Introduction
Colorectal cancer is one of the most frequently
diagnosed malignancies and one of the leading causes of
cancer-related deaths worldwide (1,2). In the
United States, colorectal cancer affects ~140,000 new cases and
causes more than 50,000 deaths per year (3). In developing countries, such as China,
changes in people's dietary habits in have lead to an increase in
the incidence rate of this disease (4,5).
Although the survival rates of patients with colon cancer have
improved significantly over the past several decades, successful
and complete treatment is complicated by the high prevalence of
distant tumor metastasis at the first diagnosis (6).
The role inflammation plays during the pathogenesis
of colon cancer and inflammatory bowel disease is well established
and is considered to be a significant risk factor for the
development and progression of colon cancer (7). As a pro-inflammatory cytokine,
interleukin (IL)-17A participates in tumor growth in different
types of malignancies (8,9). Previous studies have shown that IL-17A
achieves its functions through interactions with both proteins and
non-coding RNAs, including miRNAs and long non-coding RNAs
(lncRNAs) (10,11). However, the interactions between
IL-17A and long non-coding RNAs have rarely been studied, to the
best of our knowledge. Previous studies demonstrated that cervical
carcinoma high-expressed long non-coding RNA 1 (CCHE1) promoted
several different types of cancer (12–15);
however its involvement in colon cancer has only been recently
established (16). The present
demonstrated that CCHE1 may have promoted the growth of colon
adenocarcinoma through interactions with IL-17A and may be used a
potential biomarker for early detection of colon
adenocarcinoma.
Materials and methods
Human samples and cell lines
Blood was extracted from 62 patients with colon
adenocarcinoma (patient group) and 36 healthy volunteers (control
group) to extract the plasma. These participants were admitted to
The Inner Mongolia People's Hospital (Inner Mongolia, China)
between July 2015 and July 2018. The inclusion criteria were: i)
Diagnosed by pathological examinations; and ii) patients and their
families understood the experimental protocol and signed informed
consent. The exclusion criteria were: i) Patients who were
diagnosed with multiple diseases; ii) patients who failed to
cooperate with researchers; and iii) patients who were treated
within the 3 months prior to their admission at The Inner Mongolia
People's Hospital. Distant tumor metastasis was observed in 30
cases. A total of 19 patients were in American Joint Committee on
Cancer (AJCC) stage I and II, both of which are considered as the
early stages of cancer (17). The
patient group consisted of 33 males and 29 females, with an age
range of 28–67 years, and a mean age of 47.5±5.3 years. The control
group consisted of 20 males and 18 females, with an age range of
27–68 years, and a mean age of 46.9±4.9 years. The patient and the
control groups had a similar age range and sex distributions. The
present study was approved by The Ethics Committee of Inner
Mongolia People's Hospital. All patients signed written form
informed consent.
Hs 698.T and SNU-C1 human colon adenocarcinoma cell
lines were purchased from The American Type Culture Collection
(ATCC; Manassas, VA, USA). Cells were cultured in RPMI-1640 medium
(ATCC) containing 10% fetal bovine serum (ATCC) at 37°C with 5%
CO2 (95% humidity).
Transfection
IL-17A and CCHE1 expression vectors were purchased
from GeneCopoeia, Inc. (Rockville, MD, USA). IL-17A small
interfering (si)RNA (5′-CCUACGUUGUUUGCUACUU-3′) and scrambled siRNA
control (5′-UUCUCCGAACGUGUCACGUdTdT-3′) were purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China).
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) was used to transfect 50 nM vector and 15
nM siRNA, respectively. Untransfected cells were treated as the
control and the cells transfected with scrambled siRNA control or
an empty vector were treated as the negative control. The interval
between transfection and subsequent experimentation was 24 h.
Reverse transcription-quantitative
(RT-q)PCR
The TRIzol® Plus RNA Purification kit
(Thermo Fisher Scientific, Inc.) was used to extract total RNA from
plasma, Hs 698.T and SNU-C1 cells. Following reverse transcription
using the RevertAid RT kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol, PCR reaction systems were
prepared using qScript One-Step RT-qPCR kit (Quantabio, Beverly,
MA, USA). Sequences of primers used in the PCR reactions were:
CCHE1 forward, 5′-AAGGTCCCAGGATACTCGC-3′, and reverse,
5′-GTGTCGTGGACTGGCAAAAT-3′; β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′, and reverse,
5′-AGTACTTGCGCTCAGGAGGA-3′; IL-17 forward,
5′-CTGGAGGATAACACTGTGAGAG-3, and reverse
5′-GCTGAATGGCGACGGAGTTC-3′. IL-17 primer pairs were purchased from
SinoBiological (Wayne, PA, USA) The thermocycling conditions used
were: 95°C for 45 sec, followed by 40 cycles of 95°C for 14 sec and
58.5°C for 38 sec. All data were processed using the
2−ΔΔCq method (18).
Cell proliferation assay
Proliferation assays were performed when CCHE1 and
IL-17A overexpression rates were between 200–250% and the IL-17A
knockdown rate was >50%. Cell suspensions were prepared with a
cell density of 4×104 cells/ml. Cells were cultured at
37°C in a 5% CO2 incubator, followed by addition of 1 µl
Cell Counting Kit-8 solution (cat. no. ab228554; Abcam, Cambridge,
UK) 4, 48, 72 and 96 h later. Subsequently, cells were cultured at
37°C in a 5% CO2 incubator for an additional 4 h and a
Fisherbrand™ accuSkan™ GO UV/Vis Microplate Spectrophotometer
(Thermo Fisher Scientific, Inc.) was used to measure optical
density values at 450 nm.
Western blotting
Western blotting was only performed if the CCHE1 and
IL-17A overexpression rates were between 200–250% or the IL-17A
knockdown rate was <50%. A Total Protein Extraction kit (Merck
KGaA, Darmstadt, Germany) was used to extract the total protein
from cells. Protein samples were quantified using a bicinchoninic
acid assay kit (Sangon, Shanghai, China). Following denaturing in
boiling water for 5 min, electrophoresis was performed using a 10%
SDS-PAGE gel (35 µg per lane). Following transfer to PVDF membranes
and blocking in 5% non-fat milk for 2 h at room temperature,
western blotting was performed using conventional methods; briefly,
the membranes were incubated with the following primary antibodies
at 37°C for 15 h: Rabbit anti-human IL-17A (1:2,000; cat. no.
ab136668) and GAPDH (1:1,000; cat. no. ab8245) (both from Abcam,
Cambridge, UK). A subsequent incubation of 15 h at 37°C was
performed with the secondary, goat anti-rabbit Immunoglobulin
G-horseradish peroxidase-conjugated antibody (1:1,000; cat. no.
MBS435036; MyBioSource, Inc., San Diego, CA, USA). Signals were
developed using enhanced chemiluminescence (Sigma-Aldrich; Merck
KGaA) and were detected using a MYECL™ Imager (Thermo Fisher
Scientific, Inc.). Densitometry analysis was performed using ImageJ
version 1.46 software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All the data are expressed as the mean ± standard
deviation of three experimental repeats. Statistical analysis was
performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
Correlations between expression levels of CCHE1 and IL-17A were
analyzed using Pearson's rank correlation coefficient. The
diagnostic value of plasma CCHE1 expression levels for the
detection of early stage disease was evaluated using a receiver
operating characteristic curve. Comparisons between two groups were
performed by a Student's t-test. Comparisons between multiple
groups were performed by one-way analysis of variance followed by a
post-hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Plasma CCHE1 and IL-17A levels are
upregulated in patients with colon adenocarcinoma, but are not
affected by the presence of distant tumor metastases
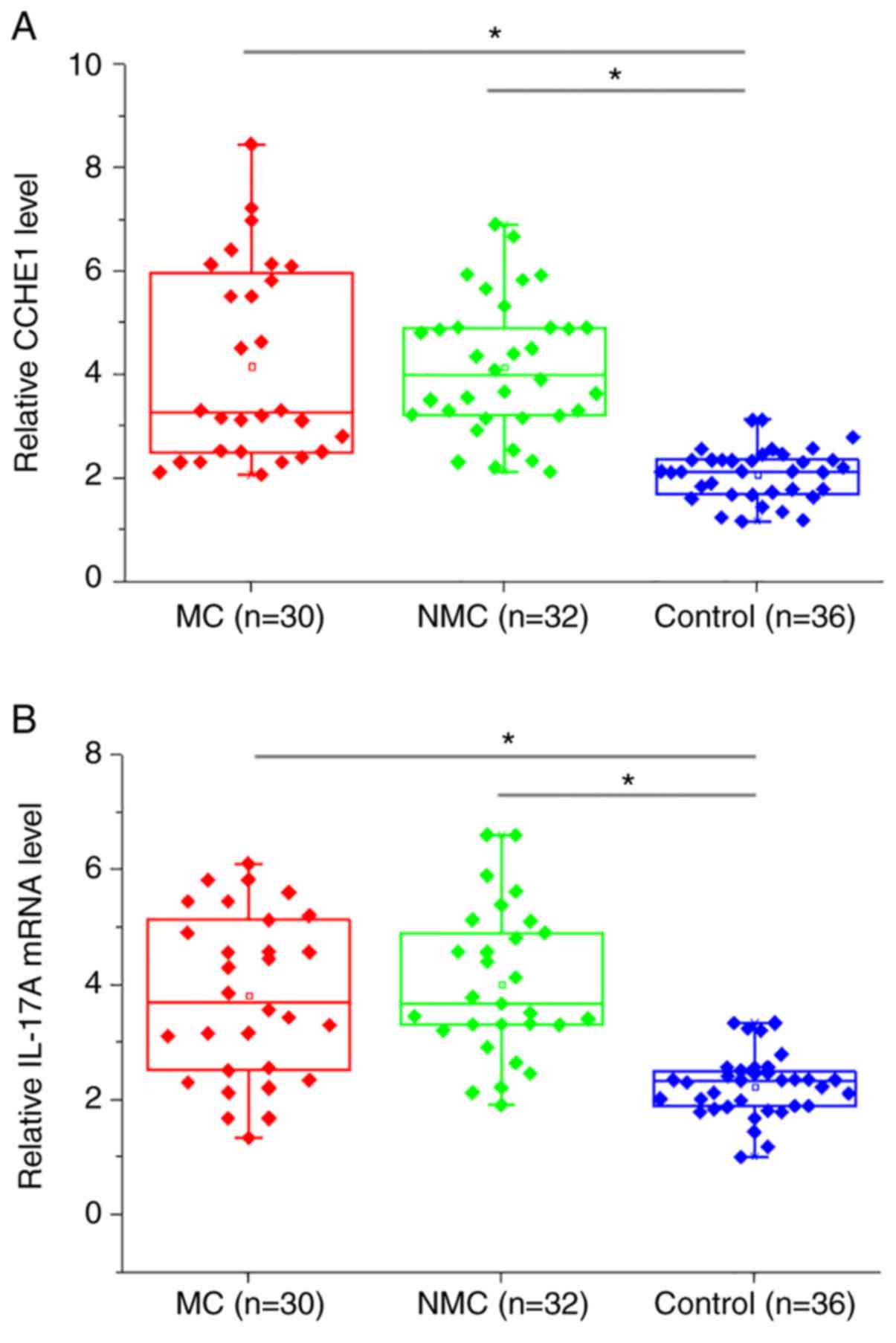
RT-qPCR results showed that, compared with healthy
controls, plasma levels of CCHE1 (Fig.
1A) and IL-17A mRNA (Fig. 1B)
were significantly increased in patients with metastatic colon
adenocarcinoma (MC) and non-metastatic colon adenocarcinoma (NMC;
all P<0.05). However, there were no significant differences in
plasma levels of CCHE1 (Fig. 1A) and
IL-17A mRNA (Fig. 1B) MC and NMC
groups (both P>0.05).
Plasma levels of CCHE1 and IL-17A are
positively associated with the primary tumor diameter
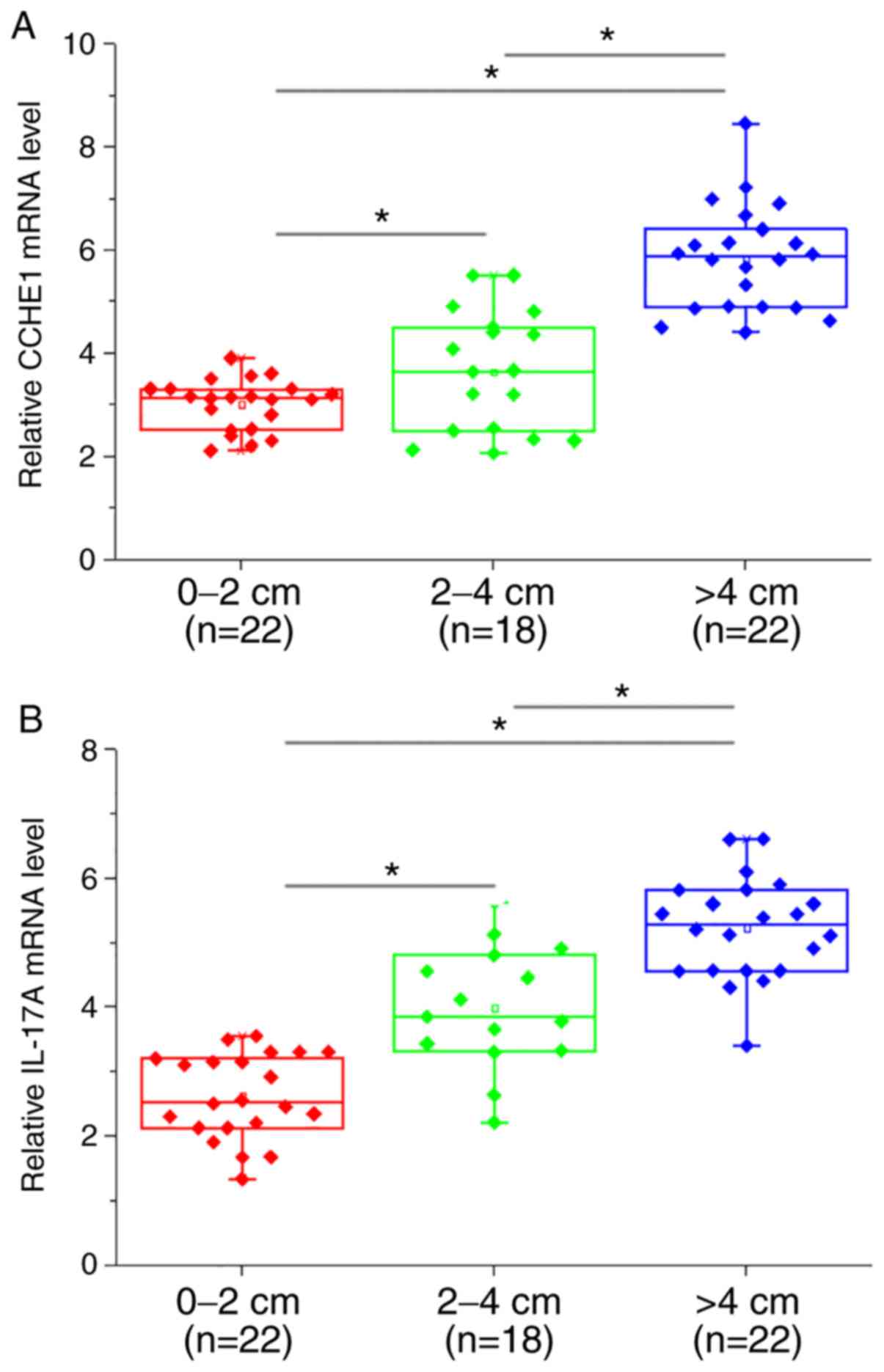
Based on the diameter of primary tumors, colon
adenocarcinoma were divided into 0–2 cm group (n=22), 2–4 cm group
(n=18) and >4 cm group (n=22). The plasma expression levels of
CCHE1 (Fig. 2A) and IL-17A (Fig. 2B) were significantly increased, in
each instance, as the primary tumor diameter increased (all
P<0.05).
Plasma levels of CCHE1 and IL-17A are
positively correlated in patients with colon adenocarcinoma, but
not in healthy controls
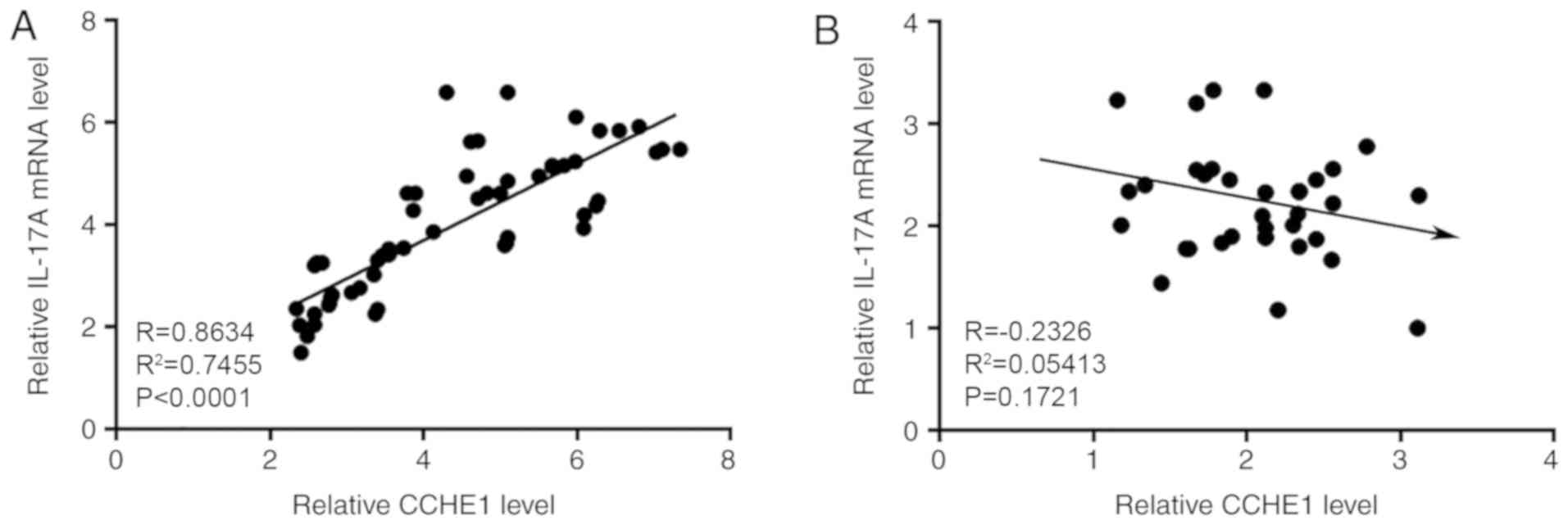
Pearson correlation coefficient analysis showed that
plasma levels of CCHE1 and IL-17A were positively correlated in
patients with colon adenocarcinoma (Fig.
3A; P<0.0001). In contrast, the correlation between plasma
levels of lncRNA CCHE1 and IL-17A was not significant in healthy
controls (Fig. 3B; P=0.1721).
Downregulation of plasma CCHE1
distinguishes patients with early stage colon adenocarcinoma from
healthy controls
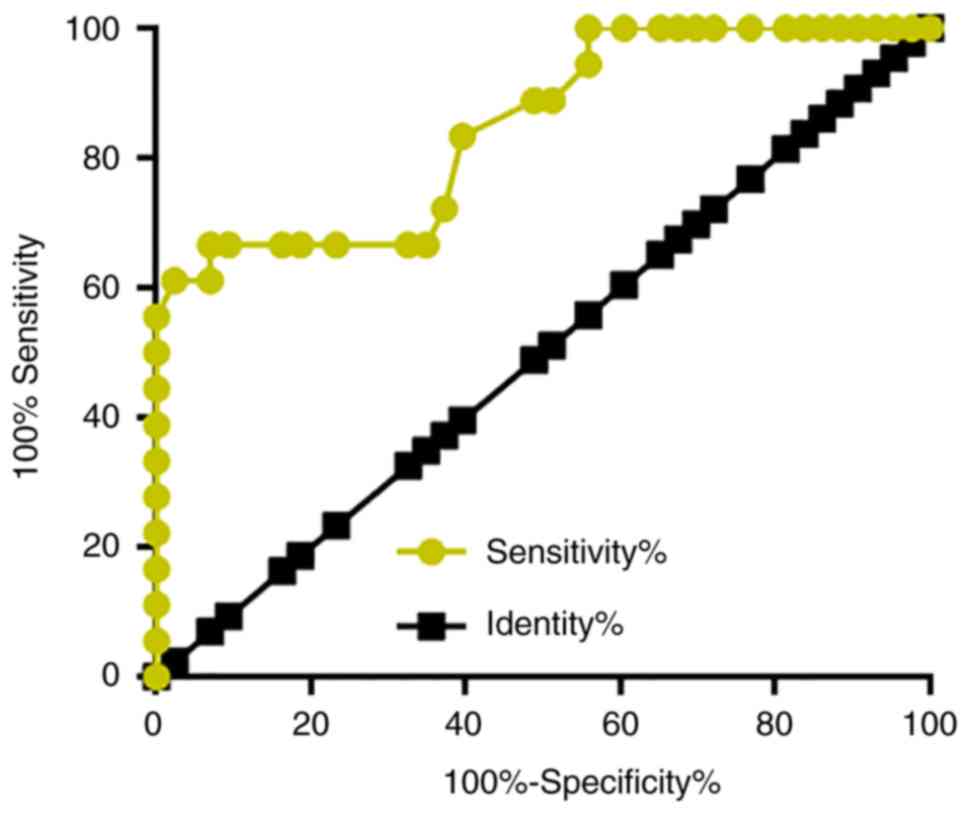
The present study included 19 patients with colon
adenocarcinoma in AJCC stages I and II, which are considered the
early stages of cancer. ROC curve analysis was performed to
evaluate the diagnostic value of plasma CCHE1 for early stage colon
adenocarcinoma. As shown in Fig. 4,
the area under the curve was 0.8745, with standard error of 0.05599
and 95% confidence interval of 0.7378–0.9573.
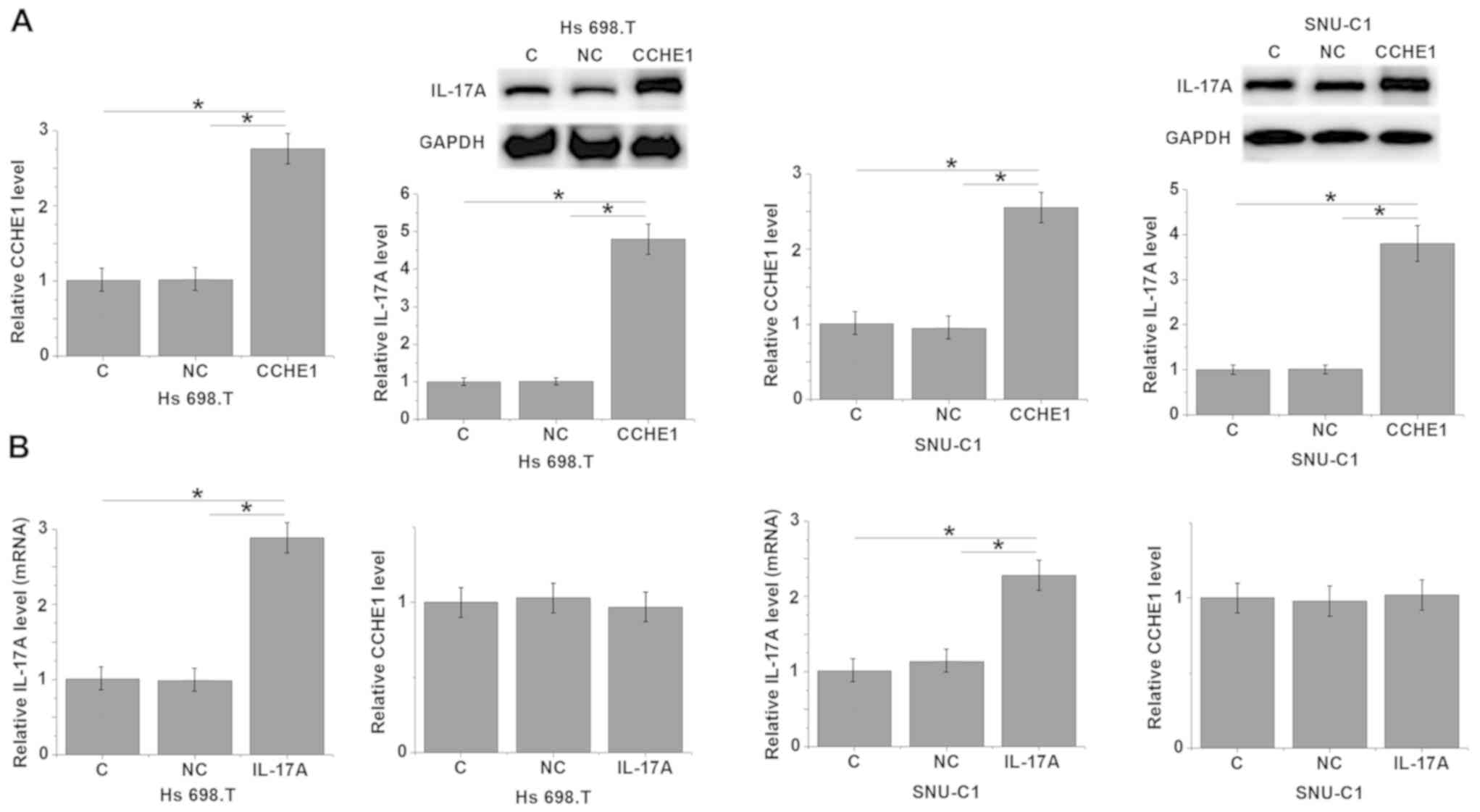
CCHE1 overexpression increases IL-17A
expression in colon adenocarcinoma cell lines Hs 698.T and
SNU-C1
Compared with the control group and negative control
group, cells transfected with CCHE1 showed a significantly
upregulated expression of CCHE1 in cells of both colon
adenocarcinoma cell lines Hs 698.T and SNU-C1 (Fig. 5A, P<0.05). Similarly, CCHE1
overexpression significantly increased the mRNA levels of IL-17A
(Fig. 5A; P<0.05). In contrast,
whilst IL-17A overexpression did significantly increase the
relative IL-17A mRNA levels in both cell lines (Fig. 5B; P<0.05), there was no
significant increase in the expression of CCHE1 mRNA levels
following IL-17A overexpression in either cell line (Fig. 5B; P>0.05).
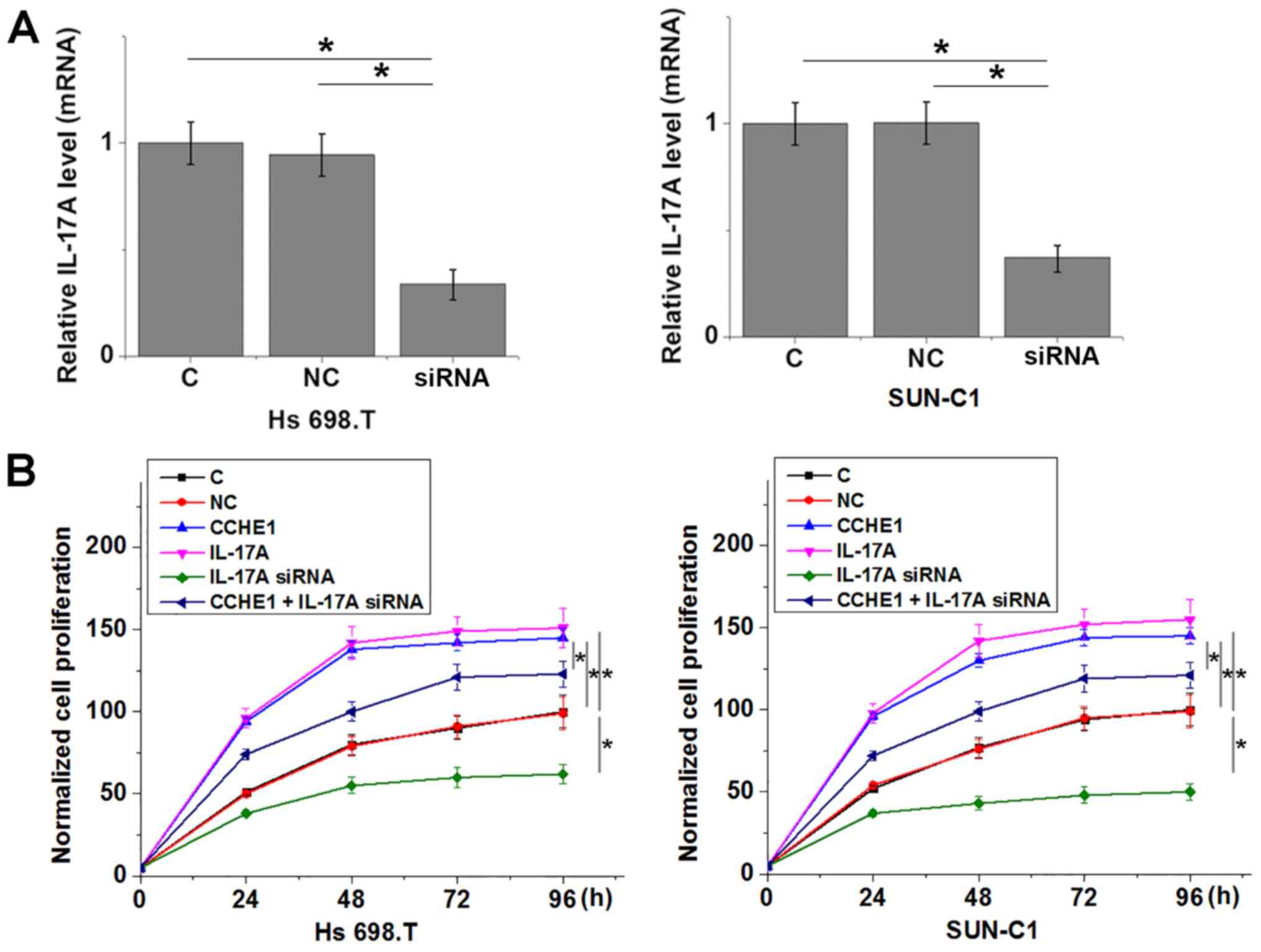
CCHE1 overexpression promotes colon
adenocarcinoma cell proliferation through IL-17A
siRNA-mediated knockdown of IL-17A significantly
reduced relative IL-17A mRNA expression levels compared with the
control and negative control groups in both cell lines (Fig. 6A; P<0.05). Compared with the
control group and the negative control group, proliferation was
increased in cells overexpressing CCHE1 or IL-17A in both cell
lines (Fig. 6B; P<0.05). In
addition, transfection with IL-17A siRNA significantly reduced cell
proliferation and attenuated the increase in proliferation when
co-transfected with CCHE1 in both cell lines (Fig. 6; P<0.05 vs. CCHE1 alone).
Discussion
The functionality of CCHE1 as an oncogene has been
characterized in cervical cancer (14), non-small lung cancer, gastric cancer
and hepatocellular carcinoma (12,13,15),
while its roles in other malignancies are unknown. The key finding
of the present study was that CCHE1 was also an oncogenic lncRNA in
colon adenocarcinoma, the major type of colon cancer accounting for
more than 95% of all colon cancer cases. The CCHE1-indcued
oncogenicity in colon adenocarcinoma was mediated, at least in
part, through interactions with IL-17A.
It has been demonstrated that the development of
colon adenocarcinoma is accompanied by changes in expression levels
of a large set of lncRNAs (19). The
altered expression of those lncRNAs reflects the development,
progression and prognosis of this disease (20). However, most of the differentially
expressed lncRNAs were likely involved in the promoting a number of
aspects of cancer development and progression (19,20).
lncRNAs only involved in a specific aspect of cancer development,
such as growth or metastasis are rare by comparison (19,20).
CCHE1 as an oncogene is overexpressed in cervical cancer, non-small
lung cancer, gastric cancer and hepatocellular carcinoma (12–15).
Based on the results of the present study, CCHE1 may also be
upregulated in colon cancer and the expression levels of CCHE1 were
affected by tumor size, but not the number of metastases. In
vitro cell proliferation experiments additionally demonstrated
that CCHE1 overexpression promoted proliferation of colon
adenocarcinoma cell lines. Therefore, CCHE1 may specifically
participate in the growth, but not metastasis of colon
adenocarcinoma. However, it has been reported that CCHE1 is
involved in the metastasis of non-small lung cancer (12). Therefore, CCHE1 may serve different
roles in different types of malignancies.
As a pro-inflammatory cytokine, IL-17A promotes
tumor growth in different types of human malignancies (8,9). Our
study also showed that IL-17A overexpression promoted, while
siRNA-mediated silencing inhibited, proliferation of human colon
adenocarcinoma cell lines. Therefore, anti-IL-17A agents, such as
Secukinumab, may be used to treat human colon adenocarcinoma.
However, further studies are required to test this hypothesis. The
present study additionally demonstrated that CCHE1 is likely an
upstream inhibitor of IL-17A in the regulation of colon
adenocarcinoma cell proliferation. However, the upstream regulation
may be through indirect mechanisms, as there was a lack of
correlation between CHE1 and IL-17A in the healthy patients. Future
studies should investigate the role of CCHE and IL-17A function in
in vivo models of colon adenocarcinoma.
The clinical relevance of CCHE1 as a potential
biomarker was also demonstrated in the present study, effectively
distinguishing patients with early stage colon adenocarcinoma from
the healthy controls. Therefore, circulating CCHE1 may potentially
be used to assist the early screening of colon adenocarcinoma.
However, more clinical trials are needed to evaluate this
possibility, particularly the diagnostic specificity.
It was previously reported that IL-17A interacts
with the IL-6-Stat3 signaling pathway to promote tumor growth
(21). Therefore, future studies
should investigate the involvement of the IL-6-Stat3 signaling
pathway, as a potential downstream effector of IL-17A in colon
adenocarcinoma. However, IL-17A plays a complex role in
tumorigenesis. IL-17A inhibits anti-tumor immunity by recruiting
myeloid derived suppressor cells (22). In contrast, IL-17 knockout in mice
increases the risk of metastatic lung melanoma (23), suggesting that IL-17A may stimulate
cytotoxic T cells to produce the potent antitumor cytokine
interferon-γ. The complex role of IL-17A in colon adenocarcinoma
requires further study.
Although the functionality of CCHE1 in cancer
biology has been extensively studied in different types of cancers
(12–15), the interactions between lncRNA CCHE1
and chemotherapeutic drugs is still unknown. Therefore, future
studies are required to elucidate the role of CCHE1 in
chemotherapy.
In conclusion, CCHE1 and IL-17A were both
upregulated in colon adenocarcinoma. CCHE1 was involved in growth
possibly through indirect interactions with IL-17A, but may not be
involved in the metastasis of colon adenocarcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW performed the majority of the experiments,
analyzed all data and was a major contributor in writing the
manuscript. HL, CZ, LX and ZC all performed some of the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Inner Mongolia People's Hospital (Inner Mongolia,
China). All patients signed written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maddams J, Utley M and Moller H:
Projections of cancer prevalence in the United Kingdom, 2010–2040.
Br J Cancer. 107:1195–1202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Wilson SE, Stewart DB and
Hollenbeak CS: Marital status and colon cancer outcomes in us
Surveillance, epidemiology and end results registries: Does
marriage affect cancer survival by gender and stage? Cancer
Epidemiol. 35:417–422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiu BC, Ji BT, Dai Q, Gridley G,
McLaughlin JK, Gao YT, Fraumeni JF Jr and Chow WH: Dietary factors
and risk of colon cancer in Shanghai, China. Cancer Epidemiol
Biomarkers Prev. 12:201–208. 2003.PubMed/NCBI
|
|
5
|
Hou L, Ji BT, Blair A, Dai Q, Gao YT and
Chow WH: Commuting physical activity and risk of colon cancer in
Shanghai, China. Am J Epidemiol. 160:860–867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benson AB III, Bekaii-Saab T, Chan E, Chen
YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton
MJ, et al: Metastatic colon cancer, version 3.2013: Featured
updates to the NCCN guidelines. J Natl Compr Canc Netw. 11:141–152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terzic J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology.
138:2101–2114.e5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rei M, Goncalves-Sousa N, Lanca T,
Thompson RG, Mensurado S, Balkwill FR, Kulbe H, Pennington DJ and
Silva-Santos B: Murine CD27(−) Vgamma6(+) gammadelta T cells
producing IL-17A promote ovarian cancer growth via mobilization of
protumor small peritoneal macrophages. Proc Natl Acad Sci USA.
111:E3562–E3570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X,
Shi L, Wu D, Dong C and Liu H: IL-17A produced by gammadelta T
cells promotes tumor growth in hepatocellular carcinoma. Cancer
Res. 74:1969–1982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Srivastava A, Nikamo P, Lohcharoenkal W,
Li D, Meisgen F, Xu Landén N, Ståhle M, Pivarcsi A and Sonkoly E:
MicroRNA-146a suppresses IL-17-mediated skin inflammation and is
genetically associated with psoriasis. J Allergy Clin Immunol.
139:550–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yanagisawa N, Maeda K, Ajisawa A, Imamura
A, Suganuma A, Ando M, Takayama N and Okuno Y: Reduced immune
response to influenza A (H1N1) 2009 monovalent vaccine in
HIV-infected Japanese subjects. Vaccine. 29:5694–5698. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao Y, Cheng S, Xiang J and Luo C: lncRNA
CCHE1 increased proliferation, metastasis and invasion of non-small
lung cancer cells and predicted poor survival in non-small lung
cancer patients. Eur Rev Med Pharmacol Sci. 22:1686–1692.
2018.PubMed/NCBI
|
|
13
|
Xu G, Zhang Y, Li N, Zhang JB and Xu R:
LncRNA CCHE1 in the proliferation and apoptosis of gastric cancer
cells. Eur Rev Med Pharmacol Sci. 22:2631–2637. 2018.PubMed/NCBI
|
|
14
|
Yang M, Zhai X, Xia B, Wang Y and Lou G:
Long noncoding RNA CCHE1 promotes cervical cancer cell
proliferation via upregulating PCNA. Tumour Biol. 36:7615–7622.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng W and Fan H: Long noncoding RNA CCHE1
indicates a poor prognosis of hepatocellular carcinoma and promotes
carcinogenesis via activation of the ERK/MAPK pathway. Biomed
Pharmacother. 83:450–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaballah HH, Gaber RA, Elrashidy MA,
Elshahat DA, Hablus MA and Ebeid AM: Expression of long non-coding
RNA CCHE1 in colorectal carcinoma: Correlations with
clinicopathological features and ERK/COX-2 pathway. Mol Biol Rep.
46:657–667. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mace AG, Pai RK, Stocchi L and Kalady MF:
American joint committee on cancer and college of american
pathologists regression grade: A new prognostic factor in rectal
cancer. Dis Colon Rectum. 58:32–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue Y, Ma G, Gu D, Zhu L, Hua Q, Du M, Chu
H, Tong N, Chen J, Zhang Z and Wang M: Genome-wide analysis of long
noncoding RNA signature in human colorectal cancer. Gene.
556:227–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi J, Li X, Zhang F, Zhang C, Guan Q, Cao
X, Zhu W, Zhang X, Cheng Y, Ou K, et al: Circulating lncRNAs
associated with occurrence of colorectal cancer progression. Am J
Cancer Res. 5:2258–2265. 2015.PubMed/NCBI
|
|
21
|
Wang L, Yi T, Kortylewski M, Pardoll DM,
Zeng D and Yu H: IL-17 can promote tumor growth through an
IL-6-Stat3 signaling pathway. J Exp Med. 206:1457–1464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He D, Li H, Yusuf N, Elmets CA, Li J,
Mountz JD and Xu H: IL-17 promotes tumor development through the
induction of tumor promoting microenvironments at tumor sites and
myeloid-derived suppressor cells. J Immunol. 184:2281–2288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang B, Kang H, Fung A, Zhao H, Wang T and
Ma D: The role of interleukin 17 in tumour proliferation,
angiogenesis, and metastasis. Mediators Inflamm. 2014:6237592014.
View Article : Google Scholar : PubMed/NCBI
|