Introduction
Chronic right lower quadrant pain is a clinical
presentation for a spectrum of neoplastic and inflammatory diseases
that may affect the ileocecal region (1,2). A
variety of structures may be pathologically involved, including the
cecum, appendix, ileocecal valve and/or terminal ileum; thus,
clinically differentiating the source of right lower quadrant pain
is challenging (2–4).
At present, cross-sectional imaging, including
computed tomography (CT) enterography and magnetic resonance
enterography (MRE), serve important roles in the detection of bowel
lesions (5–7). Recent guidelines regarding patient
preparation and acquisition for cross-sectional small bowel and
colonic imaging have been established (8). Differentiating between neoplastic and
inflammatory diseases by their morphological appearance via imaging
may be ambiguous due to the considerable overlap in conventional CT
or MR features, including increased wall thickness, luminal
narrowing and enlarged adjacent lymph nodes (9,10).
Inflammatory bowel disease increases the risk of small bowel and
colonic adenocarcinoma, but may also mimic these malignancies upon
imaging (11); thus, diagnosing
certain cancer types in such patients is difficult (9,12).
Diffusion weighted imaging (DWI) is a promising
technique that measures the random motion of water molecules in
biological tissues (5,13–15). DWI
has been widely applied for the differentiation of neoplasms and
the evaluation of therapeutic response in the solid abdominal
viscera (16,17). Additionally, DWI may be employed in
clinical MRE examination, providing similar information to
contrast-enhanced imaging in cases where the latter is
contraindicated, including pregnancy, allergy to contrast agents
and renal insufficiency (13,18). The
b-value of a DWI sequence indicates the strength of the
diffusion-sensitizing gradient, which in part, determines image
contrast (19–21). Previous studies have demonstrated the
utility of higher b-values, >1,000 sec/mm2, for the
detection of breast (22) and
pancreatic (20) cancer; however,
the majority of MRE-based analyses have employed two or three
b-values from 0–1,000 sec/mm2 for DWI (18,23–25). At
present, higher b-values in DWI have not been investigated in this
field.
Therefore, the present study aimed to evaluate the
potential of DWI with ultra-high b-values for the identification of
neoplasms with endoscopic and surgical data as the reference
standard.
Patients and methods
Patient enrolment
The institutional review board of Tongji Hospital
(Wuhan, China) approved this retrospective study and the
requirement for informed consent was waived. Analysis of the Tongji
Hospital database identified the records from 292 MRE-based
examinations of patients with suspected gastrointestinal diseases
between September 2014 and December 2016. Among these patients, 86
MRE examinations met the following inclusion criteria: i) Ileocecal
segments were completely assessed by endoscopy or surgery along
with histopathology results; ii) multiple b-values of DWI (400,
600, 800, 1,000, 1,200, 1,500 and 3,000 sec/mm2) were
included in the MRE protocol; and iii) no bowel surgery was
performed prior to MRE examination. Of the 86 MRE examinations
meeting these criteria, 6 duplicate MRE examinations for follow-up
evaluation and 4 patients with inadequate DWI images due to
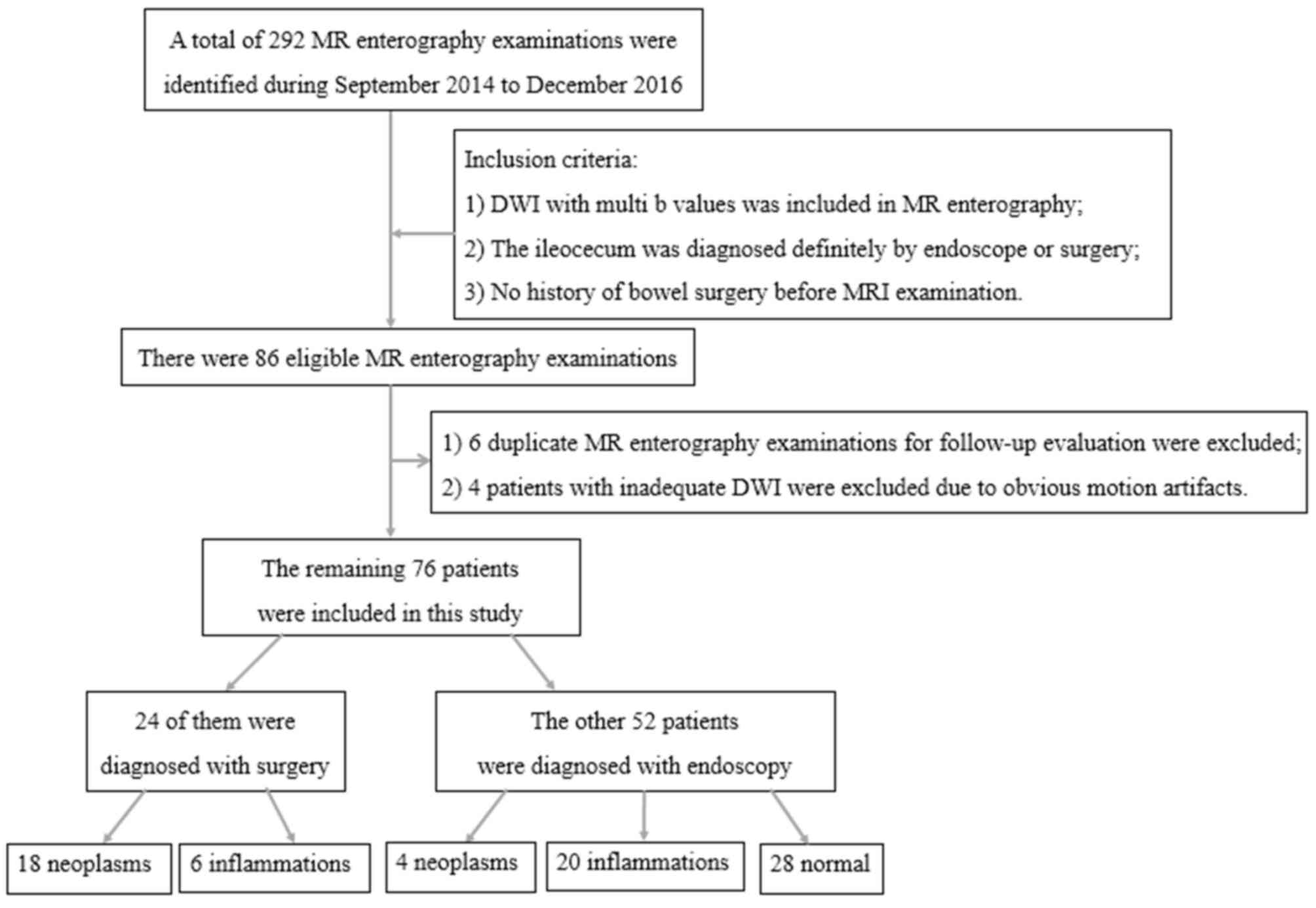
extensive motion artifacts. The remaining 76 patients were included
in the present study for analysis. A flow chart demonstrating the
selection of patients is presented in Fig. 1.
MRE protocol
The MRE examination was performed using a 3.0-T GE
MR scanner (Discovery MR750; GE Healthcare). All patients fasted
for ≥6 h prior to the examination. To achieve adequate distension
of the ileocecal segment, ~1,500 ml isosmotic mannitol solution was
administered orally to each patient ~45 min prior to MR scanning.
To reduce bowel peristalsis, 10 mg anisodamine was administered
intramuscularly 5 min prior to the examination in patients without
contraindications, including glaucoma and/or prostatic hypertrophy.
All patients were routinely scanned with a 32-channel phased-array
body coil in the supine position. The MRE protocol is summarized in
Table I. DW images were acquired in
the transverse plane using the single shot echo planar imaging
technique with parallel imaging and fat suppression (7). Diffusion-encoding gradients were
applied at seven b-values from 400–3,000 sec/mm2 (400,
600, 800, 1,000, 1,200, 1,500 and 3,000 sec/mm2). The
number of excitations was six for non-zero b-values. The total
duration of acquisition in the entire examination for every patient
was ≤30 min, depending on the respiratory rate of the patients.
 | Table I.Magnetic resonance enterography
parameter settings in a 3.0T MR scanner. |
Table I.
Magnetic resonance enterography
parameter settings in a 3.0T MR scanner.
| Parameter | Coronal/axial
SSFSE | Coronal/axial
FIESTA | Coronal/axial
LAVA | Axial
diffusion-weighted imaging |
|---|
| TR, msec | A respiratory
cycle | 3.2 | 3.8 | A respiratory
cycle |
| TE, msec | 68 | 1.2 | 1.7 | 80 |
| Flip Angel, ° | 90 | 45 | 15 | 90 |
| Matrix, pixels | 288×288 | 288×288 | 260×210 | 160×128 |
| Slice thickness,
mm | 4/5 | 4/5 | 4.2/5.0 | 6 |
| Intersection gap,
mm | 1/1 | 1/1 | 0/0 | 1 |
| Field of view,
cm2 | Variable | Variable | Variable | 38×30.4 |
| Number of
excitations | 0.7 | 1 | 0.53 | 6 |
| Fat saturation | Spectral | Spectral | N.A. | Fat |
Image analysis
All images were transferred to a workstation (AW
4.5; GE Healthcare). Apparent diffusion coefficient (ADC) maps were
generated by a mono-exponential fit using b=0 and one of the seven
b-values. Two experienced gastrointestinal radiologists
independently analyzed the image sets of DWI with the seven
b-values and corresponding ADC maps. To reduce any bias, the
radiologists were blinded to clinical details and the
ileocolonoscopy and surgical results of the patients. The seven
image sets were reviewed in a random order with a time interval of
4 weeks between readouts. Any discrepancies between the results
were resolved by consulting with a third more experienced
radiologist. The consensus of results was employed for further
statistical analysis.
For the assessment of the image sets, the b-value of
the images and the corresponding ADC maps of ileocecal segments
were evaluated as previously described (18,23) with
respect to: i) Bowel wall thickening (>3 mm); and ii) increased
signal on DW images for the lesion. Ileocolonoscopy or operative
results served as the reference standards.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 19; IBM Corp.). Sensitivities, specificities,
positive predictive values (PPVs) and negative predictive values
(NPVs) for the detection of lesions on DWI with different b-values
were calculated by comparison with the reference standards. The
Youden Index (YI) was obtained using the formula: YI=sensitivity +
specificity-1. High YI values indicated superior performances.
Diagnostic performances of DWI with different b-values were
compared using a χ2 test. P<0.05 was considered to
indicate a statistically significant difference. Interobserver
agreements between the two independent radiologists for the results
of the DWI were evaluated by κ statistical analysis (κ=0.00–0.20,
slight agreement; κ=0.21–0.40, fair agreement; κ=0.41–0.60,
moderate agreement; κ=0.61–0.80, good agreement and κ=0.81–1.00,
almost perfect agreement) (26).
Results
Patient demographics
A total of 76 patients were included in the present
study, including 41 males (mean age, 45 years; age range, 20–81
years) and 35 females (mean age, 57 years; age range, 20–80 years),
with an overall mean age of 51 years (age range, 20–81 years).
Among these 76 patients, 22 patients were diagnosed with neoplasms
and 26 patients were diagnosed with areas of inflammation according
to histopathological analysis of samples obtained following
endoscopy or surgery. The ileocecal segments of the remaining 28
patients were normal. A summary of the diagnoses for the 76
patients is presented in Table II.
Interobserver agreements from the results of the two radiologists
were rated as good to perfect. The κ values were 0.688–0.881 for
DWI with different b-values (Table
III).
 | Table II.Diagnosis of patients included in the
present study. |
Table II.
Diagnosis of patients included in the
present study.
| Diagnosis | Number of
patients | Sex (male:female),
n |
|---|
| Neoplasms | 22 | 9:13 |
| Stromal
tumor | 2 | 0:2 |
|
Adenocarcinoma | 13 | 4:9 |
|
Lymphoma | 5 | 3:2 |
|
Sarcoma | 1 | 1:0 |
|
Appendix myxoma | 1 | 1:0 |
| Inflammations | 26 | 19:7 |
| Crohn's
disease | 11 | 9:2 |
|
Intestinal tuberculosis | 4 | 2:2 |
|
Ulcerative colitis | 1 | 0:1 |
| Mucosal
chronic inflammation | 5 | 4:1 |
|
Appendicitis | 5 | 4:1 |
| Normal ileocecal
segments | 28 | 13:15 |
| Total | 76 | 41:35 |
 | Table III.Sensitivity, specificity, PPV, NPV
and YI of diffusion-weighted imaging with different b-values for
the detection of ileocecal lesions, and interobserver agreement
between independent assessments by two radiologists. |
Table III.
Sensitivity, specificity, PPV, NPV
and YI of diffusion-weighted imaging with different b-values for
the detection of ileocecal lesions, and interobserver agreement
between independent assessments by two radiologists.
| b-value,
sec/mm2 | Sensitivity (95%
CI) | Specificity (95%
CI) | PPVs (95% CI) | NPVs (95% CI) | YI | κ (95% CI) |
|---|
| 400 | 0.958
(0.846–0.993) | 0.536
(0.342–0.720) | 0.780
(0.649–0.873) | 0.882
(0.623–0.979) | 0.494 | 0.789
(0.629–0.950) |
| 600 | 0.938
(0.818–0.984) | 0.714
(0.511–0.860) | 0.849
(0.719–0.928) | 0.870
(0.653–0.966) | 0.652 | 0.688
(0.510–0.867) |
| 800 |
0.917(0.791–0.973) | 0.786
(0.5854–0.910) | 0.880
(0.750–0.950) | 0.846
(0.643–0.950) | 0.702 | 0.858
(0.738–0.978) |
| 1,000 | 0.854
(0.766–0.961) | 0.821
(0.624–0.932) | 0.891
(0.766–0.961) | 0.767
(0.624–0.932) | 0.676 | 0.754
(0.603–0.904) |
| 1,200 | 0.792
(0.623–0.875) | 0.857
(0.706–0.972) | 0.905
(0.785–0.980) | 0.706
(0.517–0.831) | 0.649 | 0.841
(0.719–0.963) |
| 1,500 | 0.688
(0.579–0.843) | 0.929
(0.750–0.988) | 0.943
(0.805–0.991) | 0.634
(0.500–0.804) | 0.616 | 0.842
(0.720–0.963) |
| 3,000 | 0.521
(0.374–0.665) | 1
(0.850–1.000) | 1.000
(0.834–1.000) | 0.549
(0.405–0.686) | 0.521 | 0.881
(0.767–0.994) |
Performances of DWI with different
b-values for the detection of lesions
The sensitivities, specificities, PPVs, NPVs and YI
from DWI with different b-values (400, 600, 800, 1,000, 1,200,
1,500 and 3,000 sec/mm2) for detecting ileocecal lesions
are presented in Table III. DWI
with low b-values achieved higher sensitivity with a relatively
lower specificity, while the specificity was increased in DWI with
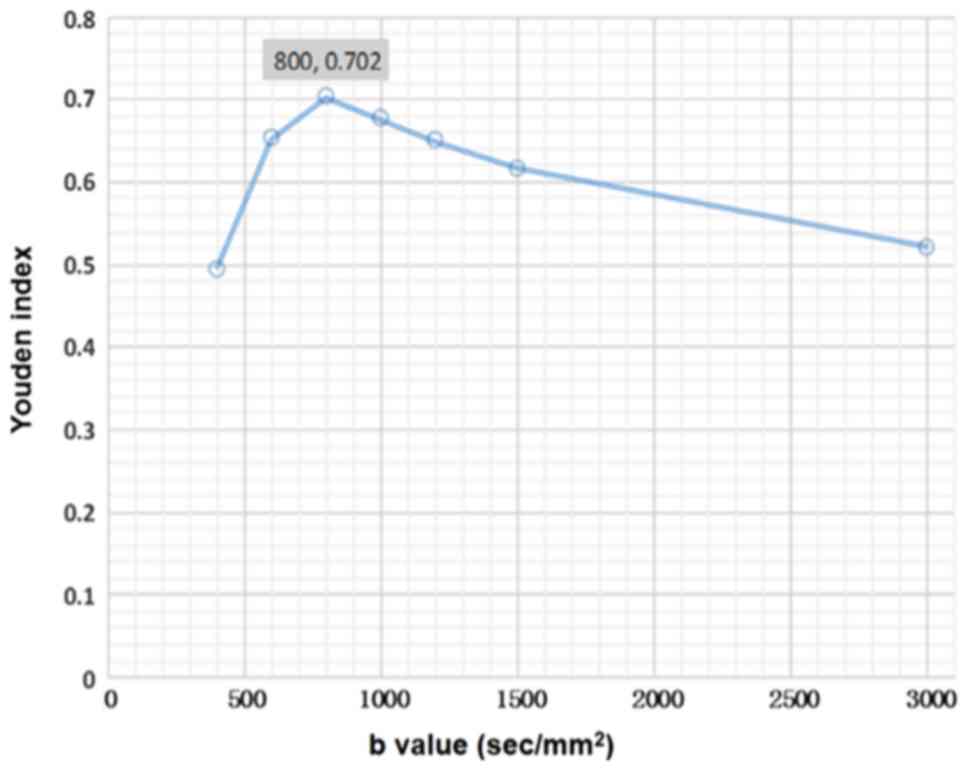
higher b-values. The YI reached the maximum (0.702) when the
b-value of DWI was 800 sec/mm2, but then decreased as
the b-value increased (Fig. 2). The
diagnostic performances of the seven b-values for DWI were
significantly different compared with each other, except for three
groups [DWI with b=600 and 800 sec/mm2 (P=0.250); DWI
with b=800 and 1,000 sec/mm2 (P=0.125); and DWI with
b=1,000 and 1,200 sec/mm2 (P=0.125)].
Effects of variable b-values on
MRI-based diagnosis
The results of DWI using different b-values are
presented in Table IV. The
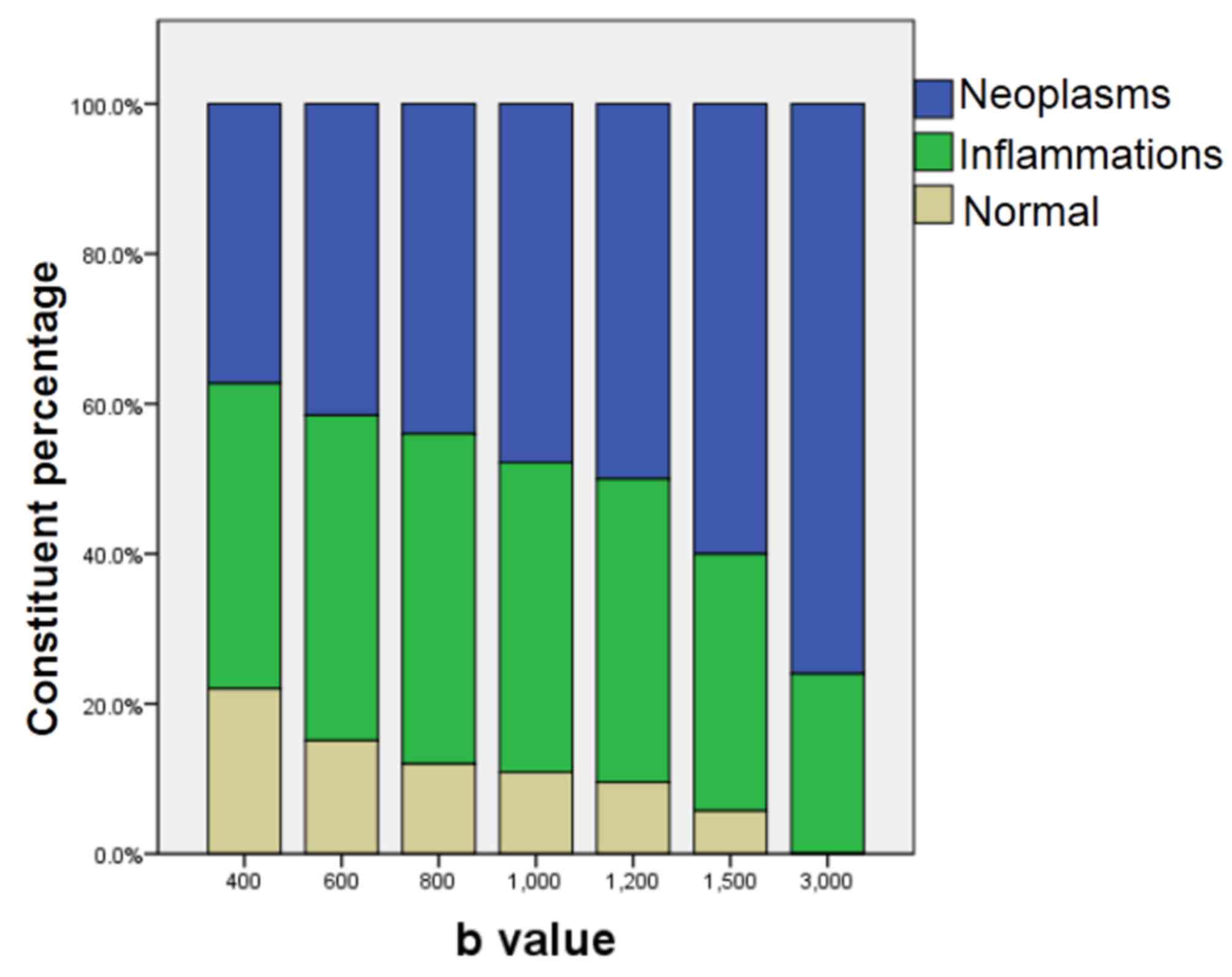
percentages of neoplastic lesions were determined via DWI with
different b-values (Fig. 3). The
signal intensities of inflammatory lesions and normal ileocecal
segments decreased to background levels with increasing b-values
(Fig. 4); however, the neoplasms
exhibited a high signal intensity relative to the background at
high b-values (Fig. 5). As presented
in Table IV and Fig. 3, the ratio of neoplasms to other
lesions detected by DWI became greater with increasing b-values.
Additionally, 76% of high signal intensity lesions on DWI with
b=3,000 sec/mm2 were neoplasms; 6 patients with
inflammatory lesions (3 patients diagnosed with Crohn's disease, 2
with chronic mucosal inflammation with acute phase alterations, and
1 patient with appendicitis and a peri-appendiceal abscess)
demonstrated a relatively high signal intensity on DWI with b=3,000
sec/mm2. An appendiceal myxoma was deemed normal when
b-values >1,200 sec/mm2 were applied and two
adenocarcinomas were not visible with b=3,000 sec/mm2
DWI.
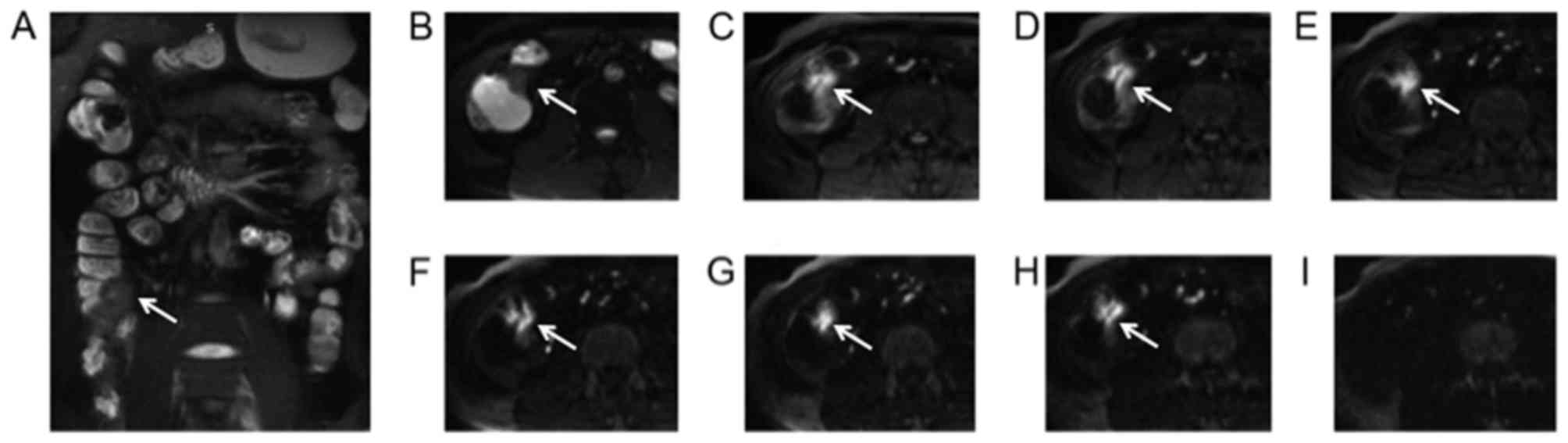
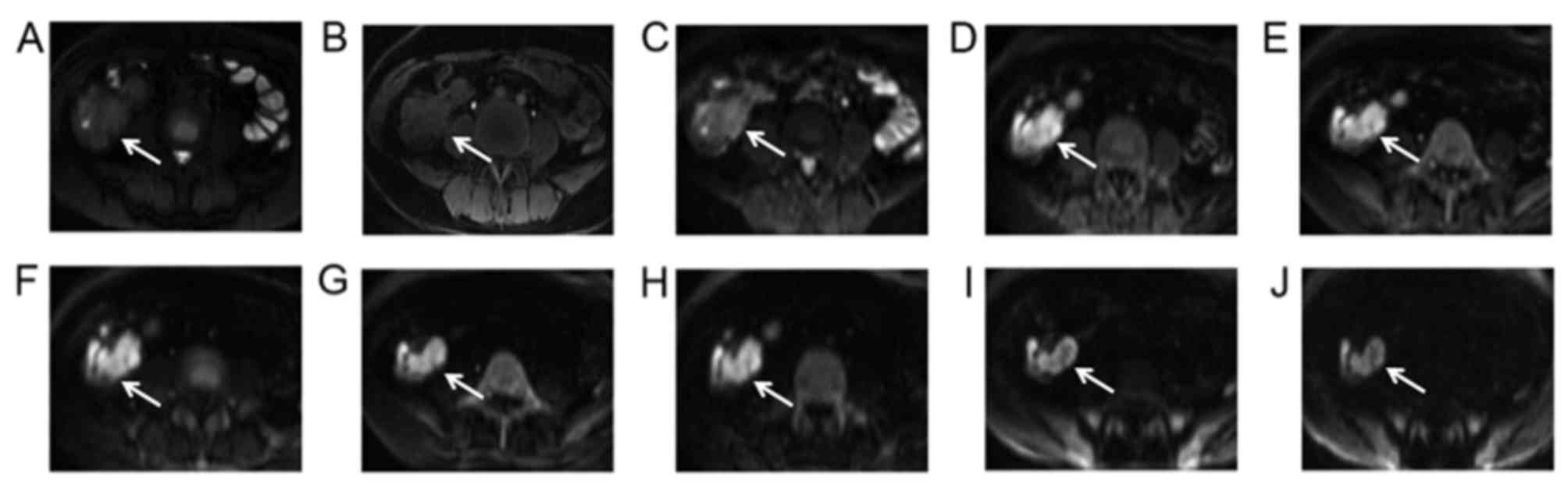 | Figure 5.A 37-year-old female with ileocecal
adenocarcinoma cancer diagnosed by pathological analysis following
surgery. The lesion (arrow) was irregular and extended to the
enteric cavity as determined via (A) conventional axial T2 weighted
image, (B) T1 weighted image and (C) DWI with b=0
sec/mm2. The lesion demonstrated a sustained high signal
intensity on DWI with increasing b-values of (D) b=400
sec/mm2, (E) b=600 sec/mm2, (F) b=800
sec/mm2, (G) b=1,000 sec/mm2, (H) b=1,200
sec/mm2, (I) b=1,500 sec/mm2 and (J) b=3,000
sec/mm2. DWI, diffusion-weighted imaging. |
 | Table IV.Number of positive results on DWI
with different b-values. |
Table IV.
Number of positive results on DWI
with different b-values.
| b-value,
sec/mm2 | Positive DWI
findings | Neoplasms,
na | Inflammatory
lesions, na | Normal,
na | % of positive
findings representing neoplasms |
|---|
| 400 | 59 | 22 | 24 | 13 | 37.3 |
| 600 | 53 | 22 | 23 | 8 | 41.5 |
| 800 | 50 | 22 | 22 | 6 | 44.0 |
| 1,000 | 46 | 22 | 19 | 5 | 47.8 |
| 1,200 | 42 | 21 | 17 | 4 | 50.0 |
| 1,500 | 35 | 21 | 12 | 2 | 60.0 |
| 3,000 | 25 | 19 | 6 | 0 | 76.0 |
Discussion
DWI is recommended for routine use by the European
Society of Gastrointestinal and Abdominal Radiology (ESGAR) and the
European Society of Pediatric Radiology (ESPR) in MRE-based
assessments of inflammatory bowel disease with an upper range of
b-values between 600–900 sec/mm2 (8). In the present study, DWI with b=800
sec/mm2 achieved the highest YI value in the detection
of neoplastic and inflammatory lesions among the seven assessed
b-values. Overall, differences in lesion detection amongst b-values
of 600–1,200 sec/mm2 were subtle, consistent with the
ESGAR/ESPR recommendations (8). A
higher b-value (3,000 sec/mm2) for DWI revealed the
specificity for distinguishing neoplasms from bowel inflammation to
be increased, with 76% of the positive findings representing
neoplasms.
DWI has been widely used for the assessment of bowel
diseases in recent years; however, the majority of studies have
investigated the detection of neoplasms or inflamed bowel loops
from normal loops using lower b-values (18,23,27–29).
However, while the differentiation between bowel inflammation and
neoplasms is clinically useful, it remains challenging when lower b
values DWI are used, as neoplasms and inflammation are detected
with high intensities at b-values between 400–1,200
sec/mm2. This is similar to the findings of a previous
assessment of pancreatic adenocarcinoma on DWI with b=1,000
sec/mm2 (30). Due to the
application of fast imaging acquisition techniques, gradient coils
and improvements in MRI hardware, the use of higher b-value DWI has
gained increasing attention in research and clinical practice
(22,31,32).
Compared with standard b values (≤1,000 sec/mm2), higher
b-values (≥1,500 sec/mm2) may enhance the image contrast
between unaffected bowel and neoplasms, as the greater cellularity
in the latter restricts free diffusion of water in the tumor
tissues (19,20).
The results of the present study demonstrated that
the signal intensity of normal and inflamed bowel decreased to
background level at b-values >1,200 sec/mm2 (Fig. 4). At such b-values, neoplastic
lesions exhibited relatively high intensity (Fig. 5) and the ratio of neoplasms in the
positive DWI findings with higher b-values increased. These
findings were consistent with previous studies (19,20,32–34),
which proposed that DWI analysis with higher b-values may provide
sufficient background suppression to reduce the false-positive
diagnoses of neoplasms. Similar findings have been reported in the
pancreas; Fukukura et al (20) revealed that b=1,500
sec/mm2 DWI improved the delineation of pancreatic
adenocarcinomas from pancreatitis. In the prostate, lesions with
increased signal intensity from higher b-value images were
predominantly tumors (82%) (34).
In the present study, there were 6 cases of bowel
inflammation demonstrating slight hyperintensity on DWI with
b=3,000 sec/mm2. The basis of restricted diffusion in
DWI is associated with the cellularity of tissues (31,35);
abscesses exhibit hyperintensity on high b-value (3,000
sec/mm2) DWI due to the abundance of inflammatory cells
with restricted motion inside the abscess cavity (36). Thus, the high signal intensity of
inflammatory lesions on high b-value DWI could be due to the
accumulation of inflammatory cells or the formation of acute micro
abscesses; however, further investigation is required. In addition,
an appendiceal myxoma and two adenocarcinomas were not visible on
the higher b-value DWI (b=3,000 sec/mm2) in the present
study. This could be associated with the abundance of mucins
(37), which was identified in these
cases via histopathological analysis. Some of these shortcomings
may be addressed by using a combination of conventional MRE
sequences alongside high b-value DWI.
In conclusion, persistent hyperintensity of
ileocecal lesions on DWI with higher b-values suggests the presence
of neoplasms rather than inflammatory bowel conditions. Thus, the
use of higher b-values for DWI in MRE may aid in the distinction of
neoplasms from other bowel-associated pathologies. Due to the
limitations in sensitivity and decreased signal to noise ratios,
high b-value DWI may be most valuable as an adjunct sequence to DWI
performed with conventional b-values (~800 sec/mm2).
Acknowledgements
The authors would like to thank Professor John
Morelli, a radiologist from St. John's Medical Center, for reading
and commenting on this manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grants nos. 81701657, 81771801
and 81571642) and the Fundamental Research Funds for the Central
Universities (grant no. NO.2017KFYXJJ126).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY wrote the main manuscript. HY, CF, ZW and JJL
searched the database and acquired patients. HY, CF, XMH, ZL, YQS
and DYH designed the study. HY, CF, ZW, JJL and YCW performed data
analysis. JJL and YCW interpreted and checked data. CF, XMH, ZL,
YQS and DYH contributed to the manuscript revisions. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
review board of Tongji Hospital and informed consent was
waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Purysko AS, Remer EM, Filho HML,
Bittencourt LK, Lima RV and Racy DJ: Beyond appendicitis: Common
and uncommon gastrointestinal causes of right lower quadrant
abdominal pain at multidetector CT. Radiographics. 31:927–947.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel NB and Wenzke DR: Evaluating the
patient with right lower quadrant pain. Radiol Clin North Am.
53:1159–1170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie X, Zhou Z, Song Y, Wang W, Dang C and
Zhang H: Differences between carcinoma of the cecum and ascending
colon: Evidence based on clinical and embryological data. Int J
Oncol. 53:87–98. 2018.PubMed/NCBI
|
|
4
|
Hoeffel C, Crema MD, Belkacem A, Azizi L,
Lewin M, Arrivé L and Tubiana JM: Multi-detector row CT: Spectrum
of diseases involving the ileocecal area. Radiographics.
26:1373–1390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rimola J, Rodriguez S, Garcia-Bosch O,
Ordás I, Ayala E, Aceituno M, Pellisé M, Ayuso C, Ricart E, Donoso
L and Panés J: Magnetic resonance for assessment of disease
activity and severity in ileocolonic Crohn's disease. Gut.
58:1113–1120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amzallag-Bellenger E, Soyer P, Barbe C,
Diebold MD, Cadiot G and Hoeffel C: Prospective evaluation of
magnetic resonance enterography for the detection of mesenteric
small bowel tumours. Eur Radiol. 23:1901–1910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dohan A, Taylor S, Hoeffel C, Barret M,
Allez M, Dautry R, Zappa M, Savoye-Collet C, Dray X, Boudiaf M, et
al: Diffusion-weighted MRI in Crohn's disease: Current status and
recommendations. J Magn Reson Imaging. 44:1381–1396. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taylor SA, Avni F, Cronin CG, Hoeffel C,
Kim SH, Laghi A, Napolitano M, Petit P, Rimola J, Tolan DJ, et al:
The first joint ESGAR/ESPR consensus statement on the technical
performance of cross-sectional small bowel and colonic imaging. Eur
Radiol. 27:2570–2582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barral M, Dohan A, Allez M, Boudiaf M,
Camus M, Laurent V, Hoeffel C and Soyer P: Gastrointestinal cancers
in inflammatory bowel disease: An update with emphasis on imaging
findings. Crit Rev Oncol Hematol. 97:30–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hristova L, Soyer P, Hoeffel C, Marteau P,
Oussalah A, Lavergne-Slove A, Boudiaf M, Dohan A and Laurent V:
Colorectal cancer in inflammatory bowel diseases: CT features with
pathological correlation. Abdom Imaging. 38:421–435. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Lerma BA, Perletti G, Bonapace IM and
Monti E: Inflammatory cues acting on the adult intestinal stem
cells and the early onset of cancer (review). Int J Oncol.
45:959–968. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Biancone L, Armuzzi A, Scribano ML, D'Inca
R, Castiglione F, Papi C, Angelucci E, Daperno M, Mocciaro F,
Riegler G, et al: Inflammatory bowel disease phenotype as risk
factor for cancer in a prospective multicentre nested case-control
IG-IBD study. J Crohns Colitis. 10:913–924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SH: DWI at MR enterography for
evaluating bowel inflammation in Crohn disease. AJR Am J
Roentgenol. 207:40–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Liang L, Li A, Hu Y, Hu D, Li Z and
Kamel IR: Monoexponential, biexponential, and stretched exponential
diffusion-weighted imaging models: Quantitative biomarkers for
differentiating renal clear cell carcinoma and minimal fat
angiomyolipoma. J Magn Reson Imaging. 46:240–247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bittencourt LK, Matos C and Coutinho AC
Jr: Diffusion-weighted magnetic resonance imaging in the upper
abdomen: Technical issues and clinical applications. Magn Reson
Imaging Clin N Am. 19:111–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huh J, Kim KJ, Park SH, Park SH, Yang SK,
Ye BD, Park SH, Han K and Kim AY: Diffusion-weighted MR
enterography to monitor bowel inflammation after medical therapy in
Crohn's disease: A prospective longitudinal study. Korean J Radiol.
18:162–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang YC, Hu DY, Hu XM, Shen YQ, Meng XY,
Tang H and Li Z: Assessing the early response of advanced cervical
cancer to neoadjuvant chemotherapy using intravoxel incoherent
motion diffusion-weighted magnetic resonance imaging: A pilot
study. Chin Med J (Engl). 129:665–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seo N, Park SH, Kim KJ, Kang BK, Lee Y,
Yang SK, Ye BD, Park SH, Kim SY, Baek S, et al: MR Enterography for
the evaluation of small-bowel inflammation in crohn disease by
using diffusion-weighted imaging without intravenous contrast
material: A prospective noninferiority study. Radiology.
278:762–772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Metens T, Miranda D, Absil J and Matos C:
What is the optimal b value in diffusion-weighted MR imaging to
depict prostate cancer at 3T? Eur Radiol. 22:703–709. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukukura Y, Shindo T, Hakamada H, Takumi
K, Umanodan T, Nakajo M, Kamimura K, Umanodan A, Ideue J and
Yoshiura T: Diffusion-weighted MR imaging of the pancreas:
Optimizing b-value for visualization of pancreatic adenocarcinoma.
Eur Radiol. 26:3419–3427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goshima S, Kanematsu M, Kondo H, Yokoyama
R, Kajita K, Tsuge Y, Watanabe H, Shiratori Y, Onozuka M and
Moriyama N: Diffusion-weighted imaging of the liver: Optimizing b
value for the detection and characterization of benign and
malignant hepatic lesions. J Magn Reson Imaging. 28:691–697. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woodhams R, Inoue Y, Ramadan S, Hata H and
Ozaki M: Diffusion-weighted imaging of the breast: Comparison of
b-values 1,000 s/mm2 and 1,500 s/mm2. Magn
Reson Med Sci. 12:229–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sirin S, Kathemann S, Schweiger B,
Hahnemann ML, Forsting M, Lauenstein TC and Kinner S: Magnetic
resonance colonography including diffusion-weighted imaging in
children and adolescents with inflammatory bowel disease: Do we
really need intravenous contrast? Invest Radiol. 50:32–39. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim KJ, Lee Y, Park SH, Kang BK, Seo N,
Yang SK, Ye BD, Park SH, Kim SY, Baek S and Ha HK:
Diffusion-weighted MR enterography for evaluating Crohn's disease:
How does it add diagnostically to conventional MR enterography?
Inflamm Bowel Dis. 21:101–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li XH, Sun CH, Mao R, Huang SY, Zhang ZW,
Yang XF, Huang L, Lin JJ, Zhang J, Ben-Horin S, et al:
Diffusion-weighted MRI enables to accurately grade inflammatory
activity in patients of ileocolonic Crohn's disease: Results from
an observational study. Inflamm Bowel Dis. 23:244–253. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bouwense SA, van Brunschot S, van
Santvoort HC, Besselink MG, Bollen TL, Bakker OJ, Banks PA,
Boermeester MA, Cappendijk VC, Carter R, et al: Describing
peripancreatic collections according to the revised atlanta
classification of acute pancreatitis: An international
interobserver agreement study. Pancreas. 46:850–857. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buisson A, Hordonneau C, Goutte M, Boyer
L, Pereira B and Bommelaer G: Diffusion-weighted magnetic resonance
imaging is effective to detect ileocolonic ulcerations in Crohn's
disease. Aliment Pharmacol Ther. 42:452–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu W, Liu J, Xiao W and Luo G: A
Diagnostic accuracy meta-analysis of CT and MRI for the evaluation
of small bowel Crohn disease. Acad Radiol. 24:1216–1225. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amzallag-Bellenger E, Soyer P, Barbe C,
Nguyen TL, Amara N and Hoeffel C: Diffusion-weighted imaging for
the detection of mesenteric small bowel tumours with magnetic
resonance-enterography. Eur Radiol. 24:2916–2926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fukukura Y, Takumi K, Kamimura K, Shindo
T, Kumagae Y, Tateyama A and Nakajo M: Pancreatic adenocarcinoma:
Variability of diffusion-weighted MR imaging findings. Radiology.
263:732–740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pramanik PP, Parmar HA, Mammoser AG, Junck
LR, Kim MM, Tsien CI, Lawrence TS and Cao Y: Hypercellularity
components of glioblastoma identified by high b-value
diffusion-weighted imaging. Int J Radiat Oncol Biol Phys.
92:811–819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agarwal HK, Mertan FV, Sankineni S,
Bernardo M, Senegas J, Keupp J, Daar D, Merino M, Wood BJ, Pinto
PA, et al: Optimal high b-value for diffusion weighted MRI in
diagnosing high risk prostate cancers in the peripheral zone. J
Magn Reson Imaging. 45:125–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Coolen J, De Keyzer F, Nafteux P, De Wever
W, Dooms C, Vansteenkiste J, Derweduwen A, Roebben I, Verbeken E,
De Leyn P, et al: Malignant pleural mesothelioma: Visual assessment
by using pleural pointillism at diffusion-weighted MR imaging.
Radiology. 274:576–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quentin M, Schimmöller L, Arsov C,
Rabenalt R, Antoch G, Albers P and Blondin D: Increased signal
intensity of prostate lesions on high b-value diffusion-weighted
images as a predictive sign of malignancy. Eur Radiol. 24:209–213.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tasaki A, Asatani MO, Umezu H, Kashima K,
Enomoto T, Yoshimura N and Aoyama H: Differential diagnosis of
uterine smooth muscle tumors using diffusion-weighted imaging:
Correlations with the apparent diffusion coefficient and cell
density. Abdom Imaging. 40:1742–1752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tomar V, Yadav A, Rathore RK, Verma S,
Awasthi R, Bharadwaj V, Ojha BK, Prasad KN and Gupta RK: Apparent
diffusion coefficient with higher b-value correlates better with
viable cell count quantified from the cavity of brain abscess. AJNR
Am J Neuroradiol. 32:2120–2125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Woodhams R, Kakita S, Hata H, Iwabuchi K,
Umeoka S, Mountford CE and Hatabu H: Diffusion-weighted imaging of
mucinous carcinoma of the breast: Evaluation of apparent diffusion
coefficient and signal intensity in correlation with histologic
findings. AJR Am J Roentgenol. 193:260–266. 2009. View Article : Google Scholar : PubMed/NCBI
|