Introduction
Gastric cancer (GC) is the third leading cause of
cancer mortality worldwide in 2012, responsible for 723,000 deaths
(1), with high incidence in Asia
(2). The vast majority (about 95%)
of gastric tumors are adenocarcinomas, which can be further
histologically classified into intestinal, diffuse and mixed types
according to the Lauren classification (3). The classification proposed by the World
Health Organization divides GCs into well to moderately
differentiated, and poorly differentiated (4). Intestinal subtype GCs are a well
differentiated and clustered sub-type, while diffuse-type is poorly
differentiated, infiltrating and scattered. Poorly cohesive gastric
carcinoma, also considered as diffuse GC, include signet-ring cells
(SRCC) and other types of poorly cohesive GC.
The incidence, distribution and characteristics of
histological subtypes of GC may vary across the globe. During the
last 50 years, the incidence and the mortality of GC have declined
worldwide, especially in developed countries (5). This decline has primarily included the
intestinal type. The intestinal-type GC predominates in high risk
geographic areas, such as East Asia, particularly in Japan and
Korea, and its incidence increases with age. In contrast, the
diffuse-type is more uniformly distributed geographically, but with
an increasing incidence in the USA and in Europe (6,7),
especially the SRCC (signet-ring cell carcinoma) (8,9). The
prognosis of diffuse adenocarcinoma has been debated and depends on
the stage of the cancer. For early GC, i.e., not extending beyond
the submucosa (mostly described in Asian countries), it is clearly
established that the prognosis of SRC-type is better than that of
non-SRC adenocarcinoma (10–15), probably because the SRC-type tumor is
more frequently confined to the mucosa and shows a lower rate of
metastasis. In contrast, numerous studies from Asia and a few
studies in Europe have demonstrated that diffuse-type GC was more
frequently diagnosed at a later stage, with a high proportion of
such tumors invading sub-serosa or serosa with lymph node
metastasis, and was associated with poorer overall survival
(16–18).
The diffuse sub-population of GC is apparently
unrelated to Helicobacter Pylori (H. pylori) and
develops from morphologically normal gastric mucosa without
atrophic gastritis; in contrast the intestinal-type GC that arises
from chronic atrophic gastritis and is associated with infectious
agents including H. pylori and Epstein-Barr virus (EBV)
(19). Interest has recently focused
on a subgroup of younger patients (aged <35 years, 1/1 sex
ratio) with higher incidence of diffuse tumor type by the Lauren
classification (13,20). At diagnosis, positive axillary node
(83% vs. 6% in intestinal-type) and peritoneal carcinomatosis
(18.6% vs. 6% in intestinal-type) are present (13,20).
Patients are usually treated when the cancer is at an advanced
stage. They are generally refractory to conventional therapeutic
approaches and their tumors are often associated with recurrence,
chemioresistance (18,21,22).
Therefore, molecular characterization and gene expression profile
of diffuse-type GC, especially those with infiltrating and
scattered growth, are critical for identifying candidate players in
GC progression.
GC is a complex and molecular heterogeneous disease
involving genetic instability and notable epigenetic modifications
(DNA methylation, microRNA and histone modifications) that have
critical roles in gastric tumorigenesis. A robust molecular
classification of GC was performed by the Cancer Genome Atlas
(TCGA) project (23). GC were
classified into four different molecular subtypes: EBV (9%), MSI
(22%), genomically stable (20%), and chromosomal instability (50%).
A small minority of GCs among the genomically stable GC was
associated with germline mutations in CDH1 (E-cadherin, a
well-known suppressor of invasion/metastasis) or in RHOA,
and correspond to the diffuse histological subtype (23,24).
There are few studies that report signaling pathways in
diffuse-subtype GCs (such as hedgehog-EMT pathway, Wnt/β catenin
signaling) or genes located downstream PI3K/Akt (2,15). Based
on expression patterns 3 molecular sub-types of GC were also
defined: proliferative, metabolic and mesenchymal (25). Our objective was to analyze molecular
characteristics of a French cohort of diffuse-type GC.
To compare signaling pathways in GC subpopulations
and identify new therapeutic targets for diffuse-type GCs, we used
quantitative RT-PCR assays of 29 gastric tumor samples to quantify
the mRNA expression of 33 genes. The list of genes was selected
from the literature (from PubMed/Medline) to be involved in various
digestive tumorigenesis and altered (mainly at the transcriptional
level) in various cancers. We also included genes reported to be
involved in diffuse-GCs according to Lauren classification,
schirrous/linitis GC, lymph node metastasis. The 33 genes encode
proteins involved in cell categories including growth factors and
receptors, epithelial-mesenchymal transition (EMT), cell
proliferation and migration, and angiogenesis. Furthermore, we
compared the expression of each gene with clinico-pathological
parameters in each subpopulation of GCs.
Materials and methods
Patients and tissue samples
A total of 29 patients underwent partial gastrectomy
for histopathologically-confirmed gastric adenocarcinoma primary
tumor tissue in the Lariboisiere Hospital (Paris, France) from 2005
to 2014. All patients provided written informed consent prior to
their inclusion in the study. Biopsies were taken for diagnosis
purposes (provided before 2014) and the present study was approved
by the Ethical Committee of Lariboisiere Hospital (Paris, France).
Eligibility criteria included: i) gastric carcinoma identified by
histopathological examination; ii) no other malignancy; iii) no
pre-operative chemotherapy or radiotherapy, and iv) availability of
complete clinical, histological and biological data. The
histological type and the number of positive axillary nodes were
determined at the time of surgery. Normal (non-malignant) samples
refer to samples harvested from the stomach, from sites distant
from the tumor. Immediately after surgery, fresh gastric tumors and
their matched normal mucosa were stored in liquid nitrogen until
mRNA extraction; other tumor samples and their adjacent normal
tissues were routinely fixed in 10% buffered formalin and embedded
in paraffin for histological analysis. The population was divided
into two groups according to the histological status of GC:
intestinal-subtype adenocarcinoma (n=16) or diffuse subtype
adenocarcinoma (n=13) according to the Lauren Classification
(Table I). A diffusely infiltrating
type of poorly differentiated gastric carcinoma associated with
extensive stromal fibrosis, called Linitis plastic carcinoma
(26), was also included in the
study. The malignancy of infiltrating carcinomas was scored
according to TNM staging system (Stage I to IV) first according to
AJCC7 (27), revised from IGCA
(28,29) and AJCC8 (30). This TNM staging includes T score in
the primary tumor (T1-T4), N score (lymph node metastasis, N1-N3
including pN3a and pN3b) and M (metastatic disease).
 | Table I.Clinicopathological characteristics
of patients with GC; diffuse-subtype and intestinal-subtype
adenocarcinomas. |
Table I.
Clinicopathological characteristics
of patients with GC; diffuse-subtype and intestinal-subtype
adenocarcinomas.
| Clincopathological
characteristic | Total, n=29 | Diffuse/poorly
cohesive GCa, n=13
(45%) | Intestinal-subtype
GCb, n=16 (55%) | P-value |
|---|
| Sex, n (%) |
|
|
| 0.9c |
|
Male | 13/29 | 6/13 (46%) | 7/16 (43%) |
|
|
Female | 16/29 | 7/13 (54%) | 9/16 (56%) |
|
| Age, years
(median) | 63+/−17 | 57 (27–71) | 75 (59–82) | 0.0004d |
| Linitis (presence
of fibrosis) |
|
|
| 0.0014c |
|
Positive | 9/29 | 8/13 (61.5%) | 1/16 (6%) |
|
|
Negative | 20/9 | 5/13 (38.5%) | 15/16 (94%) |
|
| Tumor size, mm |
|
<50 | 10/27 | 4/11 (36%) | 6/16 (37%) | 0.1d |
|
≥50 | 17/27 | 7/11 (64%) | 10/16 (63%) | 0.9c |
| Depth of tumor
invasion (T) |
|
|
| 0.5c |
|
T1-T2 | 6/29 | 2/13 (15%) | 4/16 (33 %) |
|
|
T3-T4 | 23/29 | 11/13 (85%) | 12/16 (67 %) |
|
| Lymphatic invasion,
n (%) |
|
|
| 0.006c |
|
Positive | 16/29 | 11/13 (85%) | 5/15 (33%) |
|
|
Negative | 13/29 | 2/13 (15%) | 10/15 (67 %) |
|
| Vascular invasion,
n + (%) |
|
Positive | 20/29 | 10/13 (77%) | 10/16 (62%) | 0.4c |
|
Negative | 9/29 | 3/13 (23%) | 6/16 (38%) | NS |
| Neural invasion, n
(%) |
|
|
|
|
|
Positive | 23/29 | 11/13 (68%) | 12/16 (75%) | 0.5c |
|
Negative | 6/29 | 2/13 | 4/16 | NS |
| Metastasis sites
(M), n (%) |
|
|
| 0.033c |
| Peritoneal | 6/29 | 5/13 (38%) | 1/16 (6%) |
|
| Others | 6/29 | 1 (pancreas and
colon) | 1 (liver) |
|
Total RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA extraction, cDNA synthesis and PCR conditions
were as described elsewhere (31,32). The
theoretical and practical aspects of real-time quantitative PCR
have been described in detail elsewhere (31), using ABI Prism 7900 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The precise amount of total RNA added to
each reaction mix (based on optical density) and its quality (lack
of extensive degradation) are both difficult to assess. Therefore,
we quantified transcripts of 3 endogenous RNA control genes
involved in various cellular metabolic pathways, namely TBP
(32), (Genbank accession
NM_003194), which encodes the TATA box-binding protein (a component
of the DNA-binding protein complex TFIID); RPL0 (32) (also known as 36B4; NM_001002), which
encodes human acidic ribosomal phosphoprotein P0; and PPIA
(32), which encodes peptidylprolyl
isomerase A (also known as cyclophilin A; NM_021130).
By studying the literature, we selected 33 genes
coding for the major proteins known to be involved in cancers such
as growth factors and receptors (n=10), epithelial-mesenchymal
transition (EMT, n=10), cell proliferation and migration (n=7), and
angiogenesis (n=6). Among these genes, changes in expression levels
of CDH1 (decrease) and of VIM, ZEB2 and CXCR4
(increase) have been previously suggested in diffuse sub-population
(23,33,34),
while increase in ERBB2 expression was described in
intestinal sub-type.
Primers for TBP, RPLLP0, PPIA, and the 33
target genes were chosen with the assistance of the Oligo v.6.0
computer program (National Biosciences, Plymouth, MN, USA). We
searched the dbEST and nr databases to confirm the absence of
single nucleotide polymorphisms in the primer sequences and the
total gene specificity of the nucleotide sequences chosen as
primers. The nucleotide sequence of the primers used to amplify
MKI67 and the other 32 (33 with TBP) target genes are
available on request.
Each sample was normalized on the basis of its
TBP content. Results, expressed as N-fold differences
in target gene expression relative to the TBP gene and termed
“Ntarget” were determined as Ntarget =
2ΔCtsample, where the ΔCt value of the sample is
determined by subtracting the average Ct value of the target gene
from the average Ct value of the TBP gene (31,32).
Preliminary analysis of gene expression have compared basal levels
(arbitrary values) in normal samples in the same patients as their
tumors (either or diffuse- or intestinal-subtypes). We did not
observe changes for most of the genes described in the study (ratio
for the median levels ranging from 0.8 to 1.2). The Ntarget
values of the samples were subsequently normalized such that the
median of the 11 normal gastric tissue Ntarget values was 1.
For each gene, normalized RNA values of 3 or more were considered
to represent gene overexpression in tumor samples, and values 0.33
or less represented gene underexpression.
Immunohistochemistry
Immunohistochemical labeling was performed on
paraffin sections (4 µm), as previously described (35,36).
Sections were deparaffinized, rehydrated in graded alcohol, and
subjected to antigen retrieval in citrate buffer (pH 6.0) in a high
pressure cooker. After nonspecific staining had been blocked using
a blocking agent, sections were incubated overnight with the
anti-IGF1 antibodies (rabbit polyclonal sc-9013; 1:200 dilution;
Abcam, Cambridge, UK) at 4°C using Ventana Autostainer (Roche
Diagnostics, Indianapolis, IN, USA). The antigen-antibody complex
was visualized blindly by two specialists including
pathologist.
Statistical analysis
As the mRNA levels of gene expression did not fit a
Gaussian distribution, the mRNA levels in each subgroup of samples
were characterized by their median values and ranges rather than
their mean values and coefficient of variation. For each gene,
differences of expression between tumor versus non tumoral gastric
tissues (fold change) were analyzed using the Kruskal-Wallis test
(36); differences in the number of
samples that over- (>3-fold) or and under- (<3-fold)
expressed were analyzed using the χ2 test (36). When indicated, the Mann-Whitney test
was used in some studies. The correlations (non parametric
Spearman) between expression of genes in GC (poorly
cohesive/diffuse adenocarcinoma) were determined. Relationships
between expression levels and clinical parameters were analyzed
using non parametric Kruskal-Wallis (or Mann-Whitney) and
χ2 tests, as indicated in each Table. Statistical
analyses were performed using Prism v.5.03 software (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient's characteristics
The clinicopathological characteristics of the 29
patients of the present study are shown in Table I. The distribution of the gastric
tumor subtypes was as follows: diffuse (n=13) and
intestinal-subtype (n=16) GC. The median age of patients with
diffuse GC was significantly lower 57 (27–71) years as compared
with 75 (59–82) years for intestinal-subtype (P=0.0004; Table I). Both sub-types of carcinoma have a
great tumor invasion (T3-T4); however, a higher proportion of
patients with poorly cohesive GC presented T3-T4 tumor stage.
Patients with diffuse adenocarcinoma have more lymphatic invasion
(a higher positive axillary node count) (P=0.006) and metastasis
(P=0.033) than patients with intestinal-subtype (Table I). Vascular and neural invasion were
not different (Table I). A
sub-population of diffuse GC associated with extensive fibrosis was
present in 62% of the patients (Table
I), similar to a previous report (26). In addition, when comparing the TNM
stage, diffuse GC was present at TNM stage II, III and IV (38%, 31%
and 31% respectively), while intestinal-subtype was more likely
stage I, II and III (26%, 44% and 25%).
Expression of 32 target genes and
MKI67 in diffuse GCs
We used real-time quantitative RT-qPCR to analyse
mRNA expression of 33 target genes in the 13 diffuse GC as compared
with the 11 non-tumoral gastric samples (Table II). These target genes were selected
from several important signalling pathways known to be involved in
cancer such as growth factors and associated proteins (n=10),
epithelial-mesenchymal transition (EMT, n=10), cell proliferation
and migration (n=7), and angiogenesis (n=6). The mRNA levels of the
33 target genes were high in both the non-tumoral and tumoral
gastric tissues and were thus reliably measured by real-time
RT-qPCR using fluorescence SYBR Green method (cycle threshold,
CT<35). mRNA levels in cancers were expressed relative to the
median mRNA levels observed in the 11 non-tumoral gastric tissues.
Medians and ranges of mRNA levels for the 33 target genes, along
with the percentages of overexpression and underexpression, are
shown in Table II.
 | Table II.Statistical analysis of mRNA
expression of genes in diffuse/poorly cohesive gastric cancers
relative to the peri-tumoral tissues. |
Table II.
Statistical analysis of mRNA
expression of genes in diffuse/poorly cohesive gastric cancers
relative to the peri-tumoral tissues.
| A, Growth factors
and receptors (n=10) |
|---|
|
|---|
| Genes | Nontumoral gastric
tissues(n=11)b | Diffuse/poorly
cohesive carcinoma (n=13)b |
P-valuea |
|---|
| IGF1 | 1 (0.42–2.93) | 7.37
(0.97–12.75) | 0.00037 |
| IGF2 | 1 (046–3.13) | 2.26
(0.80–19.16) | 0.024 |
| IGF1R | 1 (0.55–1.46) | 1.10
(0.63–1.55) | 0.79 (NS) |
| IGF2R | 1 (0.79–1.33) | 1.43
(0.85–1.65) | 0.0071 |
| IRS1 | 1 (0.59–1.90) | 1.50
(0.91–2.71) | 0.0084 |
| IRS2 | 1 (0.62–1.69) | 1.13
(0.52–4.44) | 0.51 (NS) |
| FGF7 | 1 (0.25–2.62) | 2.16
(1.10–3.45) | 0.0019 |
| FGFR1 | 1 (0.55–2.95) | 1.94
(0.96–3.53) | 0.060 (NS) |
| ERRB2 | 1 (0.43–1.41) | 1.62
(0.87–2.74) | 0.0016 |
| ERRB3 | 1 (0.19–1.70) | 0.91
(0.51–2.55) | 0.98 (NS) |
|
| B, EMT and
migration (n=10) |
|
| Genes | Nontumoral
gastric tissues(n=11)b | Diffuse/poorly
cohesive carcinoma (n=13)b |
P-valuea |
|
| VIM | 1 (0.65–1.64) | 1.62
(0.88–2.37) | 0.0013 |
| CDH1 | 1 (0.05–1.22) | 0.78
(0.01–1.06) | 0.04 |
| SNAI1 | 1 (0.29–2.07) | 2.39
(0.90–4.92) | 0.0019 |
| SLUG/SNAI2 | 1 (0.61–2.0) | 3.02
(1.15–4.06) | 0.0013 |
| TWIST2 | 1 (0.58–2.82) | 2.30
(0.62–3.29) | 0.046 |
| TGFB1 | 1 (0.47–1.23) | 2.05
(1.47–4.27) | 0.000034 |
| RUNX3 | 1 (0.00–2.23) | 1.77
(0.00–4.91) | 0.0065 |
| ZEB2 | 1 (0.58–1.41) | 1.70
(0.84–2.97) | 0.00046 |
| CXCR4 | 1 (0.47–3.56) | 3.14
(1.52–7.30) | 0.00086 |
| CXCL12 | 1 (0.26–3.49) | 1.05
(0.30–3.19) | 0.75 (NS) |
|
| C, Cell
proliferation and migration (n=7) |
|
| Genes | Nontumoral
gastric tissues(n=11)b | Diffuse/poorly
cohesive carcinoma (n=13)b |
P-valuea |
|
| MMP2 | 1 (0.68–1.99) | 3.21
(1.47–6.15) | 0.000057 |
| MMP9 | 1 (029–2.76) | 2.01
(0.92–4.13) | 0.015 |
| SPP1
osteopontin | 1 (0.43–2.04) | 4.12
(1.75–89.35 | 0.000057 |
| CD44 | 1 (0.57–1.89) | 1.73
(1.02–2.81) | 0.0019 |
| RHOB | 1 (0.30–2.84) | 0.52
(0.32–1.23) | 0.21 (NS) |
| RHOA | 1 (0.05–5.29) | 8.65
(0.03–20.39) | 0.0026 |
| MKI67 | 1 (0.1–3.71) | 3.76
(1.34–10.83) | 0.00078 |
|
| D, Angiogenesis
(n=6) |
|
| Genes | Nontumoral
gastric tissues(n=11)b | Diffuse/poorly
cohesive carcinoma (n=13)b |
P-valuea |
|
| VEGFA 165 | 1 (0.68–1.56) | 0.86
(0.58–2.09) | 0.71 (NS) |
| VEGFA 189 | 1 (0.49–1.63) | 0.67
(0.42–1.00) | 0.0091 |
| FLT1 | 1 (0.64–2.20) | 1.08
(0.62–2.07) | 0.91 (NS) |
| KDR | 1 (0.63–2.63) | 1.11
(0.67–1.47) | 0.40 (NS) |
| VEGFC | 1 (0.44–1.57) | 1.38
(0.86–2.60) | 0.0071 |
| NRP1 | 1 (0.57–1.87) | 1.94
(1.21–3.27) | 0.00037 |
Twenty two genes were significantly up-regulated and
2 genes were down-regulated in diffuse-GC as compared to
non-tumoral gastric samples. Up-regulated genes were: i) growth
factors: IGF1 (×7.4, P=0.0004) and IGF2 (P=0.024),
IGF2R (P=0.0071) and IRS1 (P=0.0084), FGF7
(P=0.0019) and ERBB2 (P=0.0016); ii) genes involved in EMT:
VIM (P=0.0013), SNAI1 (P=0.0019), SNAI2/SLUG
(×3, P=0.0013), TWIST2 (P=0.046), TGFβ1 (P=0.00003),
RUNX3 (P=0.0065), ZEB2 (P=0.0005), and CXCR4
(×3, P=0.0009), and iii) migration: MMP2 (×3.2, P=0.00006),
MMP9 (P=0.015), SPP1 (×4.1, P=0.00006), CD44
(P=0.002), RHOA (P=0.0026; Table
II). Other dysregulated genes include VEGF-C (P=0.0071),
NRP1 (P=0.00037), and MKI67 (×3.8, P=0.0008); this
latter gene encodes the proliferation-related antigen Ki-67. In
addition, overexpression (>3-fold) in more than 50% of the
tumors was significant for IGF1 (>75%), SLUG, CXCR4,
MMP2, SPP1, RHOA (>75%) and MKI67, as compared non
tumoral gastric tissues (Table II).
In contrast, expression of CDH1 (P=0.04) and VEGFA189
(P=0.009) were down-regulated in diffuse GC as compared to
non-tumoral gastric samples (Table
II).
Expression of 32 target genes and
MKI67 in intestinal GCs
The expression of the same 33 target genes was then
analysed in the series of 16 intestinal sub-type GC. Medians and
ranges of mRNA levels for the target genes are shown in Table S1, along with the percentages of
overexpression and underexpression. As compared to the non-tumoral
tissues, fourteen genes that were significantly up-regulated
included IGF2R (P=0.0033), ERBB2 (P=0.00006) and
ERBB3 (P=0.032), SNAI1 (P=0.0005), SNAI2/SLUG
(P=0.0031), TGFβ1 (P=0.00003), MMP2 (P=0.01),
MMP9 and SPP1 (×15, P<0.0007 and ×5, P<0.00004,
respectively), CD44 (P=0.03) and RHOA (P=0.03),
VEGFC (P=0.041) and NRP1 (P=0.023), and MKI67
(×8.5, P=0.00003; Table S1). In
contrast, expression of IGF1R (P=0.034), CXCL12
(P=0.023) and RHOB (P=0.0066) was significantly decreased
(Table S1); underexpression
(>3-fold decrease) of CXCL12 and RHOB was observed
in 44% of the intestinal-type GC.
Differential expression of genes
between the GC subtypes
Comparison of gene expression in diffuse-subtype
with respect to intestinal-GC revealed increased levels for
IGF1 (P=0.0012) and IGF1R (P=0.044), FGF7
(P=0.0001) and FGFR1 (P=0.048), ZEB2 (P=0.00008),
CXCR4 (P=0.035), whereas lower expression of CDH1
(P=0.014), MMP9 (P=0.018) and MKI67 (P=0.0057) were
observed (Table III). We also
observed higher level of RHOA in diffuse- sub-type with
respect to intestinal-sub-type (×8.7 vs. 2.8, although not
significant P=0.016), along with 85% in diffuse sub-type (vs. 50%
in intestinal-subtype) showing RHOA overexpression (>3
fold as compared to normal samples) (Table II and Table S1). The down-regulation of
CXCL12 in intestinal adenocarcinoma (P=0.013) was not
observed in the diffuse-subtype GC (Table III).
 | Table III.Statistical analysis of mRNA
expression of genes in diffuse/poorly cohesive relative to
intestinal-subtype gastric carcinoma. |
Table III.
Statistical analysis of mRNA
expression of genes in diffuse/poorly cohesive relative to
intestinal-subtype gastric carcinoma.
| A, Growth factors
and receptors (n=10) |
|---|
|
|---|
| Genes | Diffuse/poorly
cohesive adenocarcinoma (n=13)b | Intestinal
carcinoma (n=16)b |
P-valuea |
|---|
| IGF1 | 7.37
(0.97–12.75) | 1.14
(0.1–10.09) | 0.0012 |
| IGF2 | 2.26
(0.80–19.16) | 1.51
(0.18–6.18) | 0.51 (NS) |
| IGF1R | 1.10
(0.63–1.55) | 0.60
(0.39–9.33) | 0.044 |
| IGF2R | 1.43
(0.85–1.65) | 1.45
(0.81–2.63) | 0.55 (NS) |
| IRS1 | 1.50
(0.91–2.71) | 1.11 (0.56–18) | 0.079 (NS) |
| IRS2 | 1,13
(0,52–4,44) | 0.89
(0.15–1.77) | 0.10 (NS) |
| FGF7 | 2.16
(1.10–3.45) | 0.70
(0.07–2.44) | 0.00011 |
| FGFR1 | 1.94
(0.96–3.53) | 1.04
(0.31–2.68) | 0.048 |
| ERRB2 | 1.62
(0.87–2.74) | 2.20
(1.05–34.55) | 0.066 (NS) |
| ERRB3 | 0.91
(0.51–2.55) | 1.54
(0.59–3.49) | 0.066 (NS) |
|
| B, EMT and
migration (n=10) |
|
| Genes | Diffuse/poorly
cohesive adenocarcinoma (n=13)b | Intestinal
carcinoma (n=16)b |
P-valuea |
|
| VIM | 1.62
(0.88–2.37) | 1.35
(0.48–2.79) | 0.072 (NS) |
| CDH1 | 0.78
(0.01–1.06) | 1.01
(0.29–1.63) | 0.014 |
| SNAI1 | 2.39
(0.90–4.92) | 3.21
(0.52–11.62) | 0.26 (NS) |
| SLUG/SNAI2 | 3.02
(1.15–4.06) | 2.56
(1.0–10.50) | 1.00 (NS) |
| TWIST2 | 2.30
(0.62–3.29) | 1.46
(0.12–3.23) | 0.087 (NS) |
| TGFB1 | 2.05
(1.47–4.27) | 2.17
(1.05–6.51) | 1.00 (NS) |
| RUNX3 | 1.77
(0.00–4.91) | 1.66
(0.00–3.98) | 0.44 (NS) |
| ZEB2 | 1.70
(0.84–2.97) | 0.82
(0.27–1.60) | 0.000079 |
| CXCR4 | 3.14
(1.52–7.30) | 1.71
(0.5–7.16) | 0.035 |
| CXCL12 | 1.05
(0.30–3.19) | 0.43
(0.08–1.86) | 0.013 |
|
| C, Cell
proliferation and migration (n=7) |
|
| Genes | Diffuse/poorly
cohesive adenocarcinoma (n=13)b | Intestinal
carcinoma (n=16)b |
P-valuea |
|
| MMP2 | 3.21
(1.47–6.15) | 2.99
(0.46–7.95) | 0.48 (NS) |
| MMP9 | 2.01
(0.92–4.13) | 5.25
(0.80–19.27) | 0.018 |
| SPP1
osteopontin | 4.12
(1.75–89.35 | 14.51
(1.06–119.54) | 0.25 (NS) |
| CD44 | 1.73
(1.02–2.81) | 1.42
(0.75–2.55) | 0.20 (NS) |
| RHOB | 0.52
(0.32–1.23) | 0.34
(0.12–0.93) | 0.018 |
| RHOA | 8.65
(0.03–20.39) | 2.79
(0.59–23.08) | 0.16 (NS) |
| MKI67 | 3.76
(1.34–10.83) | 8.48
(1.83–17.67) | 0.0057 |
|
| D, Angiogenesis
(n=6) |
|
| Genes | Diffuse/poorly
cohesive adenocarcinoma (n=13)b | Intestinal
carcinoma (n=16)b |
P-valuea |
|
| VEGFA 165 | 0.86
(0.58–2.09) | 1.02
(0.69–4.07) | 0.15 (NS) |
| VEGFA 189 | 0.67
(0.42–1.00) | 0.70
(0.30–2.04) | 0.46 (NS) |
| FLT1 | 1.08
(0.62–2.07) | 1.06
(0.43–1.60) | 0.90 (NS) |
| KDR | 1.11
(0.67–1.47) | 1.12
(0.57–1.78) | 0.90 (NS) |
| VEGFC | 1.38
(0.86–2.60) | 1.63
(0.51–3.34) | 0.90 (NS) |
| NRP1 | 1.94
(1.21–3.27) | 1.71
(0.74–4.00) | 0.33 (NS) |
Correlations between the expressions
of five selected genes in diffuse gastric adenocarcinoma
We analysed the expression of five selected genes,
IGF1, FGF7, CDH1, ZEB2, and CXCR4 that were mostly
dysregulated (over- or under-expression) in poorly cohesive/diffuse
GC vs. non-tumoral tissue and other gastric tumors. Correlation
analysis (Table IV) show that both
IGF1 and FGF7 expression significantly correlated
primarily with FGFR1 (P=0.027 and P=0.0015, respectively),
several genes involved in EMT including VIM (P=0.017 and
P=0.007, respectively), SNAI2/SLUG (P=0.007 and P=0.012,
respectively), TWIST2 (P=0.004 and P=0.0005, respectively),
ZEB2 (P=0.0011 and P=0.0006, respectively) and MMP2
(P=0.0006 and P=0.0055, respectively), as well as NRP1
(P=0.010 and P=0.001, respectively). IGF1 expression also
correlated with CD44 (P=0.041). FGF7 expression also
correlated with IGF1 and IGF2 (P=0.012 and P=0.031,
respectively), IRS2 (P=0.041), RUNX3 (P=0.018) and
CXCL12 (P=0.027). ZEB2 expression was associated with
many genes involved in EMT and migration including VIM
(P=0.0024), SNAI2/SLUG (P=0.0005), TWIST2
(P<0.0001), TGFβ (P=0.049), RUNX3 (P=0.046),
CXCL12 (P=0.0017), and MMP2 (P=0.009; Table IV). ZEB2 expression was also
associated with IGF1 (P=0.0011), FGF7, FGFR1
(P=0.0006 and P=0.002) and NRP1 (P=0.005; Table IV). CDH1 expression was
associated with IGF2R (P<0.0001) and RHOA
(P=0.018; Table IV). No correlation
of CXCR4 expression was found with other genes (Table IV).
 | Table IV.Statistical analysis and correlation
between genes in the series of 13 diffuse/poorly cohesive gastric
cancer. |
Table IV.
Statistical analysis and correlation
between genes in the series of 13 diffuse/poorly cohesive gastric
cancer.
| A, Growth factors
and receptors (n=10) |
|---|
|
|---|
|
| IGF1 | FGF7 | CDH1 | ZEB2 | CXCR4 |
|---|
|
|
|
|
|
|
|---|
| Genes | r |
P-valuea | r |
P-valuea | r |
P-valuea | r |
P-valuea | r |
P-valuea |
|---|
| IGF1 | 1.0 | <0.0001 | 0.670 | 0.012 | 0.533 | 0.061 | 0.797 | 0.0011 | −0.049 | 0.87 |
| IGF2 | 0.335 | 0.26 | 0.599 | 0.031 | −0.170 | 0.58 | 0.528 | 0.064 | 0.467 | 0.11 |
| IGF1R | 0.412 | 0.16 | 0.440 | 0.13 | 0.418 | 0.16 | 0.445 | 0.13 | 0.401 | 0.17 |
| IGF2R | 0.434 | 0.14 | 0.088 | 0.78 | 0.901 | <0.0001 | 0.275 | 0.36 | 0.209 | 0.49 |
| IRS1 | 0.407 | 0.17 | 0.539 | 0.058 | 0.407 | 0.17 | 0.385 | 0.19 | 0.363 | 0.22 |
| IRS2 | 0.528 | 0.064 | 0.571 | 0.041 | 0.577 | 0.039 | 0.357 | 0.23 | 0.269 | 0.37 |
| FGF7 | 0.670 | 0.012 | 1.0 | <0.0001 | 0.165 | 0.59 | 0.819 | 0.0006 | 0.126 | 0.68 |
| FGFR1 | 0.610 | 0.027 | 0.786 | 0.0015 | −0.170 | 0.58 | 0.775 | 0.0019 | −0.313 | 0.30 |
| ERBB2 | 0.429 | 0.14 | 0.368 |
0.22 | 0.676 | 0.011 | 0.462 | 0.11 | 0.198 | 0.52 |
| ERBB3 | 0.434 | 0.14 | 0.500 | 0.082 | 0.478 | 0.099 | 0.522 | 0.067 | 0.396 | 0.18 |
|
| B, EMT and
migration (n=11) |
|
|
| IGF1 | FGF7 | CDH1 | ZEB2 | CXCR4 |
|
|
|
|
|
|
|
| Genes | r |
P-valuea | r |
P-valuea | r |
P-valuea | r |
P-valuea | r |
P-valuea |
|
| VIM | 0.648 | 0.017 | 0.709 | 0.0067 | 0.341 | 0.25 | 0.764 | 0.0024 | 0.038 | 0.90 |
| CDH1 | 0.533 | 0.061 | 0.165 | 0.59 | 1.0 | <0.0001 | 0.291 | 0.33 | 0.104 | 0.73 |
| SNAI1 | 0.093 | 0.76 | −0.110 | 0.72 | 0.330 | 0.27 | −0.357 | 0.23 | 0.192 | 0.53 |
| SNAI2 | 0.709 | 0.0067 | 0.670 | 0.012 | 0.423 | 0.15 | 0.830 | 0.0005 | 0.357 | 0.23 |
| TWIST2 | 0.736 | 0.0041 | 0.830 | 0.0005 | −0.029 | 0.91 | 0.874 | <0.0001 | −0.302 | 0.32 |
| TGFB1 | 0.302 | 0.32 | 0.528 | 0.064 | 0.104 | 0.73 | 0.555 | 0.049 | 0.374 | 0.21 |
| RUNX3 | 0.341 | 0.25 | 0.643 | 0.018 | −0.132 | 0.67 | 0.560 | 0.046 | 0.396 | 0.18 |
| ZEB2 | 0.797 | 0.0011 | 0.819 | 0.0006 | 0.291 | 0.33 | 1.0 | <0.0001 | 0.022 | 0.94 |
| SIP1 | 0.149 | 0.63 | −0.182 | 0.55 | 0.234 | 0.44 | 0.080 | 0.80 | −0.374 | 0.21 |
| CXCR4 | −0.049 | 0.87 | 0.126 | 0.68 | 0.104 | 0.73 | 0.022 | 0.94 | 1.0 | <0.0001 |
| CXCL12 | 0.533 | 0.061 | 0.610 | 0.027 | −0.154 | 0.62 | 0.780 | 0.0017 | −0.352 | 0.24 |
|
| C, Cell
proliferation and migration (n=7) |
|
|
| IGF1 | FGF7 | CDH1 | ZEB2 | CXCR4 |
|
|
|
|
|
|
|
| Genes | r |
P-valuea | r |
P-valuea | r |
P-valuea | r |
P-valuea | r |
P-valuea |
|
| MMP2 | 0.819 | 0.0006 | 0.720 | 0.0055 | 0.269 | 0.37 | 0.692 | 0.0087 | 0.055 | 0.86 |
| MMP9 | 0.121 | 0.69 | 0.489 | 0.090 | 0.049 | 0.87 | 0.423 | 0.15 | 0.484 | 0.094 |
| SPP1 | 0.071 | 0.82 | 0.313 | 0.30 | 0.137 | 0.65 | 0.022 | 0.94 | 0.489 | 0.090 |
| CD44 | 0.571 | 0.041 | 0.225 | 0.46 | 0.170 | 0.58 | 0.264 | 0.38 | 0.016 | 0.96 |
| RHOB | 0.203 | 0.51 | 0.121 | 0.69 | 0.016 | 0.96 | −0.132 | 0.67 | −0.203 | 0.51 |
| RHOA | 0.335 | 0.26 | 0.038 | 0.90 | 0.643 | 0.018 | 0.137 | 0.65 | 0.214 | 0.48 |
| MKI67 | 0.308 | 0.31 | 0.071 | 0.82 | 0.703 | 0.0073 | 0.038 | 0.90 | 0.005 | 0.99 |
|
| D, Angiogenesis
(n=6) |
|
|
| IGF1 | FGF7 | CDH1 | ZEB2 | CXCR4 |
|
|
|
|
|
|
|
| Genes | r |
P-valuea | r |
P-valuea | r |
P-valuea | r |
P-valuea | r |
P-valuea |
|
| VEGF165 | 0.335 | 0.26 | 0.434 | 0.14 | 0.478 | 0.099 | 0.071 | 0.82 | 0.187 | 0.54 |
| VEGF189 | 0.187 | 0.54 | 0.335 | 0.26 | 0.330 | 0.27 | 0.115 | 0.71 | 0.500 | 0.082 |
| FLT1 | 0.418 | 0.16 | 0.412 | 0.16 | 0.352 | 0.24 | 0.104 | 0.73 | 0.214 | 0.48 |
| KDR | −0.330 | 0.27 | −0.121 | 0.69 | −0.071 | 0.82 | −0.286 | 0.34 | 0.313 | 0.30 |
| VEGFC | 0.231 | 0.45 | 0.473 | 0.10 | 0.093 | 0.76 | 0.236 | 0.44 | −0.044 | 0.89 |
| NRP1 | 0.681 | 0.010 | 0.802 | 0.0010 | 0.247 | 0.42 | 0.725 | 0.0050 | 0.044 | 0.89 |
Comparison of mRNA levels of five
dysregulated genes according to clinico-pathological findings in
diffuse gastric adenocarcinoma
Diffuse subtype- GC are aggressive adenocarcinoma
associated with lymphatic invasion (a higher positive axillary node
score) and metastasis, compared with intestinal sub-type (Table I). We further analyzed the
relationships between the five selected genes and clinical
parameters in diffuse-type GC (Table
V). Interestingly, we found that CXCR4 expression was
significantly increased with TNM (IIIc-IV vs. II–IIIa, P=0.022) and
lymphatic invasion (pN2-N3 vs. pN0-N1, P=0.05; Table V). The decrease of CDH1 was
associated with tumor invasion (T3-T4) and high TNM stage (IV,
P=0.05). Increase of IGF1 was associated with lymphatic
invasion (positive vs. negative), but not with the number of
positive lymph nodes. No correlation was found between FGF7
expression and clinical parameters.
 | Table V.Correlation of selected genes with
clinical parameters in diffuse-type adenocarcinoma. |
Table V.
Correlation of selected genes with
clinical parameters in diffuse-type adenocarcinoma.
| Clinical
parameter | IGF1 | P-value | IGF2 | P-value | FGF7 | P-value | CDH1 | P-value | ZEB2 | P-value | CXCR4 | P-value | TGF-β | P-value |
|---|
| Sex |
| 0.61 |
| 0.61 |
| 0.13 |
| 0.51 |
| 0.52 |
| 0.71 |
| 0.81 |
|
Male | 7.89 |
| 2.3 |
| 2.64 |
| 0.74 |
| 2.12 |
| 3.30 |
| 2.06 |
|
|
Female | 7.37 |
| 1.93 |
| 2 |
| 0.78 |
| 1.68 |
| 3.14 |
| 2.05 |
|
| Tumor size, mm |
| 0.32 |
| 0.07 |
| 0.07 |
| 0.39 |
| 0.15 |
| 0.84 |
| 0.004a |
|
<50 | 8 |
| 1.63 |
| 2.04 |
| 0.84 |
| 1.69 |
| 2.63 |
| 1.72 |
|
|
≥50 | 8.6 |
| 2.48 |
| 2.87 |
| 0.7 |
| 2.34 |
| 2.67 |
| 2.82 |
|
| Tumor invasion,
T |
| 0.31 |
| 0.33 |
| 0.54 |
| 0.02a |
| 0.79 |
| 0.54 |
| 0.05a |
|
T1-T2 | 9.1 |
| 1.63 |
| 2.04 |
| 0.98 |
| 1.69 |
| 2.63 |
| 1.59 |
|
|
T3-T4 | 7.18 |
| 2.29 |
| 2.4 |
| 0.7 |
| 1.9 |
| 3.37 |
| 2.28 |
|
| Lymphatic invasion,
N |
| 0.29 |
| 0.14 |
| 0.90 |
|
|
|
|
| 0.05 |
| 0.81 |
| N0-N1
vs. N2-N3 | 8.83 vs. |
| 2.32 vs. |
| 2.42 vs. |
| 0.7 vs. | 0.02a | 1.9 vs. | 0.90 | 2.54 vs. |
| 2.05 vs. |
|
|
| 4.77 |
| 1.63 |
| 2.04 |
| 0.81 |
| 1.69 |
| 4.14 |
| 2.25 |
|
| N1-N2
vs. N3 |
|
|
|
|
|
| 0.7 vs. | 0.003a | 1.55 vs. | 0.41 |
|
|
|
|
|
|
|
|
|
|
|
| 0.86 |
| 2.02 |
|
|
|
|
|
| Vascular
invasion |
| 0.83 |
| 0.02a |
| 0.93 |
| 0.09 |
| >0.99 |
| 0.60 |
| 0.71 |
|
Negative (n=3) | 5.76 |
| 3.38 |
| 2.42 |
| 0.43 |
| 1.5 |
| 2.54 |
| 2.28 |
|
|
Positive (n=10) | 7.99 |
| 1.81 |
| 2.12 |
| 0.78 |
| 1.8 |
| 3.25 |
| 1.99 |
|
| Peritoneal
metastasis |
|
<0.02a |
| 0.26 |
| 0.10 |
| 0.054 |
| 0.03 |
| 0.50 |
| 0.5 |
|
Negative (n=8) | 8.83 |
| 1.93 |
| 2.42 |
| 0.79 |
| 2.07 |
| 2.67 |
| 2.28 |
|
|
Positive (n=4) | 3.13 |
| 9.6 |
| 1.66 |
| 0.33 |
| 1.39 |
| 3.6 |
| 1.99 |
|
| TNM |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| II–IIIa
vs. IIIc-IV | 8.8 vs. | 0.29 | 1.93 vs. | 0.52 | 2.16 vs. | >0.999 | 0.78 vs. | 0.46 | 1.90 vs. | 0.35 | 2.13 vs. | 0.022a | 1.85 vs. | 0.36 |
|
| 4.77 |
| 2.44 |
| 2.37 |
| 0.60 |
| 1.51 |
| 4.14 |
| 2.31 |
|
| II–II
vs. IV | 9
vs. | 0.028a | 2.29 vs. | 0.64 | 2.29 vs. | 0.1 | 0.79 vs. | 0.05 | 2.1 vs. | 0.034a | 2.67 vs. | 0.5 | 2.28 vs. | 0.045a |
|
| 3.13 |
| 1.81 |
| 1.81 |
| 0.33 |
| 1.39 |
| 3.6 |
| 1.99 |
|
| Linitis |
| 0.50 |
| 0.12 |
| 0.41 |
| 0.50 |
| 0.88 |
| 0.21 |
| 0.045a |
|
Negative (n=5) | 6.56 |
| 1.14 |
| 2.08 |
| 0.78 |
| 1.70 |
| 2.13 |
| 1.82 |
|
|
Positive (n=8) | 8.83 |
| 2.30 |
| 2.75 |
| 0.74 |
| 1.81 |
| 3.6 |
| 2.62 |
|
TGFβ expression is increased in a
sub-population of diffusely infiltrating gastric carcinoma
Linitis Plastica is a diffusely infiltrating
type of diffuse-GC associated with extensive stromal fibrosis
(26). In our series of GCs, linitis
represents 61% of the diffuse gastric carcinoma (Table I). These linitis tumors were both
larger (90 mm vs. 42 mm, P=0.005), had high tumor invasion score
(T3-T4) and were associated with fibrosis. The majority of linitis
were also graded TNM IV and associated with metastasis (66%) as
compared to other diffusely infiltrating tumors. We then analyzed
the expression of TGFβ, a known factor for fibrosis, in diffuse GC.
As shown in Table V, TGFβ1
expression was significantly correlated with tumor size (P=0.004)
and tumor invasion (T3-T4, P=0.05). Interestingly, when linitis was
compared to other (non-linitis) diffusely infiltrating GC,
TGFβ1 expression was significantly increased (×2.6 vs. 1.8,
P=0.04) and positively associated with tumor invasion (T3-T4,
P=0.004).
IGF1 protein is present in
diffuse-subtype GC tissues
In line with the objective of the study, we assessed
the localization of IGF1 protein using immunohistochemistry on
paraffin sections from a total of 29 gastric tumors. Within the
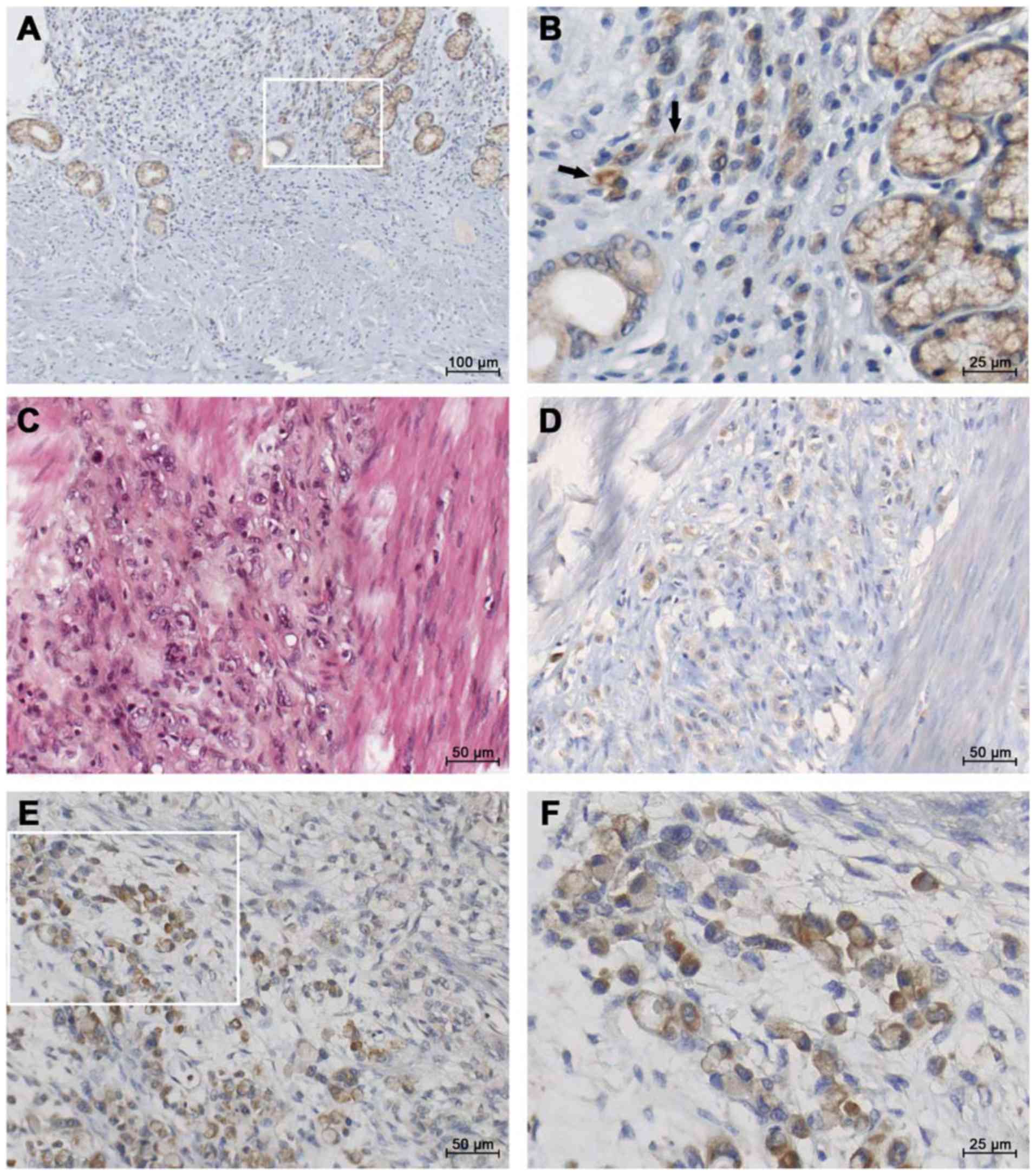
diffuse sub-type GC (Fig. 1), IGF1
staining was found in the gastric mucosa (Fig. 1A and B). Within diffuse sub-type GCs
such as linitis (associated with fibrosis, Fig. 1C), moderate IGF1 staining was
observed in single ring cells (Fig.
1D). Strong IGF1 immunostaining was observed in the most
advanced diffuse sub-type GCs (Fig. 1E
and F). In contrast, lower staining may be observed in
glandular structures in intestinal GC (Fig. S1).
Discussion
Diffuse-type gastric adenocarcinoma is an aggressive
and infiltrating carcinoma with substantially increasing incidence
in Europe and USA (6,7). In agreement with previous studies
(13,20,37), we
found that this diffuse-type GC is more common in younger patients,
with similar prevalence in both sexes, and is characterized by late
clinical presentation and aggressivity (positive axillary node
count and peritoneal carcinomatosis). Using RT-qPCR, we analyzed
the expression of 33 selected genes coding for proteins involved in
four categories: growth factors, EMT, cell proliferation and
migration, and angiogenesis, in a series of 29 gastric tumors. We
found that 22 genes were upregulated in the diffuse GC compared to
normal gastric tissue. As compared with intestinal-type GC, eleven
genes in the diffuse GC showed notable differences in expression.
Of these, overexpressed genes are involved in EMT (among which
ZEB2), cell migration (CXCR4, RHOB, and MMP9),
or are growth factors (IGF1, IGF1R, FGF7 and FGFR1).
An increase of ZEB2, CXCR4 and TGFβ1, and a decrease
of CDH1 were associated with invasion and/or metastasis in
diffuse-type GC.
Among GCs, a small minority that are genomically
stable have been associated with mutated CDH1 (24) or it loss of expression (38), and by low genomic deletion of
RHOA (23). We found a
significant decrease of CDH1 expression in diffuse GC as
compared with intestinal sub-type (P=0.014) and non-tumoral gastric
tissue (P=0.04), in agreement with the studies from the group of
Sasaki (33,34). Moreover, CDH1 underexpression
significantly correlated with tumor invasion (T3-T4, P=0.025) and a
more advanced stage (IV).
The connection between loss of E-cadherin
expression in cancers and passage through an EMT has been
established by many studies (39,40).
When gene expression profiling was performed for EMT signature
genes in diffuse GC, in addition to decrease CDH1
expression, we identified numerous significant up-regulated genes
as compared to non-tumoral gastric tissues, including VIM,
SNAI1, SLUG, ZEB2, RUNX3, TGFβ1 (P<0.01); in contrast only
SNAI1, SLUG and TGFβ1 were dysregulated in
intestinal-subtype GC. Our results suggested that mesenchymal
features are more prominent in diffuse GC, resulting in tumor
aggressiveness of this subgroup of GC. The overexpression of
TWIST2, as also observed in diffuse-GC (P=0.046), extends
previous observations on the overexpression of TWIST in
gastric and lobular breast carcinoma (41). ZEB2 is also a transcriptional factor
implicated in regulation of EMT regulator. The significant
association of ZEB2 expression with many other EMT-regulated
markers including VIM, SNAI2/SLUG, TWIST2 in diffuse- vs.
intestinal- GC (Table IV) further
indicates prominent mesenchymal features in diffuse GC. In a
previous report, Ohta et al (33) identified mesenchymal-like gene
expression (including ZEB2, TWIST2 and SLUG) in
diffuse-type GC and in gastric pit cells of gastric mucosa,
indicating that the gastric pit cell exhibits the mesenchymal
phenotype, and that diffuse-type GC also maintain it. Moreover,
high ZEB2 expression was recently found to predict poor
survival for digestive cancers using databases on 24 cohort studies
(42).
In the present study, TGFβ1 expression
(TGFβ1, another signal responsible for inducing EMT) was increased
in both diffuse and intestinal GC. However, the expression levels
of TGFβ1 do not permit discrimination between the two
sub-populations GC with respect of EMT. This finding is deduced
from -the absence of correlation between TGFβ1 and some EMT
markers (CDH1, VIM, or SNAI1) in diffuse GC, and -the
finding that TGFβ1, SNAI1 and SNAI2/SLUG are
significantly increased in intestinal-type GC. Notably,
TGFβ1 expression was significantly increased in a
sub-population of diffusely infiltrating type of GC associated with
extensive fibrosis (linitis) as compared with non-linitis diffuse
GC. Our findings reinforce the documented role of TGFβ1 in
stromal cells in aggressive GCs. We also found that TGFβ1
expression was positively associated with expression of MMP9,
PD1 and PDL2 (data not shown) in diffuse GC. These
findings highlight the role of TGFβ1-signaling pathway in
the tumoral stroma from diffuse GC, including stromal cells and the
extracellular matrix. Wu et al (43) using a meta-analysis of patients with
GC, have reported a TGFβ-associated super module of
stroma-related genes associated with diffuse-type histology and
poor prognosis in patients with GC.
Little is known about the involvement of growth
factors in GC and whether they are specific for a sub-population of
GC. We show for the first time that expression of IGF1 and
FGF7 are significantly increased in diffuse GC compared to
non-tumoral gastric tissues and as compared with intestinal-subtype
GC. Among the diffuse-gastric tumors, 77% overexpressed IGF1
and 23% overexpressed FGF-7.
The IGF system promotes cancer proliferation, and
its signalling induces the EMT phenotype which contributes to the
migration, invasiveness and metastasis of epithelial tumors.
IGF1 expression was significantly increased in
diffuse-subtype (×7.4, P=0.0004). The positive association of IGF1
expression with a set of mesenchymal marker and EMT regulator genes
(VIM, SLUG, ZEB2 and TWIST2) indicates that
IGF1 is associated with the EMT process in the diffuse-type
GC. IGF1 expression was also associated with the presence of
lymph node in GCs. Using immunostaining, IGF1 protein was also
detected in epithelial cells in gastric tumors (mainly
diffuse-subtype). Previous studies have provided some evidence for
the association of circulating IGF1 levels (and/or IGF binding
proteins, IGFBPs) with cancer risk. Unfortunately, we had no
gastric tumors samples (or plasma/serum) available to analyse
circulating IGF1 levels (and/or IGFBPs) between these two types of
GC in this cohort of patients, a potential limitation of the
current study. Studies are needed to further assess the association
between circulating IGF-1 level and GC risk.
We also show that FGF7 expression is
significantly increased (P<0.002) in diffuse-gastric tumors,
while decreased in 31% of the intestinal-subtype. Most notably,
FGF7 expression strongly correlated with the expression of
FGFR1 (P=0.0001), some mesenchymal markers (VIM, ZEB2
and TWIST2), and genes expressed by the microenvironment
(MMP2, NRP1). Our findings indicate for the first time an
important role of FGF7 (a member of the fibroblast growth factor
family) in diffuse-type GCs. Two studies have reported that FGF-7
is produced by mesenchymal cells in various tissues and cell lines
which developed the characteristics of scirrhous carcinoma upon
orthotopic implantation in mice (26,44).
Using immunocytochemistry, co-expression of FGF7 with MMP9 proteins
has been previously associated with a poor prognosis in GC
(45). Altogether, these findings
suggest that patients with tumors that overexpress FGF7 may
be candidates for new target therapies, such as emerging FGFR-1
inhibitors.
Cell migration is dependent on the dynamic function
and dis-sassembly of actin filament based structures, as well as
cell-cell and cell-extracellular matrix adhesion. Decreased
CDH1 expression, as well as increased CXCR4
expression was observed in the diffuse-subtype, leading to markedly
reduced cell adhesion and increase of cellular motility, and
resulting in tumor differentiation, invasiveness and metastasis.
Differential gene expression of RHOA (highest in
diffuse-type, as previously suggested by gain of function mutation
(46), and RHOB (lowest in
intestinal-subtype) was observed for the first time in
sub-populations of GCs. In the present study, CXCR4
expression is significantly up-regulated in diffuse subtype GC as
compared to intestinal-subtype, and is significantly associated
with TNM (IIIc-IV, P=0.022) and lymphatic invasion. Various types
of cancers including breast, prostate, brain, colon and lung
overexpress levels of CXCR4 (47). On the other hand, our findings
indicated a decreased CXCL12 expression in intestinal
sub-type (not in diffuse sub-type) with respect to the
corresponding normal tissue. Our findings complement recent studies
on GCs (48–50), and suggest that CXCR4 overexpression
in diffuse GC is a biomarker of this aggressive and infiltrating
carcinoma. The nature of the chemokine which promotes invasiveness
is not fully understood.
In conclusion, the present study presents evidence
that tumor biomarkers represent a new approach to discriminate
diffuse-type and intestinal-type GC. Several major signaling
pathways have been often described in GC without discriminating the
different subtypes. The majority of the studies in GC have been
conducted in Asia, so the conclusions should be taken cautiously
when applied to other ethnic populations. In our series of European
diffuse- GCs, we identified several candidate markers including
growth factors (IGF1 and FGF7, and their receptors),
ZEB2 (associated with VIM, SNAI2/SLUG and
TWIST2), TGFβ1 and CXCR4 involved in EMT, cell
invasion and metastasis. We also emphasize the role of TGFβ1
as a main player of intratumoral remodeling, as exemplified by
fibrosis. The relatively small number of tumors (30) could be a limiting factor and could
bias for correlation and/or matching comparison. However, we
obtained similar results when we compared tumoral tissue with
normal tissue from the same patients. Our results also agree with
the few genes previously reported in diffuse-GCs. Further studies
with a larger cohort of gastric tumor samples and with different
clinical characteristics (early and advanced stages of
subpopulations) would offer opportunity to confirm genes of
interest in diffuse-GCs.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr JP Brouland
(Department of Pathology, Lariboisiere Hospital, Paris, France) for
his assistance in GC histology and Mrs. C Marty (Lariboisiere
Hospital) for her technical help.
Funding
The present study was supported by Institut
National de la Santé et de la Recherche Medicale, Centre National
de la Recherche Scientifique and ANSES (Agence Nationale de
Securité sanitaire, Environnement, Travail, Maisons-Alfort, 94701,
France).
Availability of data and materials
The datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
MPA and IB contributed to the conception and design
of the study. SV, CP, WC and MPA performed the experiments and
statistical analysis. MP and SD conducted the GC biopsies and
collected the clinical data from the patients. MPA drafted the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Lariboisiere Hospital (Paris, France). All patients
provided written informed consent prior to their inclusion in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
diffuse-GC
|
diffuse gastric cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi J, Qu YP and Hou P: Pathogenetic
mechanisms in gastric cancer. World J Gastroenterol.
20:13804–13819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe H, Jass JR and Sobin LH:
Histological Classification of Gastric Tumours. In: Histological
typing of oesophageal and gastric tumors. World Health
Organization. International Histological Classification of Tumours.
Springer. (Berlin, Heidelberg). 1990.
|
|
5
|
Hohenberger P and Gretschel S: Gastric
cancer. Lancet. 362:305–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alberts SR, Cervantes A and van de Velde
CJ: Gastric cancer: Epidemiology, pathology and treatment. Ann
Oncol. 14 (Suppl 2):ii31–ii36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu H, Rusiecki JA, Zhu K, Potter J and
Devesa SS: Stomach carcinoma incidence patterns in the United
States by histologic type and anatomic site. Cancer Epidemiol
Biomarkers Prev. 18:1945–1952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henson DE, Dittus C, Younes M, Nguyen H
and Albores-Saavedra J: Differential trends in the intestinal and
diffuse types of gastric carcinoma in the United States, 1973–2000:
Increase in the signet ring cell type. Arch Pathol Lab Med.
128:765–770. 2004.PubMed/NCBI
|
|
9
|
Jézéquel J, Bessaguet C, Verveur C, Faycal
J, Richert Z, Metges JP, Volant A, Nousbaum JB and Robaszkiewicz M:
Trends in incidence, management, and survival of gastric and cardia
carcinomas in the area of Finistere (France) between 1984 and 2003.
Eur J Gastroenterol Hepatol. 22:1412–1419. 2010.PubMed/NCBI
|
|
10
|
Ha TK, An JY, Youn HK, Noh JH, Sohn TS and
Kim S: Indication for endoscopic mucosal resection in early signet
ring cell gastric cancer. Ann Surg Oncol. 15:508–513. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JP, Kim SC and Yang HK: Prognostic
significance of signet ring cell carcinoma of the stomach. Surg
Oncol. 3:221–227. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH,
Choi SH and Min JS: Early gastric carcinoma with signet ring cell
histology. Cancer. 94:78–83. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taghavi S, Jayarajan SN, Davey A and
Willis AI: Prognostic significance of signet ring gastric cancer. J
Clin Oncol. 30:3493–3498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gronnier C, Messager M, Robb WB, Thiebot
T, Louis D, Luc G, Piessen G and Mariette C; FREGAT working
group-FRENCH, : Is the negative prognostic impact of signet ring
cell histology maintained in early gastric adenocarcinoma? Surgery.
154:1093–1099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pernot S, Voron T, Perkins G,
Lagorce-Pages C, Berger A and Taieb J: Signet-ring cell carcinoma
of the stomach: Impact on prognosis and specific therapeutic
challenge. World J Gastroenterol. 21:11428–11438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ,
Kim SK and Lee JH: Clinicopathological characteristics of signet
ring cell carcinoma of the stomach. ANZ J Surg. 74:1060–1064. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Kim S, Lai JF, Hyung WJ, Choi WH,
Choi SH and Noh SH: Advanced gastric carcinoma with signet ring
cell histology. Oncology. 72:64–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Voron T, Messager M, Duhamel A, Lefevre J,
Mabrut JY, Goere D, Meunier B, Brigand C, Hamy A, Glehen O, et al:
Is signet-ring cell carcinoma a specific entity among gastric
cancers? Gastric Cancer. 19:1027–1040. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uemura N, Okamoto S, Yamamoto S, Matsumura
N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N and Schlemper RJ:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith BR and Stabile BE: Extreme
aggressiveness and lethality of gastric adenocarcinoma in the very
young. Arch Surg. 144:506–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Messager M, Lefevre JH, Pichot-Delahaye V,
Souadka A, Piessen G, Mariette C, Arnaud JP, Balon JM, Bonnetain F,
Borie F, et al FREGAT working group - FRENCH, : The impact of
perioperative chemotherapy on survival in patients with gastric
signet ring cell adenocarcinoma: A multicenter comparative study.
Ann Surg. 254:684–693. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue K, Nakane Y, Kogire M, Fujitani K,
Kimura Y, Imamura H, Tamura S, Okano S, Kwon AH, Kurokawa Y, et al:
Phase II trial of preoperative S-1 plus cisplatin followed by
surgery for initially unresectable locally advanced gastric cancer.
Eur J Surg Oncol. 38:143–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bass AJ, Thorsson V, Shmulevich I,
Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C,
Shen H, et al Cancer Genome Atlas Research Network, : Comprehensive
molecular characterization of gastric adenocarcinoma. Nature.
513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guilford P, Hopkins J, Harraway J, McLeod
M, McLeod N, Harawira P, Taite H, Scoular R, Miller A and Reeve AE:
E-cadherin germline mutations in familial gastric cancer. Nature.
392:402–405. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei Z, Tan IB, Das K, Deng N, Zouridis H,
Pattison S, Chua C, Feng Z, Guan YK, Ooi CH, et al: Identification
of molecular subtypes of gastric cancer with different responses to
PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology.
145:554–565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakazawa K, Yashiro M and Hirakawa K:
Keratinocyte growth factor produced by gastric fibroblasts
specifically stimulates proliferation of cancer cells from
scirrhous gastric carcinoma. Cancer Res. 63:8848–8852.
2003.PubMed/NCBI
|
|
27
|
Washington K: 7th edition of the AJCC
cancer staging manual: stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shu P, Qin J, Shen K, Chen W, Liu F, Fang
Y, Wang X, Wang H, Shen Z, Sun Y, et al: The IGCA staging system is
more accurate than AJCC7 system in stratifying survival of patients
with gastric cancer in stage III. BMC Cancer. 17:2382017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sano T, Coit DG, Kim HH, Roviello F,
Kassab P, Wittekind C, Yamamoto Y and Ohashi Y: Proposal of a new
stage grouping of gastric cancer for TNM classification:
International Gastric Cancer Association staging project. Gastric
Cancer. 20:217–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji X, Bu ZD, Yan Y, Li ZY, Wu AW, Zhang
LH, Zhang J, Wu XJ, Zong XL, Li SX, et al: The 8th edition of the
American Joint Committee on Cancer tumor-node-metastasis staging
system for gastric cancer is superior to the 7th edition: results
from a Chinese mono-institutional study of 1663 patients. Gastric
Cancer. 21:643–652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bièche I, Onody P, Laurendeau I, Olivi M,
Vidaud D, Lidereau R and Vidaud M: Real-time reverse
transcription-PCR assay for future management of ERBB2-based
clinical applications. Clin Chem. 45:1148–1156. 1999.PubMed/NCBI
|
|
32
|
Bieche I, Parfait B, Le Doussal V, Olivi
M, Rio MC, Lidereau R and Vidaud M: Identification of CGA as a
novel estrogen receptor-responsive gene in breast cancer: An
outstanding candidate marker to predict the response to endocrine
therapy. Cancer Res. 61:1652–1658. 2001.PubMed/NCBI
|
|
33
|
Ohta H, Aoyagi K, Fukaya M, Danjoh I, Ohta
A, Isohata N, Saeki N, Taniguchi H, Sakamoto H, Shimoda T, et al:
Cross talk between hedgehog and epithelial-mesenchymal transition
pathways in gastric pit cells and in diffuse-type gastric cancers.
Br J Cancer. 100:389–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanabe S, Aoyagi K, Yokozaki H and Sasaki
H: Gene expression signatures for identifying diffuse-type gastric
cancer associated with epithelial-mesenchymal transition. Int J
Oncol. 44:1955–1970. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Phrakonkham P, Brouland JP, Saad HS,
Bergès R, Pimpie C, Pocard M, Canivenc-Lavier MC and
Perrot-Applanat M: Dietary exposure in utero and during lactation
to a mixture of genistein and an anti-androgen fungicide in a rat
mammary carcinogenesis model. Reprod Toxicol. 54:101–109. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vacher S, Castagnet P, Chemlali W,
Lallemand F, Meseure D, Pocard M, Bieche I and Perrot-Applanat M:
High AHR expression in breast tumors correlates with expression of
genes from several signaling pathways namely inflammation and
endogenous tryptophan metabolism. PLoS One. 13:e01906192018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thomassen I, van Gestel YR, van Ramshorst
B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE and de Hingh IH:
Peritoneal carcinomatosis of gastric origin: A population-based
study on incidence, survival and risk factors. Int J Cancer.
134:622–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Humar B, Blair V, Charlton A, More H,
Martin I and Guilford P: E-cadherin deficiency initiates gastric
signet-ring cell carcinoma in mice and man. Cancer Res.
69:2050–2056. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen H, Lu W, Huang C, Ding K, Xia D, Wu Y
and Cai M: Prognostic significance of ZEB1 and ZEB2 in digestive
cancers: A cohort-based analysis and secondary analysis.
Oncotarget. 8:31435–31448. 2017.PubMed/NCBI
|
|
43
|
Wu Y, Grabsch H, Ivanova T, Tan IB, Murray
J, Ooi CH, Wright AI, West NP, Hutchins GG, Wu J, et al:
Comprehensive genomic meta-analysis identifies intra-tumoural
stroma as a predictor of survival in patients with gastric cancer.
Gut. 62:1100–1111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takemura S, Yashiro M, Sunami T, Tendo M
and Hirakawa K: Novel models for human scirrhous gastric carcinoma
in vivo. Cancer Sci. 95:893–900. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Q, Wang P, Shao M, Chen SW, Xu ZF,
Xu F, Yang ZY, Liu BY, Gu QL, Zhang WJ, et al: Clinicopathological
correlation of keratinocyte growth factor and matrix
metalloproteinase-9 expression in human gastric cancer. Tumori.
101:566–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kakiuchi M, Nishizawa T, Ueda H, Gotoh K,
Tanaka A, Hayashi A, Yamamoto S, Tatsuno K, Katoh H, Watanabe Y, et
al: Recurrent gain-of-function mutations of RHOA in diffuse-type
gastric carcinoma. Nat Genet. 46:583–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao H, Guo L, Zhao H, Zhao J, Weng H and
Zhao B: CXCR4 over-expression and survival in cancer: A system
review and meta-analysis. Oncotarget. 6:5022–5040. 2015.PubMed/NCBI
|
|
48
|
Izumi D, Ishimoto T, Miyake K, Sugihara H,
Eto K, Sawayama H, Yasuda T, Kiyozumi Y, Kaida T, Kurashige J, et
al: CXCL12/CXCR4 activation by cancer-associated fibroblasts
promotes integrin β1 clustering and invasiveness in gastric cancer.
Int J Cancer. 138:1207–1219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qiao J, Liu Z, Yang C, Gu L and Deng D:
SRF promotes gastric cancer metastasis through stromal fibroblasts
in an SDF1-CXCR4-dependent manner. Oncotarget. 7:46088–46099. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiang Z, Zhou ZJ, Xia GK, Zhang XH, Wei
ZW, Zhu JT, Yu J, Chen W, He Y, Schwarz RE, et al: A positive
crosstalk between CXCR4 and CXCR2 promotes gastric cancer
metastasis. Oncogene. 36:5122–5133. 2017. View Article : Google Scholar : PubMed/NCBI
|