Colorectal cancer (CRC) was the third most common
cancer type worldwide and the fourth leading cause of human
cancer-associated mortality in 2012 (1). The incidence of CRC was highest in
Europe, North America and Oceania, and lowest in South Asia,
Central Asia and Africa between 1998–2002 (2). In China, according to epidemiological
data in recent years, the five-year incidence was 74.6/100,000 and
58.3/100,000 in males and females in 2011, respectively. Each year,
>376,000 people are diagnosed with CRC and ~191,000 patients
succumb to CRC in China (3,4).
The pathogenesis of CRC is complex. In terms of
environmental factors, epidemiological studies have indicated that
high-fat diet, obesity and a western lifestyle increase the risk of
CRC (5–7). In terms of genetic factors, the
majority of CRC cases exhibit genomic instability, including
microsatellite and chromosomal instability (8,9). In
addition, gene mutations, including tumor suppressor gene
inactivation and oncogene activation, serve an important role in
the pathogenesis of CRC (8).
However, there has been less focus on the role of microbial
infections in the pathogenesis of CRC, despite the fact that direct
evidence for an infectious cause in human cancer has been reported
in the last decades (10). According
to previous statistical data, 15–20% of all types of cancer are
caused by infections with microbial pathogens (11).
There are >1,000 types of microorganisms in the
human intestinal microbiota. A total of ~1014
microorganisms serve an important role in maintaining the
physiological functions of the intestines, including energy
metabolism, epithelial cell proliferation and apoptosis, and
protection against pathogens (12).
In addition to these beneficial roles, intestinal microbes can have
a detrimental effect on human health. In recent years, a large
number of studies have indicated that the intestinal flora is
closely associated with the occurrence of CRC (13,14).
Metagenomics and transcriptional analyses have revealed that
compared with adjacent normal tissues, the enrichment of
Bacteroidetes and Firmicutes is decreased in human CRC tissues, but
the enrichment of Fusobacterium nucleatum (Fn) is
significantly increased (15,16).
Yamamura et al (17) reported
that Fn is detected in 20, 10 and 45% of esophageal, gastric
and CRC tissues, respectively. No Fn was detected in liver
and pancreatic cancer tissues (17).
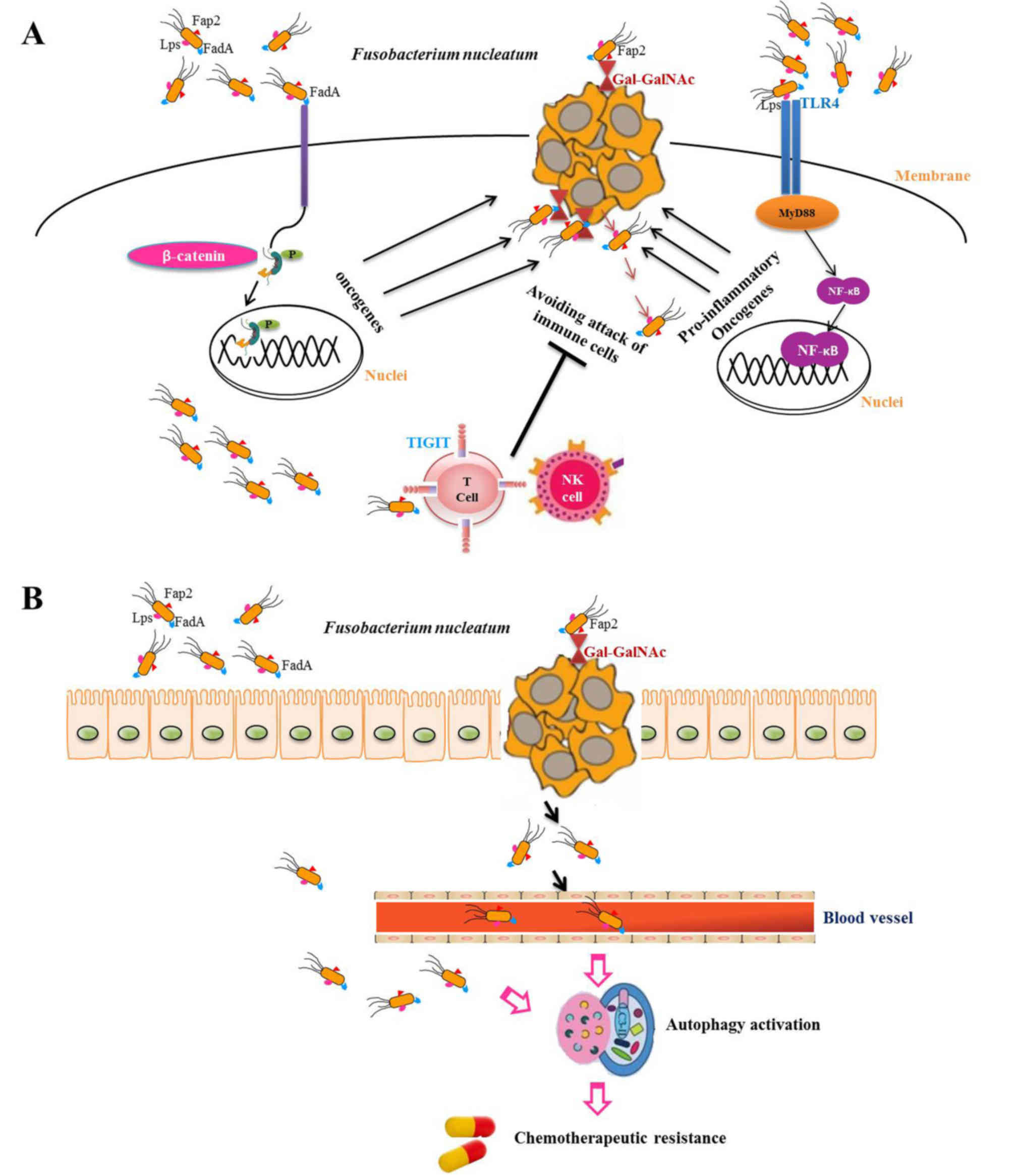
Fn adheres to and invades the intestinal mucosa through its
surface adhesion factors and virulence proteins, and ultimately
promotes the occurrence and development of CRC (18). It has previously been identified that
the absolute copy number of Fn in CRC tissues may be used as
an indicator to evaluate the prognosis of patients with CRC
(19). Recent studies have
demonstrated that Fn is not only associated with the
development of CRC, but also promotes chemotherapeutic resistance
(20,21). Therefore, in depth knowledge of the
mechanisms underlying Fn carcinogenesis in CRC may increase
the understanding of the intestinal flora and aid the development
of effective anticancer agents against the bacterium.
According to statistical data, 20–30% of treated
patients with CRC exhibit tumor recurrence and 35% of these
patients succumb to the disease within 5 years (73). Combination chemotherapy remains the
main treatment for advanced CRC (74). Therefore, studies on the mechanism of
CRC resistance to chemotherapy are important. A study by Yu et
al (75) demonstrated that
Fn abundance in recurrent CRC tissues is markedly higher
compared with that in non-recurrent CRC tissues, and high-abundance
Fn is associated with poor prognosis. Therefore, Fn
may be used as a diagnostic marker for preventing CRC recurrence
and as a prognosis predictor. In addition, a series of experiments
revealed that Fn selectively decreases miRNA-18a*/4802
expression levels through the innate immune pathway (TLR4/MYD88)
and subsequently activates cell autophagy via unc-51 like autophagy
activating kinase 1-autophagy related 7 to become resistant to
chemotherapeutic drugs. This drug resistance was overcome by the
autophagy blocker chloroquine (75).
According to a previous report, prostaglandin E
receptor 2 (PTGER2) increases the expression of NF-κβ-targeted
proinflammatory genes in neutrophils. The expression of TNF-α and
IL-6, PTGS2, C-X-C motif chemokine ligand 1, Wnt and other
cytokines in tumor lesions are significantly higher in
PTGER2-enriched compared with PTGER2-knockout mice (86). Therefore, NSAIDs and PTGER2
antagonists may be candidates for the prevention and treatment of
Fn-positive CRC.
Tumor immunotherapy is a promising area, with
significant progress being made in tumor molecular biology,
particularly with regards to the use of immune checkpoint
inhibitors, such as programmed cell death 1 (PDCD1) inhibitors
(89). The efficacy of checkpoint
inhibitors depends on the patient's gut microbiota. Complex
interactions between the gut microorganisms and the immune system
limit the effects of PDCD1 inhibitors (90). According to the OncoKB classification
system, pembrolizumab is a Food and Drug Association-approved drug
for MSI-high solid tumors (level 1) and Fn is associated
with MSI-high, CIMP and BRAF mutations (71,72,91).
Therefore, PDCD1 inhibitors may exhibit anticancer effects on
Fn-positive CRC. However, immune checkpoint inhibitors have
a number of side effects, including hepatitis, diarrhea and
enterocolitis, resulting from the complex interactions between host
genetics, immune responses, the environment and microbes (92). Therefore, the use of immune
checkpoint inhibitors has very strict indications (92). Since Fn binds to TIGIT, an
immune cell inhibitory receptor, through Fap2 to avoid attacks of
immune cells, the development of an anti-Fap2 antibody may be
beneficial for the antitumor immune response (68). Nedaeinia et al (93) revealed that inhibition of miRNA-21
suppresses metastasis of CRC cells through modulation of programmed
cell death 4. Kumar et al (94) studied host-pathogen protein-protein
interactions (HP-PPIs) and identified that Fn and
CRC-related proteins have 186 interactions, including 103 host
proteins and 76 Fn-pathogenic proteins. Therefore, the
development of drugs targeting HP-PPIs may be used to treat
Fn-positive CRC. In view of the important role of TLR4 in
Fn carcinogenesis, it may be possible to develop a drug for
the treatment of Fn-positive tumors (65). For patients with recurrent CRC, in
addition to combination chemotherapy, the benefit of autophagy
blocking agents or Fn inhibitors requires investigation in
future studies.
Previous studies have demonstrated that a diet rich
in fruits, vegetables and whole grains is associated with a lower
risk of colon cancer compared with Western dietary patterns, which
are dominated by red and processed meats (5–7,101). A high-fat diet and overconsumption
of red meat may increase the risk of CRC (102–104).
Although the mechanism of the association between diet and CRC
remains unclear, it is speculated that the gut microbiota may play
an intermediary role. It has been reported that diet influences the
composition of gut microbiota, although long-term dietary patterns
outweigh short-term changes in diet (105,106).
Considering that different groups of gut microbiota have different
metabolic capacities, particular dietary patterns may allow for the
selection of certain microbes and inhibits others (107,108).
A previous study demonstrated that certain microflora may become
extinct. By providing low-fiber diets to mice, reversible changes
are observed in the microbiota. However, following low-fiber diets
for several successive generations, the diversity of microbiota is
gradually lost and certain microbiota are undetectable (108).
Not applicable.
No funding was received.
The datasets used and/or analyzed during the
present study are available from the corresponding author upon
reasonable request.
GJ designed the study, and ZY drafted the
manuscript and revised the manuscript. All authors read and
approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng R, Zeng H, Zhang S, Chen T and Chen
W: National estimates of cancer prevalence in China, 2011. Cancer
Lett. 370:33–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berg A: Nutrition, development, and
population growth. Popul Bull. 29:3–37. 1973.PubMed/NCBI
|
|
6
|
Alsheridah N and Akhtar S: Diet, obesity
and colorectal carcinoma risk: Results from a national cancer
registry-based middle-eastern study. BMC Cancer. 18:12272018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mafiana RN, Al Lawati AS, Waly MI, Al
Farsi Y, Al Kindi M and Al Moundhri M: Association between dietary
and lifestyle indices and colorectal cancer in Oman: A case-control
study. Asian Pac J Cancer Prev. 19:3117–3122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dienstmann R, Vermeulen L, Guinney J,
Kopetz S, Tejpar S and Tabernero J: Consensus molecular subtypes
and the evolution of precision medicine in colorectal cancer. Nat
Rev Cancer. 17:2682017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brower V: Connecting viruses to cancer:
How research moves from association to causation. J Natl Cancer
Inst. 96:256–257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, . Biological agents. Volume 100 B. A
review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum.
100:1–441. 2012.
|
|
12
|
Tremaroli V and Bäckhed F: Functional
interactions between the gut microbiota and host metabolism.
Nature. 489:242–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashiro Y: Gut microbiota in health and
disease. Ann Nutr Metab. 71:242–246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sears CL and Garrett WS: Microbes,
microbiota, and colon cancer. Cell Host Microbe. 15:317–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kostic AD, Gevers D, Pedamallu CS, Michaud
M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, et
al: Genomic analysis identifies association of Fusobacterium with
colorectal carcinoma. Genome Res. 22:292–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Repass J, Maherali N and Owen K;
Reproducibility Project, : Cancer Biology; Reproducibility Project
Cancer Biology: Registered report: Fusobacterium nucleatum
infection is prevalent in human colorectal carcinoma. ELife.
5(pii): e100122016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamura K, Baba Y, Miyake K, Nakamura K,
Shigaki H, Mima K, Kurashige J, Ishimoto T, Iwatsuki M, Sakamoto Y,
et al: Fusobacterium nucleatum in gastroenterological
cancer: Evaluation of measurement methods using quantitative
polymerase chain reaction and a literature review. Oncol Lett.
14:6373–6378. 2017.PubMed/NCBI
|
|
18
|
Han YW: Fusobacterium nucleatum: A
commensal-turned pathogen. Curr Opin Microbiol. 23:141–147. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaoka Y, Suehiro Y, Hashimoto S, Hoshida
T, Fujimoto M, Watanabe M, Imanaga D, Sakai K, Matsumoto T,
Nishioka M, et al: Fusobacterium nucleatum as a prognostic
marker of colorectal cancer in a Japanese population. J
Gastroenterol. 53:517–524. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramos A and Hemann MT: Drugs, Bugs, and
Cancer: Fusobacterium nucleatum promotes chemoresistance in
colorectal cancer. Cell. 170:411–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Yang Y, Weng W, Guo B, Cai G, Ma
Y and Cai S: Fusobacterium nucleatum promotes
chemoresistance to 5-fluorouracil by upregulation of BIRC3
expressio n in colorectal cancer. J Exp Clin Cancer Res. 38:142019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han YW, Shi W, Huang GT, Kinder Haake S,
Park NH, Kuramitsu H and Genco RJ: Interactions between periodontal
bacteria and human oral epithelial cells: Fusobacterium
nucleatum adheres to and invades epithelial cells. Infect
Immun. 68:3140–3146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heckmann JG, Lang CJ, Hartl H and Tomandl
B: Multiple brain abscesses caused by Fusobacterium
nucleatum treated conservatively. Can J Neurol Sci. 30:266–268.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han YW, Fardini Y, Chen C, Iacampo KG,
Peraino VA, Shamonki JM and Redline RW: Term stillbirth caused by
oral Fusobacterium nucleatum. Obstet Gynecol. 115:442–445.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wells CD, Balan V and Smilack JD: Pyogenic
liver abscess after colonoscopy in a patient with ulcerative
colitis. Clin Gastroenterol Hepatol. 3:xxiv2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Buhimschi CS, Temoin S, Bhandari
V, Han YW and Buhimschi IA: Comparative microbial analysis of
paired amniotic fluid and cord blood from pregnancies complicated
by preterm birth and early-onset neonatal sepsis. PLoS One.
8:e561312013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hill GB: Investigating the source of
amniotic fluid isolates of fusobacteria. Clin Infect Dis. 16 (Suppl
4):S423–S424. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han YW, Redline RW, Li M, Yin L, Hill GB
and McCormick TS: Fusobacterium nucleatum induces premature
and term stillbirths in pregnant mice: Implication of oral bacteria
in preterm birth. Infect Immun. 72:2272–2279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han YW: Can oral bacteria cause pregnancy
complications? Womens Health (Lond). 7:401–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han YW: Oral health and adverse pregnancy
outcomes-what's next? J Dent Res. 90:289–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu H, Redline RW and Han YW:
Fusobacterium nucleatum induces fetal death in mice via
stimulation of TLR4-mediated placental inflammatory response. J
Immunol. 179:2501–2508. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bachrach G, Ianculovici C, Naor R and
Weiss EI: Fluorescence based measurements of Fusobacterium
nucleatum coaggregation and of fusobacterial attachment to
mammalian cells. FEMS Microbiol Lett. 248:235–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shang FM and Liu HL: Fusobacterium
nucleatum and colorectal cancer: A review. World J Gastrointest
Oncol. 10:71–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Castellarin M, Warren RL, Freeman JD,
Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P,
Allen-Vercoe E, Moore RA and Holt RA: Fusobacterium
nucleatum infection is prevalent in human colorectal carcinoma.
Genome Res. 22:299–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li YY, Ge QX, Cao J, Zhou YJ, Du YL, Shen
B, Wan YJ and Nie YQ: Association of Fusobacterium nucleatum
infection with colorectal cancer in Chinese patients. World J
Gastroenterol. 22:3227–3233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mima K, Sukawa Y, Nishihara R, Qian ZR,
Yamauchi M, Inamura K, Kim SA, Masuda A, Nowak JA, Nosho K, et al:
Fusobacterium nucleatum and T cells in colorectal carcinoma.
JAMA Oncol. 1:653–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nosho K, Sukawa Y, Adachi Y, Ito M,
Mitsuhashi K, Kurihara H, Kanno S, Yamamoto I, Ishigami K, Igarashi
H, et al: Association of Fusobacterium nucleatum with
immunity and molecular alterations in colorectal cancer. World J
Gastroenterol. 22:557–566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mima K, Cao Y, Chan AT, Qian ZR, Nowak JA,
Masugi Y, Shi Y, Song M, da Silva A, Gu M, et al: Fusobacterium
nucleatum in colorectal carcinoma tissue according to tumor
location. Clin Transl Gastroenterol. 7:e2002016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Komiya Y, Shimomura Y, Higurashi T, Sugi
Y, Arimoto J, Umezawa S, Uchiyama S, Matsumoto M and Nakajima A:
Patients with colorectal cancer have identical strains of
Fusobacterium nucleatum in their colorectal cancer and oral
cavity. Gut. Jun 22–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bullman S, Pedamallu CS, Sicinska E,
Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T,
et al: Analysis of Fusobacterium persistence and antibiotic
response in colorectal cancer. Science. 358:1443–1448. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McCoy AN, Araújo-Pérez F, Azcárate-Peril
A, Yeh JJ, Sandler RS and Keku TO: Fusobacterium is
associated with colorectal adenomas. PLoS One. 8:e536532013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Amitay EL and Brenner H: Response to
comments on ‘Fusobacterium and colorectal cancer: Causal
factor or passenger? Results from a large colorectal cancer
screening study’. Carcinogenesis. 39:852018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ito M, Kanno S, Nosho K, Sukawa Y,
Mitsuhashi K, Kurihara H, Igarashi H, Takahashi T, Tachibana M,
Takahashi H, et al: Association of Fusobacterium nucleatum
with clinical and molecular features in colorectal serrated
pathway. Int J Cancer. 137:1258–1268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Koi M, Okita Y and Carethers JM:
Fusobacterium nucleatum infection in colorectal cancer:
Linking inflammation, DNA mismatch repair and genetic and
epigenetic alterations. J Anus Rectum Colon. 2:37–46. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang S, Cai S and Ma Y: Association
between Fusobacterium nucleatum and colorectal cancer:
Progress and future directions. J Cancer. 9:1652–1659. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Coppenhagen-Glazer S, Sol A, Abed J, Naor
R, Zhang X, Han YW and Bachrach G: Fap2 of Fusobacterium
nucleatum is a galactose-inhibitable adhesin involved in
coaggregation, cell adhesion, and preterm birth. Infect Immun.
83:1104–1113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
West NR, McCuaig S, Franchini F and Powrie
F: Emerging cytokine networks in colorectal cancer. Nat Rev
Immunol. 15:615–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Aggarwal BB, Vijayalekshmi RV and Sung B:
Targeting inflammatory pathways for prevention and therapy of
cancer: Short-term friend, long-term foe. Clin Cancer Res.
15:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Balkwill FR and Mantovani A:
Cancer-related inflammation: Common themes and therapeutic
opportunities. Semin Cancer Biol. 22:33–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kostic AD, Chun E, Robertson L, Glickman
JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold
GL, et al: Fusobacterium nucleatum potentiates intestinal
tumorigenesis and modulates the tumor-immune microenvironment. Cell
Host Microbe. 14:207–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ma N, Liu Q, Hou L, Wang Y and Liu Z:
MDSCs are involved in the protumorigenic potentials of GM-CSF in
colitis-associated cancer. Int J Immunopathol Pharmacol.
30:152–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Quah SY, Bergenholtz G and Tan KS:
Fusobacterium nucleatum induces cytokine production through
Toll-like-receptor-independent mechanism. Int Endod J. 47:550–559.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dharmani P, Strauss J, Ambrose C,
Allen-Vercoe E and Chadee K: Fusobacterium nucleatum
infection of colonic cells stimulates MUC2 mucin and tumor necrosis
factor alpha. Infect Immun. 79:2597–2607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Trinchieri G: Cancer and inflammation: An
old intuition with rapidly evolving new concepts. Annu Rev Immunol.
30:677–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fardini Y, Wang X, Témoin S, Nithianantham
S, Lee D, Shoham M and Han YW: Fusobacterium nucleatum
adhesin FadA binds vascular endothelial cadherin and alters
endothelial integrity. Mol Microbiol. 82:1468–1480. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Han YW, Ikegami A, Rajanna C, Kawsar HI,
Zhou Y, Li M, Sojar HT, Genco RJ, Kuramitsu HK and Deng CX:
Identification and characterization of a novel adhesin unique to
oral fusobacteria. J Bacteriol. 187:5330–5340. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xu M, Yamada M, Li M, Liu H, Chen SG and
Han YW: FadA from Fusobacterium nucleatum utilizes both
secreted and nonsecreted forms for functional oligomerization for
attachment and invasion of host cell. J Biol Chem. 282:25000–25009.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Temoin S, Wu KL, Wu V, Shoham M and Han
YW: Signal peptide of FadA adhesin from Fusobacterium
nucleatum plays a novel structural role by modulating the
filament's length and width. FEBS Lett. 586:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G
and Han YW: Fusobacterium nucleatum promotes colorectal
carcinogenesis by modulating E-cadherin/β-catenin signaling via its
FadA adhesin. Cell Host Microbe. 14:195–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen Y, Peng Y, Yu J, Chen T, Wu Y, Shi L,
Li Q, Wu J and Fu X: Invasive Fusobacterium nucleatum
activates beta-catenin signaling in colorectal cancer via a
TLR4/P-PAK1 cascade. Oncotarget. 8:31802–31814. 2017.PubMed/NCBI
|
|
65
|
Wu Y, Wu J, Chen T, Li Q, Peng W, Li H,
Tang X and Fu X: Fusobacterium nucleatum potentiates
intestinal tumorigenesis in mice via a Toll-like receptor
4/p21-activated kinase 1 cascade. Dig Dis Sci. 63:1210–1218. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jiang WG, Sanders AJ, Katoh M, Ungefroren
H, Gieseler F, Prince M, Thompson SK, Zollo M, Spano D, Dhawan P,
et al: Tissue invasion and metastasis: Molecular, biological and
clinical perspectives. Semin Cancer Biol. 35 (Suppl):S244–S275.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Abed J, Emgård JE, Zamir G, Faroja M,
Almogy G, Grenov A, Sol A, Naor R, Pikarsky E, Atlan KA, et al:
Fap2 Mediates Fusobacterium nucleatum colorectal
adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc.
Cell Host Microbe. 20:215–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gur C, Ibrahim Y, Isaacson B, Yamin R,
Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N,
Coppenhagen-Glazer S, et al: Binding of the Fap2 protein of
Fusobacterium nucleatum to human inhibitory receptor TIGIT
protects tumors from immune cell attack. Immunity. 42:344–355.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shenker BJ and Datar S: Fusobacterium
nucleatum inhibits human T-cell activation by arresting cells
in the mid-G1 phase of the cell cycle. Infect Immun. 63:4830–4836.
1995.PubMed/NCBI
|
|
70
|
Yang Y, Weng W, Peng J, Hong L, Yang L,
Toiyama Y, Gao R, Liu M, Yin M, Pan C, et al: Fusobacterium
nucleatum increases proliferation of colorectal cancer cells
and tumor development in mice by activating Toll-like receptor 4
signaling to nuclear factor-κB, and up-regulating expression of
microRNA-21. Gastroenterology. 152:851–866.e24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tahara T, Yamamoto E, Suzuki H, Maruyama
R, Chung W, Garriga J, Jelinek J, Yamano HO, Sugai T, An B, et al:
Fusobacterium in colonic flora and molecular features of
colorectal carcinoma. Cancer Res. 74:1311–1318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mima K, Nishihara R, Qian ZR, Cao Y,
Sukawa Y, Nowak JA, Yang J, Dou R, Masugi Y, Song M, et al:
Fusobacterium nucleatum in colorectal carcinoma tissue and
patient prognosis. Gut. 65:1973–1980. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ryuk JP, Choi GS, Park JS, Kim HJ, Park
SY, Yoon GS, Jun SH and Kwon YC: Predictive factors and the
prognosis of recurrence of colorectal cancer within 2 years after
curative resection. Ann Surg Treat Res. 86:143–151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cartwright TH: Treatment decisions after
diagnosis of metastatic colorectal cancer. Clin Colorectal Cancer.
11:155–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yu T, Guo F, Yu Y, Sun T, Ma D, Han J,
Qian Y, Kryczek I, Sun D, Nagarsheth N, et al: Fusobacterium
nucleatum promotes chemoresistance to colorectal cancer by
modulating autophagy. Cell. 170:548–563.e16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lasry A, Zinger A and Ben-Neriah Y:
Inflammatory networks underlying colorectal cancer. Nat Immunol.
17:230–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Huang WW, Hsieh KP, Huang RY and Yang YH:
Role of cyclooxygenase-2 inhibitors in the survival outcome of
colorectal cancer patients: A population-based cohort study.
Kaohsiung J Med Sci. 33:308–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Williams CS, Shattuck-Brandt RL and DuBois
RN: The role of COX-2 in intestinal cancer. Expert Opin Investig
Drugs. 8:1–12. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Waddell WR, Ganser GF, Cerise EJ and
Loughry RW: Sulindac for polyposis of the colon. Am J Surg.
157:175–179. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Rothwell PM, Wilson M, Elwin CE, Norrving
B, Algra A, Warlow CP and Meade TW: Long-term effect of aspirin on
colorectal cancer incidence and mortality: 20-year follow-up of
five randomised trials. Lancet. 376:1741–1750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tsoi KK, Chan FC, Hirai HW and Sung JJ:
Risk of gastrointestinal bleeding and benefit from colorectal
cancer reduction from long-term use of low-dose aspirin: A
retrospective study of 612 509 patients. J Gastroenterol Hepatol.
33:1728–1736. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Johnson CC, Jankowski M, Rolnick S, Yood
MU and Alford SH: Influence of NSAID use among colorectal cancer
survivors on cancer outcomes. Am J Clin Oncol. 40:370–374. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sandler RS, Halabi S, Baron JA, Budinger
S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi
CL, et al: A randomized trial of aspirin to prevent colorectal
adenomas in patients with previous colorectal cancer. N Engl J Med.
348:883–890. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ng K, Meyerhardt JA, Chan AT, Sato K, Chan
JA, Niedzwiecki D, Saltz LB, Mayer RJ, Benson AB III, Schaefer PL,
et al: Aspirin and COX-2 inhibitor use in patients with stage III
colon cancer. J Natl Cancer Inst. 107:3452014.PubMed/NCBI
|
|
85
|
Bos CL, Kodach LL, van den Brink GR, Diks
SH, van Santen MM, Richel DJ, Peppelenbosch MP and Hardwick JC:
Effect of aspirin on the Wnt/beta-catenin pathway is mediated via
protein phosphatase 2A. Oncogene. 25:6447–6456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ma X, Aoki T, Tsuruyama T and Narumiya S:
Definition of prostaglandin E2-EP2 signals in the colon tumor
microenvironment that amplify inflammation and tumor growth. Cancer
Res. 75:2822–2832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Haak BW, Lankelma JM, Hugenholtz F, Belzer
C, de Vos WM and Wiersinga WJ: Long-term impact of oral vancomycin,
ciprofloxacin and metronidazole on the gut microbiota in healthy
humans. J Antimicrob Chemother. 74:782–786. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yu YN, Yu TC, Zhao HJ, Sun TT, Chen HM,
Chen HY, An HF, Weng YR, Yu J, Li M, et al: Berberine may rescue
Fusobacterium nucleatum-induced colorectal tumorigenesis by
modulating the tumor microenvironment. Oncotarget. 6:32013–32026.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Bever KM and Le DT: An expanding role for
immunotherapy in colorectal cancer. J Natl Compr Canc Netw.
15:401–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bhatt AP, Redinbo MR and Bultman SJ: The
role of the microbiome in cancer development and therapy. CA Cancer
J Clin. 67:326–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chakravarty D, Gao J, Phillips SM, Kundra
R, Zhang H, Wang J, Rudolph JE, Yaeger R, Soumerai T, Nissan MH, et
al: OncoKB: A precision oncology knowledge base. JCO Precis Oncol
2017. 2017. View Article : Google Scholar
|
|
92
|
Cramer P and Bresalier RS:
Gastrointestinal and hepatic complications of immune checkpoint
inhibitors. Curr Gastroenterol Rep. 19:32017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nedaeinia R, Sharifi M, Avan A, Kazemi M,
Nabinejad A, Ferns GA, Ghayour-Mobarhan M and Salehi R: Inhibition
of microRNA-21 via locked nucleic acid-anti-miR suppressed
metastatic features of colorectal cancer cells through modulation
of programmed cell death 4. Tumour Biol. 39:10104283176922612017.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kumar A, Thotakura PL, Tiwary BK and
Krishna R: Target identification in Fusobacterium nucleatum
by subtractive genomics approach and enrichment analysis of
host-pathogen protein-protein interactions. BMC Microbiol.
16:842016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Brook I and Frazier EH: Immune response to
Fusobacterium nucleatum and Prevotella intermedia in the
sputum of patients with acute exacerbation of chronic bronchitis.
Chest. 124:832–833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Velsko IM, Chukkapalli SS, Rivera-Kweh MF,
Chen H, Zheng D, Bhattacharyya I, Gangula PR, Lucas AR and Kesavalu
L: Fusobacterium nucleatum alters atherosclerosis risk
factors and enhances inflammatory markers with an atheroprotective
immune response in ApoE(null) mice. PLoS One. 10:e01297952015.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang HF, Li LF, Guo SH, Zeng QY, Ning F,
Liu WL and Zhang G: Evaluation of antibody level against
Fusobacterium nucleatum in the serological diagnosis of
colorectal cancer. Sci Rep. 6:334402016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hundt S, Haug U and Brenner H: Comparative
evaluation of immunochemical fecal occult blood tests for
colorectal adenoma detection. Ann Intern Med. 150:162–169. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wong SH, Kwong TNY, Chow TC, Luk AKC, Dai
RZW, Nakatsu G, Lam TYT, Zhang L, Wu JCY, Chan FKL, et al:
Quantitation of faecal Fusobacterium improves faecal
immunochemical test in detecting advanced colorectal neoplasia.
Gut. 66:1441–1448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Huang S, Yang Z, Zou D, Dong D, Liu A, Liu
W and Huang L: Rapid detection of nusG and fadA in Fusobacterium
nucleatum by loop-mediated isothermal amplification. J Med
Microbiol. 65:760–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Song M, Garrett WS and Chan AT: Nutrients,
foods, and colorectal cancer prevention. Gastroenterology.
148:1244–1260.e16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Norat T, Bingham S, Ferrari P, Slimani N,
Jenab M, Mazuir M, Overvad K, Olsen A, Tjønneland A, Clavel F, et
al: Meat, fish, and colorectal cancer risk: The European
prospective investigation into cancer and nutrition. J Natl Cancer
Inst. 97:906–916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Larsson SC and Wolk A: Meat consumption
and risk of colorectal cancer: A meta-analysis of prospective
studies. Int J Cancer. 119:2657–2664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Magalhães B, Peleteiro B and Lunet N:
Dietary patterns and colorectal cancer: Systematic review and
meta-analysis. Eur J Cancer Prev. 21:15–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
David LA, Maurice CF, Carmody RN,
Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y,
Fischbach MA, et al: Diet rapidly and reproducibly alters the human
gut microbiome. Nature. 505:559–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Xu Z and Knight R: Dietary effects on
human gut microbiome diversity. Br J Nutr. 113 (Suppl):S1–S5. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Haro C, García-Carpintero S, Rangel-Zúñiga
OA, Alcalá-Díaz JF, Landa BB, Clemente JC, Pérez-Martínez P,
López-Miranda J, Pérez-Jiménez F and Camargo A: Consumption of two
healthy dietary patterns restored microbiota dysbiosis in obese
patients with metabolic dysfunction. Mol Nutr Food Res. 61:2017.
View Article : Google Scholar
|
|
108
|
Sonnenburg ED, Smits SA, Tikhonov M,
Higginbottom SK, Wingreen NS and Sonnenburg JL: Diet-induced
extinctions in the gut microbiota compound over generations.
Nature. 529:212–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tabung FK, Liu L, Wang W, Fung TT, Wu K,
Smith-Warner SA, Cao Y, Hu FB, Ogino S, Fuchs CS and Giovannucci
EL: Association of dietary inflammatory potential with colorectal
cancer risk in men and women. JAMA Oncol. 4:366–373. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Tabung FK, Smith-Warner SA, Chavarro JE,
Wu K, Fuchs CS, Hu FB, Chan AT, Willett WC and Giovannucci EL:
Development and validation of an empirical dietary inflammatory
index. J Nutr. 146:1560–1570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Liu L, Tabung FK, Zhang X, Nowak JA, Qian
ZR, Hamada T, Nevo D, Bullman S, Mima K, Kosumi K, et al: Diets
that promote colon inflammation associate with risk of colorectal
carcinomas that contain Fusobacterium nucleatum. Clin
Gastroenterol Hepatol. 16:1622–1631.e3. 2018. View Article : Google Scholar : PubMed/NCBI
|