Introduction
Sustained virological response (SVR) is defined as
undetectable serum hepatitis C virus (HCV) RNA 24 weeks after
completion of antiviral therapy for chronic HCV infection. In the
1990's to the 2000's, Interferon (IFN) was used as the main
antiviral therapy for HCV infection. About 50% of patients with HCV
genotype type 1 have a SVR using peginterferon alfa-2a plus
ribavirin (1). Recently, the
development of an IFN-free regimen using direct acting antivirals
(DAA) has been a revolution in the treatment of patients with
chronic hepatitis C. More than 95% of patients achieved a SVR using
DAA (2,3).
HCV infection is a significant risk factor for
progressive hepatic fibrosis, subsequent liver cirrhosis and the
development of hepatocellular carcinoma (HCC). Among HCV-infected
patients, achieving SVR was associated with a reduced risk of the
development of HCC (4). However, the
risk of developing HCC does not completely disappear even after
SVR. It was reported that the 5- and 10-year HCC incidence rates
after achieving SVR by IFN-based therapy were 0.8–5.8% and
2.8–11.1%, respectively (5–10). There were differences in gene
mutation between patients who developed HCC after achieving SVR and
patients who developed HCC with chronic HCV infection (10,11).
Many HCV-infected patients will be able to obtain SVR, so
identification of biological characteristics in HCC after achieving
SVR is very important. However, the molecular biological feature of
HCC after achieving SVR was not yet clear.
In the present study, we aimed at characterizing
molecular pathological feature of surgically resected HCC after
achieving SVR and determining its relationship with prognosis and
pathological features, as well as programmed death-ligand 1 (PD-L1)
expression, markers of progenitor cells [cytokeratin 19 (CK19),
epithelial cell adhesion molecule (EpCAM)], and portal vein
invasion associated marker [regulator of G-protein signaling 5
(RGS5)].
Patients and methods
Patients
We studied the clinical and pathological findings of
372 patients associated HCV infection who were underwent
hepatectomy for initial HCC at Kurume University Hospital between
January 2003 and April 2017. Of these, 48 patients who developed
initial HCC after achieving SVR. In 48 patients, there are 35
patients with HCC after SVR treated by IFN (IFN group) and 13
patients with HCC after SVR treated by DAA (DAA group). They were
underwent hepatectomy for initial HCC at the Kurume University
Hospital between January 2003 and April 2017.
Histopathological examination
Each surgically resected liver tissue was fixed with
10% formalin, embedded in paraffin, cut into 5-μm sections, and
then used for histological analyses. The specimens were stained
with hematoxylin and eosin (H&E), reticulin, and Azan. We
evaluated all specimens, histopathologically (e.g., HCC tissue:
Size, tumor differentiation, vascular invasion, nuclear grade;
non-HCC liver tissue: Fibrosis, inflammation, steatosis). The
histological features of HCC were evaluated according to the World
Health Organization classification (12). We classified the degree of nuclear
grade into 3 levels according to nuclear atypia and mitosis
(Table I). The degrees of liver
fibrosis and hepatic inflammation were scored according to the New
Inuyama Classification from F0 to F4, and A0 to A3 (13). The liver fibrosis stage was
classified as follows: F0 (no fibrosis), F1 (fibrous portal
expansion), F2 (bridging fibrosis), F3 (bridging fibrosis with
lobular distortion) and F4 (liver cirrhosis), and the inflammatory
grade was classified as A0 (no necro-inflammatory reaction), A1
(mild necro-inflammatory reaction), A2 (moderate necro-inflammatory
reaction), or A3 (severe necro-inflammatory reaction) (13). Steatosis was defined as fat deposits
of 5% or more hepatocytes. Histopathological diagnosis and
classification were performed by three pathologists (R.K, J.A, and
O.N).
 | Table I.Assessment of nuclear grade. |
Table I.
Assessment of nuclear grade.
| A, Nuclear grade |
|---|
|
|---|
| Grade/Score | Result |
|---|
| Grade 3 | Atypia score +
Mitosis score =5 or 6 |
| Grade 2 | Atypia score +
Mitosis score =4 |
| Grade 1 | Atypia score +
Mitosis score =2 or 3 |
|
| B, Nuclear atypia
score |
|
|
Grade/Score | Result |
|
| Score 3 | Multinucleated and
pleomorphic cells |
| Score 2 | Other than score 1
and 3 |
| Score 1 | Homogenous small
round nuclear cells |
|
| C,
Mitosis |
|
|
Grade/Score | Result |
|
| Score 3 | >10 mitotic
cells/10 HPFs |
| Score 2 | 5–10 mitotic cells/10
HPFs |
| Score 1 | <5 mitotic
cells/10 HPFs |
Immunohistochemical analysis
Immunostaining was performed on paraffin-embedded
materials. The following primary antibodies were used: Anti-PD-L1
(clone 28-8; Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA), anti-CK19 (clone RCK108; Dako; Agilent Technologies, Inc.),
anti-EpCAM (clone VU1D9; Cell Signaling Technology, Inc., Danvers,
MA, USA), and anti-RGS5 (clone 1C1; Novus Biologicals, LLC,
Littleton, CO, USA). Immunohistochemical examination of PD-L1 was
performed using a fully automated DAKO system (Dako; Agilent
Technologies, Inc.), according to the manufacturer's instructions.
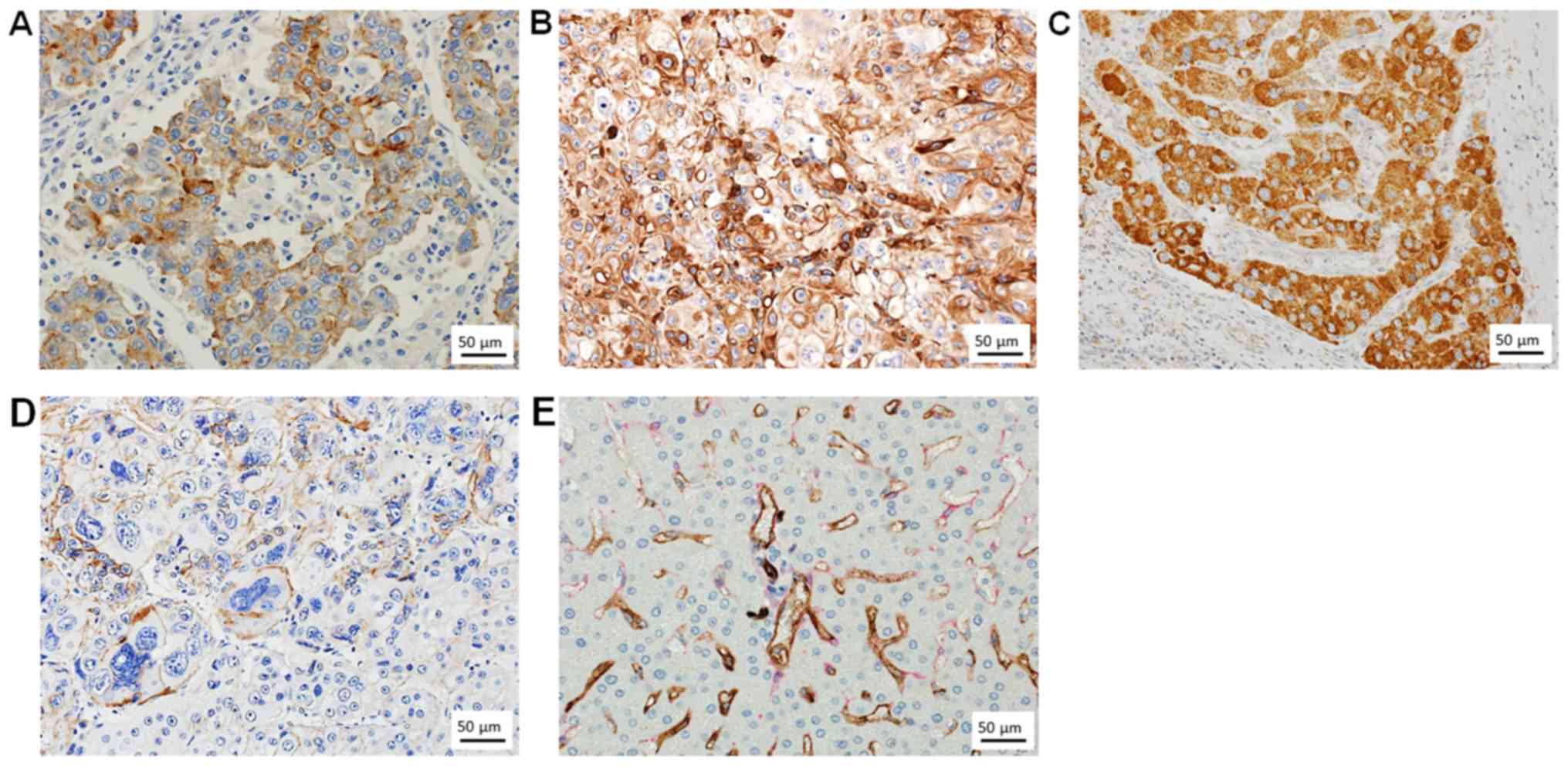
PD-L1 expression was observed in both neoplastic HCC cells and
intratumoral inflammatory cells. For neoplastic HCC cells, the
percentage of cells displaying unequivocal membranous staining was
recorded. Immunohistochemical examination of CK19 and EpCAM were
processed on an automated immunostainer (BenchMark ULTRA; Ventana
Automated Systems, Inc., Tucson, AZ, USA), according to the
manufacturer's instructions. Immunohistochemical examination of
RGS5 was performed according to our previous report (14). The positive expression in tumor cells
was shown in Fig. 1. The expression
of tumor cells were scored as follows: No expression, score 0; 1 to
4% of positive cells, score 1+; more than 5% of positive cells,
score 2+. In addition, we performed double immunostaining for CD34
and α-smooth muscle actin (αSMA). We used monoclonal antibodies
against CD34 (mouse, clone QBEnd/10, 1:100; Leica Biosystems,
Newcastle, United Kingdom) and αSMA (mouse, clone 1A4, 1:10; Dako;
Agilent Technologies, Inc.). The primary antibody for CD34 labeled
horseradish peroxidase was visualized using DAB resulting in a
brown/black target signal. The second antibody for αSMA labeled
alkaline phosphatase was visualized using Fast Red/Naphthol
resulting in a bright red target signal. The CD34 and αSMA
expression in sinusoidal mesenchymal cells was scored as follows:
No positive sinusoidal mesenchymal cells, 0; positive sinusoidal
mesenchymal cells in less than four fields under low power
magnification (×4 objective), score 1+; positive sinusoidal
mesenchymal cells in five or more fields under low power
magnification (×4 objective), score 2+. In chronic liver disease,
perisinusoidal mesenchymal cells express CD34 as transformed
hepatic endothelial cells (HECs) and perisinusoidal mesenchymal
cells express αSMA as transformed hepatic stellate cells (HSCs).
The positive expression of perisinusoidal mesenchymal cells in
non-cancerous liver tissue was shown in Fig. 1.
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD). Comparisons between 2 groups were performed using
Welch's t-test for continuous variables, and the χ2 test
for discrete variables. Kaplan-Meier survival and tumor recurrence
analyses were performed using the log-rank test. Multivariate
analyses were performed using linear and logistic regressions.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using JMP software
package (v.13.0; SAS Institute, Inc., Cary, NC, USA).
Results
Pathological findings
The clinicopathological findings in the IFN group
and the DAA group are shown in Table
II. In all cases, there were solitary HCC without hepatic vein
invasion, hepatic artery invasion, bile duct invasion, peritoneal
dissemination, and lymph node metastasis. Surgical margin was
negative in all cases.
 | Table II.Summary of patients. |
Table II.
Summary of patients.
| A, Clinical
findings |
|---|
|
|---|
| Characteristics | IFN (n=35) | DAA (n=13) | P-value |
|---|
| Age (year) | 68.8±8 | 68.1±7.4 | 0.76 |
| Male/Female | 24/11 | 5/8 | 0.1 |
| Diabetes (prevalence
rate, %) | 11 (31%) | 7 (54%) | 0.32 |
| Observation period
after resection (median) | 1,080 days | 501 days | <0.01 |
| Serum total bililubin
(mg/dl) | 0.8±0.3 | 0.7±0.3 | 0.12 |
| Prothrombin
activity (%) | 90.1±15 | 92.7±12 | 0.54 |
| Serum albumin
(g/dl) | 4.2±0.3 | 4.2±0.2 | 0.86 |
| Serum AFP (ng/ml,
median) | 6.7 | 6.4 | 0.11 |
| Serum PIVKA
(mAU/ml, median) | 118 | 32 | 0.93 |
|
| B, HCC
findings |
|
|
Characteristics | IFN
(n=35) | DAA
(n=13) | P-value |
|
| Tumor size
(mm) | 29±19 | 19±4 | <0.01 |
| Tumor
differentiation (well/moderate/poor) | 2/30/3 | 3/9/1 | 0.23 |
| Nuclear grade | 2±0.9 | 1.9±0.9 | 0.79 |
| Atypia score | 2.3±0.5 | 2.5±0.5 | 0.39 |
| Mitosis score | 1.7±0.8 | 1.7±0.9 | 0.85 |
| Portal vein
invasion (prevalence rate, %) | 21 (60%) | 5 (38%) | 0.21 |
| PD-L1 score
(0/1+/2+) | 29/3/3 | 9/3/1 | 0.55 |
| CK19 score
(0/1+/2+) | 35/0/0 | 12/1/0 | 0.34 |
| EpCAM score
(0/1+/2+) | 29/4/2 | 9/2/2 | 0.33 |
| RGS5 score
(0/1+/2+) | 6/22/7 | 5/7/1 | 0.11 |
|
| C, Non-HCC
findings |
|
|
Characteristics | IFN
(n=35) | DAA
(n=13) | P-value |
|
| Fibrosis (New
Inuyama Classification, F) | 2.3±1.3 | 3.5±1 | <0.01 |
| Inflammation (New
Inuyama Classification, A) | 1.2±0.5 | 1.3±0.5 | 0.65 |
| Steatosis
(prevalence rate, %) | 9 (26%) | 6 (46%) | 0.29 |
| CD34 score
(0/1+/2+) | 0/4/31 | 0/2/11 | 0.74 |
| αSMA score
(0/1+/2+) | 18/9/8 | 3/6/4 | 0.16 |
In the IFN group, there were 24 men and 11 women.
The mean age was 68.8±8 years. Diabetes was present in 11 (31%)
patients of the IFN group, respectively. Histologically, among the
HCC tissues in the IFN group, 2 cases were well differentiated
HCCs, 30 cases were moderately differentiated HCCs, and 3 case was
poorly differentiated HCC. The mean tumor size and nuclear grade
were 29±19 mm and 2±0.9, respectively. Portal vein invasion was
present in 21 cases (60%). In non-HCC tissues of the IFN group, the
degree of hepatic inflammation (A) was 1.2±0.5, and the degree of
liver fibrosis (F) was 2.3±1.3. Between the IFN group and the DAA
group, there were significant differences in the observation period
after resection, the tumor size, and the degree of liver
fibrosis.
In non-cancerous liver tissues of both IFN group and
DAA group, mixed with progressive fibrosis and regressive fibrosis
were observed. The progressive fibrosis defined as fibroseptal
stroma showing wide/broad, loosely aggregated collagen fibers,
which are moderately to markedly cellular containing, variably,
inflammatory cells, and ductular reactions (Fig. 2A-C) (15). The regressive fibrosis defined as
fibroseptal stroma showing thin, densely compacted stroma, which
are largely acellular (Fig. 2D-F)
(15). Two patients (6%) of the IFN
group and 2 patients (18%) of the DAA group were identified as
having non-alcoholic steatohepatitis (NASH)-like pathological
features, such as steatosis, ballooning hepatocytes and
Mallory-Denk bodies.
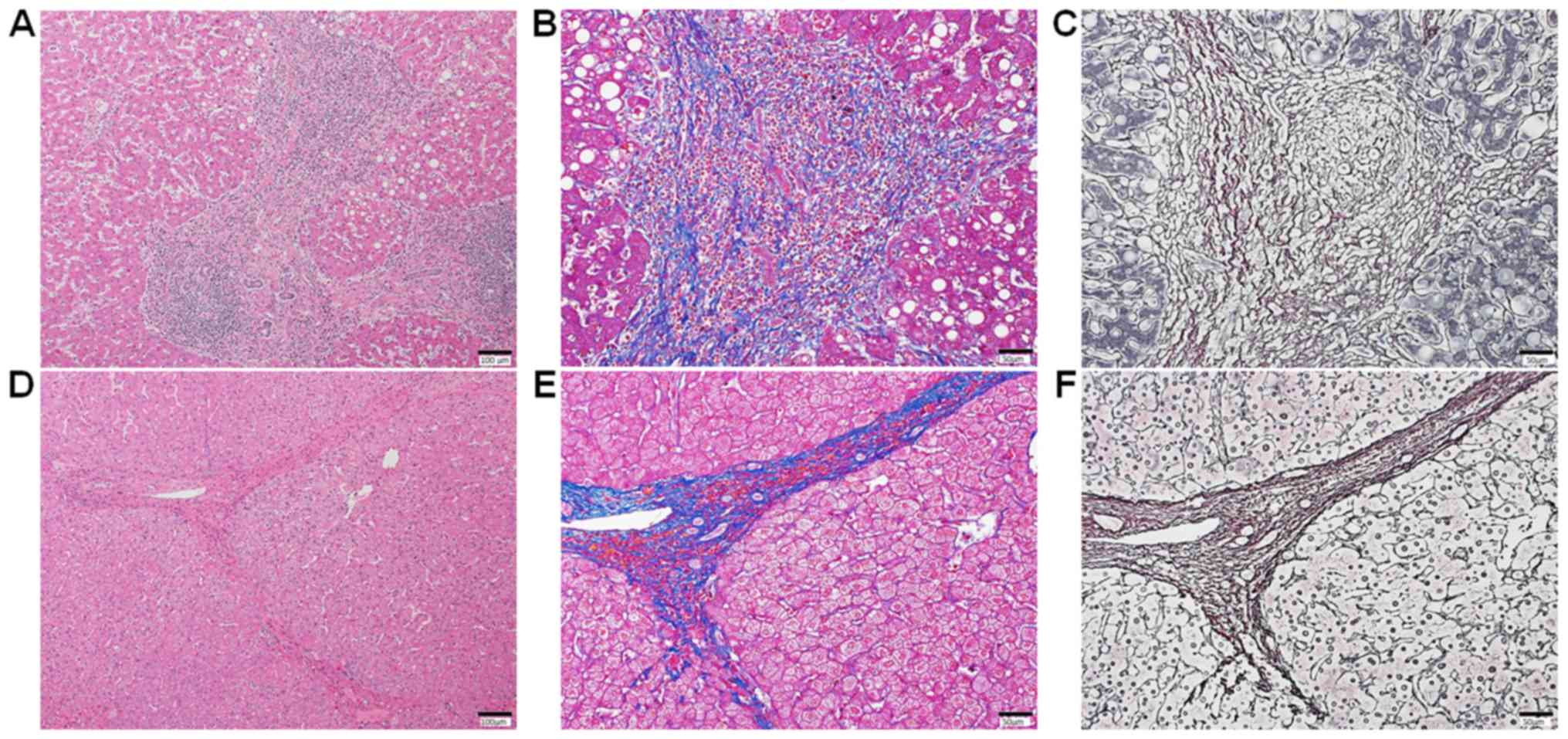 | Figure 2.Liver fibrosis in non-cancerous liver
tissue. (A) Stained with H&E. Mild fibrosis (New Inuyama
Classification, F1) with moderately inflammatory reactions (New
Inuyama Classification, A2) and mild steatosis is seen. There are
no ballooning hepatocytes, or Mallory-denk bodies. Staining with
(B) Azan and (C) reticulin. The fibrosis septa are wide, loosely
aggregated collagen fibers. (D) Stained with H&E. Moderate
fibrosis (New Inuyama Classification, F2) with mild inflammatory
reactions (New Inuyama Classification, A1) is seen. There is no
steatosis, ballooning hepatocytes, or Mallory-denk bodies. Staining
with (E) Azan and (F) reticulin. The fibroseptal stroma showing
features of thin, densely compacted stroma is present. H&E,
hematoxylin and eosin. |
Immunohistochemically, PD-L1 positive reaction was
found inside the tumor not interface of the tumor. In the IFN
group, 3, 3, and 29 cases showed a PD-L1 Score of 2+, 1+, and 0,
respectively. All cases were negative for CK19. Two, 4, and 29
cases showed an EpCAM Score of 2+, 1+, and 0, respectively. In the
DAA, 1, 3, and 9 cases showed a PD-L1 Score of 2+, 1+, and 0,
respectively. In the IFN group, CD34 and αSMA expression in
sinusoidal mesenchymal cells were present in 35 (100%) and 17 (49%)
patients, respectively. The CD34 expressions in sinusoidal
mesenchymal cells were observed in the periportal area with
regionality (Rappaport zone 1 to zone 2). The αSMA expression area
was close to the CD34 expression area (Fig. 1).
PD-L1 expression and pathological
findings
The clinicopathological findings of the
PD-L1-positive HCC and the PD-L1-negative HCC are shown in Table III. In the HCC tissues, mitosis was
more frequently observed in the PD-L1-positive HCC than in the
PD-L1-negative HCC (P<0.01). The Nuclear grade was higher in the
PD-L1-positive HCC than in the PD-L1-negative HCC (P<0.01). RGS5
and EpCAM expression levels were higher in the PD-L1 positive-HCC
than that in the PD-L1-negative HCC (P<0.05). In the
non-cancerous liver tissues, the degree of liver fibrosis was
higher in the patients with PD-L1-positive HCC than in those with
PD-L1-negative HCC.
 | Table III.Clinicopathological findings of PD-L1
positive HCC. |
Table III.
Clinicopathological findings of PD-L1
positive HCC.
| A, Clinical
findings |
|---|
|
|---|
|
| PD-L1 expression of
HCC |
|
|---|
|
|
|
|
|---|
|
Characteristics | Positive
(n=10) | Negative
(n=38) | P-value |
|---|
| Age (year) | 71±8 | 68.3±8.1 | 0.69 |
| Male/Female | 4/6 | 25/13 | 0.16 |
| IFN/DAA | 6/4 | 29/9 | 0.43 |
| Diabetes
(prevalence rate, %) | 1 (10%) | 17 (45%) | 0.06 |
| Serum total
bililubin (mg/dl) | 0.8±0.3 | 0.9±0.2 | 0.21 |
| Prothrombin
activity (%) | 85.9±10.8 | 92.2±14.7 | 0.15 |
| Serum albumin
(g/dl) | 4.1±0.38 | 4.2±0.29 | 0.41 |
| Serum AFP (ng/ml,
median) | 84 | 4.5 | 0.46 |
| Serum PIVKA
(mAU/ml, median) | 63 | 100 | 0.11 |
|
| B, HCC
findings |
|
|
| PD-L1 expression
of HCC |
|
|
|
|
|
|
Characteristics | Positive
(n=10) | Negative
(n=38) | P-value |
|
| Tumor size
(mm) | 26±14 | 26±17 | 0.92 |
| Tumor
differentiation (well/moderate/poor) | 0/8/2 | 5/31/2 | 0.06 |
| Nuclear grade | 2.7±0.5 | 1.8±0.8 | <0.01 |
| Atypia score | 2.6±0.5 | 2.3±0.5 | 0.11 |
| Mitosis score |
2.5±0.7 | 1.5±0.7 | <0.01 |
| Portal vein
invasion (prevalence rate, %) | 5 (50%) | 21 (55%) | 1 |
| CK19 score
(0/1+/2+) | 9/1/0 | 38/0/0 | 0.34 |
| EpCAM score
(0/1+/2+) | 5/3/2 | 33/3/2 | 0.04 |
| RGS5 score
(0/1+/2+) | 0/6/4 | 11/23/4 | <0.01 |
|
| C, Non-HCC
findings |
|
|
| PD-L1 expression
of HCC |
|
|
|
|
|
|
Characteristics | Positive
(n=10) | Negative
(n=38) | P-value |
|
| Fibrosis (New
Inuyama Classification, F) | 3.3±0.9 | 2.4±1.3 | 0.03 |
| Inflammation (New
Inuyama Classification, A) | 1.5±0.5 | 1.2±0.5 | 0.11 |
| Steatosis
(prevalence rate, %) | 5 (50%) | 10 (26%) | 0.24 |
| CD34 score
(0/1+/2+) | 0/1/9 | 0/5/33 | 0.79 |
| αSMA score
(0/1+/2+) | 3/4/3 | 18/11/9 | 0.43 |
Univariate and multivariate analysis
for prognosis
The observation period after resection was longer in
the IFN group than in the DAA group (P<0.01). In the IFN group,
univariate analysis for recurrence free survival after surgery
(RFS) revealed that PD-L1 expression was significant predictor for
recurrence (positive vs. negative; HR=6.01, 95% CI=1.45–23.3,
P=0.02) (Table IV). Multivariate
analysis demonstrated that PD-L1 expression was an independent poor
prognostic factor for recurrence in the IFN group (positive vs.
negative; HR=5.01, 95% CI=1.14–21.08, P=0.03) (Table IV). In the DAA group, there were not
significant prognostic factors by univariate and multivariate
analyzes.
 | Table IV.Univariate and multivariate analysis
for recurrence free survival in IFN group. |
Table IV.
Univariate and multivariate analysis
for recurrence free survival in IFN group.
| A, Clinical
findings |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (>70
years) | 3.96
(1.23–13.16) | 0.02 | 3.07
(0.79–11.27) | 0.1 |
| Male | 1.31
(0.51–3.79) | 0.59 |
|
|
| Diabetes | 0.37
(0.06–1.37) | 0.15 |
|
|
| Serum T. Bililubin
(>0.8 mg/dl) | 1.14
(0.44–2.95) | 0.78 |
|
|
| Prothrombin
activity (<90%) | 1.67
(0.59–4.53) | 0.32 |
|
|
| Serum albumin
(<4.2 g/dl) | 0.92
(0.34–2.4) | 0.87 |
|
|
| Serum AFP (>6.7
ng/ml) | 1.46
(0.55–3.93) | 0.44 |
|
|
| Serum PIVKA
(>118 mAU/ml) | 1.21
(0.46–3.09) | 0.69 |
|
|
|
| B, HCC
findings |
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Characteristics | HR (95%
CI) | P-value | HR (95%
CI) | P-value |
|
| Tumor size (>30
mm) | 1.72
(0.54–4.8) | 0.34 |
|
|
| Poor
differentiation | 5.78
(0.83–26.96) | 0.07 | 2.95
(0.39–16.19) | 0.26 |
| Nuclear grade
(>grade 2) | 1.61
(0.62–4.69) | 0.34 |
|
|
| Portal vein
invasion | 1.57
(0.61–4.31) | 0.34 |
|
|
| PD-L1 positive | 6.01
(1.45–23.3) | 0.02 | 5.01
(1.14–21.08) | 0.03 |
| CK19 or/and EpCAM
positive | Unparsable |
|
|
|
| RGS5 positive | 3.46
(0.7–62.57) | 0.15 |
|
|
|
| C, Non-HCC
findings |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Characteristics | HR (95%
CI) | P-value | HR (95%
CI) | P-value |
|
| Liver fibrosis
(>F3) | 0.92
(0.35–2.35) | 0.86 |
|
|
| Hepatic
inflammation (>A2) | 0.68
(0.15–2.13) | 0.53 |
|
|
| Liver
steatosis | 0.52
(0.14–1.5) | 0.24 |
|
|
| CD34 expression
(>score 2+) | 3.13
(0.63–56.68) | 0.19 |
|
|
| αSMA positive
(>score 2+) | 2.21
(0.82–5.75) | 0.11 |
|
|
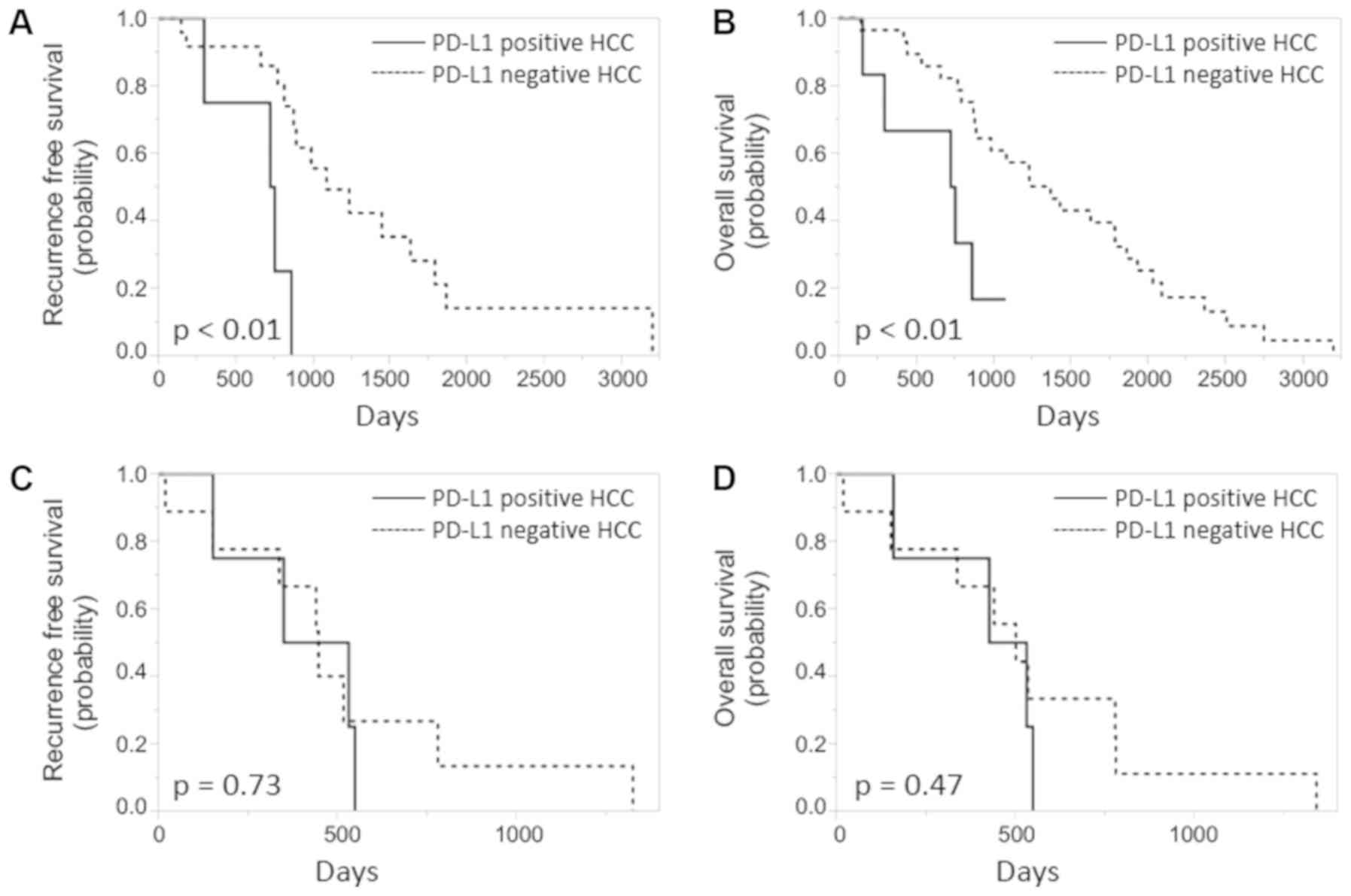
The survival curves
In the IFN group, the Kaplan-Meier curves
demonstrate that both RFS and overall survival time were
significantly shorter for the PD-L1-positive HCC, compared with the
PD-L1-negative HCC (log-rank test P<0.01; Fig. 3).
Discussion
We studied the molecular pathological
characteristics of patients who developed initial HCC after
achieving SVR. The increasing understanding of HCC biology is
necessary for future targeted therapies and personalized care.
We demonstrated that PD-L1 expression was an
independent unfavorable prognostic factor of surgically-resected
HCC after archiving SVR treated by IFN. Recent studies have
demonstrated that PD-L1 positive HCC display biological and
pathological markers of aggressiveness (e.g., high serum AFP
levels, poor differentiation, and vascular invasion) (16). PD-L1, the major ligand for PD-1,
plays a crucial role in PD-1-dependent immune suppression, which is
mediated by an antigen-specific T-cell response. PD-L1 expression
might contribute to aggressive behavior by blocking antitumor
immunity, but the specific details remain unclear. There is very
limited information on the expression of PD-L1 in HCC (16,17), and
its relationship with the clinical and histopathological features
remains unknown. In our study, PD-L1 positive HCC also display
biological and pathological markers of aggressiveness (e.g., high
mitotic activity, RGS5 expression, and EpCAM expression). RGS5 is a
member of the RGS protein family and RGS proteins act
GTPase-activating proteins for heterotrimeric G protein α subunit,
negatively regulating G-protein signaling. With regard to RGS5 and
HCC, RGS5 expression has been demonstrated to enhanced portal vein
invasion (14,18). Tsujikawa et al (19), reported that immunohistochemical
molecular analysis revealed B/S group expressing CK19, SALL4, and
EpCAM shows frequent portal vein invasion, high Ki67 labeling
index, and worse prognosis. HCCs with TP53 mutation are more
proliferative and aggressive and show CK19 and EpCAM expressions,
frequent microvascular invasion, and poor survival (20,21). Our
data suggest that PD-L1 positive HCC may have higher malignant
potential. The timing of PD-L1 positivity initiation is not clear.
However, the PD-L1 positive reaction was observed in 0% (none of 5
cases) of well differentiated HCC, 21% (8 cases of 39 cases) of
moderately differentiated HCC, and 50% (2 cases of 4 cases) in
poorly differentiated HCC. According to these our results, we
considered that the PD-L1 positivity initiation of HCC may be
occurred during dedifferentiation process.
It has been reported that both hepatic inflammation
and fibrosis are reversible and decrease after achieving SVR by
IFN-based therapy. George et al (22), reported on 49 patients with HCV
infection who had undergone liver biopsy before treatment and
4-years after achieving SVR by IFN-based therapy. Forty of these
patients (82%) showed a decrease in fibrosis score, and 45 (92%)
showed a decrease in inflammation score. Ten patients (20%) had
healthy or nearly healthy livers on long-term follow-up biopsy. In
this study, uncertain mixed both progressive fibrosis and
regressive fibrosis were observed in non-cancerous liver tissues of
patients with HCC after achieving SVR. Recently, regressive
fibrosis in chronic hepatitis B has been reported (15). Predominately regressive was defined
as most (more than 50%) fibroseptal stroma in the liver biopsy
specimens showing features of thin, densely compacted stroma,
largely darkly staining on trichrome, which are largely acellular
(15). It has been reported that
serum levels of Wisteria floribunda agglutinin-positive human Mac-2
binding protein glycosylation isomer (M2BPGi) can be used to
predict the development of HCC in patients with HCV SVR (23). M2BPGi is a serum marker of liver
fibrosis that indicates the extent of liver fibrosis in patients
with chronic liver disease. Bekki et al (24), reported that M2BP is secreted by
activated HSCs. Liver fibrosis that does not decrease sufficiently
after achieving SVR may be an important contributory factor to
liver carcinogenesis in the patients after achieving SVR. However,
in this study, the liver fibrosis was not associated with a poor
prognosis in surgically resected HCC after archiving SVR.
In this study, the degree of inflammation and
fibrosis was higher in the DAA group than those in the IFN group.
In Japan, the IFN therapy cannot be used as an anti-HCV treatment
for cirrhotic patients. On the other hand, the DAA therapy can be
used as an anti-HCV treatment for cirrhotic patients. Advanced
liver fibrosis cases were seen in the patients with achieving SVR
treated by DAA more frequently than in the patients with achieving
SVR treated by IFN. Besides, the observation period of the DAA
group was shorter than those of the IFN group because DAA was new
drug developed in 2011. Advanced liver fibrosis cases should
require clinical follow-up even after achieving SVR.
Immunohistochemically, in patients developed HCC
after achieving SVR, expression of CD34 and αSMA in sinusoidal
mesenchymal cells of non-cancerous liver tissue were frequently
observed. In chronic liver disease, hepatic fibrosis starts with
the stimulation of HSCs. Activated HSCs transform into
myofibroblasts and increase of the extracellular matrix. As a
result, capillarization of the hepatic sinusoidal endothelial cells
(HECs) occurs (25). αSMA is
frequently used as a marker of activated and myofibroblast-like
HSCs (26). Capillarized sinusoids
are lined by a continuous endothelial lining surrounded by a
complete basement membrane (27).
Immunophenotyping studies of human HECs have demonstrated that HECs
of healthy livers are characterized by the expression of the CD32
and CD16 for the Fc fragment of IgG, but lack CD34 (28). In liver cirrhosis, HECs of
capillarized sinusoids displayed changes in the positive expression
of the CD34 (29). It has been
reported that capillarized sinusoids contribute to sinusoidal
portal hypertension in liver cirrhosis (30). In capillarized sinusoids, metabolism
between the blood circulation and hepatocytes is impaired, because
the continuous endothelial lining is surrounded by a complete
basement membrane. Further molecular biological study of sinusoidal
capillarization and activation of HSCs after achieving SVR will
contribute to improving the treatment of fibrosis after achieving
SVR.
The current study has several limitations. First,
this was a retrospective study. Second, while the statistical
signification of this study was sufficient, the number of cases was
relatively small. Third, we were not able to investigate the
relationship of PD-L1 with prognosis of the DAA group enough,
because the sample size was small and the observation period after
resection was very short. These may affect the statistical results.
We tried to add the samples in the DAA group, but we cannot add the
samples because DAA was new drug developed in 2011. Further
prospective studies with larger numbers of patients and long
observation period is needed to confirm the results of this
study.
In conclusion, we found that PD-L1 expression was
associated with a poor prognosis in surgically resected HCC after
archiving SVR. We considered that PD-L1 positive HCC had blocking
antitumor immunity but also high mitotic activity. In addition, we
demonstrated liver fibrosis in non-cancerous liver tissues of
patients with HCC after achieving SVR. We consider that advanced
liver fibrosis may be a contributory factor of liver carcinogenesis
after achieving SVR. The mechanism of fibrosis and fibrinolysis in
the liver tissue after achieving SVR is not clearly. Further
molecular biological study of sinusoidal capillarization, i.e.,
CD34 expression, and activation of HSCs, i.e., CD34 expression,
after achieving SVR is necessary. These may associate with hepatic
fibrosis after achieving SVR.
Acknowledgements
The authors would like to thank Ms A Tanaka (Kurume
University School of Medicine) and Ms S Maeda (Kurume University
School of Medicine) for their technical assistance.
Funding
The present study was supported by Japan Society for
the Promotion of Science KAKENHI (grant nos. 16K19094 and
18K15105).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
RK, JA, ON and HY designed the research. RK, SO, YN,
HK, YM and MT performed the research. RK analyzed the data and
wrote the paper. HY and JA critically revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee at Kurume University (approval no. 16060). The Ethical
Committee waived the requirement for written informed consent for
the cases as the data for these patients were retrospectively
analyzed.
Patient consent for publication
Written informed consent was obtained from all
participants for the publication of any data or associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fried MW, Shiffman ML, Reddy KR, Smith C,
Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G,
Dhumeaux D, et al: Peginterferon alfa-2a plus ribavirin for chronic
hepatitis C virus infection. N Engl J Med. 347:975–982. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mizokami M, Yokosuka O, Takehara T,
Sakamoto N, Korenaga M, Mochizuki H, Nakane K, Enomoto H, Ikeda F,
Yanase M, et al: Ledipasvir and sofosbuvir fixed-dose combination
with and without ribavirin for 12 weeks in treatment-naive and
previously treated Japanese patients with genotype1 hepatitis C: An
open-label, randomised, phase 3 trial. Lancet Infect Dis.
15:645–653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumada H, Suzuki Y, Ikeda K, Toyota J,
Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, et
al: Daclatasvir plus Asunaprevir for chronic HCV Genotype1b
infection. Hepatology. 59:2083–2091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgan RL, Baack B, Smith BD, Yartel A,
Pitasi M and Falck-Ytter Y: Eradication of hepatitis C virus
infection and the development of hepatocellular carcinoma: A
meta-analysis of observational studies. Ann Intern Med.
158:329–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arase Y, Kobayashi M, Suzuki F, Suzuki Y,
Kawamura Y, Akuta N, Kobayashi M, Sezaki H, Saito S, Hosaka T, et
al: Effect of type 2 diabetes on risk for malignancies includes
hepatocellular carcinoma in chronic hepatitis C. Hepatology.
57:964–973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oze T, Hiramatsu N, Yakushijin T, Miyazaki
M, Yamada A, Oshita M, Hagiwara H, Mita E, Ito T, Fukui H, et al:
Post-treatment levels of α-fetoprotein predict incidence of
hepatocellular carcinoma after interferon therapy. Clin
Gastroenterol Hepatol. 12:1186–1195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asahina Y, Tsuchiya K, Nishimura T,
Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K,
Nakanishi H, et al: α-fetoprotein levels after interferon therapy
and risk of hepatocarcinogenesis in chronic hepatitis C.
Hepatology. 58:1253–1262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Makiyama A, Itoh Y, Kasahara A, Imai Y,
Kawata S, Yoshioka K, Tsubouchi H, Kiyosawa K, Kakumu S, Okita K,
et al: Characteristics of patients with chronic hepatitis C who
develop hepatocellular carcinoma after a sustained response to
interferon therapy. Cancer. 101:1616–1622. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirakawa M, Ikeda K, Arase Y, Kawamura Y,
Yatsuji H, Hosaka T, Sezaki H, Akuta N, Kobayashi M, Saitoh S, et
al: Hepatocarcinogenesis following HCV RNA eradication by
interferon in chronic hepatitis patients. Intern Med. 47:1637–1643.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayashi T, Tamori A, Nishikawa M, Morikawa
H, Enomoto M, Sakaguchi H, Habu D, Kawada N, Kubo S, Nishiguchi S
and Shiomi S: Differences in molecular alterations of
hepatocellular carcinoma between patients with a sustained
virological response and those with hepatitis C virus infection.
Liver Int. 29:126–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuura K, Sawai H, Ikeo K, Ogawa S, Iio
E, Isogawa M, Shimada N, Komori A, Toyoda H, Kumada T, et al:
Genome-wide association study identifies TLL1 variant associated
with development of hepatocellular carcinoma after eradication of
hepatitis C virus infection. Gastroenterol. 152:1383–1394. 2017.
View Article : Google Scholar
|
|
12
|
Theise ND, Park YN, Curado MP, Sakamoto M,
Franceschi S, Torbenson M, Hytiroglou P, Wee A and Kudo M: WHO
classification of tumours of the digestive system. Lyon: IARC
Press; pp. 205–216. 2010
|
|
13
|
Ichida F, Tsuji T, Omata M, Inoue K,
Kamimura T, Yamada G, Hino K, Yokosuka O and Suzuki H: New Inuyama
classification; new criteria for histological assessment of chronic
hepatitis. Int Hepatol Commun. 6:112–119. 1996. View Article : Google Scholar
|
|
14
|
Umeno Y, Ogasawara S, Akiba J, Hattori S,
Kusano H, Nakashima O, Koga H, Torimura T, Yamakawa R and Yano H:
Regulator of G-protein signaling 5 enhances portal vein invasion in
hepatocellular carcinoma. Oncol Lett. 15:1763–1770. 2018.PubMed/NCBI
|
|
15
|
Sun Y, Zhou J, Wang L, Wu X, Chen Y, Piao
H, Lu L, Jiang W, Xu Y, Feng B, et al: New classification of liver
biopsy assessment for fibrosis in chronic hepatitis B patients
before and after treatment. Hepatology. 65:1438–1450. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calderaro J, Rousseau B, Amaddeo G, Mercey
M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay
D, et al: Programmed death ligand 1 expression in hepatocellular
carcinoma: Relationship with clinical and pathological features.
Hepatology. 64:2038–2046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M,
Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al: Overexpression
of PD-L1 significantly associates with tumor aggressiveness and
postoperative recurrence in human hepatocellular carcinoma. Clin
Cancer Res. 15:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu M, Chen X, Zhang J, Wang D, Fang X,
Wang X, Wang G, Chen G, Jiang X, Xia H and Wang Y: Over-expression
of regulator of G protein signaling 5 promotes tumor metastasis by
including ephithelial-mesenchymal transition in hepatocellular
carcinoma cells. J Surg Oncol. 108:192–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsujikawa H, Masugi Y, Yamazaki K, Itano
O, Kitagawa Y and Sakamoto M: Immunohistochemical molecular
analysis indicates hepatocellular carcinoma subgroups that reflect
tumor aggressiveness. Hum Pathol. 50:24–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calderaro J, Couchy G, Imbeaud S, Amaddeo
G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage
P, et al: Histological subtypes of hepatocellular carcinoma are
related to gene mutations and molecular tumour classification. J
Hepatol. 67:727–738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goossens N, Sun X and Hoshida Y: Molecular
classification of hepatocellular carcinoma: Potential therapeutic
implications. Hepatol Oncol. 2:371–379. 2015. View Article : Google Scholar
|
|
22
|
George SL, Bacon BR, Brunt EM,
Mihindukulasuriya KL, Hoffmann J and Di Bisceglie AM: Clinical,
virologic histologic, and biochemical outcomes after successful HCV
therapy: A 5-year follow-up of 150 patients. Hepatology.
49:729–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sasaki R, Yamasaki K, Abiru S, Komori A,
Nagaoka S, Saeki A, Hashimoto S, Bekki S, Kugiyama Y, Kuno A, et
al: Serum wisteria floribunda agglutinin-positive Mac-2 binding
protein values predict the development of hepatocellular carcinoma
among patients with chronic hepatitis C after sustained virological
response. PLoS One. 10:e1290532015. View Article : Google Scholar
|
|
24
|
Bekki Y, Yoshizumi T, Shimoda S, Itoh S,
Harimoto N, Ikegami T, Kuno A, Narimatsu H, Shirabe K and Maehara
Y: Hepatic stelleate cells secreting WFA+-M2BP: Its role
in biological interactions with Kupffer cells. J Gastroenterol
Hepatol. 32:1387–1393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friedman SL: Hepatic stellate cells:
Protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Motoyama H, Komiya T, Thuy le TT, Tamori
A, Enomoto M, Morikawa H, Iwai S, Uchida-Kobayashi S, Fujii H,
Hagihara A, et al: Cytoglobin is expressed in hepatic stellate
cells, but not in myofibroblasts, in normal and fibrotic human
liver. Lab Invest. 94:192–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Couvelard A, Scoazec JY and Feldmann G:
Expression of cell-cell and cell-matrix adhesion proteins by
sinusoidal endothelial cells in the normal and cirrhotic human
liver. Am J Pathol. 143:738–752. 1993.PubMed/NCBI
|
|
28
|
Nonaka H, Tanaka M, Suzuki K and Miyajima
A: Development of murine hepatic sinusoidal endothelial cells
characterized by the expression of hyaluronan receptors. Dev Dyn.
236:2258–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martinez-Hernandez A and Martinez J: The
role of capillarization in hepatic failure: Studies in carbon
tetrachloride-induced cirrhosis. Hepatology. 14:864–874. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blanc JF, Bioulac-Sage P and Rosenbaum J:
Hepatic stellate cells and liver fibrogenesis. Gastroenterol Clin
Biol. 21:869–879. 1997.(In French). PubMed/NCBI
|