Introduction
Non-small cell lung cancer (NSCLC) accounts for ~85%
of all lung cancer cases which is the leading cause of cancer
related mortality in the world and the second most common cause of
death in developed countries, after cardiovascular diseases
(1). Several meta-analyses of data
from large randomized controlled trials support the view that
cisplatin-based adjuvant chemotherapy alone or in combination with
neoadjuvant treatment regimens provides a significant survival
advantage for stage IB-III NSCLC patients (2–5).
However, individual patient outcomes for any given regimen is still
highly uncertain and overall survival remains only 15% across all
stages. One of the main reasons for unsatisfactory survival rates
in patients with lung cancer is intrinsic or acquired multidrug
resistance (MDR). Multiple cellular mechanisms are involved in MDR
in vivo and these are partially reflected by in vitro
chemoresistance profiles of NSCLC cells (6–11).
Research on MDR mechanisms has been mainly on proteins involved in
membrane transport, cell cycle and DNA repair pathways but
recently, lipid metabolites including sphingolipids, have emerged
as an important player in a number of fundamental biological
processes with relevance to cancer pathogenesis and therapy
(12).
Sphingolipids are a family of membrane lipids with
structural roles in the regulation of the fluidity and sub-domain
structure of the lipid bilayers (13). They are metabolized, giving, rise to
signaling molecules such as ceramide, sphingosine and sphingosine
1-phosphate (S1P) that are associated with cellular activities
crucial for health and disease, notably in cancer (14). The generation of endogenous ceramide
and/or sphingosine in response to stress stimuli is associated with
senescence, growth arrest and apoptosis (15,16). In
contrast, S1P plays a key role in mediating cell proliferation,
survival, migration and angiogenesis (17–19). It
is one of most important sphingolipid metabolites as it is involved
in the onset or progression of pathological conditions such as
autoimmune diseases, cardiovascular conditions, diabetes and cancer
(20).
By converting sphingosine into S1P, the sphingosine
kinase-1 isoform (SphK1) (21)
alters the ceramide/sphingosine/S1P balance (22). It effectively regulates drug-induced
apoptosis and serves as a chemotherapy/radiotherapy sensor in both
cell cultures and animal models of various tumors (23–28)
including NSCLC (29,30).
Several studies have examined the prognostic and
predictive value of SphK1 in solid tumors. In a series of 48
malignant astrocytomas, SphK1 mRNA expression levels correlated
with patient survival, with a three-fold increase in median
survival in patients with low compared to high expression (31). A recent meta-analysis including
thirty-four studies of SphK1 expression in 4,673 patients showed
that there was a significant difference in SphK1 expression between
cancer, normal tissue adjacent to cancer and benign tissues, as
well as different cancer types (32). In addition, SphK1 expression was
associated with 5-year and overall survival rates in breast,
gastric and other cancers (32). The
prognostic value of SphK1 was confirmed in breast cancer where the
upper quartile of mRNA SphK1 expression correlated with poor
prognosis, irrespective of the estrogen receptor status (33). Assessing S1P content has also been
postulated to have diagnostic potential in ovarian cancer, as shown
by a significant increase in the product of its activity, in
ascites (34,35). A significant increase in both SphK1
expression and enzymatic activity has also been found to be
correlated with aggressiveness in prostate cancer specimens at the
time of surgery (34,35).
In lung cancer tissue, increased expression of mRNA
and protein levels of SphK1 is also seen, compared to adjacent
normal lung tissue, and increased SphK1 expression was
significantly correlated with tumor progression and poor survival
in patients with NSCLC (30). In
NSCLC cell cultures, enforced expression of SphK1 significantly
inhibited doxorubicin- and docetaxel-induced apoptosis, and is
associated with upregulation of the antiapoptotic proteins Bcl-xl,
c-IAP1, c-IAP2, and TRAF1 (30). In
contrast, silencing SphK1 expression or inhibiting SphK1 activity
with a pharmacological inhibitor significantly enhanced the
sensitivity of NSCLC cells to apoptosis induced by
chemotherapeutics both in vitro and in vivo (30). Moreover, overexpression of SphK1 is
associated with activation of the PI3K/Akt/NF-κB pathway,
inhibition of which abrogates the antiapoptotic effect of SphK1 in
NSCLC cells (30).
S1P can be irreversibly degraded by the S1P lyase
(S1P lyase) which is highly conserved throughout evolution and is
required for the maintenance of physiological levels of S1P and
other sphingolipid intermediates (36). S1P lyase expression potentiates
apoptosis in response to DNA damage and other stressful stimuli
through a cascading mechanism that involves p53, PIDD and caspase-2
(37). Enforced expression of S1P
lyase in HEK293 and A549 human lung cancer cells increased
sensitivity to cisplatin and carboplatin (38). The first piece of evidence of the
loss of S1P lyase expression in a human neoplasm was reported in
prostate cancer patients where an inverse correlation was found
between both SphK1 and S1P lyase expression and activity,
suggesting an overall tumor increase in S1P (27).
As the prognostic role of SphK1 in NSCLC needs
further validation and given the dearth of literature on SphK1
expression in patients with adjuvant platinum-based chemotherapy,
our primary aim was evaluate the prognostic and predictive value of
SphK1 expression. We also analyzed S1P lyase expression for the
first time in NSCLC patients.
Patients and methods
Patients and samples
A total of 176 archival formalin-fixed,
paraffin-embedded (FFPE) tissue samples from an NSCLC patient
cohort were acquired from the University Hospital in Olomouc.
Informed, written consent for the use of tissues and clinical data
was obtained from all participants and the studies were carried out
according to the latest Declaration of Helsinki. In addition, the
present study was approved by the Ethics Committee of University
Hospital Olomouc and Medical Faculty, Palacký University (Olomouc,
Czech Republic) on June 2011. Patients were diagnosed and underwent
subsequent radical surgery between 1996 and 2000 and 2005–2011.
Slides, with routinely stained sections from each case, were
re-examined by two independent pathologists and re-classified
according to the WHO classification of tumors (2015). The cohort
consisted of 122 men and 54 women, of whom, 51 patients were in
clinical stage I, 23 in stage II, and 84 in stage III and 8 in
stage IV. A total of 95 patients had received adjuvant chemotherapy
(aCHT), of whom 21 were treated with the combination of cisplatin
and navelbine (18 patients with 4× cycles, 2 patients with 3×
cycles an 1 patient with 1× cycle), 71 patients were treated with
the combination of carboplatin and navelbine (64 patients with 4×
cycles, 5 patients with 3× cycles and 2 patients with 1× cycle). Of
the remaining three patients, one was treated with the combination
of carboplatin and gemcitabine (4× cycles), one with carboplatin
and doxorubicin (4× cycles) and one with carboplatin and paclitaxel
(4× cycles). Detailed characteristics of patients are given in
Tables I and II. Disease-free survival (DFS) was
determined as the interval from diagnosis to disease recurrence,
with a median of 27 months (25% and 75% quartiles; 8 and 130
months, respectively). Overall survival (OS) was determined as the
time from diagnosis to disease specific death (median, 38 months;
25% and 75% quantiles; 9 and 140 months, respectively).
 | Table I.Clinicopathological characteristics
of NSCLC patients treated with surgery only. |
Table I.
Clinicopathological characteristics
of NSCLC patients treated with surgery only.
| Clinicopathological
characteristics | Total n (n=78) | Percent (%) |
|---|
| Age, years |
|
≤64 | 44 | 56.4 |
|
>64 | 34 | 43.6 |
| Gender |
|
|
|
Female | 19 | 24.4 |
|
Male | 59 | 75.6 |
| Histology |
|
ADC | 39 | 50.0 |
|
SCC | 28 | 35.9 |
|
LCC | 11 | 14.1 |
| Grade |
| G1 | 14 | 17.9 |
| G2 | 27 | 34.6 |
| G3 | 34 | 43.6 |
|
ANP | 3 | 3.8 |
| TNM stage |
| I | 23 | 29.5 |
| II | 6 | 7.7 |
|
III | 34 | 43.6 |
| IV | 5 | 6.4 |
|
Missing | 10 | 12.8 |
| T |
| 1 | 28 | 35.9 |
| 2 | 25 | 32.1 |
| 3 | 9 | 11.5 |
| 4 | 6 | 7.7 |
|
Missing | 10 | 12.8 |
| N |
| 0 | 43 | 55.1 |
| 1 | 10 | 12.8 |
| 2 | 14 | 17.9 |
| 3 | 1 | 1.3 |
|
Missing | 10 | 12.8 |
| M |
| 0 | 63 | 80.8 |
| 1 | 4 | 5.1 |
| 2 | 1 | 1.3 |
|
Missing | 10 | 12.8 |
 | Table II.Clinicopathological characteristics
of NSCLC patients treated with adjuvant chemotherapy. |
Table II.
Clinicopathological characteristics
of NSCLC patients treated with adjuvant chemotherapy.
| Clinicopathological
characteristics | Total n (n=95) | Percent (%) |
|---|
| Age, years |
|
|
|
≤64 | 48 | 50.5 |
|
>64 | 47 | 49.5 |
| Gender |
|
|
|
Female | 35 | 36.8 |
|
Male | 60 | 63.2 |
| Histology |
|
|
|
ADC | 26 | 27.4 |
|
SCC | 47 | 79.5 |
|
LCC | 22 | 23.1 |
| Grade |
|
|
| G1 | 9 | 9.5 |
| G2 | 20 | 21.1 |
| G3 | 62 | 65.3 |
|
ANP | 4 | 4.2 |
| TNM stage |
|
|
| I | 28 | 29.5 |
| II | 17 | 17.9 |
|
III | 47 | 49.5 |
| IV | 3 | 3.2 |
| T |
|
|
| 1 | 11 | 11.6 |
| 2 | 63 | 66.3 |
| 3 | 13 | 13.7 |
| 4 | 8 | 8.4 |
| N |
|
|
| 0 | 44 | 46.3 |
| 1 | 20 | 21.1 |
| 2 | 30 | 31.6 |
| 3 | 1 | 1.1 |
| M |
|
|
| 0 | 93 | 97.9 |
| 1 | 2 | 2.1 |
Immunohistochemistry
Formalin-fixed and paraffin-embedded (FFPE)
specimens were cut in 4 µm sections, mounted on silane-coated
slides, deparaffinized in xylene and rehydrated by washing in
serial dilutions of ethanol. Antigen retrieval was performed in an
automatic multifunctional microwave tissue processor (T/T MEGA) at
95°C for 5 min, using citrate buffer at pH 6.0. Endogenous
peroxidase activity was blocked with 0.3% hydrogen peroxide for 15
min. Nonspecific binding was blocked with 5% horse serum in
phosphate buffered saline (PBS). Sections were incubated in primary
antibody against SphK1 (38) and S1P
lyase (cat. no. HPA023086; Sigma-Aldrich). Specific binding was
visualized using the Envision dual link system (Dako).
IHC stained slides were evaluated by three
independent pathologists (J.S., T.T., M.G.) and scored according to
the histoscore method (H score method). The histoscore grades
staining intensity as negative (0), weak (1), moderate (2) and strong (3) and then multiplies the percentage of
tumor cells within each category. The histoscore range is from 0
(minimum) to 300 (maximum). Agreement between observers was
calculated using an interclass correlation coefficient.
Statistical analysis
Correlations between the levels of examined proteins
and survival parameters of the patients and their
clinicopathological features were analyzed using statistical
software IBM SPSS statistics v.22 (IBM Corp., Armonk, NY, USA). The
Kruskal-Wallis test with Bonferroni's correction was applied for
nonparametric comparisons of independent groups. For survival
analysis, Kaplan-Meier curves were calculated, and tests of
statistical significance were based on log-rank statistics.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Immunohistochemical distribution of
SphK1 and S1P lyase in normal adjacent and NSCLC tissue
Immunohistochemical staining of normal adjacent lung
tissue and the distribution of SphK1 and S1P lyase were examined in
several formalin-fixed paraffin-embedded tissue sections from non
tumoral regions of the lung. Normal pseudo-stratified columnar
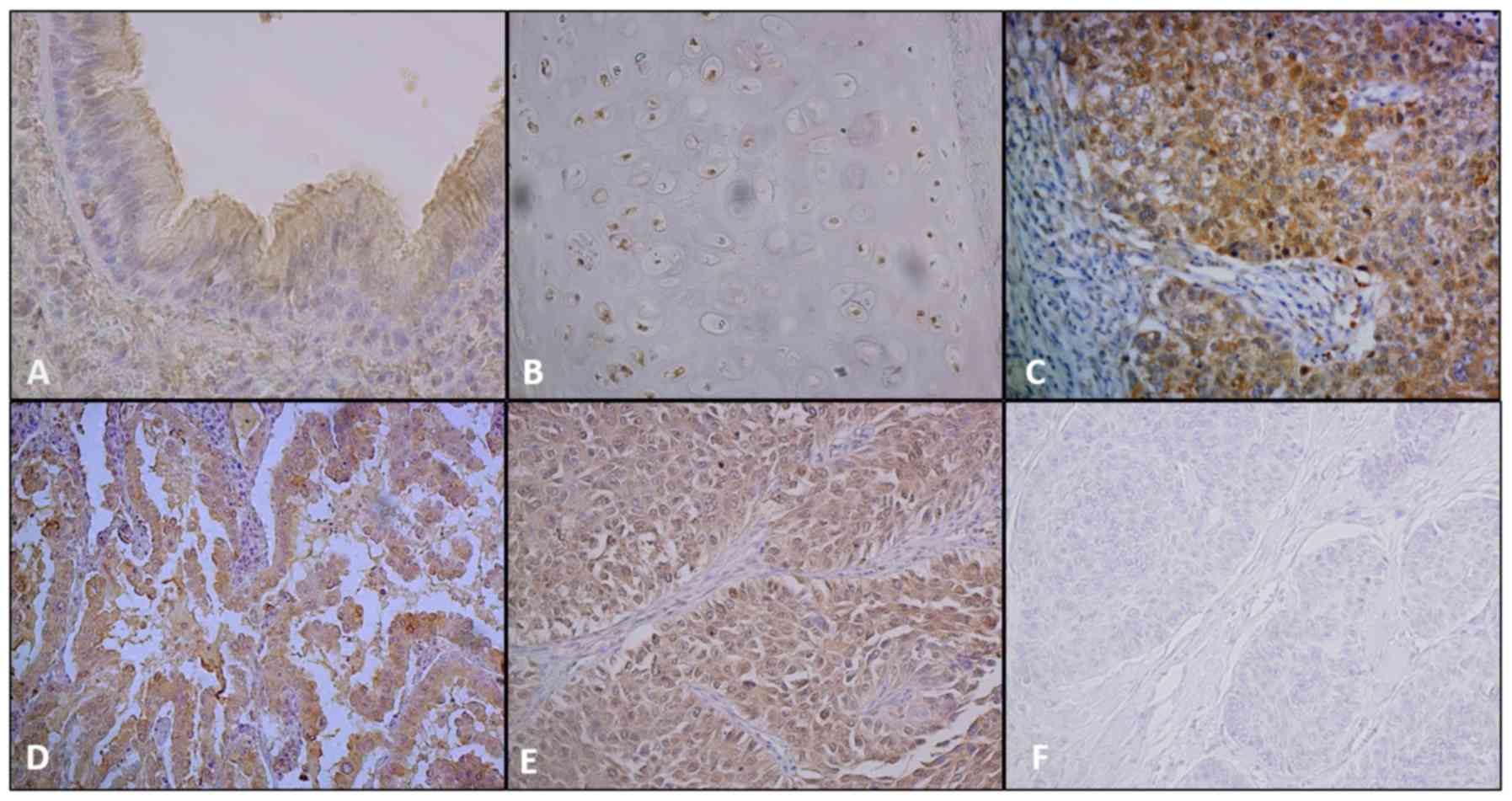
epithelial cells in bronchiole (Fig.
1A) stained very intensely on the apical surface at the point
of ciliary attachment for SphK1. The staining of SphK1 in
bronchiolar cartilage, is shown in Fig.
1B. Interestingly, SphK1 levels appear to be related to
chondrocyte maturation. Although immature chondrocytes showed
moderate staining for SphK1, mature chondrocytes were devoid of
staining. Alveolar parenchyma and type II pneumocytes exhibited a
weak staining for SphK1 (not shown). Moderate to strong staining of
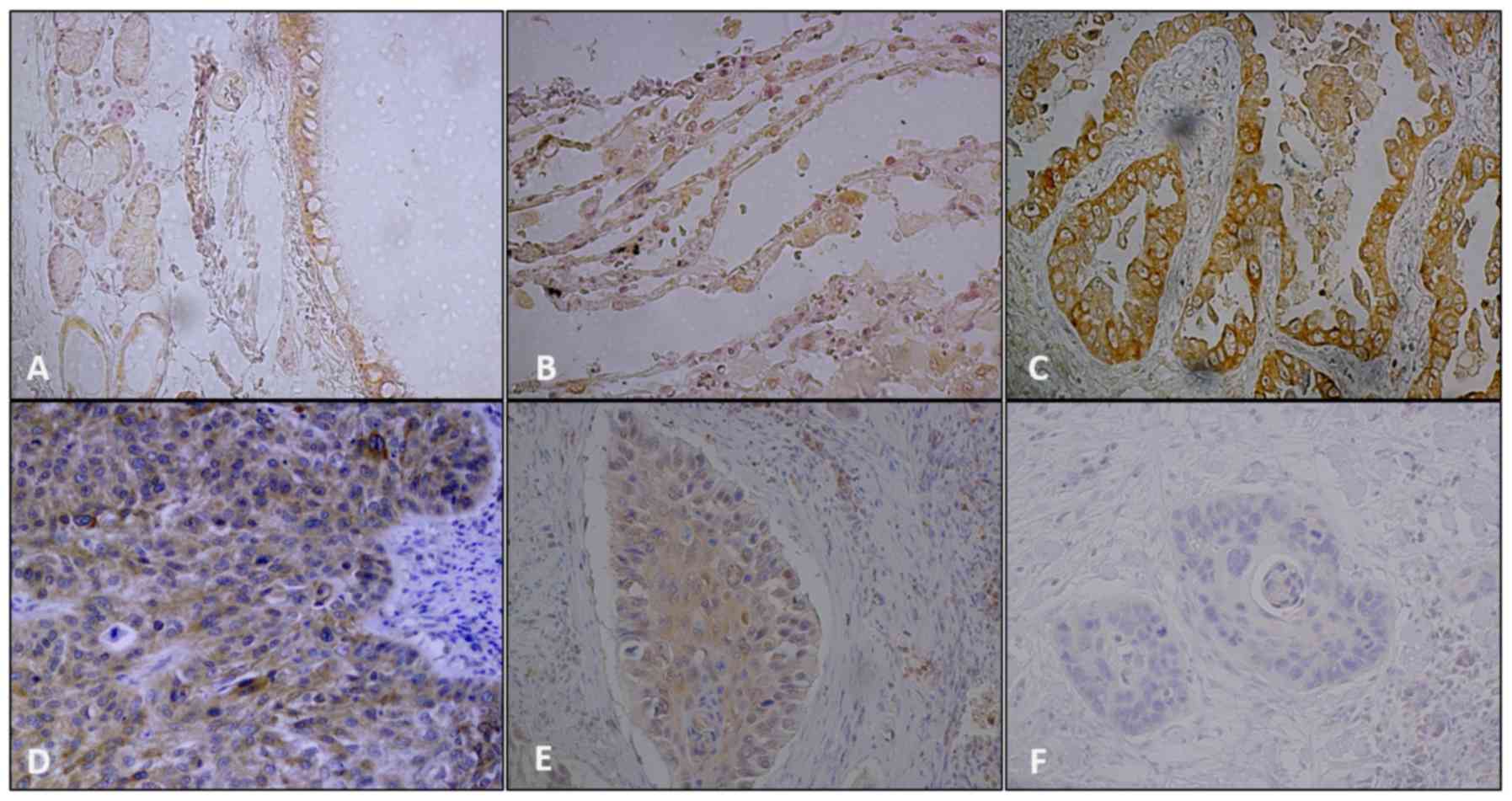
S1P lyase was shown in normal pseudo-stratified columnar epithelial
cells and type II pneumocytes (Fig. 2A
and B).
NSCLC samples exhibited various immunostaining
patterns for SphK1 (Fig. 1C-E) and
S1P lyase (Fig. 2C-E). Staining for
both markers were mainly cytoplasmic and membranous and varied from
weak to strong. Nuclear positivity of SphK1 was also seen in some
cases. Adenocarcinomas showed the strongest expression of both
SphK1 and S1P lyase (P<0.0001 and P=0.001 respectively; Fig. 3A-B). Staining for both markers was
conspicuously absent in the surrounding stroma. IgG control
staining for SphK1 and S1P lyase respectively are shown in Fig. 1F and Fig.
2F. Overexpression of SphK1 was significantly associated with
more advanced disease stage (P=0.008; Fig. 3C), while there was a non-significant
trend of S1P lyase association with advanced stage (P=0.06;
Fig. 3D).
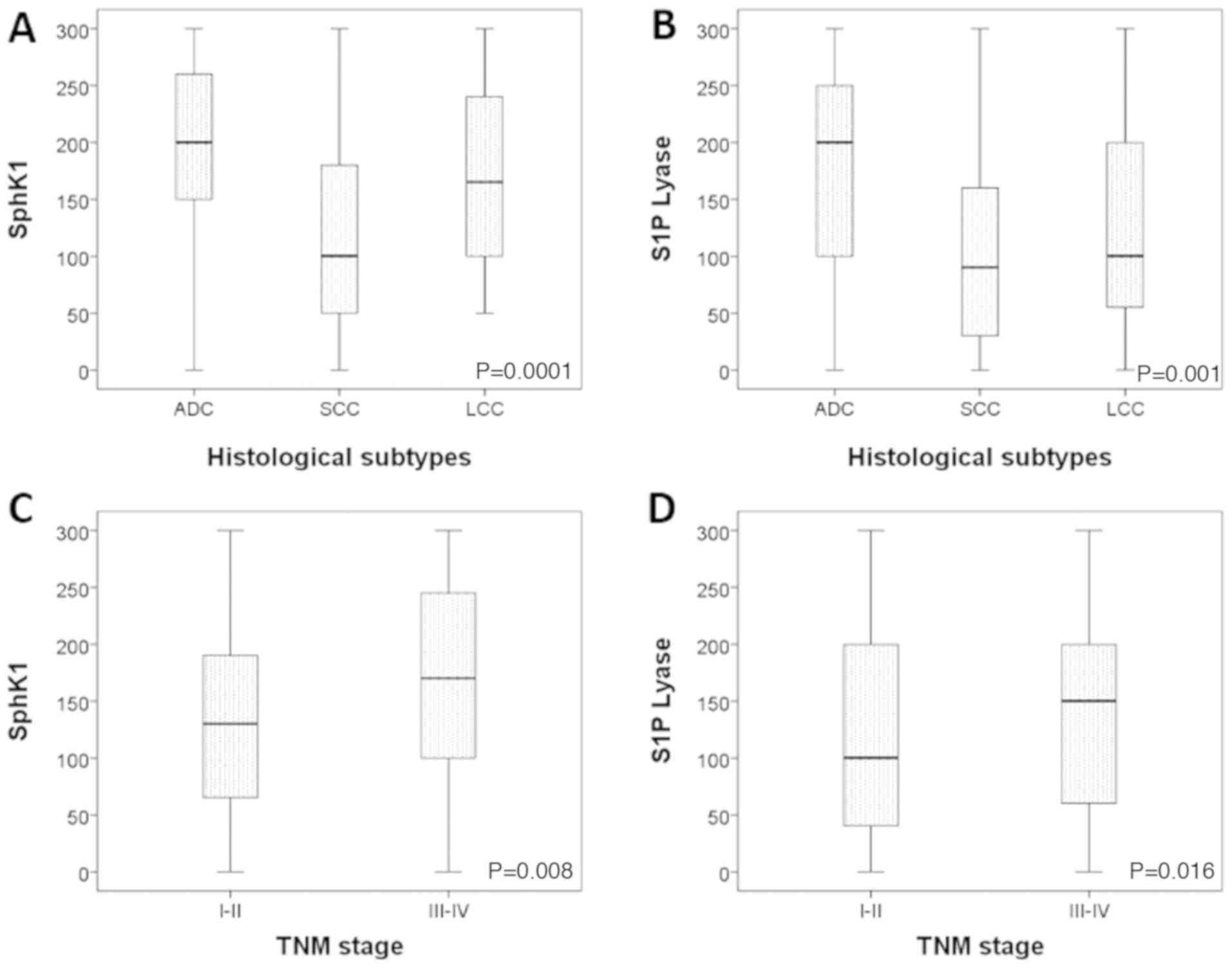 | Figure 3.Distribution of SphK1 and S1P lyase
in different histological subtypes and grades of NSCLC. (A) The
highest expression of SphK1 was observed in ADC, followed by LCC
and SCC (P<0.0001). (B) The highest expression of S1P lyase was
observed in ADC, followed by LCC and SCC (P=0.001). (C) SphK1
expression is higher in stage III–IV patients when compared with
stage I–II ones (P=0.008). (D) S1P lyase expression is higher in
stage III–IV, but not significantly difference (P=0.016). NSCLC,
non-small cell lung cancer; ADC, adenocarcinoma; SCC, squamous cell
carcinoma; LCC, large cell carcinoma; TNM, tumor, node, metastases;
SphK1, sphingosine kinase-1; S1P, sphingosine 1-phosphate. |
The predictive value of SphK1 and S1P
lyase in NSCLC patients
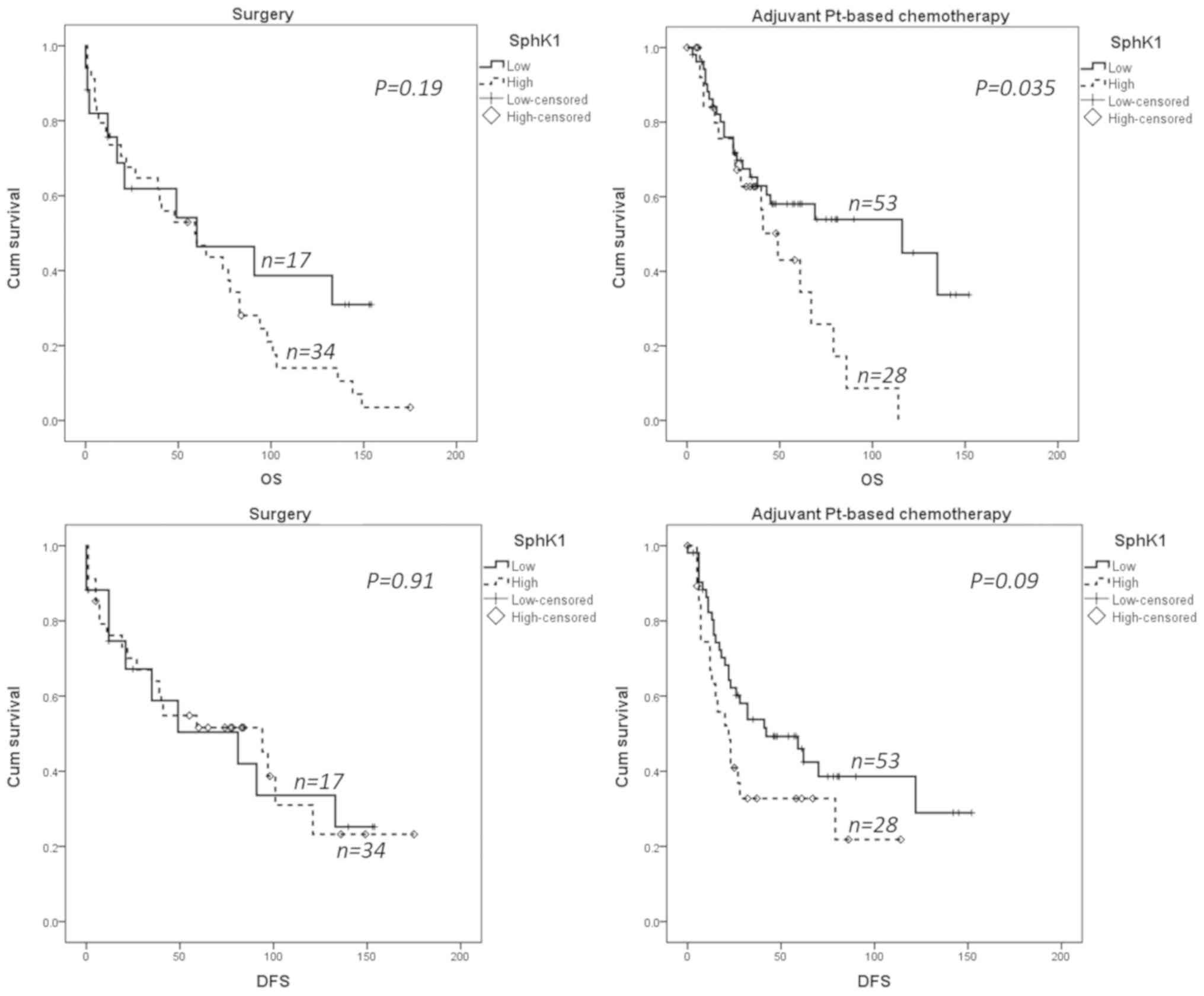
The Kaplan-Meier survival analysis showed that
overexpression of SphK1 (dichotomized for median) was significantly
associated with poor overall survival in patients treated with
platinum based chemotherapy (P=0.035). However, the overall
survival difference was not significant in patients treated with
surgery only. With regards to disease free survival, there was a
trend to high SphK1 expression association with poor outcome
(P=0.09), which was not seen in patients treated by surgery alone
(Fig. 4).
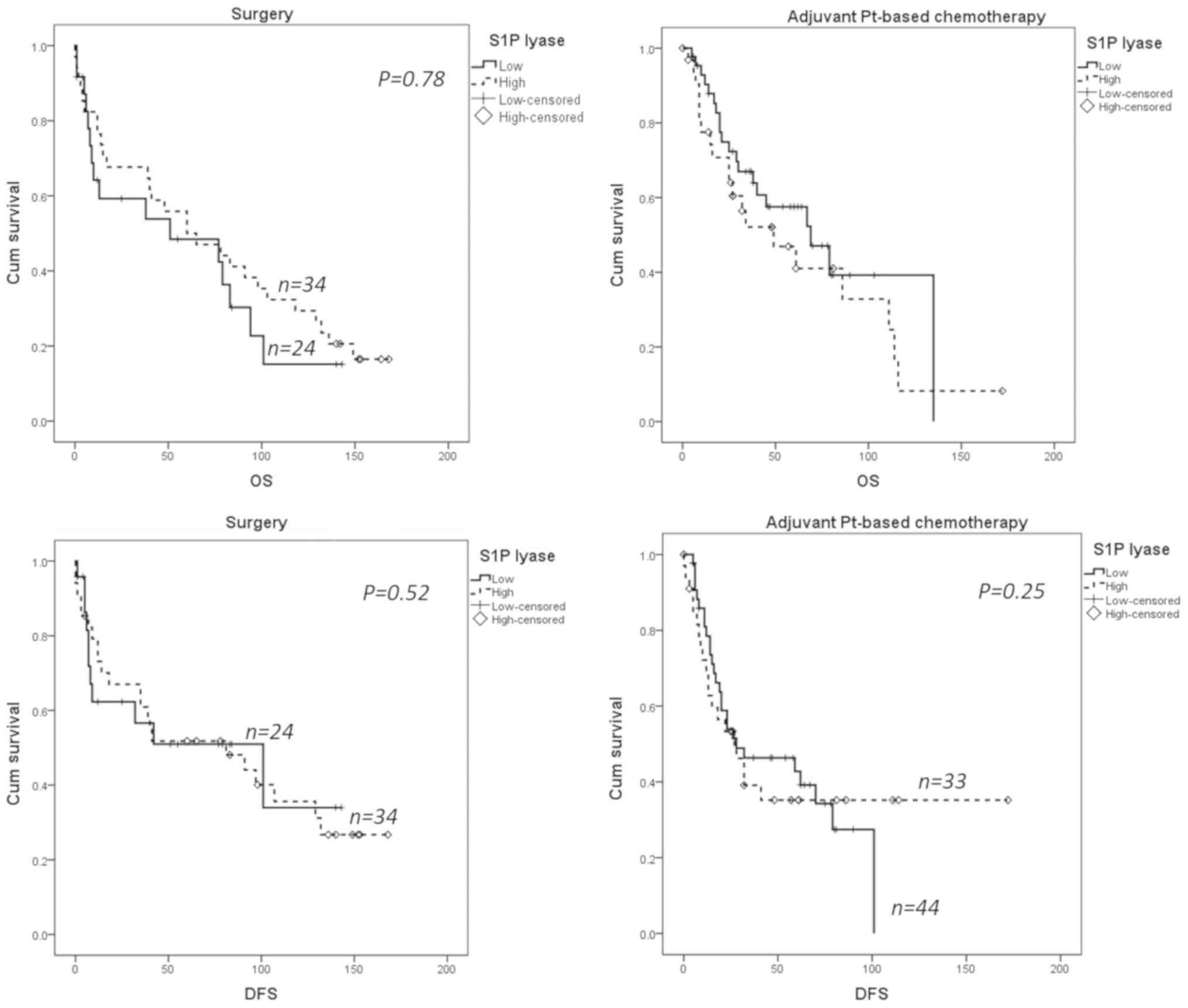
We found no statistically significant association
between S1P lyase expression and survival outcomes (Fig. 5) and no inverse correlation between
SphK1 and S1P lyase as reported for prostate cancer (27).
Discussion
In the present study, we analyzed the prognostic and
predictive value of SphK1 and S1P lyase, two key enzymes that
control S1P content in cells, in patients with NSCLC treated with
adjuvant chemotherapy based on carboplatin and navelbine. NSCLC
samples exhibited various immunostaining patterns for both SphK1
and S1P lyase.
We found that SphK1 staining was mainly cytosolic
and membranous with the highest expression seen in adenocarcinomas.
Our observations are in line with the findings of Johnson et
al (39), who originally
evaluated the expression of SphK1 in normal and cancerous lung
tissue. Nuclear positivity of SphK1 has rarely been observed,
however the biological significance of this expression is not
known.
So far, only one study appears to have examined the
prognostic and predictive role of SphK1 in NSCLC. In 2011, Song
et al (30) showed that
immunohistochemical expression of SphK1 was markedly increased in
NSCLC and, in relation to clinical stage and TNM classification. In
agreement with this study, we found a statistically significant
correlation between SphK1 expression and clinical stage.
Significantly, overall survival of patients with high SphK1
expression was found to be shorter than in patients with low SphK1
expression (30). However, these
authors did not stratify patients according to adjuvant
chemotherapy. To the best of our knowledge, no clinical studies
have ever examined the role of SphK1 in platinum-based chemotherapy
resistance in patients. We show for the first time that high SphK1
expression is associated with shorter overall survival and
increased risk of disease relapse in patients with NSCLC treated
with adjuvant chemotherapy.
Several studies have examined the relationship
between SphK1 and platinum sensitivity in vitro. In colon
cancer cells, it has been shown that downregulation of SphK1
enhances cisplatin sensitivity (40). In gastroesophageal cancer cells,
cisplatin resistance is correlated with increased SphK1 mRNA
expression (41). With regards to
lung cancer, cisplatin-resistant lung cancer cell line H460/DDP has
been characterized by overexpression of SphK1 compared to the
parental cell line (42).
Collectively, these data suggest an important role of SphK1 in
mediating cisplatin sensitivity, at least in vitro.
In conclusion, the present study is the first to
examine the immunohistochemical expression of both S1P lyase and
SphK1 in NSCLC in relationship to survival in patients treated with
adjuvant chemotherapy. Our data validate the prognostic role of
SphK1 expression in patients with NSCLC, including those treated
with adjuvant platinum-based chemotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Ministry of Education Czech Republic (grant nos. MSMT 61875921, NPS
I LO1304 and RVO:61989592), the Ministry of Health Czech Republic
(grant nos. IGA MZ CR 10259-3, 9959-3, AZV NV19-03-00069 and
RVO:FNOL00098892), by internal grants from Palacký University
(grant nos. 91110281 and LF_2019_004), the National Health and
Medical Research Council of Australia, the Programme VLTAVA (French
Embassy, Prague), the CNRS (France) and the Ligue Nationale contre
le Cancer (France).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
JS, MG, LC and TT conducted the histopathological
diagnoses and the re-classification of tumor samples, and evaluated
and scored cases for SphK1 and S1P lyase immunohistochemical
expression. GM and GK performed the major statistical analyses of
the data. MJ optimized the initial IHC staining and performed the
preliminary analyses. VK, IG and PS diagnosed the patients with
lung cancer, collected the clinical data and obtained the follow-up
information. JK performed the surgery and provided the samples for
the study. SP and MM provided and validated the molecular biology
tools for the study; SP made the antibodies and MM assessed the
antibodies. JS and MG contributed to the design of the study, wrote
the manuscript and performed the statistical analysis. OC
contributed to the design of the study, analyzed the data and wrote
the manuscript.
Ethics approval and consent to
participate
Informed, written consent for the use of tissues and
clinical data was obtained from all participants and the studies
were carried out according to the latest Declaration of Helsinki.
The procedures were approved by the local Ethics Committee on
06/29/2011.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arriagada R, Bergman B, Dunant A, Le
Chevalier T, Pignon JP and Vansteenkiste J; International Adjuvant
Lung Cancer Trial Collaborative Group, : Cisplatin-based adjuvant
chemotherapy in patients with completely resected non-small-cell
lung cancer. N Engl J Med. 350:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al LACE Collaborative Group, : Lung adjuvant
cisplatin evaluation: A pooled analysis by the LACE Collaborative
Group. J Clin Oncol. 26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douillard JY, Rosell R, De Lena M,
Carpagnano F, Ramlau R, Gonzáles-Larriba JL, Grodzki T, Pereira JR,
Le Groumellec A, Lorusso V, et al: Adjuvant vinorelbine plus
cisplatin versus observation in patients with completely resected
stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine
International Trialist Association [ANITA]): A randomised
controlled trial. Lancet Oncol. 7:719–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zatloukal P, Petruzelka L, Zemanova M,
Havel L, Janku F, Judas L, Kubik A, Krepela E, Fiala P and Pecen L:
Concurrent versus sequential chemoradiotherapy with cisplatin and
vinorelbine in locally advanced non-small cell lung cancer: A
randomized study. Lung Cancer. 46:87–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scheper RJ, Broxterman HJ, Scheffer GL,
Kaaijk P, Dalton WS, van Heijningen TH, van Kalken CK, Slovak ML,
de Vries EG, van der Valk P, et al: Overexpression of a M(r)
110,000 vesicular protein in non-P-glycoprotein-mediated multidrug
resistance. Cancer Res. 53:1475–1479. 1993.PubMed/NCBI
|
|
7
|
Marchetti S, de Vries NA, Buckle T, Bolijn
MJ, van Eijndhoven MA, Beijnen JH, Mazzanti R, van Tellingen O and
Schellens JH: Effect of the ATP-binding cassette drug transporters
ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva)
disposition in in vitro and in vivo pharmacokinetic studies
employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type
mice. Mol Cancer Ther. 7:2280–2287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ozvegy-Laczka C, Cserepes J, Elkind NB and
Sarkadi B: Tyrosine kinase inhibitor resistance in cancer: Role of
ABC multidrug transporters. Drug Resist Updat. 8:15–26. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berger W, Setinek U, Hollaus P, Zidek T,
Steiner E, Elbling L, Cantonati H, Attems J, Gsur A and Micksche M:
Multidrug resistance markers P-glycoprotein, multidrug resistance
protein 1, and lung resistance protein in non-small cell lung
cancer: Prognostic implications. J Cancer Res Clin Oncol.
131:355–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikuta K, Takemura K, Sasaki K, Kihara M,
Nishimura M, Ueda N, Naito S, Lee E, Shimizu E and Yamauchi A:
Expression of multidrug resistance proteins and accumulation of
cisplatin in human non-small cell lung cancer cells. Biol Pharm
Bull. 28:707–712. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berger W, Elbling L, Hauptmann E and
Micksche M: Expression of the multidrug resistance-associated
protein (MRP) and chemoresistance of human non-small-cell lung
cancer cells. Int J Cancer. 73:84–93. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yano K: Lipid metabolic pathways as lung
cancer therapeutic targets: A computational study. Int J Mol Med.
29:519–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Futerman AH and Hannun YA: The complex
life of simple sphingolipids. EMBO Rep. 5:777–782. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cuvillier O and Levade T: Enzymes of
sphingosine metabolism as potential pharmacological targets for
therapeutic intervention in cancer. Pharmacol Res. 47:439–445.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morad SA and Cabot MC:
Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer.
13:51–65. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cuvillier O: Sphingosine in apoptosis
signaling. Biochim Biophys Acta. 1585:153–162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pitson SM: Regulation of sphingosine
kinase and sphingolipid signaling. Trends Biochem Sci. 36:97–107.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maceyka M, Harikumar KB, Milstien S and
Spiegel S: Sphingosine-1-phosphate signaling and its role in
disease. Trends Cell Biol. 22:50–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cuvillier O, Ader I, Bouquerel P, Brizuela
L, Gstalder C and Malavaud B: Hypoxia, therapeutic resistance, and
sphingosine 1-phosphate. Adv Cancer Res. 117:117–141. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mendelson K, Evans T and Hla T:
Sphingosine 1-phosphate signalling. Development. 141:5–9. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cuvillier O: Downregulating sphingosine
kinase-1 for cancer therapy. Expert Opin Ther Targets.
12:1009–1020. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cuvillier O, Pirianov G, Kleuser B, Vanek
PG, Coso OA, Gutkind S and Spiegel S: Suppression of
ceramide-mediated programmed cell death by sphingosine-1-phosphate.
Nature. 381:800–803. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonhoure E, Pchejetski D, Aouali N,
Morjani H, Levade T, Kohama T and Cuvillier O: Overcoming
MDR-associated chemoresistance in HL-60 acute myeloid leukemia
cells by targeting sphingosine kinase-1. Leukemia. 20:95–102. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pchejetski D, Golzio M, Bonhoure E, Calvet
C, Doumerc N, Garcia V, Mazerolles C, Rischmann P, Teissié J,
Malavaud B, et al: Sphingosine kinase-1 as a chemotherapy sensor in
prostate adenocarcinoma cell and mouse models. Cancer Res.
65:11667–11675. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guillermet-Guibert J, Davenne L,
Pchejetski D, Saint-Laurent N, Brizuela L, Guilbeau-Frugier C,
Delisle MB, Cuvillier O, Susini C and Bousquet C: Targeting the
sphingolipid metabolism to defeat pancreatic cancer cell resistance
to the chemotherapeutic gemcitabine drug. Mol Cancer Ther.
8:809–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pchejetski D, Bohler T, Brizuela L, Sauer
L, Doumerc N, Golzio M, Salunkhe V, Teissié J, Malavaud B, Waxman
J, et al: FTY720 (fingolimod) sensitizes prostate cancer cells to
radiotherapy by inhibition of sphingosine kinase-1. Cancer Res.
70:8651–8661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brizuela L, Ader I, Mazerolles C, Bocquet
M, Malavaud B and Cuvillier O: First evidence of sphingosine
1-phosphate lyase protein expression and activity downregulation in
human neoplasm: Implication for resistance to therapeutics in
prostate cancer. Mol Cancer Ther. 11:1841–1851. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gstalder C, Ader I and Cuvillier O: FTY720
(Fingolimod) Inhibits HIF1 and HIF2 Signaling, Promotes Vascular
Remodeling, and Chemosensitizes in Renal Cell Carcinoma Animal
Model. Mol Cancer Ther. 15:2465–2474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito H, Yoshida K, Murakami M, Hagiwara K,
Sasaki N, Kobayashi M, Takagi A, Kojima T, Sobue S and Suzuki M:
Heterogeneous sphingosine-1-phosphate lyase gene expression and its
regulatory mechanism in human lung cancer cell lines. Biochim
Biophys Acta. 1811:119–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song L, Xiong H, Li J, Liao W, Wang L, Wu
J and Li M: Sphingosine kinase-1 enhances resistance to apoptosis
through activation of PI3K/Akt/NF-κB pathway in human non-small
cell lung cancer. Clin Cancer Res. 17:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Guan HY, Gong LY, Song LB, Zhang N,
Wu J, Yuan J, Zheng YJ, Huang ZS and Li M: Clinical significance of
sphingosine kinase-1 expression in human astrocytomas progression
and overall patient survival. Clin Cancer Res. 14:6996–7003. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Wang Y, Wan Z, Liu S, Cao Y and
Zeng Z: Sphingosine kinase 1 and cancer: A systematic review and
meta-analysis. PLoS One. 9:e903622014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruckhäberle E, Karn T, Denkert C, Loibl S,
Ataseven B, Reimer T, Becker S, Holtrich U, Rody A, Darb-Esfahani
S, et al: Predictive value of sphingosine kinase 1 expression in
neoadjuvant treatment of breast cancer. J Cancer Res Clin Oncol.
139:1681–1689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sutphen R, Xu Y, Wilbanks GD, Fiorica J,
Grendys EC Jr, LaPolla JP, Arango H, Hoffman MS, Martino M, Wakeley
K, et al: Lysophospholipids are potential biomarkers of ovarian
cancer. Cancer Epidemiol Biomarkers Prev. 13:1185–1191.
2004.PubMed/NCBI
|
|
35
|
Malavaud B, Pchejetski D, Mazerolles C, de
Paiva GR, Calvet C, Doumerc N, Pitson S, Rischmann P and Cuvillier
O: Sphingosine kinase-1 activity and expression in human prostate
cancer resection specimens. Eur J Cancer. 46:3417–3424. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Serra M and Saba JD: Sphingosine
1-phosphate lyase, a key regulator of sphingosine 1-phosphate
signaling and function. Adv Enzyme Regul. 50:349–362. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oskouian B, Sooriyakumaran P, Borowsky AD,
Crans A, Dillard-Telm L, Tam YY, Bandhuvula P and Saba JD:
Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and
p38-dependent pathways and is down-regulated in colon cancer. Proc
Natl Acad Sci USA. 103:17384–17389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Min J, Van Veldhoven PP, Zhang L, Hanigan
MH, Alexander H and Alexander S: Sphingosine-1-phosphate lyase
regulates sensitivity of human cells to select chemotherapy drugs
in a p38-dependent manner. Mol Cancer Res. 3:287–296. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Johnson KR, Johnson KY, Crellin HG,
Ogretmen B, Boylan AM, Harley RA and Obeid LM: Immunohistochemical
distribution of sphingosine kinase 1 in normal and tumor lung
tissue. J Histochem Cytochem. 53:1159–1166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qin L, Liu S, Qin M, Wu W, Qin N, Fu Z, Xu
C, Huang J and Lai M: Down-regulation of sphingosine kinase 1
(SphK1) enhances the chemosensitivity to cisplatin in human colon
cancer RKO cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 33:623–629.
2017.(In Chinese). PubMed/NCBI
|
|
41
|
Matula K, Collie-Duguid E, Murray G,
Parikh K, Grabsch H, Tan P, Lalwani S, Garau R, Ong Y, Bain G, et
al: Regulation of cellular sphingosine-1-phosphate by sphingosine
kinase 1 and sphingosine-1-phopshate lyase determines chemotherapy
resistance in gastroesophageal cancer. BMC Cancer. 15:7622015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao J, Tian ML and Song LP: The role of
SPHK-1 in non-small cell lung cancer drug-resistant cell line H460.
J Xi'an Jiaotong Univ. 38:172–175. 2017.(In Chinese).
|