Introduction
Colorectal cancer (CRC) is one of the most common
malignancies worldwide and the second most common cause of
cancer-associated mortality (1).
Despite advances in the treatment of CRC, the mortality rate of
this disease remains high (2).
Therefore, more reliable molecular prognostic markers are required
to improve CRC diagnosis and treatment.
The nectin protein family belongs to the
immunoglobulin superfamily, and its members are involved in the
formation and maintenance of adherens junctions in cooperation with
cadherin (3). At present, nectin-1,
−2, −3 and −4 have been identified (3). Nectin-4 was originally described as a
molecule homologous to the poliovirus receptor, also known as
poliovirus receptor-like-4 (4).
Unlike nectin-1 and −3, which are widely expressed in the tissues
of adults, nectin-4 is largely restricted to embryonic and
placental tissues (5). However,
studies have indicated that tracheal tissue, skin and hair
follicles express low levels of this protein (5,6). In
addition, a number of studies have revealed that nectin-4
overexpression is fundamental for the invasion and metastasis of
ovarian (7), breast (8), and lung cancer (9). The role of nectin-4 in cancer has not
been extensively studied and its expression and prognostic value in
CRC remains to be elucidated.
Integrins are cell membrane receptors that recognize
and bind to the extracellular matrix and participate in multiple
aspects of metastasis (10),
including tumor angiogenesis (11).
Furthermore, it has previously been reported that integrins are
associated with another type of blood supply in tumors,
vasculogenic mimicry (VM) (12–14). VM,
which differs from classical tumor angiogenesis, is an alternative
means of increasing the blood supply to tumors. In VM, tumor cells
and a channel consisting of a basement membrane that stains
positively to periodic acid-Schiff (PAS) are present; however this
channel does not contain endothelial cells (15). It has been reported that VM, which is
commonly observed in malignancies, is associated with poor
differentiation, advanced clinical stage and poor prognosis
(16). Further insight into the
mechanisms of underlying the formation VM may provide novel insight
for the development of anti-tumor therapy. A study demonstrated
that short fibers and small pores in the matrix environment induce
the formation of VM; specifically, an upregulation of the conserved
transcription module has been reported in tumor cells, resulting in
enhanced invasion and metastasis regulated by integrin β-1 (ITGB1)
(14). Therefore, ITGB1 may serve a
vital role in VM formation; however, further investigation is
required.
The purpose of the present study was to evaluate the
expression and prognostic value of nectin-4, ITGB1 and VM in CRC by
performing a retrospective study based on data from The Cancer
Genome Atlas (TCGA) cohort, and data obtained from another cohort
of 68 non-overlapping patients with CRC.
Materials and methods
Bioinformatics analysis of TCGA
data
Data for nectin-4 mRNA expression in CRC and normal
tissues was downloaded from TCGA (https://cancergenome.nih.gov/) to examine the role of
nectin-4 expression in CRC and its association with the
clinicopathological features of patients. A total of 372 CRC
samples and 31 normal samples from TCGA database were downloaded
using the R package TCGA-Assembler 2.0 (17). This cohort of 372 patients with CRC
consisted of 204 male and 168 female patients; 199 cases ≥65 years
old, 173 <65 years old; 282 were located in the colon, 90 were
located in the rectum; 67 were Tumor-Node-Metastasis (TNM) I/TII
stage, 304 were TNM III/TIV; 204 had no lymph node metastasis, 165
had lymph node metastases; 254 had no distant metastasis, and 50
had distant metastases.
Patients and tissue samples
The present study was approved by the Ethics
committee of The First Affiliated Hospital of Guangxi Medical
University (Nanning, China) and performed in accordance with the
guidelines of the Declaration of Helsinki (no. BBMCEC2012063). All
patients provided written informed consent to participate in the
study. Between September 2013 and September 2016, a total of 68 CRC
paraffin embedded tissues and 15 normal mucosal tissues were
obtained from The First Affiliated Hospital of Guangxi Medical
University. The cohort constituted 39 (57.4%) males and 29 (42.6%)
females, and the median age of patients was 56 years (range, 26–81
years). Pathological staging was accorded to the National
Comprehensive Cancer Network CRC classification (18). Detailed clinical and pathological
data were also collected. Patients who had received preoperative
chemo- or radiotherapy, or any other anti-cancer therapy were
excluded from the study.
Immunohistochemical (IHC) staining and
cluster of differentiation (CD)34/PAS dual staining
All tissue samples were fixed in 10% formalin for 24
h in room temperature and embedded in paraffin. Tissue sections
(4–5 µm) were deparaffinized in xylene and rehydrated in ethanol
(100, 95, 80 and 70%), and slides were soaked in methanol (98%),
containing 3% H2O2 for 10 min to block
endogenous peroxidase activity. For antigen retrieval the slides
were heated in the microwave for 30 min in citric acid buffer (pH
6.4). The slides were blocked with 10% normal goat serum (OriGene
Technologies, Inc.) in PBS for 30 min at room temperature, and
further incubated with primary antibodies for nectin-4 (cat. no.
21903-1-AP, 1:100, Proteintech Group, Inc., Chicago, IL, USA),
ITGB1 (cat. no. 12594-1-AP, 1:200, Proteintech Group) and CD34
(cat. no. ZM-0046, OriGene Technologies, Inc.) at 4°C overnight.
The following day the slides were incubated with appropriate
horseradish peroxidase-conjugated secondary antibodies (OriGene
Technologies, Inc.) for 30 min at room temperature. Visualization
of the IHC reaction was performed using 3,3′-diaminobenzidine for 5
min at room temperature. After IHC staining of CD34, the sections
were washed with running distilled water for 5 min and incubated
with periodic acid for 20 min and Schiff reagent for 8 min at room
temperature. CD34/PAS double-positive staining was used to
characterize VM structures. Following staining with hematoxylin for
1 min at room temperature the slides were examined under an Olympus
BX53 light microscope (magnification, ×200 and ×400; Olympus
Corporation, Tokyo, Japan).
IHC evaluation
The slides were independently evaluated by two
pathologists, and IHC staining was quantified using the Remmele
immunoreactive score (IRS). IRS=staining intensity (SI) ×
percentage of positive cells (PP). SI was defined as: i) 0,
Negative; ii) 1, weak; iii) 2, moderate; and iv) 3, strong. PP was
defined as: i) 0, Negative; ii) 1, <25% positive cells; iii) 2,
26–50% positive cells; iv) 3, 51–75% positive cells; and v) 4,
>75% positive cells. A total of ten visual fields from different
areas of each tumor were used for IRS evaluation. The staining
scoring system was defined as follows: i) 0, Negative staining (−);
ii) 1–4 as weakly positive (1+); iii) 5–8 as moderately positive
(2+); and iv) 9–12 as strongly positive staining (3+). The negative
and weakly positive categories (− and 1+) were defined as negative,
and moderate and strong positive categories (2+ and 3+) were
recorded as positive results (19).
Statistical analysis
An independent sample t-test was used to compare
continuous variables between CRC tissues and normal tissues. The
χ2 test was used to determine the association between
nectin-4, ITGB1 and VM formation. The association between nectin-4,
ITGB1, VM formation and the clinicopathological parameters of
patients was analyzed using the two-tailed χ2 test. All
statistical analyses were performed using the SPSS software package
(version 17.0; SPSS, Chicago, IL, USA), and P<0.05 was
considered to indicate a statistically significant difference.
Results
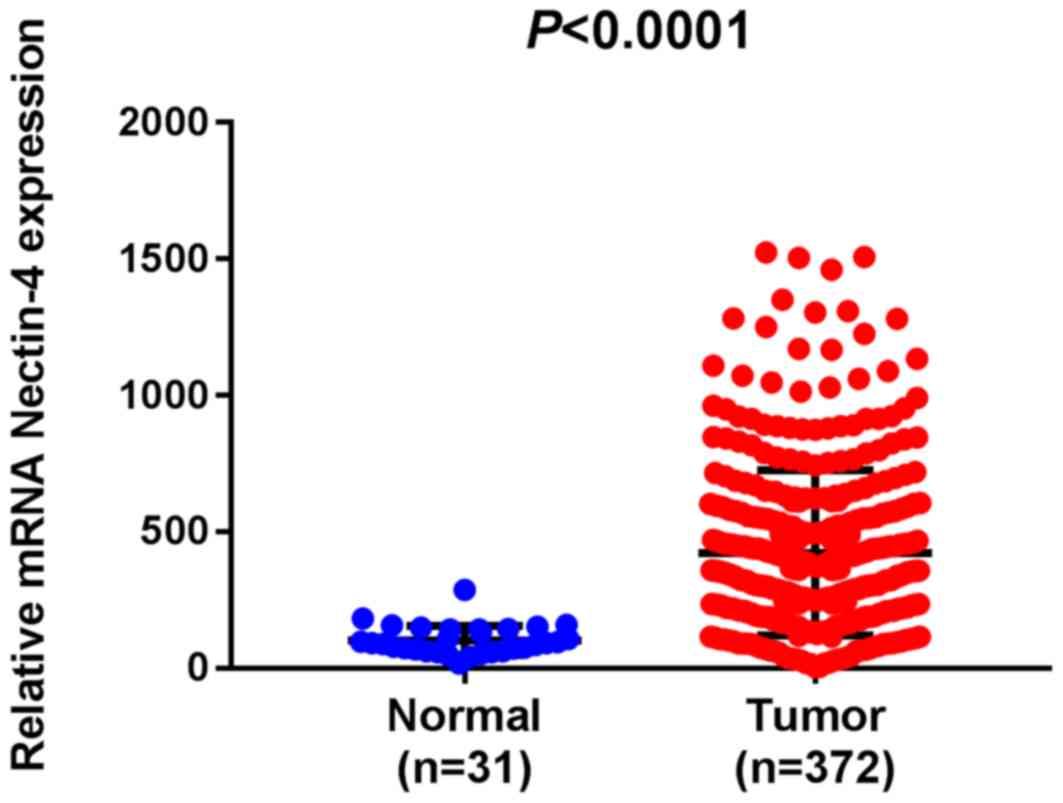
Upregulation of nectin-4 mRNA
expression is associated with aggressive CRC
Nectin-4 mRNA expression data from 372 patients with
CRC, and 31 normal cases were downloaded from the TCGA database.
P-values were calculated using the t-test. Nectin-4 mRNA expression
in CRC tissues and normal mucosal tissues was analyzed. As
presented in Fig. 1, nectin-4 mRNA
expression was upregulated in CRC tissues compared with that in
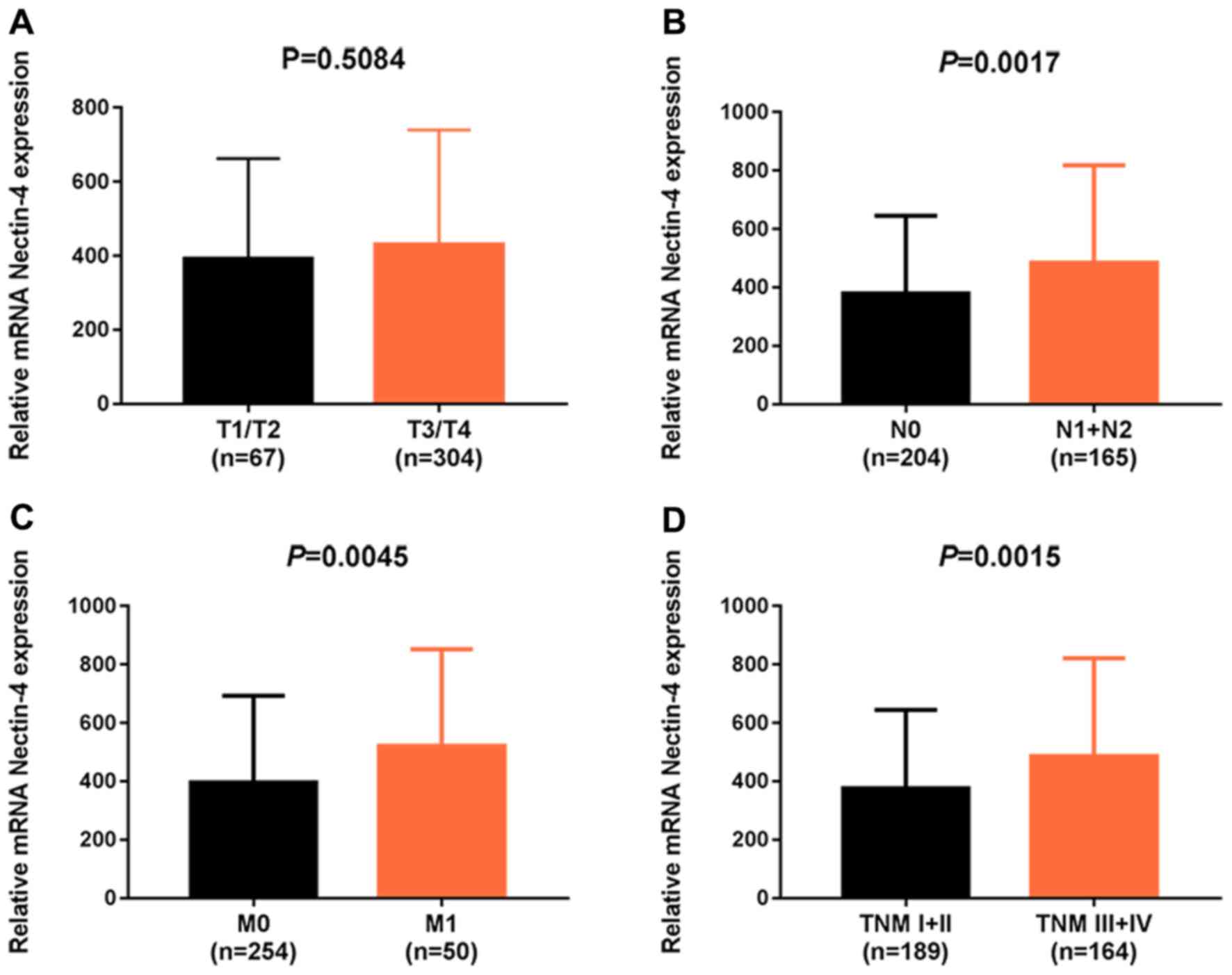
normal mucosal tissues (P<0.0001; Fig. 1). The association between nectin-4
mRNA expression and patients' clinicopathological parameters were
also assessed. Nectin-4 mRNA expression in tumor tissues was
significantly associated with lymph node metastasis (N stage;
Fig. 2B), distant metastasis (M
stage, Fig. 2C) and advanced
clinical stage (TNM stage, Fig. 2D).
By contrast, no significant differences were identified between
nectin-4 mRNA expression levels and tumor (T) stage (T stage,
Fig. 2A), patient's age, tumor type,
tumor site, presence of polyps (data not shown). Collectively these
results suggested that the overexpression of nectin-4 mRNA was
associated with the clinical progression of CRC.
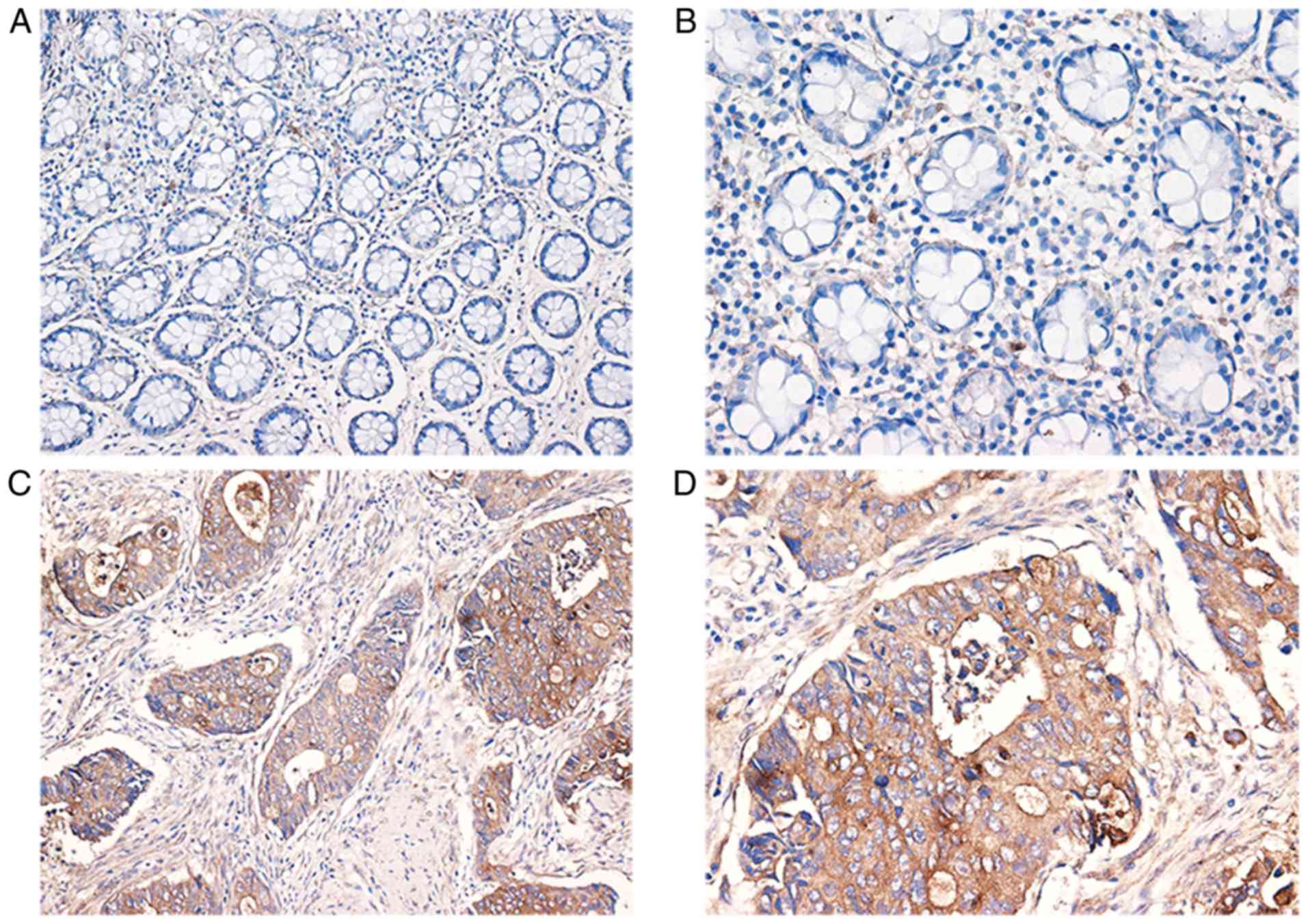
Nectin-4 protein expression is higher
in CRC tumors compared with normal mucosal tissues
To further confirm the results obtained from TCGA
database analysis, a non-overlapping cohort of 68 patients with CRC
were recruited, and nectin-4 protein expression levels were
determined using IHC staining. A total of 68 CRC and 15 normal
mucosal tissue samples were collected. According to the National
Comprehensive Cancer Network CRC classification, 7 patients were
classified as stage I, 25 as stage II, 19 as stage III and 17 as
stage IV. Consistent with the results obtained from the TCGA
cohort, high nectin-4 protein expression was more frequently
observed in tumor tissues when compared with normal tissues
(Fig. 3). Only 3 (20%) normal
mucosal samples displayed high nectin-4 protein expression levels,
while 48 (70.6%) of the CRC tissues displayed high levels (Table I, P<0.01).
 | Table I.Nectin-4 expression in colorectal
cancer and normal mucosal tissues. |
Table I.
Nectin-4 expression in colorectal
cancer and normal mucosal tissues.
| Immunohistochemical
staining | Colorectal cancer
tissues n=68 (%) | Normal tissues n=15
(%) | P-value |
|---|
| Negative (−) | 20 (29.4) | 12 (80) | 0.001a |
| Positive (+) | 48 (70.6) | 3 (20) |
|
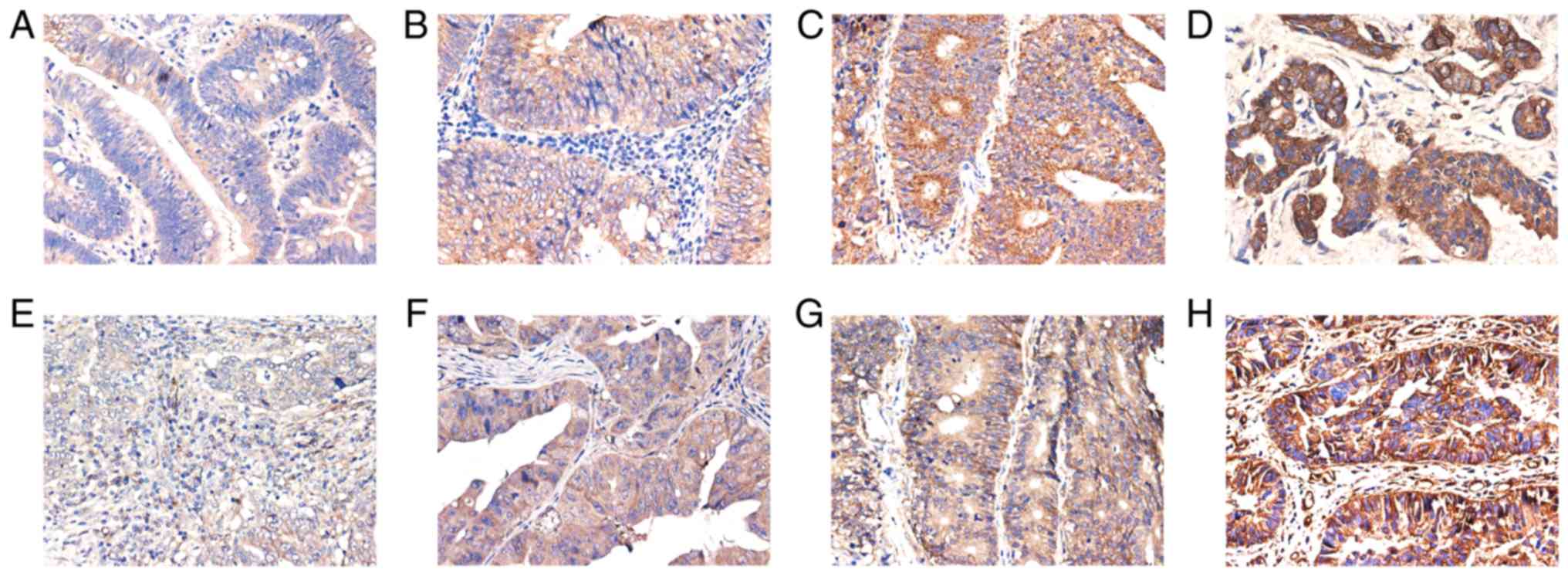
Association between nectin-4, ITGB1
and VM formation in CRC
In addition to nectin-4 protein expression, the
expression of ITGB1 and VM formation were also determined. Of the
68 cases analyzed, 48 (70.6%) were positive for nectin-4 protein
expression, 46 (67.6%) were positive for ITGB1 protein expression
and in 17 (25%) cases, VM formation was observed (Table II). Nectin-4 and ITGB1 protein
expression were higher in later TNM stages (TNM III and IV)
compared with early cancer stages (TNM I and II; Fig. 4). Notably, statistical analysis
revealed that nectin-4 was positively associated with ITGB1
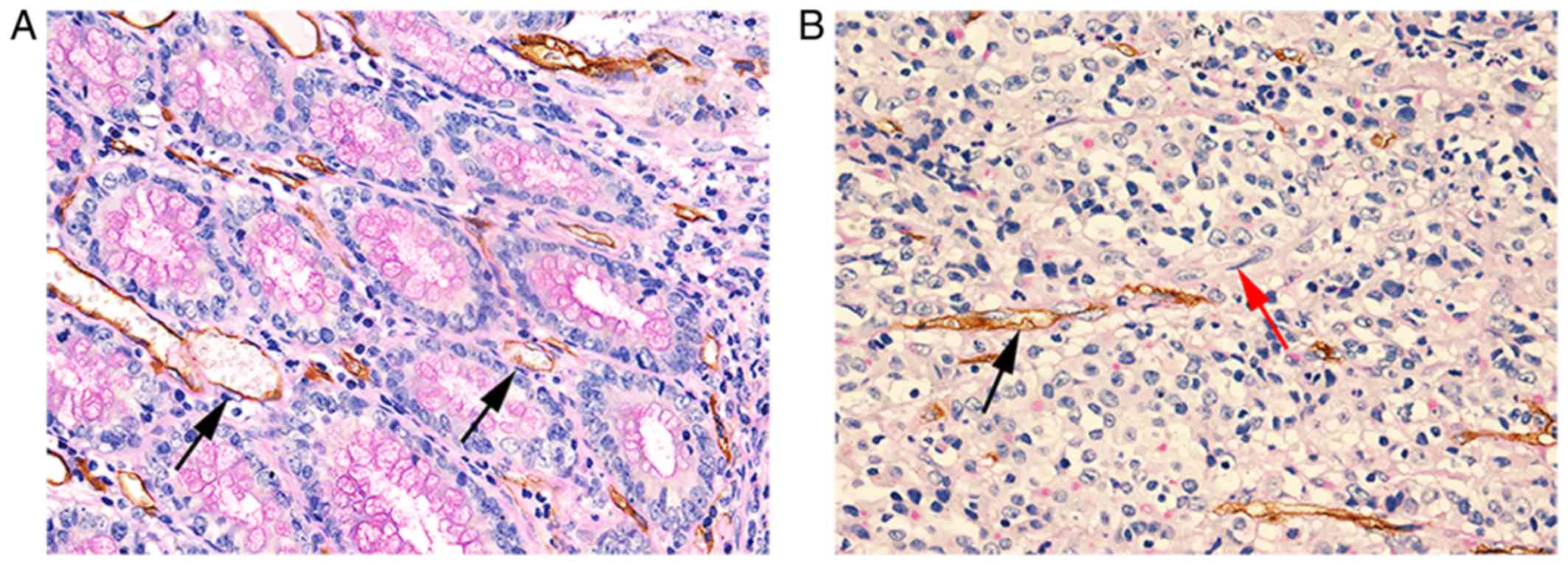
expression (Table II). CD34/PAS
double staining was used to detect VM formation. Structures
characterized by CD34−/PAS+ staining, with
red blood cells in the vascular-like tube, and surrounded by tumor
cells, were identified as VM (Fig.
5, red arrow). Structures with CD34+ and
PAS+ staining were identified as endothelium dependent
vasculature (Fig. 4, black arrow).
Statistical analysis revealed that nectin-4 protein expression was
positively associated with VM formation (Table II).
 | Table II.Correlation between nectin-4 and ITGB1
or VM in colorectal cancer. |
Table II.
Correlation between nectin-4 and ITGB1
or VM in colorectal cancer.
|
| ITGB1 (n) |
|
| VM (n) |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Nectin-4 (n) | − | + | χ2
value | P-value | − | + |
χ2-value | P-value |
|---|
| − | 17 | 3 | 32.556 |
<0.001b | 19 | 1 | 4.628 | 0.014a |
| + | 5 | 43 |
|
| 32 | 16 |
|
|
Associations between nectin-4, ITGB1
and VM, and the clinicopathological parameters of patients with
CRC
To evaluate the influences of nectin-4, ITGB1, and
VM on CRC, the obtained results were further compared with patient
clinicopathological characteristics. The expression of nectin-4
(48/68, 70.6%) and ITGB1 (46/68, 67.6%), in addition to VM
formation 17/68, 25%), were all positively associated with distant
metastasis stage (M stage; P=0.031, P=0.017, and P=0.034,
respectively), TNM stage (P=0.033, P=0.020, and P=0.023,
respectively), but not with patient sex, age, tumor size or lymph
node metastasis stage (N stage; Table
III). Compared with the early stages (TNM I and II), the
expression of nectin-4 and ITGB1, and VM formation were more
frequently observed in the later stages of CRC (TNM III and IV).
These results indicated that nectin-4 and ITGB1 protein expression,
and VM formation were positively associated with CRC progression
and may be indicators of poor prognosis.
 | Table III.Relationship between nectin-4, ITGB1,
VM and clinicopathological features in colorectal cancer. |
Table III.
Relationship between nectin-4, ITGB1,
VM and clinicopathological features in colorectal cancer.
|
| Nectin-4 |
| ITGB1 |
| VM |
|
|---|
|
|
|
|
|
|
|
|
|---|
|
Characteristics | − | + | P-value | − | + | P-value | − | + | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
Male | 10 | 28 | 0.528 | 11 | 27 | 0.499 | 24 | 11 | 0.207 |
|
Female | 10 | 20 |
| 11 | 19 |
| 27 | 6 |
|
| Age |
|
|
|
|
|
|
|
|
|
|
≥65 | 5 | 15 | 0.403 | 6 | 14 | 0.789 | 16 | 4 | 0.760 |
|
<65 | 17 | 31 |
| 16 | 32 |
| 35 | 13 |
|
| Size (cm) |
|
|
|
|
|
|
|
|
|
|
≥5.0 | 10 | 25 | 0.876 | 9 | 26 | 0.344 | 25 | 8 | 0.889 |
|
<5.0 | 10 | 23 |
| 13 | 20 |
| 26 | 9 |
|
| N stage |
|
|
|
|
|
|
|
|
|
| N0 | 13 | 27 | 0.504 | 14 | 26 | 0.577 | 30 | 10 | 0.909 |
| N1 +
N2 | 7 | 21 |
| 8 | 20 |
| 21 | 7 |
|
| M stage |
|
|
|
|
|
|
|
|
|
| M0 | 19 | 32 | 0.031a | 21 | 30 | 0.017a | 42 | 9 | 0.034a |
| M1 | 1 | 16 |
| 1 | 16 |
| 11 | 8 |
|
| TNM stage |
|
|
|
|
|
|
|
|
|
| I +
II | 14 | 20 | 0.033a | 16 | 18 | 0.020a | 30 | 4 | 0.023a |
| III +
IV | 6 | 28 |
| 6 | 28 |
| 21 | 13 |
|
Discussion
CRC is a common gastrointestinal malignancy with a
high incidence of metastasis. Once metastasis occurs, the outcomes
of surgery, radiotherapy or chemotherapy on patient prognosis are
unsatisfactory, and the mortality rate remains high. Therefore the
identification of novel molecular prognostic and predictive markers
is required.
Nectin-4, a cell adhesion molecule that interacts
with the cadherins, serves a key role in the formation and
maintenance of adherens junctions (6,20).
Nectin-4 consists of three immunoglobulin-like domains, which
constitute transmembrane and extracellular domains, and a short
cytoplasmic tail (20). It has been
reported that nectin-4 promotes the anchorage-independent growth of
human mammary epithelial cells by driving cell-to-cell attachment
and activating integrin β4/Src homology region 2-containing protein
tyrosine phosphatase 2/c-Src signaling (21). In addition, nectin-4 regulates
epithelial-mesenchymal transition, tumor invasion and metastasis in
breast cancer through its influence on the Wnt/β-catenin signaling
pathway and the phosphoinositide 3-kinase/protein kinase B
signaling axis (22). In non-small
cell lung cancer, nectin-4 promotes tumor invasion and metastasis
by activating the Rho-related protein racL (10). Furthermore, it has been reported that
VM, as an alternative blood supply to tumors, is associated with a
malignant phenotype and poor patient prognosis (23–25).
Traditional anti-angiogenic therapy is aimed at
endothelium-dependent blood vessels. Although this treatment delays
the progression of tumors in a short period of time, recurrence and
metastasis are issues for a number of patients (26). Therefore, further insight into the
mechanisms of VM may aid developments in the field of anti-tumor
therapeutics. Recent studies have reported that ITGB1 may promote
proliferation, invasion and metastasis in a variety of tumor types,
and may therefore be associated with poor prognosis (27,28).
Additional studies have also revealed that ITGB1 is crucial for the
formation of VM, where ITGB1 promoted migrational persistence and
influenced the shape of VM structures by regulating specific
aspects of the transcriptional module associated with the VM
network-forming phenotype (15).
In the present study, nectin-4 mRNA expression data
from 372 patients with CRC were downloaded from a TCGA database. In
this cohort, the overexpression of nectin-4 mRNA was strongly
associated with lymphatic metastasis, distant metastasis and TNM
classification. Collectively these findings suggest that nectin-4
overexpression is associated with the progression of CRC, which is
consistent with findings for nectin-4 expression in other tumor
types (8,29). To further confirm the results
obtained from the TCGA cohort, nectin-4 protein expression was
assessed using IHC staining in a separate cohort of patients. The
results revealed that nectin-4 protein expression was significantly
upregulated in CRC tissues compared with normal mucosal tissues. In
addition, an association between nectin-4, and ITGB1 protein
expression and VM formation was observed in this cohort. Positive
IHC staining results for these parameters were associated with M
and TNM stage, but not patient sex, age, tumor size or N stage.
Therefore, the results of the present study suggested that the
expression of nectin-4 and ITGB1, and VM formation may facilitate
the progression of CRC.
In conclusion, we aimed to combine the data obtained
from a TCGA database with that obtained from a separate patient
cohort in order to examine nectin-4 expression at the mRNA and
protein levels. The results concluded that nectin-4 was upregulated
in CRC tissues compared with normal mucosal tissues, and that
nectin-4 expression was positively associated with ITGB1 expression
and VM formation. Furthermore, all three parameters (nectin-4,
ITGB1 and VM) were significantly associated with M and TNM stage,
characteristic features of highly invasive CRCs. Therefore,
nectin-4, ITGB1 and VM combined may be useful in identifying the
progression of CRC I patients, and those with poor prognosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81760516),
the 2018 Innovation Project of Guangxi Graduate Education (grant
no. YCBZ2018046) and the Guangxi Zhuang Autonomous Region Health
and Family Planning Commission Self-financing research projects
(grant no. Z20170086).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ contributed to the study design, data analysis
and drafted the manuscript. KL and YS performed the experiments and
the bioinformatics analysis. PP and ShL contributed to the clinical
data acquisition and analysis. SiL and ZY performed the
experiments. MQ and JH contributed to the study design, reviewed
and edited the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
committee of the First Affiliated Hospital of Guangxi Medical
University, and performed in accordance with the guidelines of the
Declaration of Helsinki (no. BBMCEC2012063). All patients admitted
to the study provided written informed consent for their
participation.
Patient consent for publication
All patients admitted to the study provided informed
consent for their participation and publication of the data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takai Y, Miyoshi J, Ikeda W and Ogita H:
Nectins and nectin-like molecules: Roles in contact inhibition of
cell movement and proliferation. Nature reviews. Nat Rev Mol Cell
Biol. 9:603–615. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajc J, Gugić D, Fröhlich I, Marjanović K
and Dumenčić B: Prognostic role of Nectin-4 expression in luminal B
(HER2 negative) breast cancer. Pathol Res Pract. 213:1102–1108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reymond N, Fabre S, Lecocq E, Adelaide J,
Dubreuil P and Lopez M: Nectin4/PRR4, a new afadin-associated
member of the nectin family that trans-interacts with nectin1/PRR1
through V domain interaction. J Biol Chem. 276:43205–43215. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brancati F, Fortugno P, Bottillo I, Lopez
M, Josselin E, Boudghene-Stambouli O, Agolini E, Bernardini L,
Bellacchio E, Iannicelli M, et al: Mutations in PVRL4, encoding
cell adhesion molecule nectin-4, cause ectodermal
dysplasia-syndactyly syndrome. Am J Hum Genet. 87:265–273. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Derycke MS, Pambuccian SE, Gilks CB,
Kalloger SE, Ghidouche A, Lopez M, Bliss RL, Geller MA, Argenta PA,
Harrington KM and Skubitz AP: Nectin 4 overexpression in ovarian
cancer tissues and serum: Potential role as a serum biomarker. Am J
Clin Pathol. 134:835–845. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fabre-Lafay S, Monville F, Garrido-Urbani
S, Berruyer-Pouyet C, Ginestier C, Reymond N, Finetti P, Sauvan R,
Adélaïde J, Geneix J, et al: Nectin-4 is a new histological and
serological tumor associated marker for breast cancer. BMC Cancer.
7:732007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takano A, Ishikawa N, Nishino R, Masuda K,
Yasui W, Inai K, Nishimura H, Ito H, Nakayama H, Miyagi Y, et al:
Identification of nectin-4 oncoprotein as a diagnostic and
therapeutic target for lung cancer. Cancer Res. 69:6694–6703. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bianconi D, Unseld M and Prager GW:
Integrins in the spotlight of cancer. Int J Mol Sci. 17(pii):
E20372016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duro-Castano A, Gallon E, Decker C and
Vicent MJ: Modulating angiogenesis with integrin-targeted
nanomedicines. Adv Drug Deliv Rev. 119:101–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vartanian A, Stepanova E, Grigorieva I,
Solomko E, Belkin V, Baryshnikov A and Lichinitser M: Melanoma
vasculogenic mimicry capillary-like structure formation depends on
integrin and calcium signaling. Microcirculation. 18:390–399. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Camorani S, Crescenzi E, Gramanzini M,
Fedele M, Zannetti A and Cerchia L: Aptamer-mediated impairment of
EGFR-integrin αvβ3 complex inhibits vasculogenic mimicry and growth
of triple-negative breast cancers. Sci Rep. 7:466592017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Velez DO, Tsui B, Goshia T, Chute CL, Han
A, Carter H and Fraley SI: 3D collagen architecture induces a
conserved migratory and transcriptional response linked to
vasculogenic mimicry. Nat Commun. 8:16512017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu
D, Yu X and Tian Y: Advanced research on vasculogenic mimicry in
cancer. J Cell Mol Med. 19:315–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei L, Jin Z, Yang S, Xu Y, Zhu Y and Ji
Y: TCGA-assembler 2: Software pipeline for retrieval and processing
of TCGA/CPTAC data. Bioinformatics. 34:1615–1617. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon cancer, version 1.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sadeghi MR, Jeddi F, Soozangar N, Somi MH,
Shirmohamadi M, Khaze V and Samadi N: Nrf2/P-glycoprotein axis is
associated with clinicopathological characteristics in colorectal
cancer. Biomed Pharmacother. 104:458–464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurita S, Ogita H and Takai Y: Cooperative
role of nectin-nectin and nectin-afadin interactions in formation
of nectin-based cell-cell adhesion. J Biol Chem. 286:36297–36303.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pavlova NN, Pallasch C, Elia AE, Braun CJ,
Westbrook TF, Hemann M and Elledge SJ: A role for PVRL4-driven
cell-cell interactions in tumorigenesis. ELife. 2:e003582013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siddharth S, Goutam K, Das S, Nayak A,
Nayak D, Sethy C, Wyatt MD and Kundu CN: Nectin-4 is a breast
cancer stem cell marker that induces WNT/β-catenin signaling via
Pi3k/Akt axis. Int J Biochem Cell Biol. 89:85–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao Z, Bao M, Miele L, Sarkar FH, Wang Z
and Zhou Q: Tumour vasculogenic mimicry is associated with poor
prognosis of human cancer patients: A systemic review and
meta-analysis. Eur J Cancer. 49:3914–3923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Qiao L, Liang N, Xie J, Luo H,
Deng G and Zhang J: Vasculogenic mimicry and tumor metastasis. J
BUON. 21:533–541. 2016.PubMed/NCBI
|
|
25
|
Sun B, Zhang D, Zhao N and Zhao X:
Epithelial-to-endothelial transition and cancer stem cells: Two
cornerstones of vasculogenic mimicry in malignant tumors.
Oncotarget. 8:30502–30510. 2017.PubMed/NCBI
|
|
26
|
Dey N, De P and Brian LJ: Evading
anti-angiogenic therapy: Resistance to anti-angiogenic therapy in
solid tumors. Am J Transl Res. 7:1675–1698. 2015.PubMed/NCBI
|
|
27
|
Chen MB, Lamar JM, Li R, Hynes RO and Kamm
RD: Elucidation of the roles of tumor integrin beta1 in the
extravasation stage of the metastasis cascade. Cancer Res.
76:2513–2524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Z, Zou L, Ma G, Wu X, Huang F, Feng T,
Li S, Lin Q, He X, Liu Z and Cao X: Integrin β1 is a critical
effector in promoting metastasis and chemo-resistance of esophageal
squamous cell carcinoma. Am J Cancer Res. 7:531–542.
2017.PubMed/NCBI
|
|
29
|
Zhang Y, Liu S, Wang L, Wu Y, Hao J, Wang
Z, Lu W, Wang XA, Zhang F, Cao Y, et al: A novel PI3K/AKT signaling
axis mediates Nectin-4-induced gallbladder cancer cell
proliferation, metastasis and tumor growth. Cancer Lett.
375:179–189. 2016. View Article : Google Scholar : PubMed/NCBI
|