Introduction
Testicular seminoma is one of the most common solid
cancer types in young men and accounts for almost half of
testicular germ cell tumors (1). The
vast majority of seminoma patients are cured by current therapy
concepts, including orchiectomy, radiotherapy and/or platinum-based
chemotherapy (2–4). However, survival is poor in these
patients with platinum refractory disease relapsing after high-dose
chemotherapy (5). Given the high
density of immune cells typically seen in seminomas, it is
intuitive, that immune checkpoint inhibitors may represent a
therapeutic option in these tumors. Clinical response and
non-response of advanced germ cell cancers after therapy with
Nivolumab or Pembrolizumab have indeed been reported and clinical
trials systematically evaluating these drugs in platinum resistant
germ cell tumors are ongoing (6,7).
The clinical success of checkpoint inhibitors
targeting the PD-1/PD-L1 system is stunning in many cancers
(8–10), and earlier studies reported a
prognostic role of PD-L1 expressing tumor infiltrating lymphocytes
in seminoma samples (11,12). Moreover, there is much hope that the
use of combinatorial drugs targeting not only a single, but several
immune checkpoint receptors, might further improve therapeutic
results. T cell immunoreceptor with Ig and ITIM domain (TIGIT), a
co-inhibitory transmembrane glycoprotein of the poliovirus receptor
(PVR)/nectin superfamily, is another interesting candidate for
novel checkpoint therapies (13,14). In
mouse models and ongoing clinical studies, blockade or ablation of
TIGIT, alone or in combination with blockade of PD-1, can restore
tumor suppressive effects (15–19).
These findings indicate that TIGIT, similar to PD-1, has a crucial
role in inhibiting the tumor-directed immune response and, thus,
might be a suitable and relevant target for novel immune-modulating
therapies. Several drugs targeting TIGIT are currently under
development (20). TIGIT expressing
lymphocytes have so far been demonstrated in acute myeloid
leukemia, non-small cell lung cancer, colorectal carcinoma and
melanoma (16,17,21).
While it appears possible that the selection of the
optimal immune checkpoint inhibitor may depend on the role of the
respective target in a cancer's associated immune cells, we were
interested in the expression of TIGIT and PD-1 on lymphocytes in
seminomas. In this study, the patterns of TIGIT and PD-1 were
analyzed by immunohistochemistry in 78 seminomas in a tissue
microarray (TMA) format.
Materials and methods
Patients and tissues
Formalin-fixed paraffin embedded tissue samples from
78 anonymized patients with seminoma were retrieved from the
archives of the Institute of Pathology of the University Medical
Center, Hamburg Eppendorf. On average, the mean age was 38±9 years
and the median and mean tumor sizes were 30 mm (range:10 to 70 mm)
and 33±14 mm. This patient cohort contained two 0.6 mm tumor
punches per patient were assembled in a TMA. The TMA manufacturing
process has been described earlier (22).
Immunohistochemistry
Three freshly cut consecutive TMA sections were
immunoassayed for CD3, TIGIT and PD-1. Slides were deparaffinized
and exposed to heat-induced antigen retrieval for 5 min in an
autoclave at 121°C in pH 6 buffer for PD-1, pH 7.8 buffer for TIGIT
and pH 9 buffer for CD3. Primary antibody specific for CD3 (rabbit
polyclonal antibody, undiluted, cat. no. IR503; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA), TIGIT (mouse monoclonal
antibody, Dianova GmbH, Hamburg, Germany; cat. no. DIA-TG1; 1:70)
and PD-1 [mouse monoclonal (NAT105) antibody, Abcam, Cambridge, UK;
cat. no. ab52587; 1:50] was applied at 37°C for 60 min. Bound
antibody was then visualized using the EnVision Kit (Dako; Agilent
Technologies, Inc.) according to the manufacturer's directions.
Scoring of CD3, TIGIT and PD-1
immunostaining
To assure that the quantity of tissue analyzed per
patient was identical, only the first spot per patient that was
interpretable for all three markers was analyzed. The total number
of CD3+, TIGIT+ and PD-1+ cells
was manually counted in each TMA slide and tissue spot. The
TIGIT:CD3 and PD-1:CD3 ratio was calculated for each tissue spot to
determine the fraction of TIGIT and PD-1 positive T
lymphocytes.
Statistical analysis
The JMP 12.0 software package (23) (SAS Institute Inc., NC, USA) was used
to calculate the mean and standard deviation of the fraction of
TIGIT and PD-1 positive cells.
Results
All 78 seminomas included in this study harbored
tumor infiltrating CD3+ T lymphocytes. Their number was
variable between individual cancers and ranged from 16 to 2,113
(mean: 623, standard deviation: 509). All tumors also showed TIGIT
and PD-1 staining in a variable number of immune cells. This number
was overall somewhat higher for TIGIT than for PD-1: The number of
TIGIT+ lymphocytes per 0.6 mm tissue spot ranged from 2
to 1,147 (mean: 194, standard deviation: 185), the number of
PD-1+ lymphocytes ranged from 2 to 424 (mean: 107,
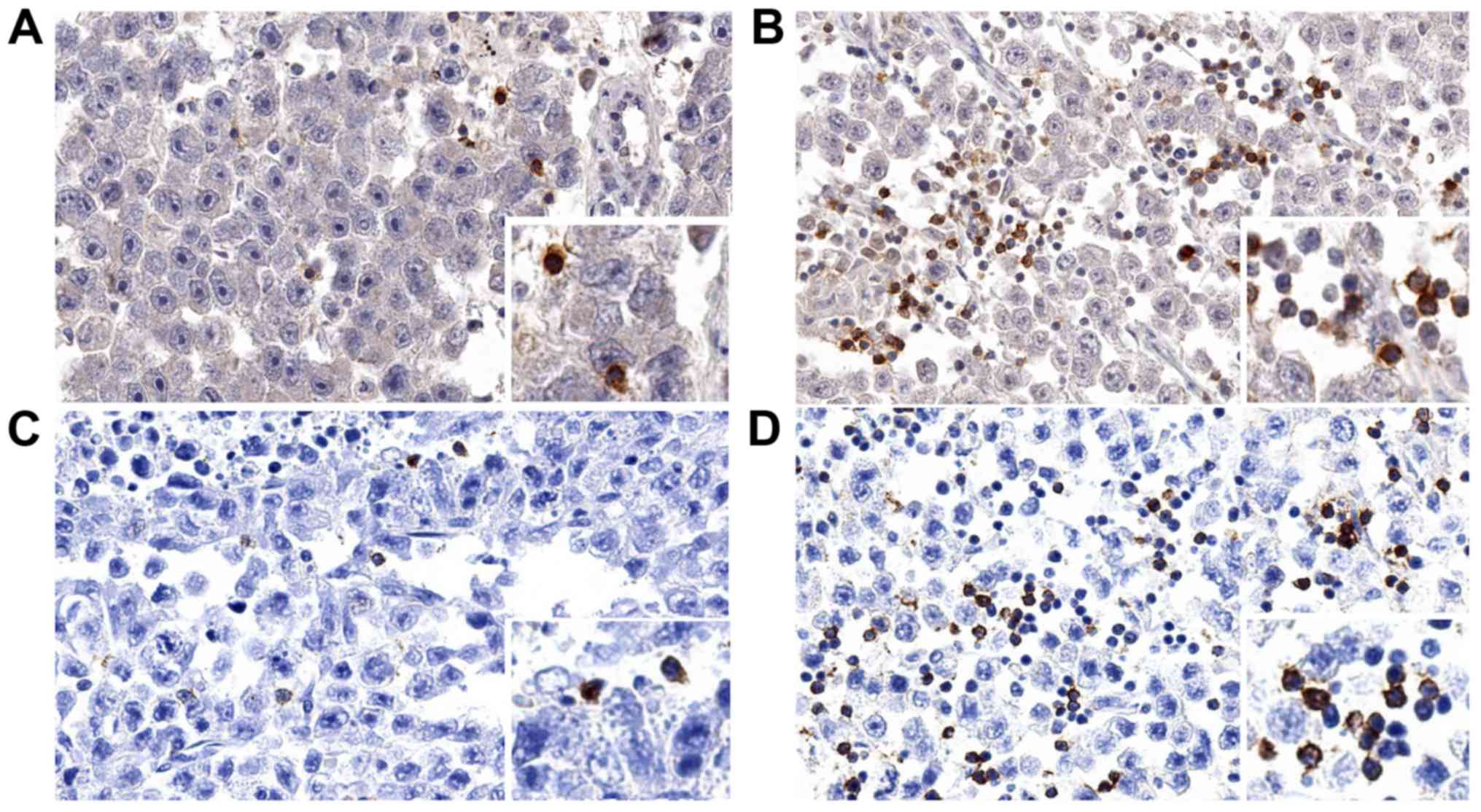
standard deviation: 93). Representative images of immunostainings
are shown in Fig. 1.
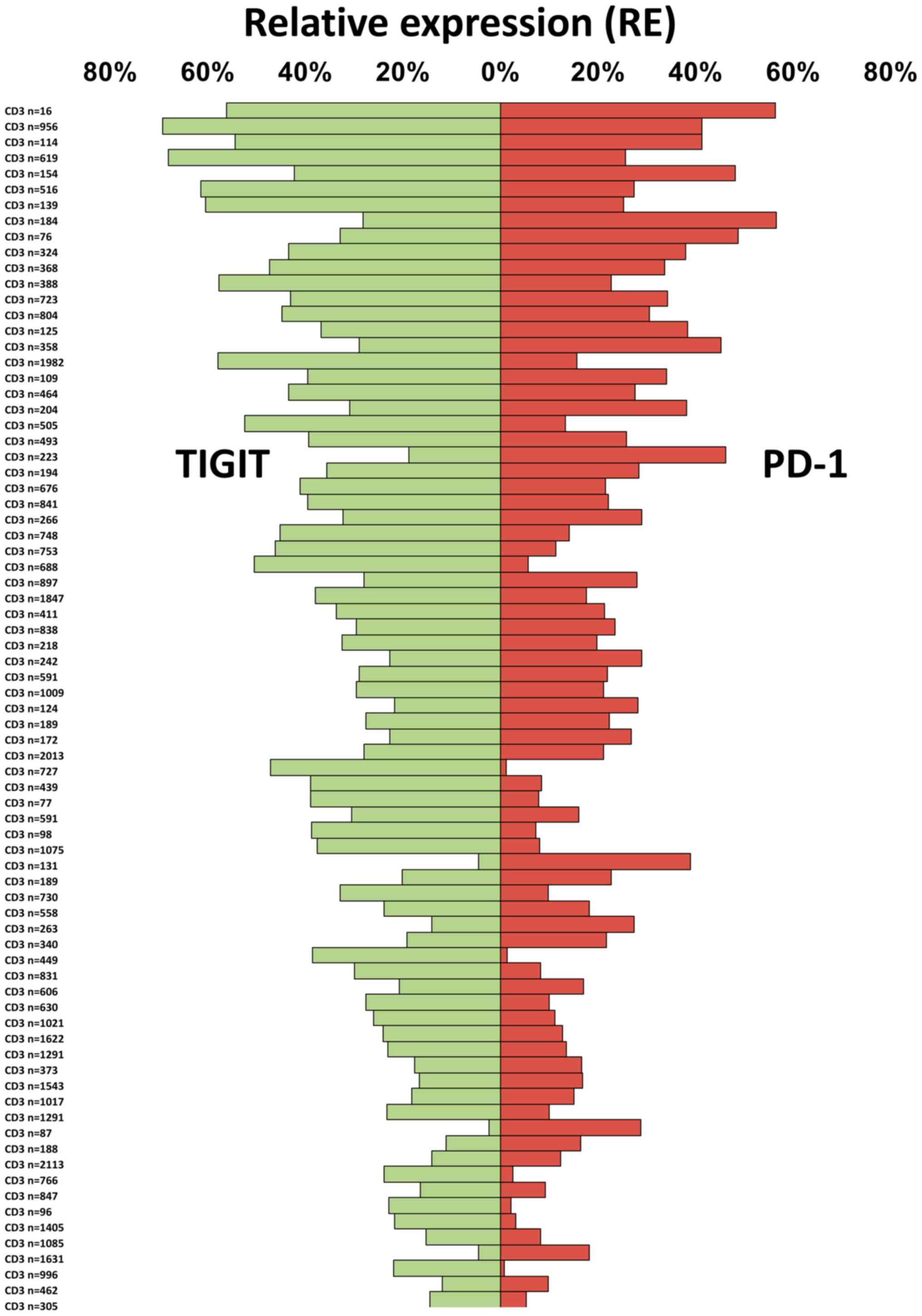
To compare the relative abundance of TIGIT and PD-1
expressing T cells in individual tumor samples, we used the number
of CD3+ T cells per tissue spot as a reference. It
showed that both the fraction of TIGIT+ T cells and of
PD-1+ T cells was variable among the 78 seminoma
patients. The fraction of TIGIT+ T cells (mean:
32.2±14.7%) ranged from 2.3 to 69.4% and that of PD-1 (mean:
21.6±13.2%) from 0.8 to 56.5% (Fig.
2).
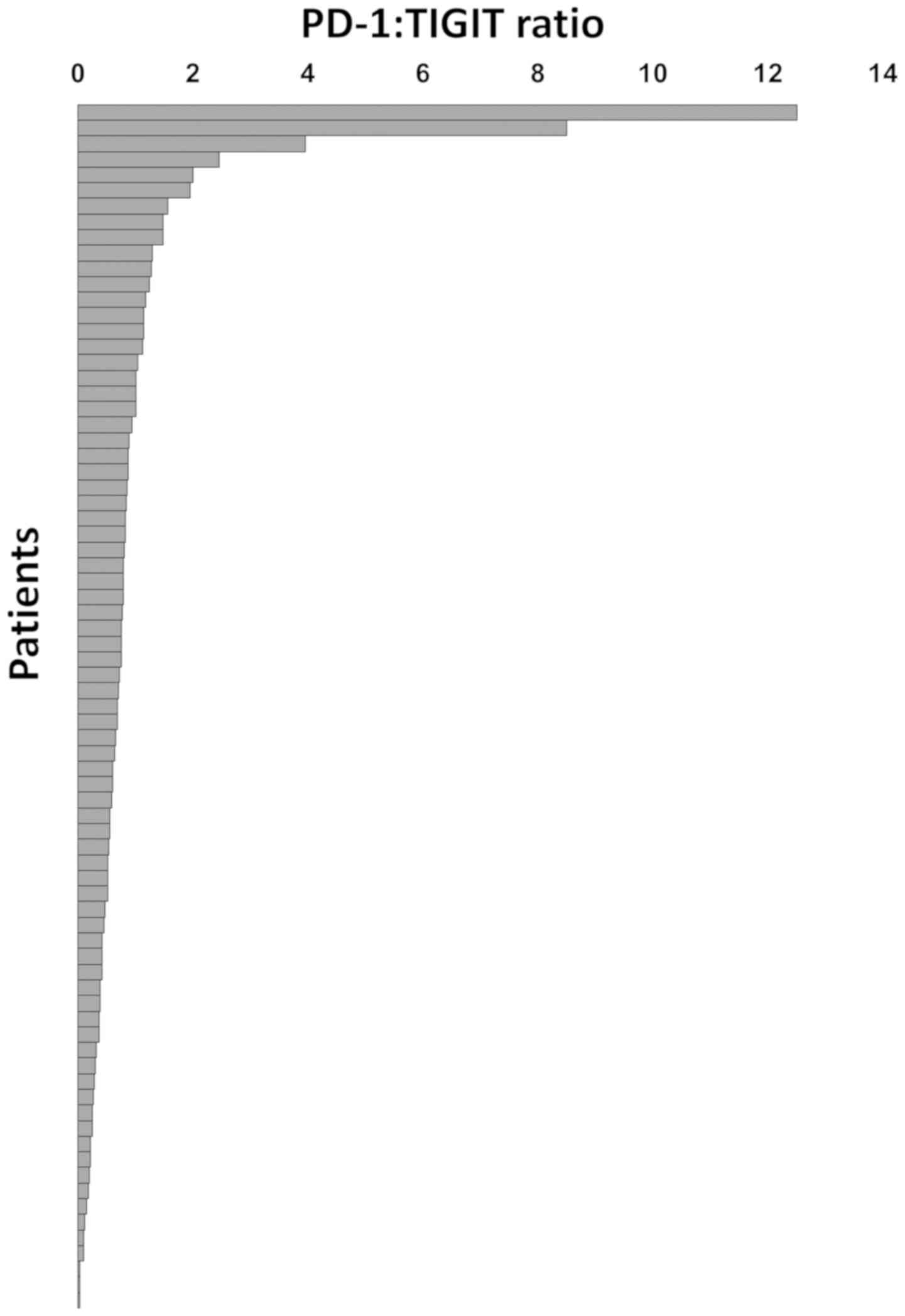
In individual cancers, TIGIT expression largely but
not fully paralleled that of PD-1. However, the relative importance
of PD-1 and TIGIT appeared to be rather variable. TIGIT expression
exceeded that of PD-1 in 58 cancers while 18 tumors had PD-1 levels
beyond that of TIGIT. In these cancers, the PD-1: TIGIT ratio
ranged from 0.02 to 12.5 (Fig.
3).
Discussion
The data from the present study demonstrate that not
only the fraction of TIGIT+ and/or PD-1+ T
cells, but also their relative abundance, is highly variable
between individual seminomas.
TIGIT+ T lymphocytes were detected in all
tumors in our study. This was expected because in an earlier study
using a comparable experimental set-up, we had also regularly found
TIGIT expression in a considerable fraction of lymphocytic cells in
healthy lymphatic organs, various inflammatory diseases and in
samples of lung and colorectal cancers (19). Taken together, these findings
strongly support the concept that TIGIT expression is an inherent
feature of T cell lymphocytic infiltrations in normal, inflammatory
and cancerous tissues (19). Earlier
studies using flow cytometry have also described regular presence
of TIGIT+ T cells in these immune microenvironments
(13,17,21,24,25). For
example, Johnston et al (16), detected TIGIT expression among
CD8+ cytotoxic T cells in colon and breast cancer.
Josefsson et al (24),
described TIGIT expressing cells in follicular lymphomas. Luo et
al (25), showed increased TIGIT
expression in the autoimmune environment of rheumatoid arthritis
(26). Drugs targeting TIGIT are
currently developed by various companies (15,20).
Although there is some evidence for a lack of response to PD-L1
inhibitory drugs in more than 90% of the treated patients (27), further therapy attempts using
combined or single anti-TIGIT and/or anti-PD-1 therapies are still
lacking in testicular seminoma.
Overall, the existing data on the prevalence of
TIGIT expression seems to suggest that such drugs may potentially
be applicable to a very broad range of different tumor types.
The high interest in TIGIT emanates from its analogy
to PD-1, which has become a major therapeutic ‘host target’ in a
multitude of human tumor types (8,28,29).
That PD-1 expression was seen in a fraction of T lymphocytes in all
analyzed seminomas is in line with a recent study using multiplex
fluorescence immunohistochemistry (30). In this study, Siska et al
found a variable T cell infiltration and immune checkpoint
expression in almost all analyzed large sections of seminomas and
non-seminomas. That comparable absolute and relative numbers were
found in our study using brightfield immunohistochemistry
represents an indirect validation of our experimental approach.
The high numbers of intratumoral CD3+,
TIGIT+ and PD-1+ cells per 0.6 mm tissue spot
(0.28 mm2) demonstrate that immune cells play a
particularly strong role in seminoma. Adjusted numbers per square
millimeter (CD3: Average 2,203±1,799 per mm2) are higher
than what we found in urinary bladder cancer (CD3: 625±800,
cells/mm2) (31) or what
was earlier described in breast (150 to 300 CD3+
cells/mm2) (32) or
colorectal cancer (400 to 700 CD3+ cells/mm2)
(33). The potential significance of
these immune cells for anti-tumor activity is best demonstrated by
cases of ‘burned out seminomas’ (34). In these patients-sometimes
extensive-metastatic seminoma spread occurs in the absence of vital
tumor tissue in the testis. Instead, circumscribed scar formation
indicates the location of a ‘self-healed’ testicular seminoma.
Based on this, it is tempting to speculate that treatment with
immune checkpoint inhibitors-perhaps even first line-might be
particularly successful in testicular germ cell tumors. Currently
used platinum-based therapies are highly efficient (35) but there are only inadequate treatment
options available for chemotherapy refractory or relapsed
metastatic testicular seminomas (36). However, because of the young age,
patients often develop long-term sequelae of treatment, such as
cardiovascular disease, renal insufficiency or secondary
malignancies (35,37,38).
Therapies targeting immune checkpoint receptors may exert
comparable little long-term side effects (39,40).
The most striking observation in our study was the
high variability of the relative fraction of TIGIT+ and
PD-1+ lymphocytes in seminomas. We earlier reported a
similar diversity of the relative role of TIGIT and PD-1 in a
cohort of 40 Hodgkin's lymphomas (41). If it holds true that the different
checkpoint receptors are so variably expressed in individual cancer
patients, the analysis of the inflammatory cells may proof
instrumental to select the optimal immune checkpoint inhibitor for
a given patient.
In conclusion, the results of our study demonstrate
frequent expression of immune checkpoints receptors in human
seminomas. This argues for a potential benefit of drugs targeting
immune checkpoint molecules in these tumors. The high variability
of the relative prevalence of TIGIT+ and
PD-1+ cells between patients raises the hypothesis that
a thorough analysis of checkpoint proteins in tumor infiltrating
lymphocytes may in the future assist the choice of therapy.
Acknowledgements
The authors would like to thank Mrs Christina
Möller-Koop, Mrs Inge Brandt, Mrs Melanie Witt and Mrs Janett
Lüttgens from the Department of Pathology, University Medical
Centre Hamburg-Eppendorf, for their technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW, GS, RS, NB and KF undertook study conception and
design. AH, NB, WL, RS, GS, KF were responsible for development of
the methodology. Acquisition of data, including providing animals,
acquiring and managing patients, providing facilities, was the
responsibility of AH, WL, NB, TM, BW, ND, WW, DH, ML, JI, SM, FB,
RU, DD, TK, AL, CW, FJ, EB and SS. Analysis and interpretation of
data, including statistical analysis, biostatistics, computational
analysis was performed by AH, WW, WL, NB, RS and GS. Writing,
review, and/or revision of the manuscript was performed by RS, GS,
WW and NB. Administrative, technical, or material support (i.e.,
reporting or organizing data, tissue processing, antibody
development) was performed by MK, CHM, GMF, DH, ML, JI, SM, FB, RU,
DD, TK, AL, CW, FJ, EB, SS and WW. GS, RS, WW and AH supervised the
study.
Ethics approval and consent to
participate
Archived diagnostic leftover tissues for
manufacturing of TMAs was pseudo-anonymized and used without
informed consent according to the local law (HmbKHG, §12a). The
study was approved by the local ethic committee (Ethics commission
of the Ärztekammer Hamburg no. WF-049/09) and in line with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hayes-Lattin B and Nichols CR: Testicular
cancer: A prototypic tumor of young adults. Semin Oncol.
36:432–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krege S, Beyer J, Souchon R, Albers P,
Albrecht W, Algaba F, Bamberg M, Bodrogi I, Bokemeyer C,
Cavallin-Ståhl E, et al: European consensus conference on diagnosis
and treatment of germ cell cancer: A report of the second meeting
of the European Germ Cell Cancer Consensus group (EGCCCG): Part I.
Eur Urol. 53:478–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanna N and Einhorn LH: Testicular cancer:
A reflection on 50 years of discovery. J Clin Oncol. 32:3085–3092.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adra N and Einhorn LH: Salvage therapy for
relapsed testicular cancer. Oncotarget. 8:69200–69201. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oing C, Alsdorf WH, von Amsberg G, Oechsle
K and Bokemeyer C: Platinum-refractory germ cell tumors: An update
on current treatment options and developments. World J Urol.
35:1167–1175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zschäbitz S, Lasitschka F, Hadaschik B,
Hofheinz RD, Jentsch-Ullrich K, Grüner M, Jäger D and Grüllich C:
Response to anti-programmed cell death protein-1 antibodies in men
treated for platinum refractory germ cell cancer relapsed after
high-dose chemotherapy and stem cell transplantation. Eur J Cancer.
76:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adra N, Einhorn LH, Althouse SK,
Ammakkanavar NR, Musapatika D, Albany C, Vaughn D and Hanna NH:
Phase II trial of pembrolizumab in patients with platinum
refractory germ-cell tumors: A hoosier cancer research network
study GU14-206. Ann Oncol. 29:209–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8:5612017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chovanec M, Cierna Z, Miskovska V,
Machalekova K, Svetlovska D, Kalavska K, Rejlekova K, Spanik S,
Kajo K, Babal P, et al: Prognostic role of programmed-death ligand
1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular
germ cell tumors. Oncotarget. 8:21794–21805. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cierna Z, Mego M, Miskovska V, Machalekova
K, Chovanec M, Svetlovska D, Hainova K, Rejlekova K, Macak D,
Spanik S, et al: Prognostic value of programmed-death-1 receptor
(PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann
Oncol. 27:300–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu XG, Hou M and Liu Y: TIGIT, a novel
therapeutic target for tumor immunotherapy. Immunol Invest.
46:172–182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manieri NA, Chiang EY and Grogan JL:
TIGIT: A key inhibitor of the cancer immunity cycle. Trends
Immunol. 38:20–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dougall WC, Kurtulus S, Smyth MJ and
Anderson AC: TIGIT and CD96: New checkpoint receptor targets for
cancer immunotherapy. Immunol Rev. 276:112–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnston RJ, Comps-Agrar L, Hackney J, Yu
X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al:
The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T
cell effector function. Cancer Cell. 26:923–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chauvin JM, Pagliano O, Fourcade J, Sun Z,
Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ and
Zarour HM: TIGIT and PD-1 impair tumor antigen-specific
CD8+ T cells in melanoma patients. J Clin Invest.
125:2046–2058. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kurtulus S, Sakuishi K, Ngiow SF, Joller
N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK and Anderson AC: TIGIT
predominantly regulates the immune response via regulatory T cells.
J Clin Invest. 125:4053–4062. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blessin NC, Simon R, Kluth M, Fischer K,
Hube-Magg C, Li W, Makrypidi-Fraune G, Wellge B, Mandelkow T,
Debatin NF, et al: Patterns of TIGIT expression in normal lymphatic
tissue, inflammation, and cancer. Dis Markers. 2019:51605652019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garber K: Industry ‘road tests’ new wave
of immune checkpoints. Nat Biotechnol. 35:487–488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong Y, Zhu L, Schell TD, Zhang J, Claxton
DF, Ehmann WC, Rybka WB, George MR, Zeng H and Zheng H: T-cell
immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T-Cell
exhaustion and poor clinical outcome in AML patients. Clin Cancer
Res. 22:3057–3066. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kononen J, Bubendorf L, Kallioniemi A,
Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G
and Kallioniemi OP: Tissue microarrays for high-throughput
molecular profiling of tumor specimens. Nat Med. 4:844–847. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
JMP® V: SAS Institute Inc.; Cary, NC:
https://www.jmp.com/1989-2019
|
|
24
|
Josefsson SE, Huse K, Kolstad A, Beiske K,
Pende D, Steen CB, Inderberg EM, Lingjærde OC, Østenstad B, Smeland
EB, et al: T cells expressing checkpoint receptor TIGIT are
enriched in follicular lymphoma tumors and characterized by
reversible suppression of T-cell receptor signaling. Clin Cancer
Res. 24:870–881. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo Q, Deng Z, Xu C, Zeng L, Ye J, Li X,
Guo Y, Huang Z and Li J: Elevated expression of immunoreceptor
tyrosine-based inhibitory motif (TIGIT) on T lymphocytes is
correlated with disease activity in rheumatoid arthritis. Med Sci
Monit. 23:1232–1241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu X, Harden K, Gonzalez LC, Francesco M,
Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al: The
surface protein TIGIT suppresses T cell activation by promoting the
generation of mature immunoregulatory dendritic cells. Nat Immunol.
10:48–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Necchi A, Giannatempo P, Raggi D, Mariani
L, Colecchia M, Farè E, Monopoli F, Calareso G, Ali SM, Ross JS, et
al: An open-label randomized phase 2 study of durvalumab alone or
in combination with tremelimumab in patients with advanced germ
cell tumors (APACHE): Results from the first planned interim
analysis. Eur Urol. 75:201–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Farkona S, Diamandis EP and Blasutig IM:
Cancer immunotherapy: The beginning of the end of cancer? BMC Med.
14:732016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siska PJ, Johnpulle RAN, Zhou A, Bordeaux
J, Kim JY, Dabbas B, Dakappagari N, Rathmell JC, Rathmell WK,
Morgans AK, et al: Deep exploration of the immune infiltrate and
outcome prediction in testicular cancer by quantitative multiplexed
immunohistochemistry and gene expression profiling. Oncoimmunology.
6:e13055352017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mandelkow T, Blessin NC, Lueerss E, Pott
L, Simon R, Li W, Wellege B, Debatin NF, Hoflmayer D, Izbicki JR,
et al: Immune exclusion is frequent in small cell carcinoma of the
bladder. Disease Markers. Article ID 2532518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garcia-Martinez E, Gil GL, Benito AC,
González-Billalabeitia E, Conesa MA, García García T, García-Garre
E, Vicente V and Ayala de la Peña F: Tumor-infiltrating immune cell
profiles and their change after neoadjuvant chemotherapy predict
response and prognosis of breast cancer. Breast Cancer Res.
16:4882014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fabre E, Jira H, Izard V, Ferlicot S,
Hammoudi Y, Theodore C, Di Palma M, Benoit G and Droupy S:
‘Burned-out’ primary testicular cancer. BJU Int. 94:74–78. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hanna NH and Einhorn LH: Testicular
cancer-discoveries and updates. N Engl J Med. 371:2005–2016. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feldman DR, Patil S, Trinos MJ, Carousso
M, Ginsberg MS, Sheinfeld J, Bajorin DF, Bosl GJ and Motzer RJ:
Progression-free and overall survival in patients with
relapsed/refractory germ cell tumors treated with single-agent
chemotherapy: Endpoints for clinical trial design. Cancer.
118:981–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abouassaly R, Fossa SD, Giwercman A,
Kollmannsberger C, Motzer RJ, Schmoll HJ and Sternberg CN: Sequelae
of treatment in long-term survivors of testis cancer. Eur Urol.
60:516–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wethal T, Kjekshus J, Røislien J, Ueland
T, Andreassen AK, Wergeland R, Aukrust P and Fosså SD:
Treatment-related differences in cardiovascular risk factors in
long-term survivors of testicular cancer. J Cancer Surviv. 1:8–16.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ribas A, Puzanov I, Dummer R, Schadendorf
D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD,
et al: Pembrolizumab versus investigator-choice chemotherapy for
ipilimumab-refractory melanoma (KEYNOTE-002): A randomised,
controlled, phase 2 trial. Lancet Oncol. 16:908–918. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li W, Blessin NC, Simon R, Kluth M,
Fischer K, Hube-Magg C, Makrypidi-Fraune G, Wellge B, Mandelkow T,
Debatin NF, et al: Expression of the immune checkpoint receptor
TIGIT in Hodgkin's lymphoma. BMC Cancer. 18:12092018. View Article : Google Scholar : PubMed/NCBI
|