Introduction
Lung cancer is a common type of malignancy with
significant mortality (1). Some
researchers hold the opinion that small cell lung cancer (SCLC) is
systemic from initial development (2). Due to its fast progression, poor
prognosis and the tendency towards whole body metastasis at an
early stage, the majority of patients are diagnosed at an advanced
state of the disease (2). Only
30–40% of patients are in the limited stage (LS), which means in
the ipsilateral hemithorax and within a single radiotherapy region,
prior to treatment (3). By combining
surgery, radiotherapy and chemotherapy, LS patients may achieve a
15–20 month median survival time, and 20–40% have a 2-year survival
time (3). Certain extensive stage
(ES) patients may only receive supportive treatment due to
extensive metastasis and a poor performance status, thus resulting
in an even shorter survival time (4). The median survival time for patients
with SCLC is 2–4 months if the disease is left untreated, and the
2-year survival rate is ~5% (3).
Over the past 15 years, the median and 5-year survival rate of
patients with SCLC has not significantly improved (5).
At present, etoposide/carboplatin (EC) and
etoposide/cisplatin (EP) therapy regimens are the two first line
regimens for the treatment of SCLC (6). Although similar in efficacy, the two
regimens have different toxicity profiles. Cisplatin is associated
with adverse gastrointestinal effects, neurotoxicity and renal
function impairment, and its administration requires a hydration
regimen. Carboplatin is associated with myelosuppression (7–9).
The occurrence and progression of tumors is often
accompanied by an intratumoral inflammatory response, which
suppresses anti-tumor immunity (10). It has been demonstrated that this
inflammatory response may serve an important role in the
development and progression of lung cancer (10). The neutrophil to lymphocyte ratio
(NLR) in peripheral blood serves as an inflammatory marker,
representing the absolute value of the ratio of neutrophil count to
lymphocyte count. As a routine hematological test, it is
convenient. Additionally, it is associated with patient prognosis
in different types of tumors, such as bladder cancer,
hepatocellular carcinoma and non-small cell lung cancer (11–16).
However, the prognostic value of NLR in SCLC requires further
research. Currently, indicators that aid in the selection of first
line treatment for patients with SCLC are limited. The present
study retrospectively analyzed the relationship between the NLR and
the progression-free survival (PFS) in 73 cases of SCLC in order to
investigate the significance of the NLR when selecting a first-line
treatment.
Materials and methods
Clinical data
A total of 73 SCLC cases with relatively complete
clinical data were reviewed using the electronic medical records
and registration database at the Fujian Medical University Union
Hospital (Fuzhou, China). The patients had sought treatment at the
aforementioned hospital between January 2014 and May 2016. The
present study was approved by the Institutional Review Board (IRB)
of Fujian Medical University Union Hospital (IRB no. 2017KY084) and
informed consent was obtained from the patients. All patients were
diagnosed with SCLC by pathological examination; lung tumor
histopathological examination was used to confirm small cell
carcinoma and immunohistochemical examination confirmed compliance
with small cell lung cancer. The exclusion criteria were as
follows: i) Second primary tumor; ii) diseases that may result in
hematological changes (including lymphoma, leukemia and bone marrow
dysplasia syndrome); iii) chronic diseases (including
cardiovascular disease, diabetes, asthma and chronic obstructive
pulmonary disease); iv) histological mixed-type tumors with
adenocarcinoma and other histological types (Such as lung squamous
cell carcinoma, alveolar carcinoma, mesenchymal sarcoma, and large
cell carcinoma); and v) no exposure to prophylactic cranial
irradiation. Among the 73 patients, 69 were male (94.50%, mean age
61.43 years, age range 39.00–83.00 years) and 4 were female (5.50%,
mean age 65.25 years, age range 53.00–75.00 years). Data were
collected from routine blood tests, including neutrophil and
lymphocyte counts and levels of serum albumin and lactic
dehydrogenase, performed during the initial diagnosis.
Additionally, the first-line therapeutic regimen used was recorded
for each patient. The follow-up time was set from the initial
diagnosis to August 31st 2017. Information regarding PFS and
overall survival (OS) time was acquired via inpatient and
outpatient medical records and telephone follow-up. PFS was
calculated from the date of first diagnosis to the onset of disease
progression [According to RECIST 1.1 solid tumor efficacy
evaluation criteria (17)] or the
last follow-up. The OS time was defined as the period from
diagnosis to mortality (OS time study endpoint) or the last
follow-up.
Research methods
The PFS data were divided into two groups: Low PFS
(<4.50) and high PFS (≥4.50) according to the PFS median of
4.50. An NLR cut-off value of 3.80 was selected during initial
diagnosis by means of a receiver operating characteristic (ROC)
curve used to calculate Youden's index. The patients were
subsequently divided into two groups: Low-NLR (NLR <3.80) and
high-NLR (NLR ≥3.80). Based on the staging method of the Veterans
Administration Lung Study Group of the United States (18), patients with SCLC were further
classified into two groups: LS and ES. Statistical methods were
used to analyze the associations between NLR, the choice of
first-line therapeutic regimen, PFS and OS time.
Statistical analysis
Clinical data are presented as the mean ± standard
deviation or median (range). All analyses were performed using SPSS
software (version 19; IBM Corp., Armonk, NY, USA). ROC curve
analysis was used to determine the cut-off value for the NLR. The
Chi-square test was used to compare the different clinical
characteristics in the groups, and a t-test was used to compare the
NLR between groups. The Kaplan-Meier method was used to compare
survival time differences between the high- and low-NLR groups, and
survival differences between patient groups were analyzed using the
log-rank test. Stratified analysis was performed between LS and ES
patients in order to compare the prognoses associated with
different therapeutic regimens. P<0.05 was considered to
indicate a statistically significant difference. All P-values were
two-tailed.
Results
Association between pre-treatment NLR
and clinical characteristics
The 73 SCLC cases were divided into 39 high-NLR
cases and 34 low-NLR cases. The clinical and hematological
characteristics of the patients are presented in Table I. The differences in NLR between
groups, following stratification by several clinical
characteristics including age, sex and cancer stage, were not
statistically significant (P>0.05; Table II, Among the 73 patients, we could
not collect smoking history data, stage, ECOG performance status,
brain metastases, lung metastases, bone metastases, pleural
metastasis, adrenal metastasis situation, albumin and MKI67 from 7
patients, the CEA data from 11 patients, LDH from 9 patients, NSE
from 20 patients).
 | Table I.Clinical and hematological
characteristics of patients with small cell lung cancer. |
Table I.
Clinical and hematological
characteristics of patients with small cell lung cancer.
| Variable | Mean | Range |
|---|
| Age (years) | 61.64 | 39.00–83.00 |
| Sex
(male/female) | 61.64 | 39.00–83.00 |
| PFS (months) | 5.02 | 1.00–16.00 |
| OS time
(months) | 13.15 | 1.00–63.00 |
| BMI
(kg/m2) | 21.65 | 17.72–25.61 |
| Lymphocytes count
(109/l) | 2.01 | 0.46–26.80 |
| Neutrophil count
(109/l) | 5.41 | 1.27–11.90 |
| Hemoglobin
(g/l) | 131.75 | 93.00–169.00 |
| RBC count
(1012/l) | 4.31 | 3.14–5.87 |
| NLR | 3.81 | 0.27–13.63 |
 | Table II.Association between the NLR and the
clinicopathological characteristics of patients with SCLC. |
Table II.
Association between the NLR and the
clinicopathological characteristics of patients with SCLC.
| Characteristic | NLR | t | P-value | n | High NRL | Low NRL | χ2 | P-value |
|---|
| Age at diagnosis
(years) |
| 0.336 | 0.738 |
|
|
| 0.053 | 0.817 |
|
<60 | 3.94±3.12 |
|
| 30 | 16 | 14 |
|
|
|
≥60 | 3.72±2.31 |
|
| 43 | 23 | 18 |
|
|
| Sex |
| 0.93 | 0.761 |
|
|
| 0.42 | 0.838 |
|
Male | 3.83±2.68 |
|
| 69 | 37 | 30 |
|
|
|
Female | 3.59±2.78 |
|
| 4 | 2 | 2 |
|
|
| Smoking |
| −0.218 | 0.828 |
|
|
| 0.525 | 0.469 |
| No | 3.54±2.26 |
|
| 22 | 14 | 8 |
|
|
|
Yes | 3.69±2.64 |
|
| 44 | 25 | 21 |
|
|
| Stage |
| 0.076 | 0.940 |
|
|
| 0.053 | 0.817 |
|
LS-SCLC | 3.85±2.90 |
|
| 29 | 16 | 14 |
|
|
|
ES-SCLC | 3.79±2.52 |
|
| 37 | 23 | 18 |
|
|
| ECOG performance
status |
| 1.127 | 0.288 |
|
|
| 1.127 | 0.288 |
| 1
Point | 3.04±1.44 |
|
| 34 | 22 | 14 |
|
|
| 2
Point | 4.61±3.35 |
|
| 32 | 17 | 18 |
|
|
| Brain
metastases |
| 0.062 | 0.804 |
|
|
| 1.37 | 0.243 |
| No | 3.54±2.35 |
|
| 62 | 35 | 31 |
|
|
|
Yes | 3.84±2.70 |
|
| 4 | 4 | 1 |
|
|
| Lung
metastases |
| 0.233 | 0.816 |
|
|
| 0.059 | 0.808 |
| No | 3.86±2.89 |
|
| 46 | 27 | 23 |
|
|
|
Yes | 3.70±2.09 |
|
| 20 | 12 | 9 |
|
|
| Bone
metastases |
| 0.092 | 0.927 |
|
|
| 0.004 | 0.951 |
| No | 3.83±2.65 |
|
| 51 | 29 | 24 |
|
|
|
Yes | 3.77±2.78 |
|
| 15 | 10 | 8 |
|
|
| Pleural
metastasis |
| 1.670 | 0.397 |
|
|
| 0.59 | 0.442 |
| No | 3.70±2.60 |
|
| 63 | 38 | 30 |
|
|
|
Yes | 6.30±3.64 |
|
| 3 | 1 | 2 |
|
|
| Adrenal
metastasis |
| 0.080 | 0.936 |
|
|
| 0.42 | 0.838 |
| No | 3.82±2.71 |
|
| 63 | 37 | 30 |
|
|
|
Yes | 3.71±1.96 |
|
| 3 | 2 | 2 |
|
|
| LDH, IU/l |
| −1.602 | 0.114 |
|
|
| 1.904 | 0.168 |
|
≤245 | 3.11±2.08 |
|
| 26 | 19 | 10 |
|
|
|
>245 | 4.05±2.57 |
|
| 38 | 19 | 20 |
|
|
| Albumin, g/l |
| 1.127 | 0.288 |
|
|
| 0.342 | 0.559 |
|
<37.5 | 4.16±3.15 |
|
| 31 | 18 | 17 |
|
|
|
≥37.5 | 3.48±2.08 |
|
| 35 | 21 | 15 |
|
|
| NSE, ng/ml |
| −0.416 | 0.679 |
|
|
| 0.223 | 0.637 |
|
<16.3 | 3.53±1.87 |
|
| 13 | 6 | 7 |
|
|
|
≥16.3 | 3.86±2.65 |
|
| 40 | 22 | 19 |
|
|
| CEA, ng/ml |
| 1.130 | 0.263 |
|
|
| 2.302 | 0.129 |
|
<5.0 | 3.95±2.47 |
|
| 37 | 19 | 20 |
|
|
|
≥5.0 | 3.23±2.42 |
|
| 25 | 17 | 8 |
|
|
| MKI67a, % |
| −0.466 | 0.642 |
|
|
| 0.34 | 0.523 |
|
≤90 | 3.70±2.46 |
|
| 45 | 24 | 19 |
|
|
|
>90 | 4.00±2.99 |
|
| 21 | 15 | 13 |
|
|
Association between PFS, OS time and
pre-treatment NLR
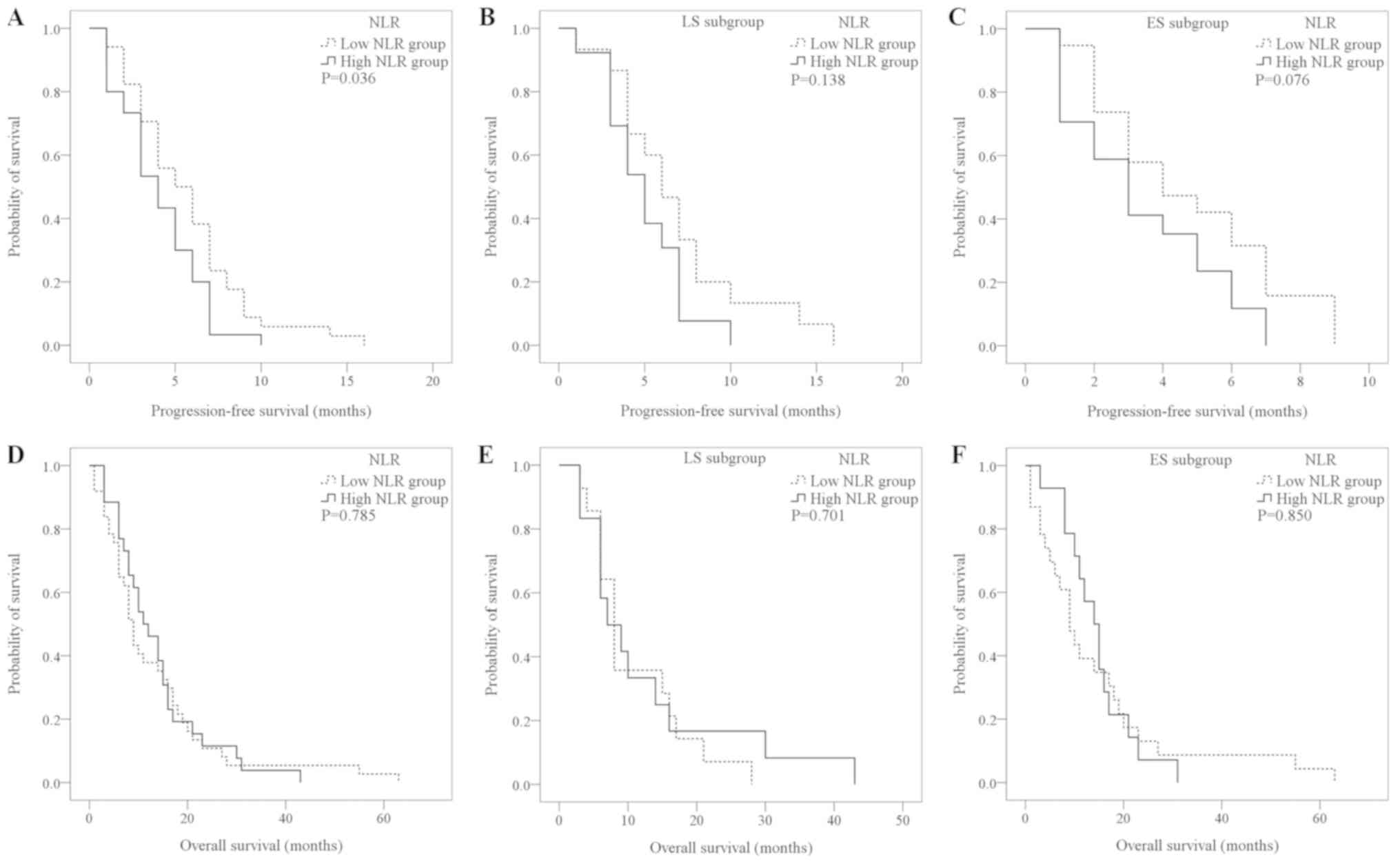
Compared with the high-NLR group, the low-NLR group
had a longer PFS (5.71±0.59 vs. 4.10±0.44 months; P=0.036; Fig. 1A). In the sub-group analysis of LS
and ES patients, the PFS of the low-NLR group was longer than that
of the high-NLR group; however, the difference was not
statistically significant (Fig. 1B and
C). The difference in OS time between the high- (n=26) and
low-NLR groups (n=34) was not significantly different (13.73±1.87
vs. 13.22±2.18 months; P=0.785; Fig.
1D). A total of 13 patients did not reach the OS time study
endpoint. There was no significant difference in the OS time
between the high- and low-NLR groups in the ES or LS populations
(Fig. 1E and F).
Association between the NLR and the
efficacy of the EC or EP regimens
Patients treated with the EP regimen (n=40) had a
PFS of 5.36±0.53 months. Patients treated with the EC regimen
(n=18) had a PFS of 4.78±0.79 months. A total of 15 patients were
treated with regimens other than EP and EC, including
etoposide/lobaplatin or gemcitabine/oxaliplatin, had a PFS of
4.60±0.68 months. The difference in survival time was not
statistically significant (P=0.515) (data not shown), when
comparing patients treated with EP, patients treated with EC and
patients treated with the other regimens.
In the low-NLR group, the PFS of the EP
regimen-treated patients (n=20) and the EC regimen-treated patients
(n=10) was similar and not significantly different (5.85±0.79 vs.
6.50±1.09 months; P=0.828; Fig. 2A).
However, in the high-NLR group, the PFS of the EP regimen-treated
group (n=20) was significantly longer compared with the EC
regimen-treated group (n=8; 4.59±0.62 vs. 2.63±0.53 months;
P=0.021; Fig. 2B). This significant
difference was also observed in the high-NLR LS group (EP
treatment, 6.50±0.85 months vs. EC treatment, 2.80±0.49 months;
P=0.002; Fig. 2C), while in the ES
group there was no statistically significant difference between the
EP and EC treatment groups (EP treatment, 3.54±0.67 months vs. EC
treatment, 2.33±1.33 months; P=0.370; Fig. 2D).
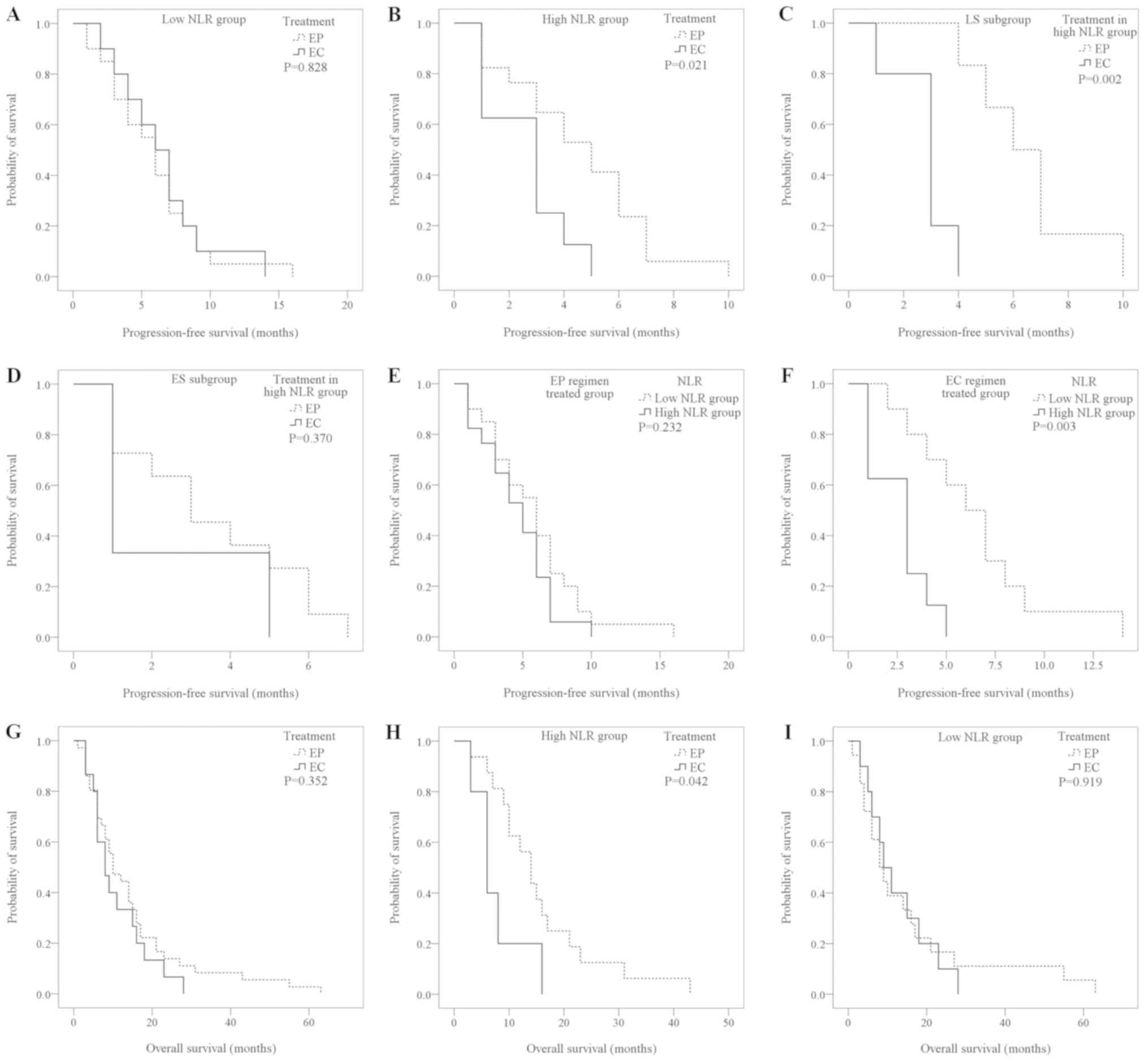 | Figure 2.Kaplan-Meier survival curves for the
PFS and OS time of patients with small cell lung cancer treated
with EP and EC. (A) In the low-NLR group, the PFS of the EP and EC
regimen-treated patients was not significantly different (P=0.828;
log-rank). (B) In the high-NLR group, the PFS in the EP treated
group was significantly longer compared with the EC treated group
(P=0.021; log-rank). (C) In the LS subgroup, the PFS of the high
NLR patients treated with EP was significantly longer than those
treated with EC (P=0.002; log-rank). (D) The PFS of the high-NLR
patients with treated with EP and EC regimens in the ES subgroup
was not significantly different (P=0.370; log-rank). (E) In the EP
regimen-treated group, the PFS of the high-group and low-NLR groups
was not statistically different (P=0.232; log-rank). (F) In
patients treated with the EC regimen, the low-NLR group had a
significantly longer PFS compared with the high-NLR group (P=0.003;
log-rank). (G) The OS time of the EP and EC treated groups was not
significantly different (P=0.352; log-rank). (H) In the high-NLR
group, the OS time of the EP regimen-treated patients was
significantly longer than that of the EC regimen-treated patients
(P=0.042; log-rank). (I) In the low-NLR group, the OS time of the
EP and EC regimen-treated patients was similar (P=0.919; log-rank).
PFS, progression-free survival; OS, overall survival; EP,
etoposide/cisplatin; EC, etoposide/carboplatin; NLR, neutrophil to
lymphocyte ratio; LS, limited stage; ES, extensive stage. |
Among the patients in the EP regimen treated group
(n=40), the PFS of the high-NLR group (n=20) and low-NLR group
(n=20) was not statistically different (4.59±0.62 vs. 5.85±0.79
months; P=0.232; Fig. 2E). However,
among the patients in the EC regimen treated group (n=18), the PFS
of the low-NLR group (n=10) was significantly longer than in the
high-NLR group (n=8; 6.50±1.09 vs. 2.63±0.53; P=0.003; Fig. 2F).
Among the total cases that reached the OS time study
endpoint, 36 cases had been treated with the EP regimen and 15
cases with the EC regimen with the other 9 patients using neither
regimen. There was no statistically significant difference in OS
time (EP, 14.86±2.34 months vs. EC, 11.00±1.93 months; P=0.352;
Fig. 2G). In the high-NLR group, the
OS time of the EP regimen-treated patients (n=18) was significantly
longer than that of the EC regimen-treated patients (n=5) EP,
15.69±2.52 months vs. EC, 7.8±2.20 months; P=0.042; Fig. 2H). However, in the low-NLR group, the
OS time of the EP regimen patients (n=18) and the EC regimen (n=10)
was similar and no statistically significant difference was
observed (15.28±4.10 vs. 12.60±2.60 months; P=0.919; Fig. 2I). No significant differences in OS
time were identified within the low-NLR patients classified as
either LS or ES when comparing treatments with an EP or an EC
regimen (LS subgroup, P=0.378; ES subgroup, P=0.052).
The association between NLR and
thoracic radiotherapy benefit
A total of 34 patients received local radiotherapy.
In the low-NLR group, the PFS of patients who received thoracic
radiotherapy (n=10) was significantly longer than for those who did
not (n=24; 8.20±1.35 vs. 4.67±0.50 months; P=0.011; Fig. 3A). The increase in PFS was greater in
the low-NLR LS patients (with thoracic radiotherapy, 8.50±1.69
months vs. without thoracic radiotherapy, 5.00±0.69 months;
P=0.049; Fig. 3B), while with the ES
patients, no statistically significant difference was determined
(7.00±0.00 vs. 4.53±0.65 months; P=0.439) (data not shown). In the
high-NLR group, there were no significant differences between
patients who received radiotherapy and those who did not (6.67±0.33
vs. 3.82±0.45 months; P=0.095) (data not shown).
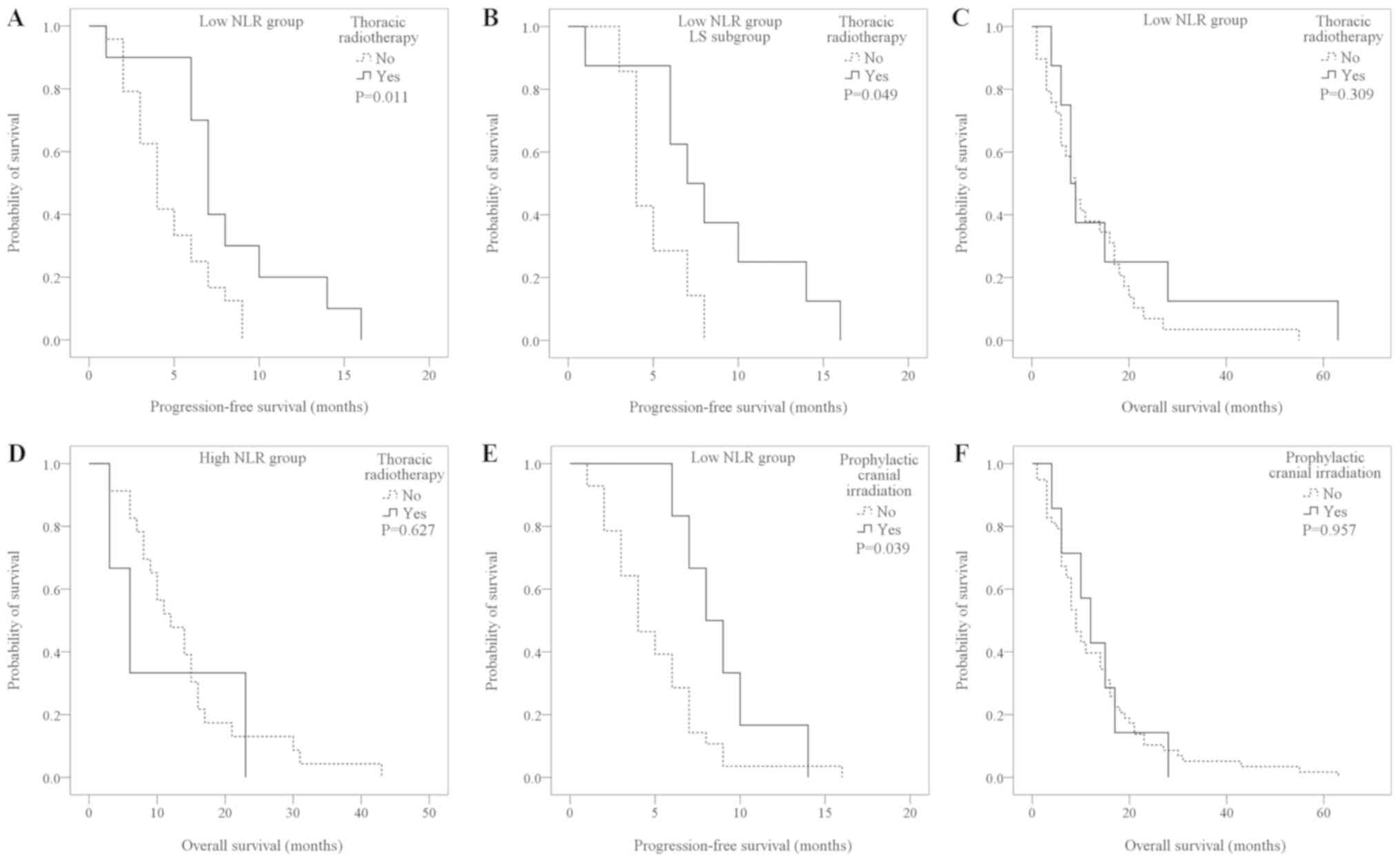 | Figure 3.Kaplan-Meier survival curves for the
PFS and OS time of patients with small cell lung cancer patients
who received radiotherapy and those that did not. (A) Patients in
the low-NLR group that received thoracic radiotherapy had a longer
PFS compared with those who did not (P=0.011; log-rank). (B) LS
patients in the low-NLR group who received radiotherapy had a
longer PFS compared with LS patients in the low-NLR group who did
not receive radiotherapy (P=0.049; log-rank). (C) Among the
patients in the low-NLR group who reached the OS time study
endpoint, those who received thoracic radiotherapy had a longer OS
than those who did not (P=0.309; log-rank). (D) In the high-NLR
group, the OS time was not statistically different between patients
who received thoracic radiotherapy and those that did not (P=0.627;
log-rank). (E) In the low-NLR group, patients who received
prophylactic cranial irradiation had a longer PFS than those who
did not (P=0.039, log-rank). (F) The OS time of patients who
received prophylactic cranial irradiation or not (P=0.957;
log-rank) in patients who received prophylactic cranial irradiation
vs. patients who did not receive prophylactic cranial irradiation.
PFS, progression-free survival; OS, overall survival; EP,
etoposide/cisplatin; EC, etoposide/carboplatin; NLR, neutrophil to
lymphocyte ratio; LS, limited stage; ES, extensive stage. |
Among the patients in the low-NLR group who reached
the OS time study endpoint, those who received thoracic
radiotherapy (n=8) had a longer OS time than those who did not
(n=26), although the difference was not statistically significant
(17.63±7.01 vs. 12.00±2.05 months; P=0.309; Fig. 3C). Receiving or not receiving
thoracic radiotherapy did not result in a statistically significant
difference in the OS time in the LS (11.50±3.63 vs. 10.62±2.28
months; P=0.804) or ES sub-groups (9.25±2.71 vs. 8.02±3.75 months;
P=0.136) (data not shown). In the high-NLR group, patients who
received radiotherapy had a similar OS time to those that did not
receive radiotherapy (10.67±6.22 vs. 14.13±1.99 months; P=0.627;
Fig. 3D).
Association between NLR, cranial
radiotherapy and PFS
Analysis of 34 patient cases without cranial
metastasis who received prophylactic cranial irradiation, a SCLC
treatment (4,19), following chemotherapy demonstrated
that low-NLR patients who received prophylactic cranial irradiation
(n=6) had a longer PFS than those who did not (n=28; 9.00±1.16 vs.
5.00±0.60 months; P=0.039; Fig. 3E).
In the high-NLR group, there were no significant differences
between patients who received prophylactic cranial irradiation
(n=2) and did not (n=28) (6.00±1.00 vs. 3.96±0.45 months; P=0.353).
There was no statistically significant difference observed in the
OS time between patients who received prophylactic cranial
irradiation and those who did not (13.14±3.03 vs. 13.16±1.59
months; P=0.957; Fig. 3F).
Discussion
The identification of cancer driver genes and the
emergence of targeted drug resulted in remarkable progress in
recent years in the field of non-small cell lung cancer treatment.
However, little has been achieved in the treatment of SCLC, with
chemotherapy still being the main therapy approach (20). SCLC is sensitive to chemotherapy and
radiotherapy, and previous studies have demonstrated that
chemotherapy relieves symptoms and improves survival time for the
majority of patients with SCLC (21,22). To
date, the regimen, which consists of etoposide in combination with
platinum, remains the first recommended regimen to treat SCLC
(23).
Carboplatin has a similar therapeutic effect to
cisplatin, but has fewer side effects and is better tolerated
(24–26). In multiple randomized controlled
clinical studies (24–26), carboplatin or cisplatin in
combination with etoposide had a similar clinical outcome. A
meta-analysis of 663 cases compared the curative effects of
carboplatin and cisplatin in SCLC, and determined that for either
LS or ES patients, the PFS (5.5 vs. 5.3 months) and the OS time
(9.6 vs. 9.4 months) of the two regimens were not significantly
different (7). In the present study,
the PFS was compared in patients receiving EP, EC and other
regimens. It was determined that the difference in PFS was not
significant (P=0.44) (data not shown). Deciding which treatment
option is optimal remains challenging.
A higher NLR level may be associated with an
increase in neutrophils and/or a decrease in lymphocytes.
Neutrophils regulate the activity of lymphocytes or natural killer
cells, thus inhibiting tumor growth (27). By contrast, lymphocytes may induce
the death of cytotoxic cells, and suppress the anti-tumor immune
response (28,29). In addition, a previous study reported
that wide deposition of abscess-induced neutrophil extracellular
traps (NETs) may segregate circulating tumor cells, promoting the
development of tumor metastasis (30). NETs may also protect circulating
tumor cells from being purged by the immune system by adhering to
and recruiting platelets (31). The
aforementioned studies serve as the theoretical basis for the
evaluation of tumor prognosis using NLR. Previous studies
investigating immunocytes in the tumor microenvironment have
demonstrated that tumor-associated macrophages (TAMs) and
tumor-associated neutrophils (TANs) are involved in tumor genesis
and development (32–34). TAMs are divided into M1- and
M2-types, while TANs are divided into N1- and N2-types, with M2-
and N2-types serving important roles in promoting tumor genesis,
development and metastasis (32–34).
Although current hematological analysis do not distinguish
tumor-associated neutrophil subtypes, an increase in the neutrophil
ratio and, therefore, an increase in the NLR has been reported when
examining blood samples from patients with a TAN ratio increase.
Thus, the NLR increase demonstrated in the current study may be a
TAN ratio increase. It would follow that the prognosis of patients
with SCLC may be affected by the increase of TANs, which may affect
tumor proliferation and drug resistance, manifesting as an NLR in
hematological analysis.
Deng et al (35) reported that a high NLR results in a
shorter PFS when investigating SCLC. Xie et al (36) and Hong et al (37) demonstrated that a high NLR may
predict poor prognosis for patients with SCLC. These results are
similar to the results obtained in the present study, in which
patients with a NLR of ≤3.80 (low NLR) achieved a significantly
better curative effect and longer PFS and OS when treated with the
EP regimen, compared with the EC regimen, with an even better PFS
observed in the LS patients. The PFS of the low-NLR patients was
longer in LS patients receiving the EC regimen. These results
suggested that EP and EC regimens had an equal effect in the
low-NLR group patients, whereas in the high-NLR group the EP
regimen was superior to the EC regimen; which was likely affected
by the tumor microenvironment. The tumor microenvironment contains
cytokines produced by TAMs and TANs that may influence carboplatin.
Wang et al (38) revealed
that interleukin 6 (IL6) decreases carboplatin treatment
sensitivity through activating the signal transducer and activator
of transcription 3 (STAT3) signaling pathway. Thus, it was
hypothesized that patients with a higher NLR had a higher ratio of
TAMs and TANs in the present study. This may resulted in the
presence of more cytokines, including IL6, to activate drug
resistance signaling pathways against carboplatin, including the
STAT3 signaling pathway, in order to induce drug resistance. This
warrants further investigation and monitoring the NLR may aid the
selection of a first line chemotherapy regimen.
Radiotherapy is another key method used to treat
patients with SCLC. Currently, thoracic radiotherapy is the
standard treatment for patients with LS SCLC (39). The present study demonstrated that
the low-NLR group patients had a longer PFS following thoracic
radiotherapy. Both thoracic and cranial radiotherapy are local
treatments, so higher NLR levels may be associated with systemic
tumor metastasis, tumor progression and tumor drug resistance.
Thus, it is possible that local treatment is more effective at
improving the prognosis of patients in the low-NLR group.
In conclusion, for the initially diagnosed patients
with SCLC, pre-treatment NLR may have prognostic prediction value.
In addition, it may aid in optimal first-line therapy selection.
The current study revealed that compared with the high-NLR group,
the low-NLR group had a longer PFS. Patients with high
pre-treatment NLR may benefit from EP over EC treatment. A
limitation of the present study was its retrospective nature and
the relatively small number of patients due to morbidity and
patients lost to follow-up. Future prospective clinical studies
with a larger sample size focusing on the underlying cellular
mechanisms are required to determine the significance of NLR in the
treatment of SCLC.
Acknowledgements
The authors would like to thank Professor Qiang Chen
(Departments of Oncology, Fujian Medical University Union Hospital)
for his contribution to the research.
Funding
The present study was supported by funding from the
Fujian Province Natural Genetic Health Joint Capital Project
awarded to Chunmei Shi (grant no. 2015J01397) and the Young and
Middle-Aged Teachers' Scientific Research Project in Fujian
Province awarded to Zhangchi Pan (grant no. JAT170226).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZCP and LZ contributed to the conception of the
study, analyzed the data and wrote the manuscript. CL, XBH, SFS,
XYL and CMS contributed to the design of the study, and conducted
additional analyses. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Institutional Review Board of Fujian Medical
University Union Hospital (Institutional Review Board no.
2017KY084). Informed consent was obtained from patients included in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klutker G, Sauer R and Fietkau R: Combined
treatment modality in small cell lung cancer: The impact of
radiotherapy on survival. Strahlenther Onkol. 184:61–66. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurup A and Hanna NH: Treatment of small
cell lung cancer. Crit Rev Oncol Hematol. 52:117–126. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Puglisi M, Dolly S, Faria A, Myerson JS,
Popat S and O'Brien ME: Treatment options for small cell lung
cancer-do we have more choices? Br J Cancer. 102:629–638. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaspar LE, Mcnamara EJ, Gay EG, Putnam JB,
Crawford J, Herbst RS and Bonner JA: Small-cell lung cancer:
Prognostic factors and changing treatment over 15 years. Clin Lung
Cancer. 10:115–122. 2009.
|
|
6
|
Murray N and Turrisi AT III: A review of
first-line treatment for small-cell lung cancer. J Thorac Oncol.
1:270–278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossi A, Di Maio M, Chiodini P, Rudd RM,
Okamoto H, Skarlos DV, Früh M, Qian W, Tamura T, Samantas E, et al:
Carboplatin- or cisplatin-based chemotherapy in first-line
treatment of small-cell lung cancer: The COCIS meta-analysis of
individual patient data. J Clin Oncol. 30:1692–1698. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Dwyer PJ and Calvert AH: Platinum
analogs. Cancer: Principles and Practice of Oncology. DeVita VT,
Lawrence TS and Rosenbery SA: 10th. Lippincott Williams &
Wilkins; Philidelphia, PA: pp. 199–207. 2015
|
|
9
|
Go RS and Adjei AA: Review of the
comparative pharmacology and clinical activity of cisplatin and
carboplatin. J Clin Oncol. 17:409–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Callaghan DS, O'Donnell D, O'Connell F
and O'Byrne KJ: The role of inflammation in the pathogenesis of
non-small cell lung cancer. J Thorac Oncol. 5:2024–2036. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gondo T, Nakashima J, Ohno Y, Choichiro O,
Horiguchi Y, Namiki K, Yoshioka K, Ohori M, Hatano T and Tachibana
M: Prognostic value of neutrophil-to-lymphocyte ratio and
establishment of novel preoperative risk stratification model in
bladder cancer patients treated with radical cystectomy. Urology.
79:1085–1091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mano Y, Shirabe K, Yamashita Y, Harimoto
N, Tsujita E, Takeishi K, Aishima S, Ikegami T, Yoshizumi T,
Yamanaka T and Maehara Y: Preoperative neutrophil-to-lymphocyte
ratio is a predictor of survival after hepatectomy for
hepatocellular carcinoma: A retrospective analysis. Ann Surg.
258:301–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng YB, Zhao W, Liu B, Lu LG, He X,
Huang JW, Li Y and Hu BS: The blood neutrophil-to-lymphocyte ratio
predicts survival in patients with advanced hepatocellular
carcinoma receiving Sorafenib. Asian Pac J Cancer Prev.
14:5527–5531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao Y, Yuan D, Liu H, Gu X and Song Y:
Pretreatment neutrophil to lymphocyte ratio is associated with
response to therapy and prognosis of advanced non-small cell lung
cancer patients treated with first-line platinum-based
chemotherapy. Cancer Immunol Immunother. 62:471–479. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Unal D, Eroglu C, Kurtul N, Oguz A and
Tasdemir A: Are neutrophil/lymphocyte and platelet/lymphocyte rates
in patients with non-small cell lung cancer associated with
treatment response and prognosis? Asian Pac J Cancer Prev.
14:5237–5242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kacan T, Babacan NA, Seker M, Yucel B,
Bahceci A, Eren AA, Eren MF and Kilickap S: Could the neutrophil to
lymphocyte ratio be a poor prognostic factor for non small cell
lung cancers? Asian Pac J Cancer Prev. 15:2089–2094. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Micke P, Faldum A, Metz T, Beeh KM,
Bittinger F, Hengstler JG and Buhl R: Staging small cell lung
cancer: Veterans administration lung study group versus
international association for the study of lung cancer-what limits
limited disease? Lung Cancer. 37:271–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naidoo J, Kehoe M, Sasiadek W, Hacking D
and Calvert P: Prophylactic cranial irradiation in small cell lung
cancer: A single institution experience. Ir J Med Sci. 183:129–132.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Byers LA and Rudin CM: Small cell lung
cancer: Where do we go from here? Cancer. 121:664–672. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ettinger DS and Aisner J: Changing face of
small-cell lung cancer: Real and artifact. J Clin Oncol.
24:4526–4527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Evans W, Shepherd FA, Feld R, Osoba D,
Dang P and Deboer G: VP-16 and cisplatin as first-line therapy for
small-cell lung cancer. J Clin Oncol. 3:1471–1477. 1885. View Article : Google Scholar
|
|
24
|
Hatfield LA, Huskamp HA and Lamont EB:
Survival and toxicity after cisplatin plus etoposide versus
carboplatin plus etoposide for extensive-stage small-cell lung
cancer in elderly patients. J Oncol Pract. 12:666–673. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skarlos DV, Samantas E, Kosmidis P,
Fountzilas G, Angelidou M, Palamidas P, Mylonakis N, Provata A,
Papadakis E, Klouvas G, et al: Randomized comparison of
etoposide-cisplatin vs. etoposide-carboplatin and irradiation in
small-cell lung cancer. A Hellenic Co-operative Oncology Group
study. Ann Oncol. 5:601–607. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okamoto H, Watanabe K, Kunikane H,
Yokoyama A, Kudoh S, Asakawa T, Shibata T, Kunitoh H, Tamura T and
Saijo N: Randomised phase III trial of carboplatin plus etoposide
vs split doses of cisplatin plus etoposide in elderly or poor-risk
patients with extensive disease small-cell lung cancer: JCOG 9702.
Br J Cancer. 97:162–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pillar J, Kamp VM, van Hoffen E, Visser T,
Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P and Koenderman
L: A subset of neutronphils in human systemic inflammation inhibits
t cell responses through mac-1. J Clin Invest. 122:327–336. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cools-Lartigue J, Spicer J, McDonald B,
Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P and Ferri L:
Neutrophil extracellular traps sequester circulating tumor cells
and promote metastasis. J Clin Invest. 123:3446–3458. 2013.
View Article : Google Scholar
|
|
31
|
Demers M and Wagner DD: Neutrophil
extracellular traps: A new link to cancer-associated thrombosis and
potential implications for tumor progression. Oncoimmunology.
2:229462013. View Article : Google Scholar
|
|
32
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunology. 11:889–896. 2010. View Article : Google Scholar
|
|
34
|
Allavena P, Sica A, Garlanda C and
Mantovani A: The Yin-Yang of tumor-associated macrophages in
neoplastic progression and immune surveillance. Immunol Rev.
222:155–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng M, Ma X, Liang X, Zhu C and Wang M:
Are pretreatment neutrophil-lymphocyte ratio and
platelet-lymphocyte ratio useful in predicting the outcomes of
patients with small-cell lung cancer? Oncotarget. 8:37200–37207.
2017.PubMed/NCBI
|
|
36
|
Xie D, Marks R, Zhang M, Jiang G, Jatoi A,
Garces Y, Mansfield A, Molina J and Yang P: Nomograms predict
overall survival for patients with small-cell lung Cancer
incorporating pretreatment peripheral blood markers. J Thorac
Oncol. 10:1213–1220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hong X, Cui B, Wang M, Yang Z, Wang L and
Xu Q: Systemic immune-inflammation index, based on platelet counts
and neutrophil-lymphocyte ratio, is useful for predicting prognosis
in small cell lung cancer. Tohoku J Exp Med. 236:297–304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang ZY, Zhang JA, Wu XJ, Liang YF, Lu YB,
Gao YC, Dai YC, Yu SY, Jia Y, Fu XX, et al: IL-6 inhibition reduces
STAT3 activation and enhances the antitumor effect of carboplatin.
Mediators Inflamm. 2016:80264942016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pignon JP, Arriagada R, Ihde DC, Johnson
DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS, Lebeau B, et
al: A meta-analysis of thoracic radiotherapy for small-cell lung
cancer. New Engl J Med. 327:1618–1624. 1992. View Article : Google Scholar : PubMed/NCBI
|