dev Neurobiol.
74:438–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bahrami A, Hasanzadeh M, ShahidSales S,
Yousefi Z, Kadkhodayan S, Farazestanian M, Joudi Mashhad M, Gharib
M, Mahdi Hassanian S and Avan A: Clinical significance and
prognosis value of Wnt signaling pathway in cervical cancer. J Cell
Biochem. 118:3028–3033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burgy O and Königshoff M: The WNT
signaling pathways in wound healing and fibrosis. Matrix Biol
68–69. 67–80. 2018. View Article : Google Scholar
|
|
36
|
Kypta RM and Waxman J: Wnt/β-catenin
signalling in prostate cancer. Nat Rev Urol. 9:418–428. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Su GY, Zhao C, Qu FZ, Wang P and
Zhao YQ: Anticancer activity and potential mechanisms of 1C, a
ginseng saponin derivative, on prostate cancer cells. J Ginseng
Res. 42:133–143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cong N, Du P, Zhang A, Shen F, Su J, Pu P,
Wang T, Zjang J, Kang C and Zhang Q: Downregulated microRNA-200a
promotes EMT and tumor growth through the wnt/β-catenin pathway by
targeting the E-cadherin repressors ZEB1/ZEB2 in gastri
adenocarcinoma. Oncol Rep. 29:1579–1587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu HG, Zheng Q, Song JX, Li J, Wang H, Liu
P, Wang J, Wang CD and Zhang XL: Intermittent cyclic mechanical
tension promotes endplate cartilage degeneration via canonical Wnt
signaling pathway and E-cadherin/β-catenin complex cross-talk.
Osteoarthritis Cartilage. 24:158–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang X, Yang M, Shi H, Hu J, Wang Y, Sun
Z and Xu S: Reduced E-cadherin facilitates renal cell carcinoma
progression by WNT/β-catenin signaling activation. Oncotarget.
8:19566–19576. 2017.PubMed/NCBI
|
|
41
|
Fang X, Yu SX, Lu Y, Bast RC Jr, Woodgett
JR and Mills GB: Phosphorylation and inactivation of glycogen
synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA.
97:11960–11965. 2000. View Article : Google Scholar : PubMed/NCBI
|