Introduction
Lung cancer is one of the most common human
malignancies in China and worldwide (1,2). The
5-year overall survival rate for lung cancer is <20%, which is
largely due to late diagnosis (3).
Patients with lung cancer are often diagnosed at advanced stages,
when treatment options are limited (4). Platinum-based chemotherapy is
recommended for patients with metastatic disease, and the
administration of concurrent chemotherapy and radiation is
beneficial for advanced lung cancer (5). Cisplatin and carboplatin are widely
used first-line clinical therapeutic drugs (6). However, lung cancer cells become
resistant to these drugs, which prevents an effective therapeutic
response (7). Thus, the
identification of novel biomarkers to predict multidrug resistance
and serve as therapeutic targets may provide alternative strategies
to improve the survival of patients with advanced lung cancer.
It has been reported that several genes are involved
in multidrug resistance to chemotherapy in patients with lung
cancer (8). Among these, members of
the multidrug resistance protein (MRP) family, including MRP1, MRP2
and MRP3, are highly expressed and associated with chemotherapy
resistance in lung cancer (9).
Similarly, increased expression levels of P-glycoprotein (P-gp),
which is encoded by the human multidrug resistant 1 (MDR1) gene,
are associated with resistance to vinca alkaloids (10), etoposide (11), and taxanes (12) in patients with lung cancer. CA916798
has been identified as a novel multidrug resistance gene in
cis-dichlorodiamine platinum (CDDP)-resistant lung adenocarcinoma
cells (13). Overexpression of
CA916798 in lung cancer cells enhances resistance to multiple
chemotherapeutic agents by regulating CDDP-induced cell growth and
apoptosis (14). Mechanistically,
CA916798 may exert its multidrug resistance functions via the
phosphoinositide 3-kinase/AKT signaling pathway (15,16).
However, the majority of previous studies have focused on the
molecular characteristics, biological functions and molecular
mechanism of CA916798 in lung cancer (14–18). It
is still unknown whether CA916798 expression is associated with
platinum-based chemotherapy in tumor tissues from patients with
lung cancer.
The present study evaluated the messenger RNA (mRNA)
and protein expression levels of CA916798 in tumor tissues prior
and subsequent to chemotherapy in patients with advanced lung
cancer. Both the mRNA and protein expression levels of CA916798
were altered in chemotherapy-sensitive lung cancer tissues. The
present study further evaluated the prognostic implications of
CA916798 mRNA and protein expression levels in patients with lung
cancer.
Materials and methods
Patients and tissue samples
Patients newly diagnosed with lung cancer at the
Southwest Hospital of the Third Military Medical University
(Chongqing, China) were enrolled between March and December 2016.
Tissue specimens were collected prior to chemotherapy and following
2 cycles (with each cycle repeated every 3 weeks) of chemotherapy
by bronchoscopy. The patients were under conscious sedation and
intubated with a flexible bronchoscope (Olympus Corporation). A
transbronchial lung biopsy was performed under fluoroscopic
guidance. The bronchopulmonary segment for obtaining specimens was
determined prior to the procedure based on computed tomography (CT)
of the chest. The demographic characteristics of the patients,
including age, sex and smoking status, were collected through a
questionnaire. The clinicopathological characteristics of the
patients, including pathological stage (TNM staging system)
(19), maximum tumor diameter and
histological type, were collected through hospital examinations,
including CT and pathological examination. The inclusion criteria
were: i) Patients who were diagnosed by histology or cytology as
exhibiting lung cancer, including lung squamous cell carcinoma,
lung adenocarcinoma and small cell lung cancer; ii) patients with
advanced lung cancer, including stage III and IV lung cancer; iii)
patients who had never received any radiation or chemotherapy; iv)
patients who had received ≥2 cycles of first-line chemotherapy; and
v) patients who were able to comply with the study and follow-up.
The exclusion criteria were: i) Patients who had received surgery;
ii) patients who refused treatment; and iii) patients who had been
diagnosed with other malignancies. According to the inclusion and
exclusion criteria, 30 patients were included in the study. Written
informed consent was obtained from all the patients. The research
protocol was approved by the Ethics Committee of the Third Military
Medical University.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from tumor tissues using
TRIzol® reagent (Takara Biotechnology Co., Ltd.)
according to the manufacturer's instructions. The concentration and
purity of the RNA samples were evaluated by an ultraviolet
spectrophotometer (Thermo Fisher Scientific, Inc.). The integrity
of the RNA samples was determined by agarose gel electrophoresis.
Upon quantification, 1 µg total RNA was reverse transcribed into
complementary DNA using PrimeScript™ RT reagent kit with gDNA
eraser (Takara Biotechnology Co., Ltd.). The temperature protocol
for the RT-PCR was as follows: 37°C for 15 min, followed by 85°C
for 5 sec. qPCR was conducted using SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.) in a CFX 96 thermal cycler (Bio-Rad
Laboratories, Inc.). The thermocycling conditions for the qPCR was
as follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 5
sec, 60°C for 30 sec and 72°C for 30 sec, followed by a final step
of 72°C for 2 min. The expression levels of GAPDH were used as the
internal control. Quantification cycle (Cq) values of GAPDH mRNA
<25 and Cq values of CA916798 mRNA <35 were considered of
good quality. The 2−ΔΔCq method (20) was used to calculate the relative
expression of CA916798 as follows:
ΔCq=Cq(CA916798)-Cq(GAPDH), where
ΔΔCq=ΔCq(post-chemotherapy)-ΔCq(pre-chemotherapy)
and fold-change=2−ΔΔCq. The primers used in RT-qPCR
analysis were synthesized by Sangon Biotech Co., Ltd., and the
sequences are as follows: CA916798 forward,
5′-GCTTCCTCCTCAACCTCGTCCT-3′ and reverse,
5′-GTAGCCCACTTATCCACCTTCTCC-3′; and GAPDH forward,
5′-AAGGTGAAGGTCGGAGTCAAC-3′ and reverse,
5′-GGGGTCATTGATGGCAACAATA-3′.
Immunohistochemistry (IHC)
analysis
Tumor tissues were obtained by bronchoscopy, fixed
in 10% buffered formalin for 24 to 48 h at room temperature,
paraffin embedded and sectioned at a thickness of 4 µm. The
paraffin-embedded lung cancer tissue sections were deparaffinized
in xylene, rehydrated through graded alcohol (95, 80 and 70%) to
water and immersed in citrate buffer (pH 6.0). Antigen retrieval
was accomplished using 0.05 M glycine-HCl buffer (pH 3.5)
containing 0.01% EDTA at 95°C for 15 min. The sections were blocked
with 5% BSA (Boster Biological Technology) and 0.1% Triton in PBS
for 30 min at 37°C. The sections were then incubated overnight at
4°C in citrate buffer with anti-CA916798 antibody (1:200). The
anti-CA916798 polyclonal antibody was produced and gifted by Dr
Haijing Wang at the Army Military Medical University (Chongqing,
China) (14). The sections were then
incubated with horseradish peroxidase-conjugated secondary antibody
(1:1,000; cat. no. ab6721; Abcam) for 30 min at room temperature. A
section incubated with PBS instead of primary anti-CA916798
antibody was used as a negative control. The positive control was a
positive biopsy from our previous study (18). Immunostaining was evaluated by 2
pathologists blinded to the results. The immunohistochemical images
were obtained at ×200 magnification under an optical microscope
(Olympus Corporation). For each section, 5 visual fields were
randomly selected to calculate the percentage of CA916798-positive
cells. The intensity of CA916798 protein expression was scored and
divided into 4 levels according to the percentage of
CA916798-positive cells: 0 (negative staining) for 0–10%; 1 (weak
positive staining) for 10–25%; 2 (moderate positive staining) for
25–75%; and 3 (strong positive staining) for 75–100%. A score of 0
was defined as negative and a score of 1–3 was defined as
positive.
Patient follow-up and therapeutic
evaluation
All patients were treated with first-line
platinum-based chemotherapy on day 1, and repeated every 3 weeks
(120 mg cisplatin, 45 mg lobaplatin or 140 mg nedaplatin in
combination with 1,800 mg gemcitabine, 120 mg docetaxel, 240 mg
irinotecan or 240 mg paclitaxel liposome). Subsequently, CT imaging
was conducted to evaluate the response to chemotherapy following 2
cycles of therapy. Response Evaluation Criteria in Solid Tumors
version 1.1 (21) was used to
classify patients into complete response (CR), partial response
(PR), stable disease (SD) and progressive disease (PD) groups.
Patients were considered to be responsive to chemotherapy when they
exhibited a CR or PR. Patients who had SD or PD were classified as
non-responsive. Progression-free survival time was defined from the
first day of chemotherapy to disease progression, tumor-induced
mortality or last follow-up. All patients were actively followed up
within 6 months of diagnosis, with subsequent annual follow-ups by
telephone interview or outpatient visit.
Statistical analysis
Statistical analyses were conducted using SPSS 22.0
software (IBM Corp.) and graphs were generated with GraphPad Prism
5.0 (GraphPad Software, Inc.). Continuous variables were compared
with either unpaired or paired Student's t-test. Fisher's exact
test was used to assess the differences in proportions between 2
groups. Progression-free survival was analyzed by the Kaplan-Meier
method with upregulated and downregulated expression of CA916798. A
multivariate Cox proportional hazards regression analysis was used
to explore the prognostic significance of CA916798 expression.
Two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographic and clinicopathological
characteristics
A total of 30 patients were included in the present
study, of which 83.3% (25/30) were male, with an overall mean age
of 59.17±8.22 years. In total, 66.7% (20/30) of patients had
non-small cell lung cancer. According to the American Joint
Committee on Cancer Tumor-Node-Metastasis staging system (19), 56.7% (17/30) of patients had stage
III cancer and 43.3% (13/30) had stage IV cancer. The mean maximum
tumor diameter was 70.06±23.52 mm (Table
I). All patients received platinum-based chemotherapy for 2
cycles. A total of 19 patients who achieved CR or PR were assigned
to the chemotherapy-sensitive group, while 11 patients who had SD
or PD were assigned to the chemotherapy-resistant group according
to CT manifestations (Fig. S1). As
shown in Table I, response to
chemotherapy was not associated with age, sex, pathological stage,
smoking status or maximum tumor diameter, while the association
between drug resistance and histological type was significant
(P=0.049).
 | Table I.Demographic and clinical
characteristics of chemotherapy-sensitive (n=19) and -resistant
(n=11) lung cancer patients in the present study. |
Table I.
Demographic and clinical
characteristics of chemotherapy-sensitive (n=19) and -resistant
(n=11) lung cancer patients in the present study.
|
|
| Response to
chemotherapy |
|
|---|
|
|
|
|
|
|---|
| Patient
characteristics | n (%) |
Chemotherapy-sensitive, CR + PR |
Chemotherapy-resistant, SD + PD | P-value |
|---|
| Total patients | 30 (100.00) |
|
|
|
| Age at diagnosis,
yearsa |
| 58.26±9.37 | 60.73±5.82 | 0.703b |
| Sex, n (%) |
|
|
|
|
| Male | 25 (83.33) | 15 (78.95) | 10 (90.91) | 0.626c |
|
Female | 5 (16.67) | 4
(21.05) | 1
(9.09) |
|
| Histological type,
n (%) |
|
|
|
|
|
NSCLC | 20 (66.67) | 10 (52.63) | 10 (90.91) | 0.049c |
|
SCLC | 10 (33.33) | 9
(47.37) | 1
(9.09) |
|
| Pathological stage,
n (%) |
|
|
|
|
|
III | 17 (56.67) | 9
(47.37) | 8
(72.73) | 0.259c |
| IV | 13 (43.33) | 10 (52.63) | 3
(27.27) |
|
| Smoking status, n
(%) |
|
|
|
|
|
Smoker | 24 (80.00) | 14 (73.68) | 10 (90.91) | 0.372c |
|
Never | 6 (20.00) | 5
(26.32) | 1
(9.09) |
|
| Maximum tumor
diametera |
| 65.52±20.28 | 77.93±27.52 | 0.553b |
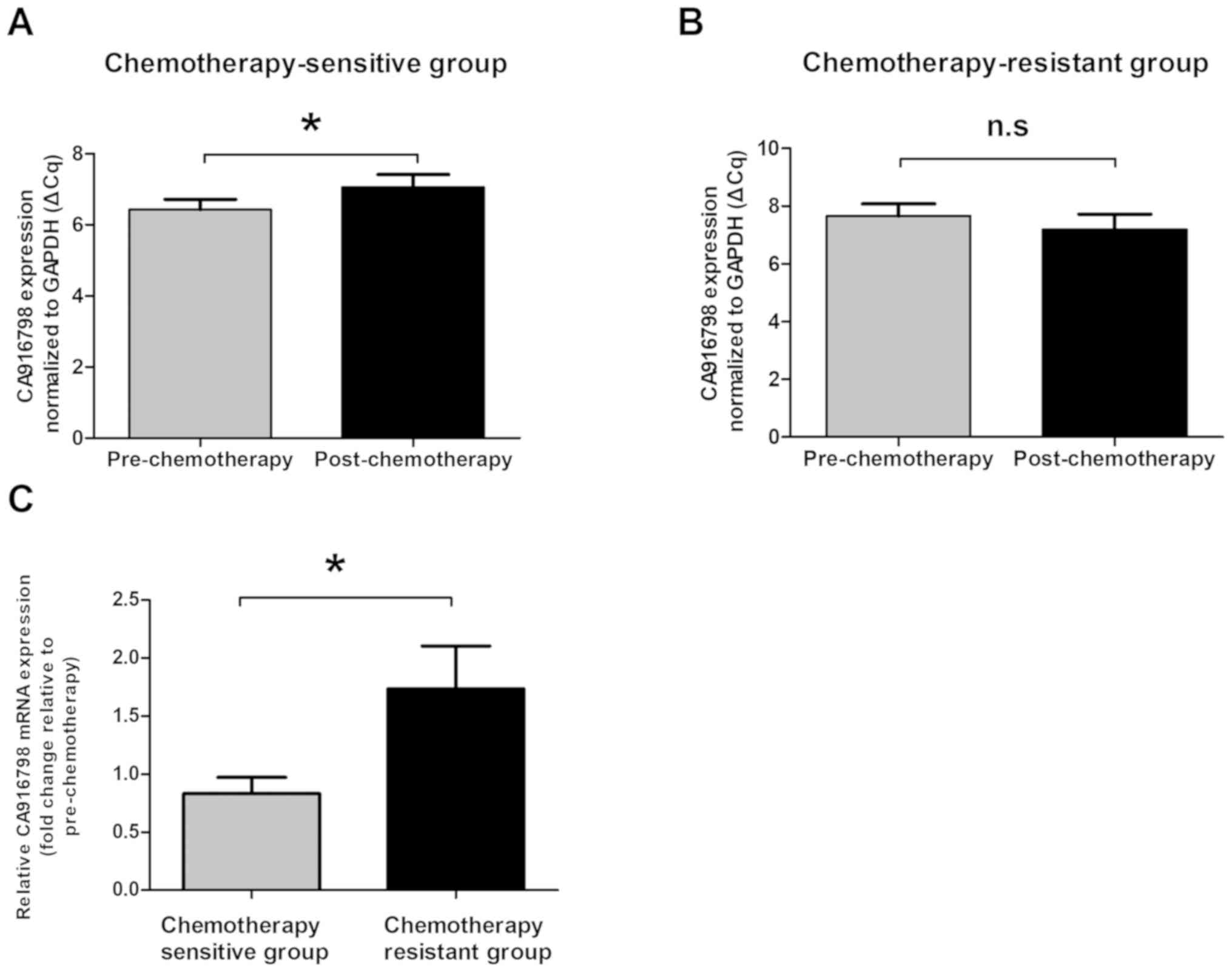
CA916798 mRNA expression is
downregulated post-chemotherapy in chemotherapy-sensitive patients
with lung cancer
Tumor samples were collected from 30 patients prior
to chemotherapy and following 2 cycles of chemotherapy.
Subsequently, the dynamic changes in CA916798 mRNA expression
levels were investigated in the 30 paired lung cancer tissues using
RT-qPCR. CA916798 mRNA expression levels were significantly
downregulated in post-chemotherapy tumor tissues compared with
those in pre-chemotherapy tissues in chemotherapy-sensitive
patients (P<0.05) (Fig. 1A).
However, CA916798 mRNA expression levels did not significantly
change in chemotherapy-resistant lung cancer samples (P>0.05)
(Fig. 1B). The fold-change in
CA916798 mRNA levels following chemotherapy, normalized to GAPDH
and relative to the expression levels prior to chemotherapy, was
calculated for each patient. The fold-change in CA916798 mRNA
expression levels in the chemotherapy-sensitive lung cancer group
of patients was significantly lower compared with those in the
chemotherapy-resistant group (P<0.05) (Fig. 1C).
CA916798 protein expression is
downregulated following chemotherapy in chemotherapy-sensitive
patients with lung cancer
To identify the protein expression levels of
CA916798 in lung cancer tissues, IHC staining of tumor specimens
was used to analyze the protein expression levels of CA916798.
CA916798 protein was positively detected in nearly all biopsy
specimens from the patients with lung cancer prior to chemotherapy.
However, the IHC staining after 2 cycles of chemotherapy showed
that CA916798 protein was only positively stained in 42.1% (8/19)
of samples from chemotherapy-sensitive patients, which was
significantly lower than the percentage exhibited by
chemotherapy-resistant patients (P<0.05) (Table II). The positive staining of
CA916798 protein was also compared before and after chemotherapy.
In chemotherapy-sensitive patients, the expression of CA916798 was
significantly decreased post-chemotherapy in 89.5% (17/19) of lung
cancer cases compared with that in chemotherapy-resistant patients
(P<0.05) (Table II).
Representative IHC staining results of CA916798 are shown in
Fig. S2.
 | Table II.Associations between CA916798 protein
expression level and chemotherapy response in
chemotherapy-sensitive (n=19) and -resistant (n=11) lung cancer
patients in the present study. |
Table II.
Associations between CA916798 protein
expression level and chemotherapy response in
chemotherapy-sensitive (n=19) and -resistant (n=11) lung cancer
patients in the present study.
|
| Response to
chemotherapy |
|
|---|
|
|
|
|
|---|
| CA916798 protein
level |
Chemotherapy-sensitive, CR + PR, n
(%) |
Chemotherapy-resistant, SD + PD, n
(%) | P-value |
|---|
| CA916798 protein
level pre-chemotherapy |
|
|
>0.05a |
|
Positive (n=28) | 18 (94.74) | 10 (90.91) |
|
|
Negative (n=2) | 1 (5.26) | 1 (9.09) |
|
| CA916798 protein
level post-chemotherapy |
|
| 0.002a |
|
Positive (n=19) | 8 (42.11) | 11 (100.00) |
|
|
Negative (n=11) | 11 (57.89) | 0 (0.00) |
|
| Changes of CA916798
protein post-chemotherapy |
|
|
<0.001a |
|
Downregulated (n=18) | 17 (89.47) | 1 (9.09) |
|
|
Upregulated (n=8) | 0 (0.00) | 8 (72.73) |
|
|
Unchanged (n=4) | 2 (10.53) | 2 (18.18) |
|
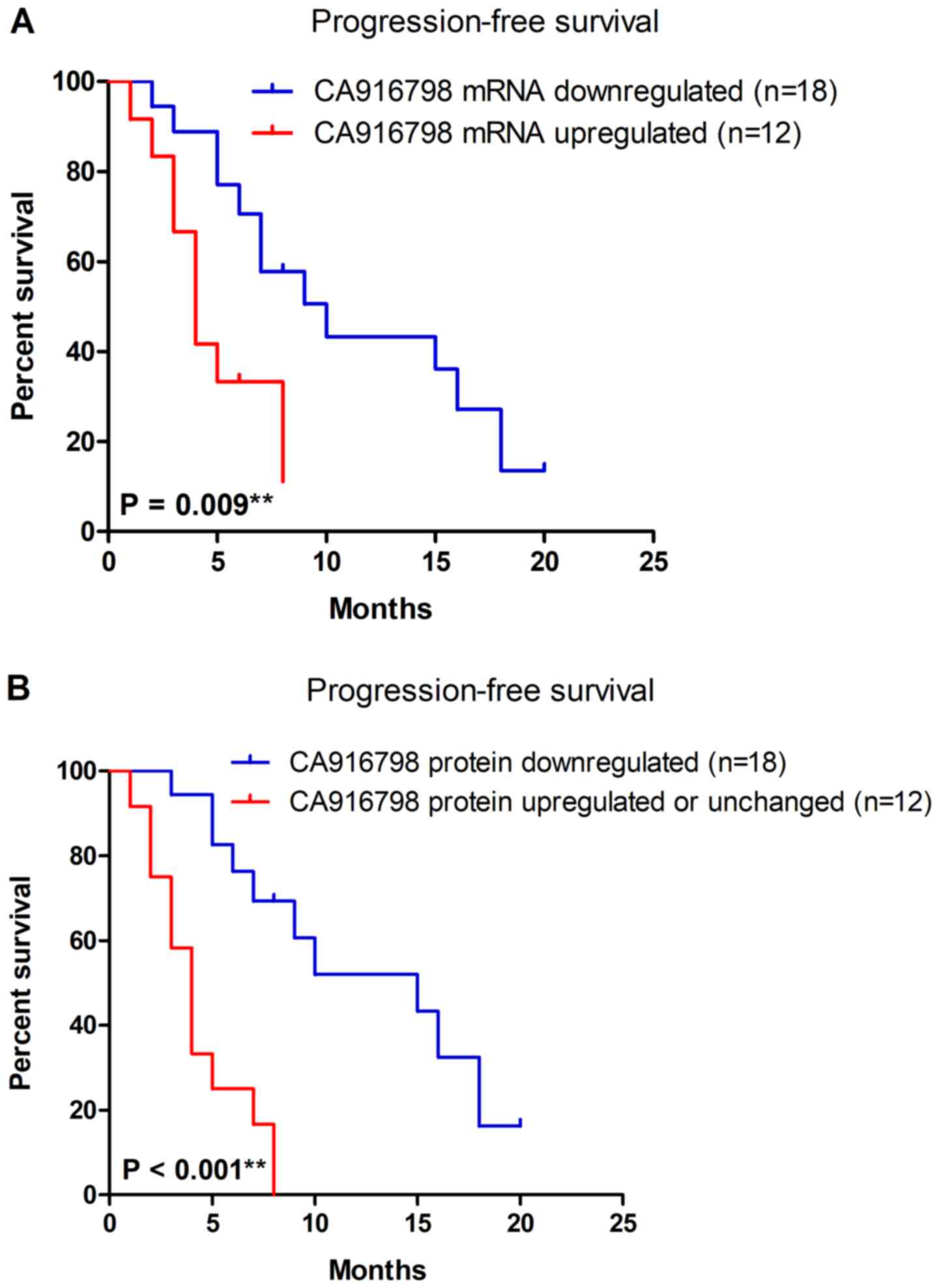
Expression of CA916798 is associated
with progression-free survival in patients with lung cancer
Since CA916798 expression levels were associated
with the response to chemotherapy, the present study further
explored the prognostic significance of CA916798 expression in lung
cancer. Kaplan-Meier survival analysis was performed to explore the
association between CA916798 mRNA expression and progression-free
survival time of the patients. The patients were divided into two
groups: A CA916798 mRNA downregulated group, which included
patients with downregulated expression of CA916798 mRNA
post-chemotherapy and a CA916798 mRNA upregulated group, which
included patients with upregulated expression of CA916798 mRNA
post-chemotherapy. The downregulated expression of CA916798 mRNA
post-chemotherapy was significantly associated with improved
progression-free survival time (P=0.009) (Fig. 2A). The association with
progression-free survival time in patients with lung cancer
remained significant in multivariate Cox proportional-hazards
regression analysis upon adjusting for age, sex, histological type,
pathological stage, smoking status and maximum tumor diameter
[n=30; hazard ratio (HR), 0.304; 95% confidence interval (CI),
0.114–0.807; P=0.017]. Consistent with the results of CA916798 mRNA
expression, Kaplan-Meier analysis also indicated that downregulated
protein expression of CA916798 post-chemotherapy was significantly
associated with improved progression-free survival time
(P<0.001) (Fig. 2B). Upon
adjusting for age, gender, histological type, pathological stage,
smoking status and maximum tumor diameter, the Cox proportional
hazard model indicated that downregulated protein expression of
CA916798 was an independent prognostic factor for lung cancer
(n=30; HR, 0.145; 95% CI, 0.050–0.418; P<0.001).
Changes in CA91679 expression are
associated with response to continued chemotherapy in patients with
lung cancer and SD
Following 2 cycles of chemotherapy, 11 patients with
lung cancer were resistant to chemotherapy. These SD patients
continued treatment with first-line platinum-based chemotherapy. Of
these, only 3 patients exhibited response to continued
chemotherapy, which was associated with changes in CA91679 protein
expression (Table III). In the SD
patients who were resistant to continued chemotherapy, 100% (8/8)
of the patients displayed upregulation of CA916798 protein
expression following 2 cycles of chemotherapy, which was
significantly higher than the percentage exhibited by
chemotherapy-sensitive patients (P=0.005) (Table III).
 | Table III.Associations between CA916798 protein
expression level and response to continued chemotherapy in
chemotherapy-sensitive (n=3) and -resistant (n=8) patients with
stable lung cancer. |
Table III.
Associations between CA916798 protein
expression level and response to continued chemotherapy in
chemotherapy-sensitive (n=3) and -resistant (n=8) patients with
stable lung cancer.
|
| Response to
continued chemotherapy |
|
|---|
|
|
|
|
|---|
| CA916798 protein
level |
Chemotherapy-sensitive, n (%) |
Chemotherapy-resistant, n (%) | P-value |
|---|
| Changes in CA916798
protein post-chemotherapy |
|
| 0.005 |
|
Upregulated (n=8) | 0 (0.00) | 8 (100.00) |
|
|
Downregulated (n=1) | 1 (33.33) | 0 (0.00) |
|
|
Unchanged (n=2) | 2 (66.67) | 0 (0.00) |
|
Discussion
The present study demonstrated that CA916798, a
novel multidrug resistance gene, was downregulated following 2
cycles of chemotherapy in chemotherapy-sensitive patients with lung
cancer. In addition, CA916798 expression level was demonstrated to
be an independent predictor for progression-free survival time in
patients with advanced lung cancer.
Primary therapeutic strategies for lung cancer
include surgical treatment, radiation therapy, targeted therapy,
immunotherapy and chemotherapy (22,23). For
early-stage lung cancer, surgery is the major treatment modality to
improve both overall survival time and progression-free survival
time (24). However, based on the
preponderance of advanced-stage diagnoses, chemotherapy is still
used for the treatment of lung cancer (25,26). For
patients receiving chemotherapy, multidrug resistance limits the
ability to treat advanced lung cancer effectively (27). Multidrug resistance is a phenomenon
that allows cancer cells to become resistant to multiple
structurally and mechanistically distinct anticancer drugs
(28). Multidrug resistance,
existing prior to chemotherapy or appearing upon exposure to
anticancer drug treatment, can be attributed to different
mechanisms (29,30). The most studied mechanism of
multidrug resistance is drug efflux, which is caused by ATP-binding
cassette transporters (31).
P-glycoprotein 1 or MDR1 and MRP2 are typical efflux transmembrane
proteins that are involved in multidrug resistance in various types
of cancers (32,33). However, these genes are not fully
responsible for the mechanism of multidrug resistance, and the
clinical evidence is inconclusive with regard to an association
between these genes and outcomes in lung cancer (34). Thus, a better understanding of novel
molecular determinants of multidrug resistance is important.
Establishment of novel predictive and prognostic biomarkers of
multidrug resistance is one of the most important strategies to
overcome chemotherapy resistance in lung cancer.
In our previous studies, CA916798 was identified as
a novel gene in a multidrug resistant cell line by suppression
subtractive hybridization (13).
Gain- and loss-of-function of CA916798 in lung cancer cell lines
were investigated, which revealed that CA916798 could enhance cell
resistance to chemotherapeutic agents (14,15).
However, the clinical value of CA916798 expression remained
uncertain. In the present study, following 2 cycles of standard
chemotherapy, 63.3% (19 out of 30) of patients responded to drug
treatments effectively. Dynamic changes in CA916798 mRNA and
protein expression levels were observed in tumor tissues following
2 cycles of standard chemotherapy. In the chemotherapy-sensitive
patients, both the CA916798 mRNA and protein expression levels were
downregulated following 2 cycles of chemotherapy. However, the
CA916798 mRNA and protein expression levels were upregulated
following chemotherapy in chemotherapy-resistant patients. A
previous study also reported that the expression of MRP1 was
significantly upregulated following treatment with platinum-based
combinations in lung cancer (35).
Similarly, increased MDR1 mRNA and protein expression levels have
been associated with chemotherapy (36,37).
These studies, alongside those on CA916798, suggest that
platinum-based chemotherapy may induce the expression of multidrug
resistance genes.
The present study has elucidated how CA916798
expression level changed following 2 cycles of platinum-based
chemotherapy. Thus, the expression of CA916798 may be a predictive
and prognostic biomarker for chemotherapy sensitivity in patients
with advanced lung cancer. The present study further explored the
prognostic significance of CA916798 expression. Downregulated
CA916798 mRNA and protein expression levels were associated with
improved progression-free survival in lung cancer. Thus, if
CA916798 expression in tumor tissue is downregulated following 2
cycles of chemotherapy, the patient will have an improved
prognosis. Conversely, if CA916798 expression is upregulated
following 2 cycles of chemotherapy, the patient will have a poor
prognosis. These results suggested that CA916798 expression could
be a novel biomarker for predicting progression of lung cancer
following platinum-based chemotherapy.
In clinical practice, it is often difficult for
doctors to select between continuing chemotherapy or replacing it
with other treatment options, such as radiotherapy or biological
therapy, for patients with SD who had no significant reduction in
lesions following 2 cycles of chemotherapy. In the present study,
11 patients who had SD following 2 cycles of chemotherapy continued
treatment with platinum-based chemotherapy. Of these, 8 patients
with upregulated CA916798 protein expression were resistant to
continued chemotherapy. However, 3 patients with downregulated (or
unchanged) CA916798 protein expression were sensitive to continued
chemotherapy. The results suggested that, if CA916798 expression
was upregulated following 2 cycles of chemotherapy, patients with
SD may remain resistant to continued chemotherapy and the type of
treatment should be changed.
Several limitations exist in the present study.
Firstly, in order to explore the dynamic changes in CA916798
expression, tumor tissues should be collected from patients prior
to chemotherapy and following 2 cycles of chemotherapy. Due to the
strict inclusion and exclusion criteria, the sample size was
limited, which may not ensure sufficient statistical power. The
results require validation in further studies with larger sample
size. Secondly, the histological type in the present study was
complex, since it included non-small cell lung cancer (lung
adenocarcinoma and lung squamous cell carcinoma) and small cell
lung cancer. In the present study, small cell lung cancer may have
been more sensitive to chemotherapy compared with non-small cell
lung cancer. Since different histological types of lung cancer may
have different sensitivities to chemotherapy (38), subgroup analysis of different
histological types of lung cancer is also required.
In conclusion, the present study demonstrated that
both CA916798 mRNA and protein expression levels are downregulated
post-chemotherapy in chemotherapy-sensitive patients with lung
cancer, but not in chemotherapy-resistant patients. Furthermore,
the dynamic change in CA916798 mRNA or protein expression level
post-chemotherapy is an independent prognostic factor for lung
cancer. These findings suggest that CA916798 may be a promising
biomarker to predict chemotherapy resistance and optimize therapy
for patients with lung cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Wu Jieping
Medical Foundation Clinical Research Funding (grant no.
320.6750.12227).
Availability of data and materials
All data generated or analyzed during the present
study are included in this article.
Authors' contributions
XZ designed the study, interpreted the results and
obtained funding. HD performed the experiments and participated in
the study design, results interpretation and manuscript writing. ZY
and LL collected the human tissue samples and provided valuable
technical support and conceptual advice. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The research protocol was approved by the Ethics
Committee of the Third Military Medical University (Chongqing,
China). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zimmermann S and Peters S: Present
standards and future perspectives in the treatment of metastatic
non-small cell lung cancer. Cancer Metastasis Rev. 34:173–182.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azzoli CG, Giaccone G and Temin S:
American society of clinical oncology clinical practice guideline
update on chemotherapy for stage IV non-small-cell lung cancer. J
Oncol Pract. 6:39–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nascimento AV, Singh A, Bousbaa H,
Ferreira D, Sarmento B and Amiji MM: Overcoming cisplatin
resistance in non-small cell lung cancer with Mad2 silencing siRNA
delivered systemically using EGFR-targeted chitosan nanoparticles.
Acta Biomater. 47:71–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nikolaou M, Pavlopoulou A, Georgakilas AG
and Kyrodimos E: The challenge of drug resistance in cancer
treatment: A current overview. Clin Exp Metastasis. 35:309–318.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Yang SH and Guo XL: New insights
into Vinca alkaloids resistance mechanism and circumvention in lung
cancer. Biomed Pharmacother. 96:659–666. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joshi P, Vishwakarma RA and Bharate SB:
Natural alkaloids as P-gp inhibitors for multidrug resistance
reversal in cancer. Eur J Med Chem. 138:273–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pesic M, Markovic JZ, Jankovic D, Kanazir
S, Markovic ID, Rakic L and Ruzdijic S: Induced resistance in the
human non small cell lung carcinoma (NCI-H460) cell line in vitro
by anticancer drugs. J Chemother. 18:66–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang CH, Wang C, Ojima I and Horwitz SB:
Taxol analogues exhibit differential effects on photoaffinity
labeling of β-tubulin and the multidrug resistance associated
P-glycoprotein. J Nat Prod. 81:600–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He J, Lan X, Duan HL, Luo H and Zhou XD:
CA916798 affects growth and metastasis of androgen-dependent
prostate cancer cells. Eur Rev Med Pharmacol Sci. 22:4477–4487.
2018.PubMed/NCBI
|
|
14
|
Wang HJ, Yang HP, Zhou XD, Dai XT, Chen YF
and Xiong W: CA916798 regulates multidrug resistance of lung cancer
cells. Asian Pac J Cancer Prev. 12:3403–3408. 2011.PubMed/NCBI
|
|
15
|
Qi Z, Wang Y and Zhou X: CA916798 gene
participates in cisplatin resistance of human lung adenocarcinoma
A549 cells through PI3K/AKT/mTOR pathway. Nan Fang Yi Ke Da Xue Xue
Bao. 32:1290–1293. 2012.(In Chinese). PubMed/NCBI
|
|
16
|
Wang YL, Zhu BJ, Qi ZZ, Wang HJ and Zhou
XD: Akt1 enhances CA916798 expression through mTOR pathway. PLoS
One. 8:e623272013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang HJ, Yang ZX, Dai XT, Chen YF, Yang HP
and Zhou XD: Bisdemethoxycurcumin sensitizes cisplatin-resistant
lung cancer cells to chemotherapy by inhibition of CA916798 and
PI3K/AKT signaling. Apoptosis. 22:1157–1168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang X, Tang C, Luo H, Wang H and Zhou X:
Shp2 confers cisplatin resistance in small cell lung cancer via an
AKT-mediated increase in CA916798. Oncotarget. 8:23664–23674.
2017.PubMed/NCBI
|
|
19
|
Travis WD, Brambilla E, Rami-Porta R,
Vallières E, Tsuboi M, Rusch V and Goldstraw P; International
Staging Committee, : Visceral pleural invasion: Pathologic criteria
and use of elastic stains: Proposal for the 7th edition of the TNM
classification for lung cancer. J Thorac Oncol. 3:1384–1390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishino M, Jackman DM, Hatabu H, Yeap BY,
Cioffredi LA, Yap JT, Jänne PA, Johnson BE and Van den Abbeele AD:
New response evaluation criteria in solid tumors (RECIST)
guidelines for advanced non-small cell lung cancer: Comparison with
original RECIST and impact on assessment of tumor response to
targeted therapy. AJR Am J Roentgenol. 195:W221–W228. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
da Costa DJ, Parrish JW, Singh NK and Hsia
DW: Lung cancer: Advances and insights in diagnosis, treatment, and
palliation. Am J Respir Crit Care Med. 198:667–669. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zappa C and Mousa SA: Non-small cell lung
cancer: Current treatment and future advances. Transl Lung Cancer
Res. 5:288–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang P: Epidemiology of lung cancer
prognosis: Quantity and quality of life. Methods Mol Biol.
471:469–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burdett SS, Stewart LA and Rydzewska L:
Chemotherapy and surgery versus surgery alone in non-small cell
lung cancer. Cochrane Database Syst Rev. CD0061572007.PubMed/NCBI
|
|
27
|
Kim ES: Chemotherapy resistance in lung
cancer. Adv Exp Med Biol. 893:189–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ullah MF: Cancer multidrug resistance
(MDR): A major impediment to effective chemotherapy. Asian Pac J
Cancer Prev. 9:1–6. 2008.PubMed/NCBI
|
|
29
|
Zahreddine H and Borden KL: Mechanisms and
insights into drug resistance in cancer. Front Pharmacol. 4:282013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kartal-Yandim M, Adan-Gokbulut A and Baran
Y: Molecular mechanisms of drug resistance and its reversal in
cancer. Crit Rev Biotechnol. 36:716–726. 2016.PubMed/NCBI
|
|
31
|
Cnubben NH, Wortelboer HM, van Zanden JJ,
Rietjens IM and van Bladeren PJ: Metabolism of ATP-binding cassette
drug transporter inhibitors: Complicating factor for multidrug
resistance. Expert Opin Drug Metab Toxicol. 1:219–232. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sodani K, Patel A, Kathawala RJ and Chen
ZS: Multidrug resistance associated proteins in multidrug
resistance. Chin J Cancer. 31:58–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi CH: ABC transporters as multidrug
resistance mechanisms and the development of chemosensitizers for
their reversal. Cancer Cell Int. 5:302005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leslie EM, Deeley RG and Cole SP:
Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2,
and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol.
204:216–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Triller N, Korosec P, Kern I, Kosnik M and
Debeljak A: Multidrug resistance in small cell lung cancer:
Expression of P-glycoprotein, multidrug resistance protein 1 and
lung resistance protein in chemo-naive patients and in relapsed
disease. Lung Cancer. 54:235–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roy S, Kenny E, Kennedy S, Larkin A,
Ballot J, Perez De Villarreal M, Crown J and O'Driscoll L:
MDR1/P-glycoprotein and MRP-1 mRNA and protein expression in
non-small cell lung cancer. Anticancer Res. 27:1325–1330.
2007.PubMed/NCBI
|
|
37
|
Melguizo C, Prados J, Luque R, Ortiz R,
Caba O, Alvarez PJ, Gonzalez B and Aranega A: Modulation of MDR1
and MRP3 gene expression in lung cancer cells after paclitaxel and
carboplatin exposure. Int J Mol Sci. 13:16624–16635. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chan BA and Coward JI: Chemotherapy
advances in small-cell lung cancer. J Thorac Dis. 5 (Suppl
5):S565–S578. 2013.PubMed/NCBI
|