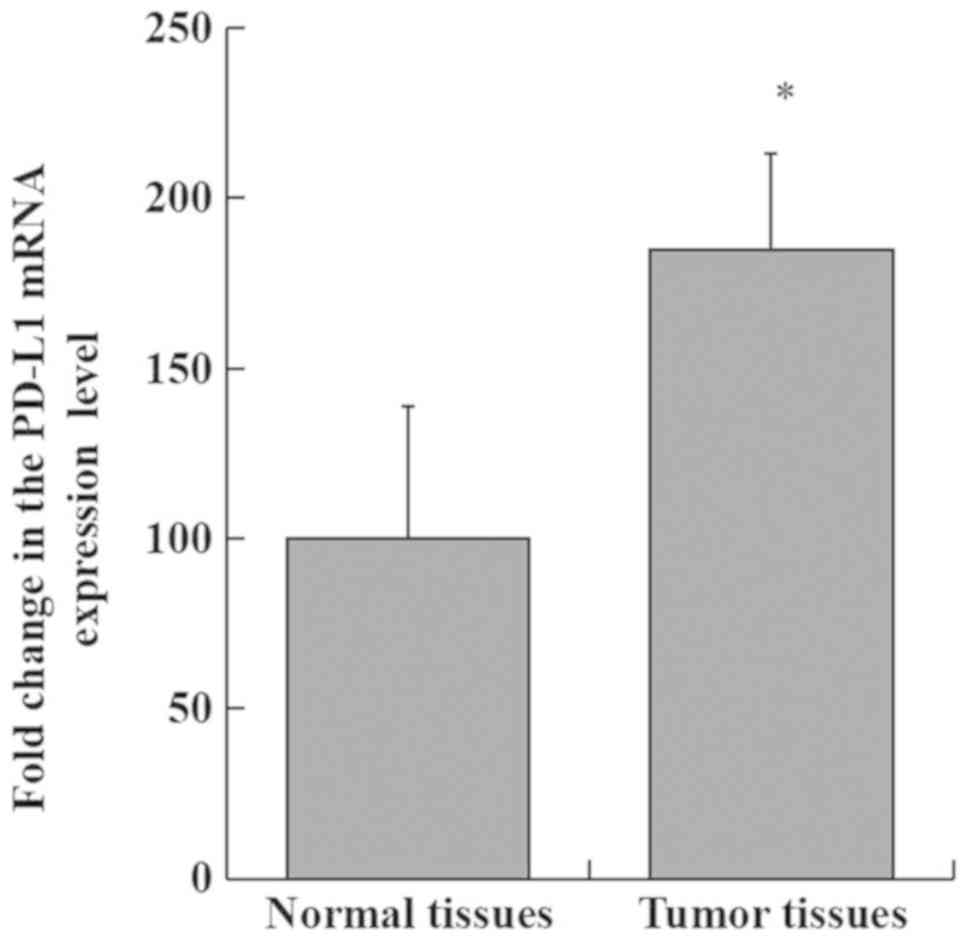
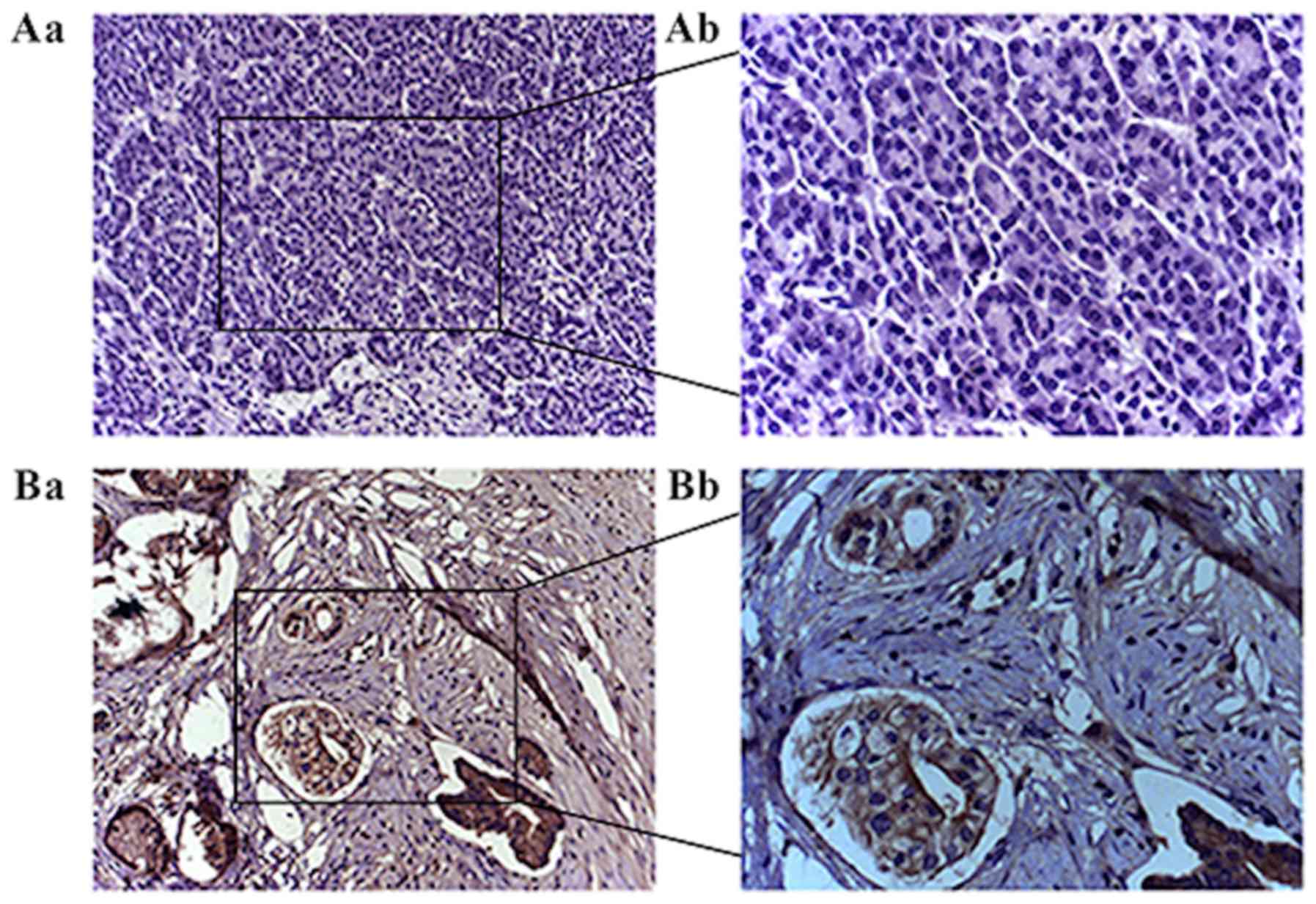
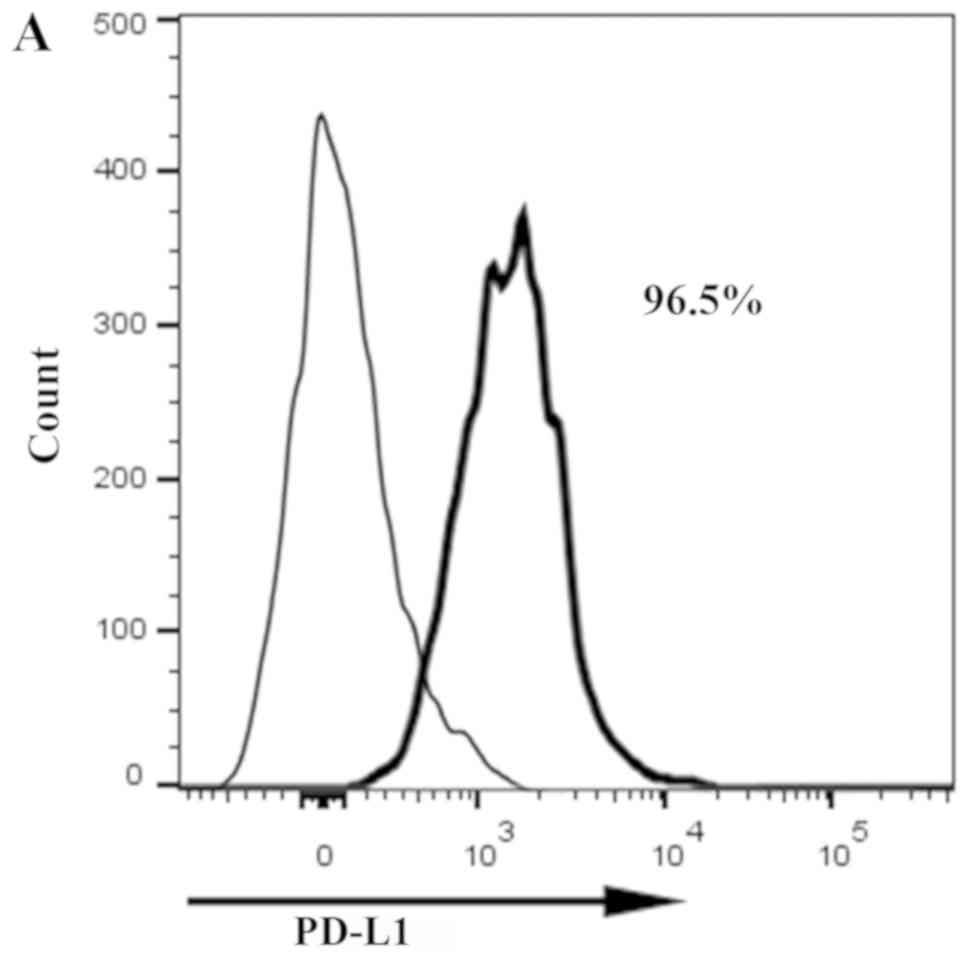
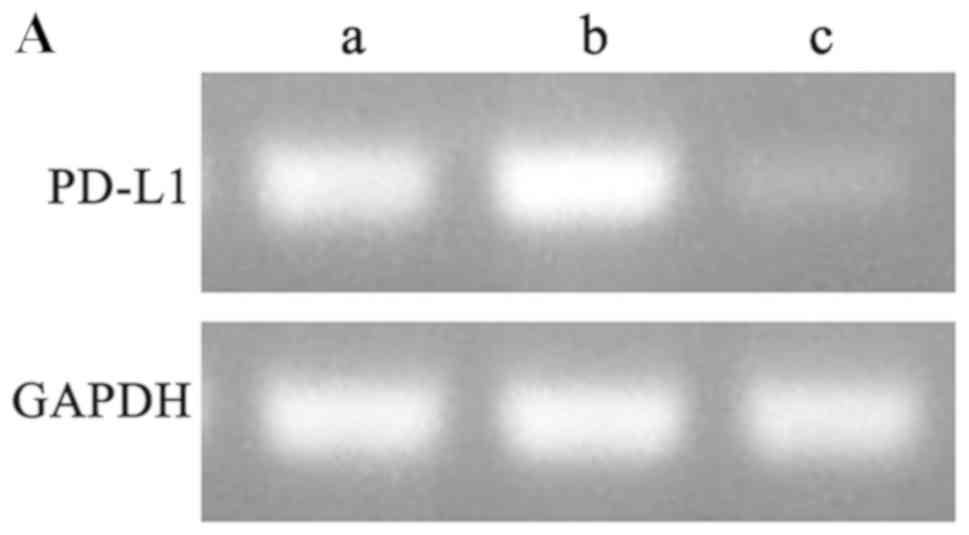
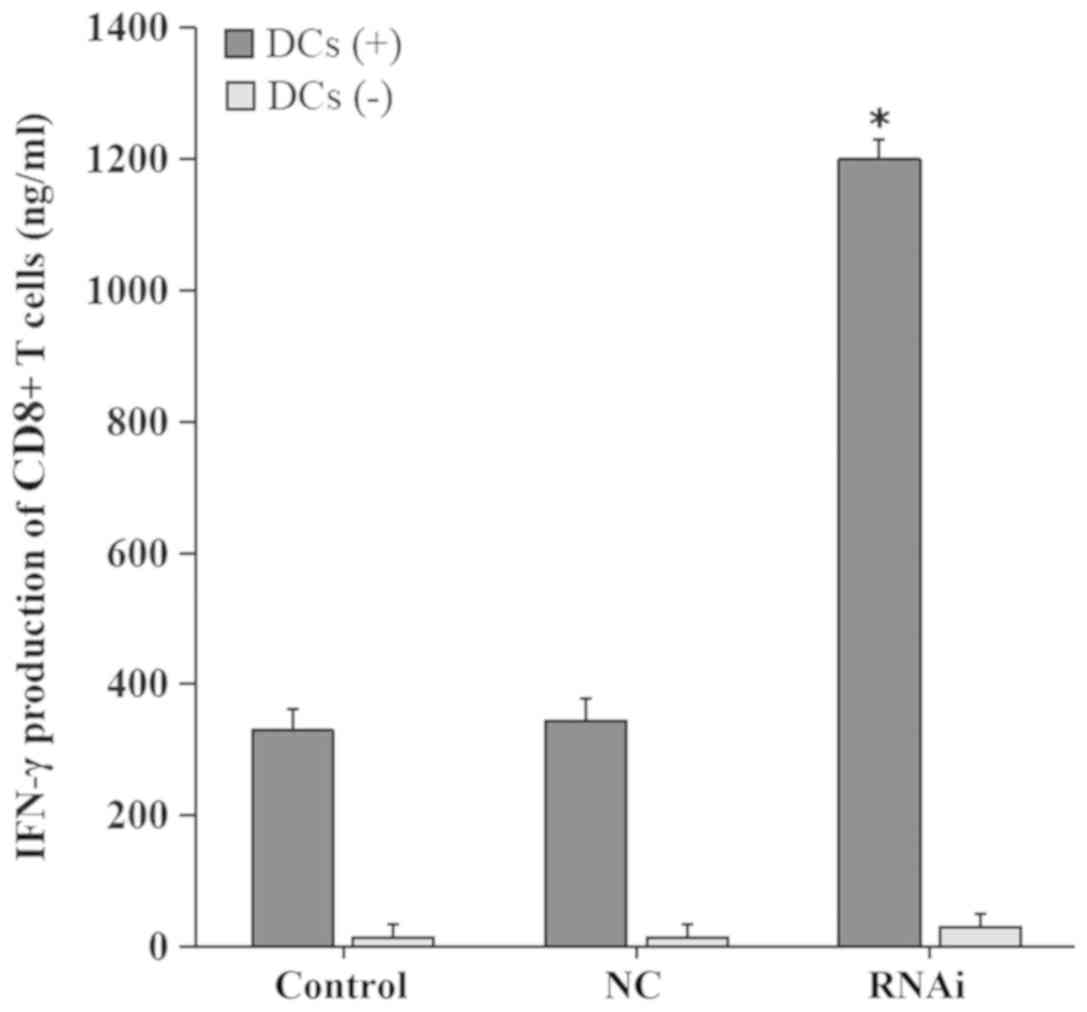
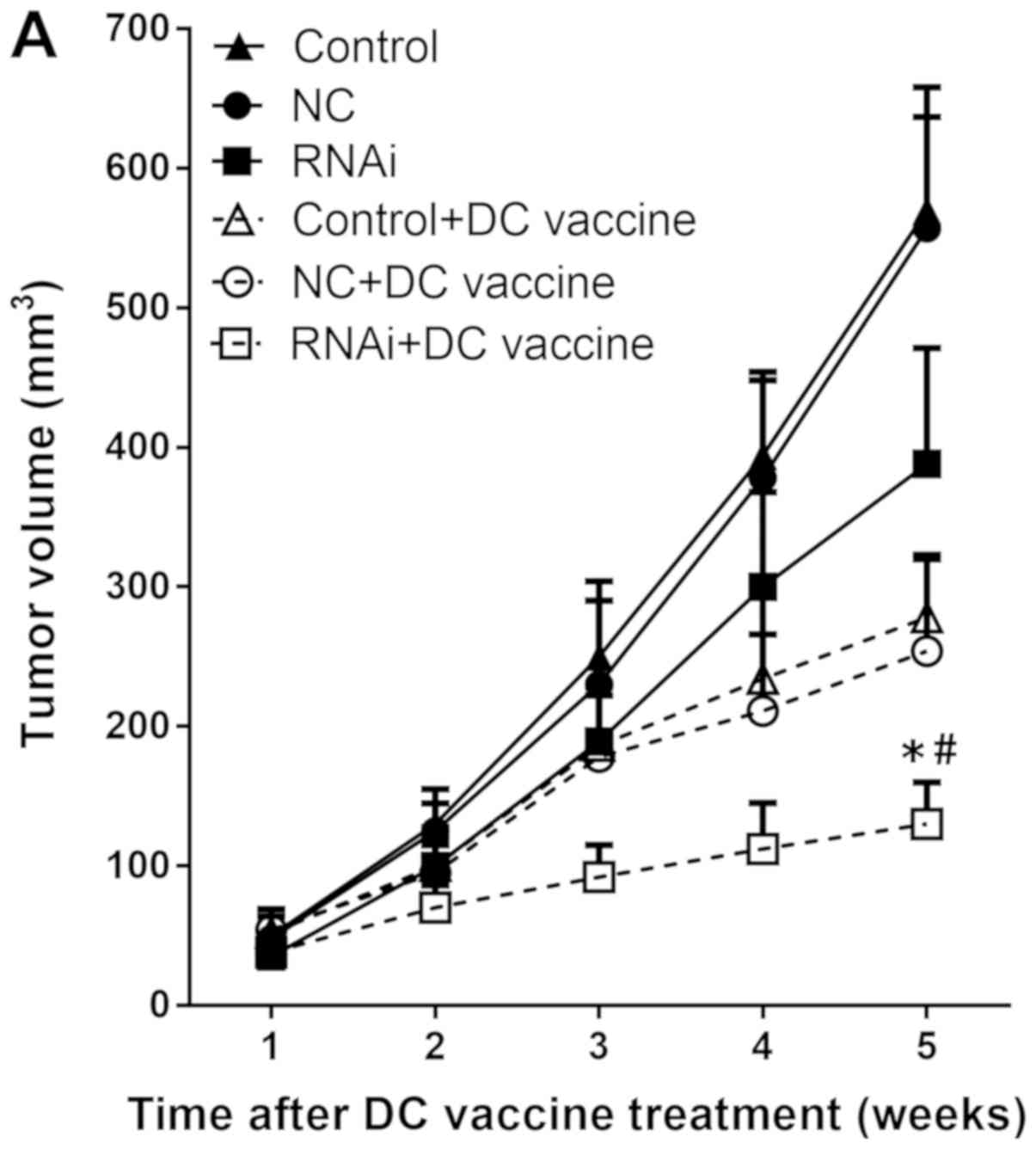
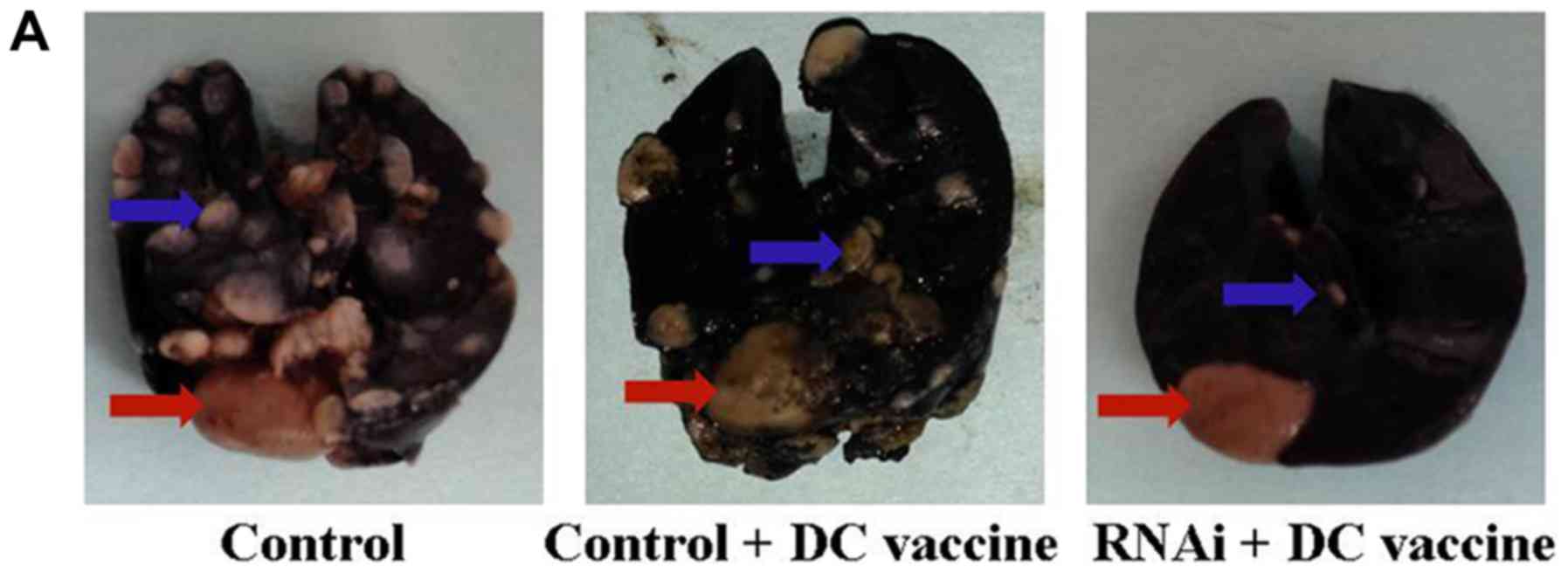
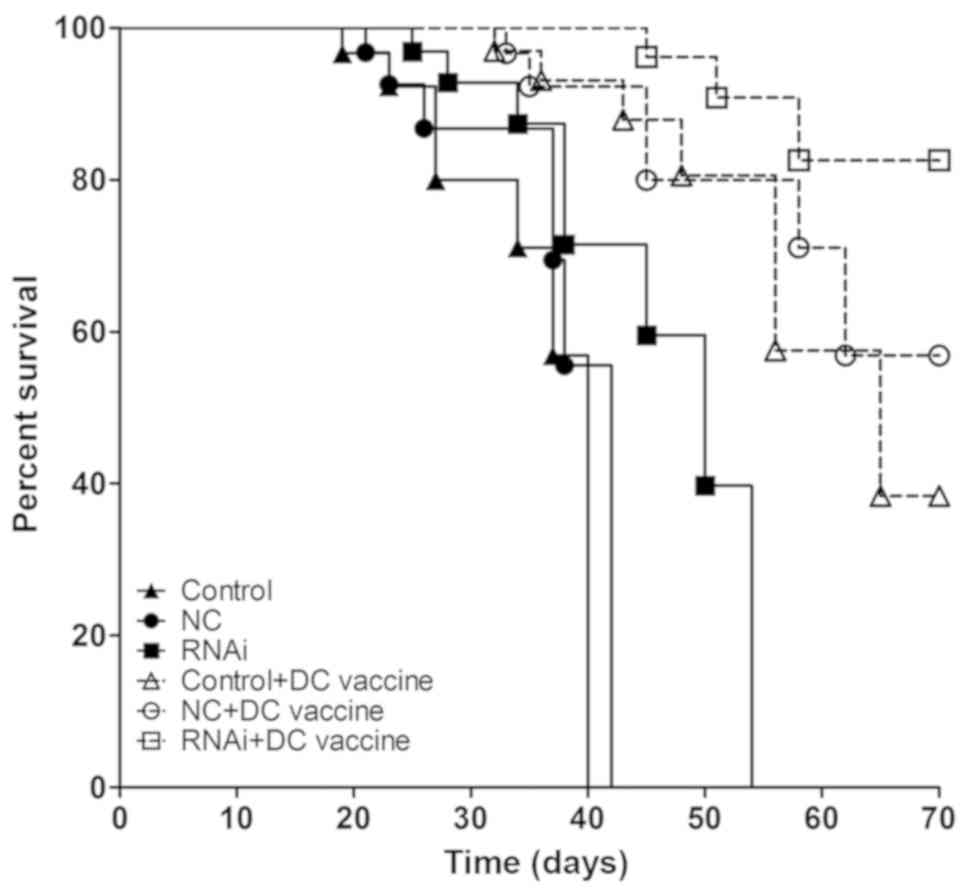
|
1
|
Chu LC, Goggins MG and Fishman EK:
Diagnosis and detection of pancreatic cancer. Cancer J. 23:333–342.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wells A, Grahovac J, Wheeler S, Ma B and
Lauffenburger D: Targeting tumor cell motility as a strategy
against invasion and metastasis. Trends Pharmacol Sci. 34:283–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vanneman M and Dranoff G: Combining
immunotherapy and targeted therapies in cancer treatment. Nat Rev
Cancer. 12:237–251. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patard JJ: Dendritic cells for the
treatment of metastatic renal cell carcinoma: At a low ebb? Eur
Urol. 50:11–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Platt CD, Ma JK, Chalouni C, Ebersold M,
Bou-Reslan H, Carano RA, Mellman I and Delamarre L: Mature
dendritic cells use endocytic receptors to capture and present
antigens. Proc Natl Acad Sci U S A. 107:4287–4292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alloatti A, Kotsias F, Magalhaes JG and
Amigorena S: Dendritic cell maturation and cross-presentation:
Timing matters. Immunol Rev. 272:97–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allgeier T, Garhmmer S, Nossner E, Wahl U,
Kronenberger K, Dreyling M, Hallek M and Mocikat R: Dendritic
cell-based immunogens for B-cell chronic lymphocytic leukemia.
Cancer Lett. 245:275–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thompson RH, Gillett MD, Cheville JC,
Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen
L, et al: Costimulatory B7-H1 in renal cell carcinoma patients:
Indicatorof tumor aggressiveness and potential therapeutic target.
Proc Natl Acad Sci U S A. 101:17174–17179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci U S A. 99:12293–12297. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blank C, Brown I, Peterson AC, Spiotto M,
Iwai Y, Honjo T and Gajewski TF: PD-L1/B7-H1 inhibits the effector
phase of tumor rejection by T cell receptor (TCR) transgenic CD81 T
cells. Cancer Res. 64:1140–1145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allen PJ, Kuk D, Castillo CF, Basturk O,
Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide
V, He J, et al: Multi-institutional validation study of the
American joint commission on cancer (8th edition) changes for T and
N staging in patients with pancreatic adenocarcinoma. Ann Surg.
265:185–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KG and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brown JA, Dorfman DM, Ma FR, Sullivan EL,
Munoz O, Wood CR, Greenfield EA and Freeman GJ: Blockade of
programmed death-1 ligands on dendritic cells enhances T cell
activation and cytokine production. J Immunol. 170:1257–1266. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ge Y, Xi H and Zhang XG: Vaccination with
immature dendritic cells combined with CD40 mAb induces protective
immunity against B lymphoma in SCID-hu mice. Biomed Pharmacother.
64:487–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng M, Xiong G, Cao Z, Yang G, Zheng S,
Song X, You L, Zheng L, Zhang T and Zhao Y: PD-1/PD-L1 and
immunotherapy for pancreatic cancer. Cancer Lett. 407:57–65. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gajewski TF, Schreiber H and Fu YX: Innate
and adaptive immune cells in the tumor microenvironment. Nat
Immunol. 14:1014–1022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y and Cao X: Immunosuppressive cells
in tumor immune escape and metastasis. J Mol Med(Berl). 94:509–522.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ito A, Kondo S, Tada K and Kitano S:
Clinical development of immune checkpoint inhibitors. Biomed Res
Int. 2015:6054782015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zitvogel L and Kroemer G: Targeting
PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology.
1:1223–1225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity and immune correlates
of anti-PD-1 antibody in cancer. N Engl J Med. 366:2443–2454. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Flies DB and Chen L: The new B7s: Playing
a pivotal role in tumor immunity. J Immunother. 30:251–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng
P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et
al: Blockade of B7-H1 improves myeloid dendritic cell-mediated
antitumor immunity. Nat Med. 9:562–567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong H and Chen L: B7-H1 pathway and its
role in the evasion of tumor immunity. J Mol Med (Berl).
81:281–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Strome SE, Dong H, Tamura H, Voss SG,
Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al:
B7-H1 blockade augments adoptive T-cell immunotherapy for squamous
cell carcinoma. Cancer Res. 63:6501–6505. 2003.PubMed/NCBI
|
|
34
|
Hirano F, Kaneko K, Tamura H, Dong H, Wang
S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al: Blockade of
B7-H1 and PD-1 by monoclonal antibodies potentiates cancer
therapeutic immunity. Cancer Res. 65:1089–1096. 2005.PubMed/NCBI
|
|
35
|
Loos M, Giese NA, Kleeff J, Giese T, Gaida
MM, Bergmann F, Laschinger M, W Büchler M and Friess H: Clinical
significance and regulation of the costimulatory molecule B7-H1 in
pancreatic cancer. Cancer Lett. 268:98–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iwai Y, Terawaki S and Honjo T: PD-1
blockade inhibits hematogenous spread of poorly immunogenic tumor
cells by enhanced recruitment of effector T cells. Int Immunol.
17:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blank C, Brown I, Marks R, Nishimura H,
Honjo T and Gajewski TF: Absence of programmed death receptor 1
alters thymic development and enhances generation of CD4/CD8
double-negative TCR-transgenic T cells. J Immunol. 171:4574–4581.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nishimura H, Agata Y, Kawasaki A, Sato M,
Imamura S, Minato N, Yagita H, Nakano T and Honjo T:
Developmentally regulated expression of the PD-1 protein on the
surface of double-negative (CD42/CD82) thymocytes. Int Immunol.
8:773–780. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carter L, Fouser LA, Jussif J, Fitz L,
Deng B, Wood CR, Collins M, Honjo T, Freeman GJ and Carreno BM:
PD-1: PD-L1 inhibitory pathway affects both CD4 (+) and CD8 (+) T
cells and is overcome by IL-2. Eur J Immunol. 32:634–643. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Selenko-Gebauer N, Majdic O, Szekeres A,
Höfler G, Guthann E, Korthäuer U, Zlabinger G, Steinberger P, Pickl
WF, Stockinger H, et al: B7-h1 (programmed death-1 ligand) on
dendritic cells is involved in the induction and maintenance of T
cell anergy. J Immunol. 170:3637–3644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gerber SA, Sedlacek AL, Cron KR, Murphy
SP, Frelinger JG and Lord EM: IFN-γ mediates the antitumor effects
of radiation therapy in a murine colon tumor. Am J Pathol.
182:2345–2354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ge Y, Xi H, Ju S and Zhang X: Blockade of
PD-1/PD-L1 immune checkpoint during DC vaccination induces potent
protective immunity against breast cancer in SCID-hu mice. Cancer
Lett. 336:253–259. 2013. View Article : Google Scholar : PubMed/NCBI
|