Introduction
Prostate, lung and colorectal cancer account for
~42% of all cancer types in men, and prostate cancer (PCa) accounts
for almost one in five newly diagnosed cancer cases in the United
States (1). In the United States,
PCa is the most common cancer in men, and PCa-specific mortality
ranks second, after that of lung cancer (2). Although four well-established risk
factors have been identified, namely increased age, ethnicity,
obesity and family history, other potential factors that determine
the risk of developing PCa are not well known (3,4).
Histologically, most cases of PCa are classified as acinar
adenocarcinoma and have a poor prognosis (5).
There is increasing awareness that cancer metastasis
plays an important role in the survival of PCa patients. Treatment
decisions for PCa patients differ according to both patient- and
disease-related factors. Radical prostatectomy (RP) is the standard
treatment for clinically localized PCa, and it provides adequate
local control in organ-confined disease (6). Traditionally, RP is discouraged in
patients with advanced disease, owing to the increased complication
rate and treatment-related morbidity (7). In recent years, it has been suggested
that prostatectomy may provide a benefit for metastatic PCa
patients (8); however, for advanced
disease with site-specific metastasis of the bone, brain, liver or
lung, there is insufficient evidence to support the efficacy of
prostatectomy, particularly RP which includes including total
prostatectomy and cystoprostatectomy.
To the best of our knowledge, analyses of the
prognostic value of organ-specific metastasis based on large
population-based data for PCa are lacking. Thus, in the present
study, the data pertaining to metastatic prostate adenocarcinoma
patients registered in the Surveillance, Epidemiology and End
Results (SEER) database was reviewed, and the prognostic outcomes
were analyzed to assess the efficacy of prostatectomy among
patients with bone metastasis only.
Materials and methods
Data collection/selection and
description of participants
The SEER-18 Regs Research Data released in November
2017 was retrieved using the SEER*Stat software version 8.3.5
(https://seer.cancer.gov/seerstat/software/) (National
Cancer Institute; National Institutes of Health, USA). Detailed
information about distant metastatic sites was updated to 2013 and
was not available before the year 2010. Therefore, the current
study was restricted to patients registered between January 2010
and December 2013. The survival data of PCa patients was monitored
until December 2017. In order to identify patients with metastatic
prostate adenocarcinoma, cases were included with the primary site
stated as ‘Prostate’ and the following codes: ICD-O-3 Hist/behave,
malignant=‘8140/3: Adenocarcinoma, NOS’. Patients with stage I, II
and III PCa according to the 7th AJCC prostate cancer
classification criteria were excluded (9). Cases with unknown race data, unknown
marital status data, unknown survival data and unknown specific
metastatic site data were excluded. Only data from patients with
single primary PCa were extracted. Extracted data included the
following: Marital status at diagnosis, age at diagnosis, sex, race
(white, black or other), grade, TNM stage according to the AJCC
(7th edition, 2010), RX Summ-Surg Prim Site (surgical information
of primary cancer site), radiation sequence with surgery, CS mets
at DX-bone (bone metastases since 2010), CS mets at DX-brain (brain
metastases since 2010), CS mets at DX-liver (liver metastases since
2010), CS mets at DX-lung (lung metastases since 2010),
cancer-specific factor 1 (serum PSA levels), survival and vital
status record (10). At present,
systemic therapy data are not available in the SEER database.
Exclusion criteria were as follows: i) Patients with unclear
derived M stage according to the AJCC (7th edition); ii) patients
with unclear RX Summ-Surg Prim Site (1998+); iii) patients
classified as clinical stage I/II or III prostate adenocarcinoma
according to the AJCC 7th edition.
Study variables
Patient characteristics were extracted from the SEER
database, including marital status, race, age at diagnosis, TNM
stage at diagnosis, primary tumor site, grade, surgery condition,
radiotherapy condition, bone metastasis, brain metastasis, liver
metastasis and lung metastasis. According to the AJCC 7th edition
criteria of TNM stage and clinical stage of prostate cancer, PCa
patients with T4, N0, M0 any T stage, N1, M0, and any T stage, any
N stage, M1 were classified as stage IV patients.
Statistical analysis
In the present study, the χ2 test was
used to compare the clinicopathological characteristics among cases
with and without bone metastasis. Kaplan-Meier analysis was used to
build survival curves and log-rank testing was employed for the
comparison of long-term survival outcomes. The Cox proportional
hazards regression model was employed to perform univariate and
multivariate analyses of the hazard ratios with corresponding 95%
confidence intervals (CIs) of the study variates. Associations
between marital status, age at diagnosis, race, histological grade
and TNM stage at diagnosis were examined by binary logistic
regression. A two-tailed P-value of <0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS software 20.0 (IBM
Corporation).
Results
Incidence of different metastatic
sites among stage IV PCa patients
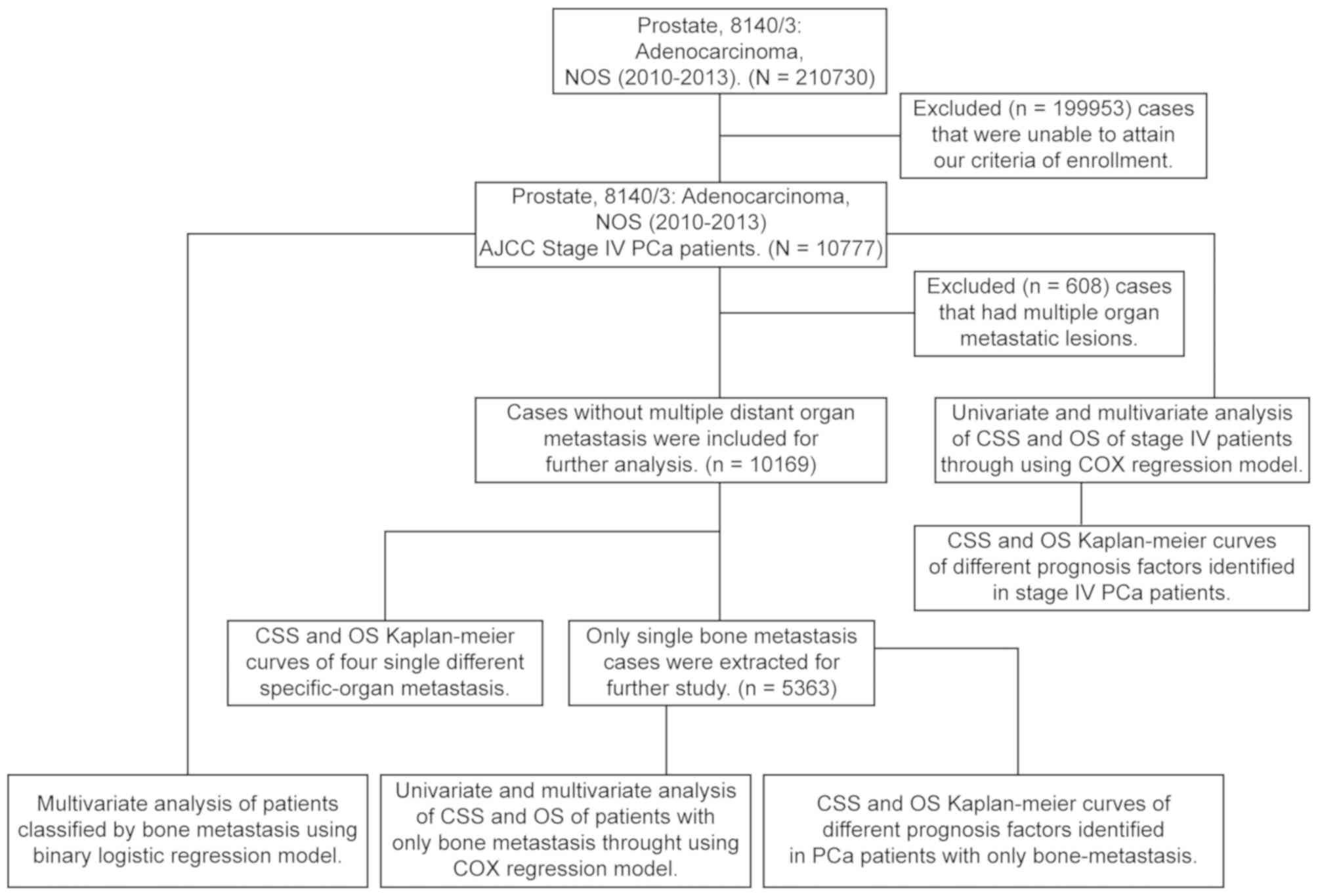
The selection criteria are shown in Fig. 1. Among 210,730 cases identified in
the SEER database, a total of 10,777 patients with stage IV
prostatic adenocarcinoma between January 2010 and December 2013
were included in the present study.
Table I summarizes
the distribution of different clinical characteristics of PCa
patients. The majority of patients (n=5,963, 55.33%) presented with
bone metastasis, followed by lung (n=512, 4.75%), liver (n=280,
2.60%) and brain (n=83, 0.77%) metastasis. Prostatectomy (RP,
including total prostatectomy and cystoprostatectomy) was performed
in 2,981 (27.66%) patients, and 1,085 (10.07%) patients received
radiation therapy. There were 4,258 (39.51%) patients with lymph
node metastasis.
 | Table I.Baseline characteristics of patients
with stage IV prostate adenocarcinoma (n=10,777) in the present
study. |
Table I.
Baseline characteristics of patients
with stage IV prostate adenocarcinoma (n=10,777) in the present
study.
| Characteristics | Number | Percentage |
|---|
| Age, years |
|
|
|
<50 | 426 | 3.95 |
| ≥50 | 10,351 | 96.05 |
| Race |
|
|
|
White | 8,179 | 75.89 |
|
Black | 1,929 | 17.90 |
|
Othersa | 669 | 6.21 |
| Marital status |
|
|
|
Married | 6,941 | 64.41 |
|
Single/unmarried | 3,836 | 35.59 |
| Grade |
|
|
| I | 21 | 0.19 |
| II | 488 | 4.83 |
| III | 8,216 | 76.24 |
| IV | 81 | 0.75 |
|
Unknown | 1,971 | 18.29 |
| Bone
metastasis |
|
|
|
Yes | 5,963 | 55.33 |
| No | 4,814 | 44.67 |
| Brain
metastasis |
|
|
|
Yes | 83 | 0.77 |
| No | 10,694 | 99.23 |
| Liver
metastasis |
|
|
|
Yes | 280 | 2.60 |
| No | 10,497 | 97.40 |
| Lung
metastasis |
|
|
|
Yes | 512 | 4.75 |
| No | 10,265 | 95.25 |
| Lymph node
metastasis |
|
|
|
Yes | 4,258 | 39.51 |
| No | 5,250 | 48.71 |
|
Unknown | 1,269 | 11.78 |
| Radiation
therapy |
|
|
|
Yes | 1,085 | 10.07 |
| No | 9,692 | 89.93 |
| Prostatectomy |
|
|
|
Yes | 2,981 | 27.66 |
| No | 7,761 | 72.01 |
|
Unknown | 35 | 0.32 |
Identification of statistically
significant variates with regard to survival outcomes in patients
with stage IV PCa
Several variates were identified by univariate and
multivariate analysis of cancer specific-survival (CSS) and overall
survival (OS) in PCa patients using Cox hazards regression models.
Single/unmarried status, age ≥50 years, black race, M1 stage, bone
metastasis, liver metastasis and lung metastasis were associated
with worse CSS and OS. Races classified as ‘other’ (American
Indian/Alaska native and Asian/Pacific Islander), radiation therapy
and prostatectomy were associated with better CSS and OS (Tables II and III). Based on multivariate Cox regression
analysis, the following factors were significantly associated with
poor OS and/or CSS: Single/unmarried status [hazard ratios (HRs),
1.164 (CSS) and 1.211 (OS), P<0.001], age ≥50 years [HRs, 1.309
(CSS), P=0.034 and 1.421 (OS), P=0.003], black race vs. white race;
[HR, 1.151 (CSS), P=0.009], M1 stage [HRs, 3.096 (CSS) and 2.419
(OS), P<0.001] and PSA level >20 ng/ml [HR, 1.27 (OS),
P=0.035]. On the other hand, ‘other’ race (American Indian/Alaska
native and Asian/Pacific Islander) was a significant predictor of
better CSS and OS (vs. white race; HRs, 0.750, P=0.005 and 0.774,
P=0.004, respectively), as was prostatectomy (HR, 0.147 and 0.143,
respectively, P<0.001). Radiation therapy was a significant
predictor of better OS only (HR, 0.756, P=0.003) (Table III). Next, Kaplan-Meier survival
analysis was performed to calculate the differences in OS and CSS
by the variates identified through multivariate Cox hazards
regression analysis (Fig. 2). The
3-year CSS rate of patients who received prostatectomy was 97.3%,
compared with 54.3% in patients who did not undergo prostatectomy
(P<0.0001). The 3-year OS rate of patients who received
prostatectomy was 96.0%, whereas that of patients who did not was
only 47.4% (P<0.001) (Fig. 2E).
Married status, age <50 years and radiation therapy also led to
higher 3-year CSS and OS rates (Fig. 2A,
B and D). By contrast, M1 stage, bone metastasis, liver
metastasis, lung metastasis and black race were associated with
reduced survival in stage IV PCa patients (Fig. 2C and F-I).
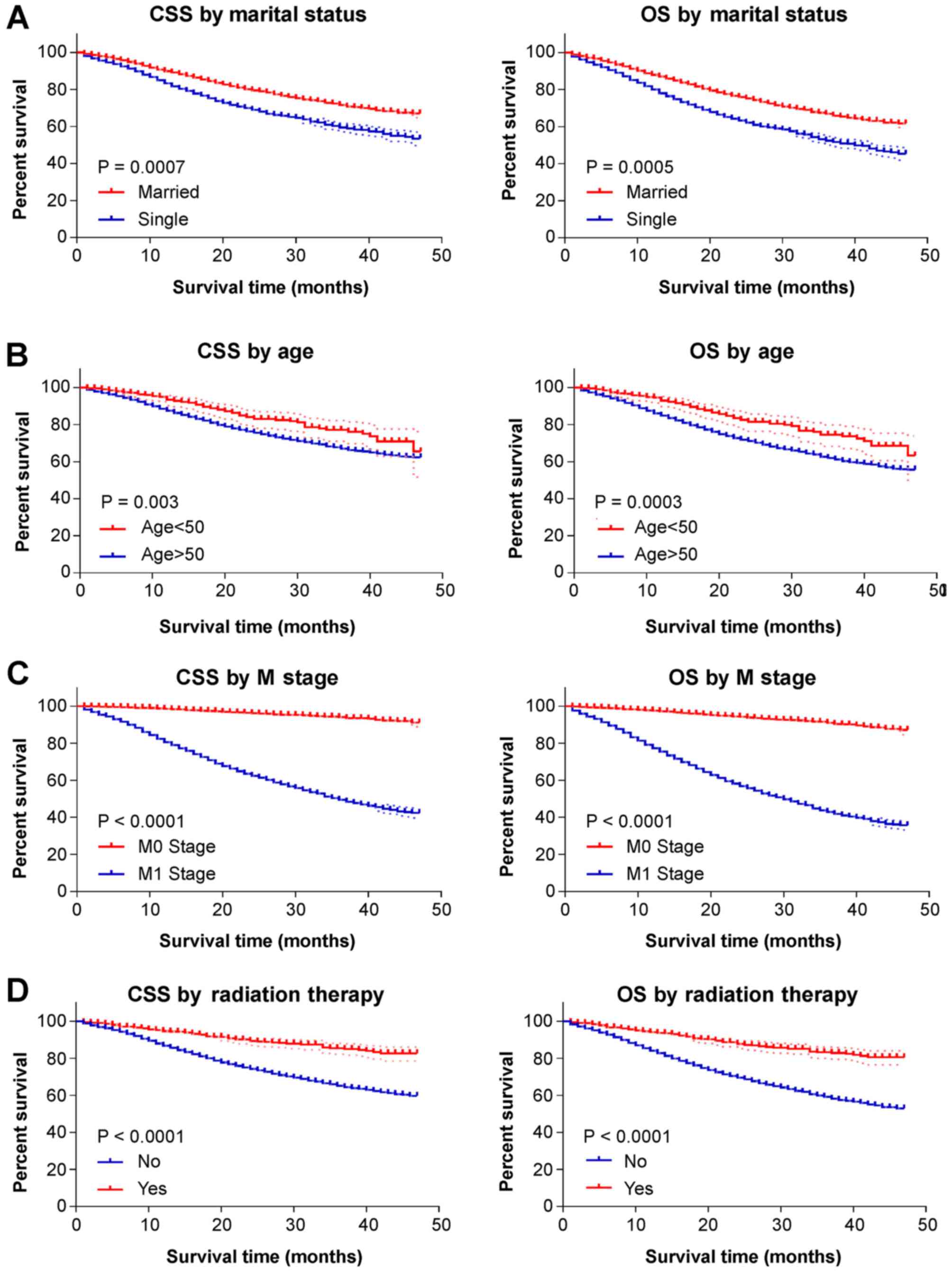 | Figure 2.Kaplan-Meier curves for CSS and OS by
different study variates. (A-D) The dotted lines reveal the 95%
confidence interval of each points on the Kaplan-Meier curve. The
marital status ‘single’ includes divorced, single (never married),
separated and widowed. Prostatectomy includes: Radical
prostatectomy, NOS; total prostatectomy, NOS; excised prostate,
prostatic capsule, ejaculatory ducts, seminal vesicle and including
a narrow cuff of bladder neck; Prostatectomy, NOS. CSS,
cancer-specific survival; OS, overall survival; NOS, not otherwise
specified. Kaplan-Meier curves for CSS and OS by different study
variates. (E-I) The dotted lines reveal the 95% confidence interval
of each points on the Kaplan-Meier curve. The marital status
‘single’ includes divorced, single (never married), separated and
widowed. Prostatectomy includes: Radical prostatectomy, NOS; total
prostatectomy, NOS; excised prostate, prostatic capsule,
ejaculatory ducts, seminal vesicle and including a narrow cuff of
bladder neck; Prostatectomy, NOS. CSS, cancer-specific survival;
OS, overall survival; NOS, not otherwise specified. |
 | Table II.Univariate analysis of CSS and OS in
10,777 patients with advanced prostate cancer. |
Table II.
Univariate analysis of CSS and OS in
10,777 patients with advanced prostate cancer.
|
| CSS | OS |
|---|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Marital status |
|
|
|
|
|
|
|
|
|
Married |
| 1.000 |
|
|
| 1.000 |
|
|
|
Single | <0.001 | 1.608 | 1.479 | 1.748 | <0.001 | 1.647 | 1.528 | 1.776 |
| Age, years |
|
|
|
|
|
|
|
|
|
<50 |
| 1.000 |
|
|
| 1.000 |
|
|
|
≥50 | <0.001 | 1.620 | 1.263 | 2.077 | <0.001 | 1.775 | 1.404 | 2.243 |
| Race |
|
|
|
|
|
|
|
|
|
White |
| 1.000 |
|
|
| 1.000 |
|
|
|
Black | 0.016 | 1.137 | 1.024 | 1.262 | 0.003 | 1.152 | 1.048 | 1.265 |
|
Othera | 0.005 | 0.754 | 0.618 | 0.920 | 0.01 | 0.795 | 0.667 | 0.947 |
| Grade |
|
|
|
|
|
|
|
|
| Well
differentiated |
| 1.000 |
|
|
| 1.000 |
|
|
|
Moderately differentiated | 0.168 | 0.440 | 0.136 | 1.416 | 0.006 | 0.305 | 0.132 | 0.707 |
| Poorly
differentiated | 0.934 | 0.953 | 0.307 | 2.961 | 0.198 | 0.591 | 0.265 | 1.316 |
|
Undifferentiated | 0.144 | 2.418 | 0.740 | 7.897 | 0.486 | 1.360 | 0.573 | 3.228 |
| Tumor stage |
|
|
|
|
|
|
|
|
| T0 |
| 1.000 |
|
|
| 1.000 |
|
|
| T1 | <0.001 | 0.434 | 0.274 | 0.686 | 0.004 | 0.519 | 0.332 | 0.811 |
| T2 | <0.001 | 0.411 | 0.26 | 0.648 | 0.002 | 0.488 | 0.313 | 0.761 |
| T3 | <0.001 | 0.152 | 0.095 | 0.244 | <0.001 | 0.175 | 0.111 | 0.276 |
| T4 | <0.001 | 0.272 | 0.172 | 0.430 | <0.001 | 0.32 | 0.205 | 0.501 |
| Node stage |
|
|
|
|
|
|
|
|
| N0 |
| 1.000 |
|
|
| 1.000 |
|
|
| N1 | <0.001 | 0.627 | 0.569 | 0.692 | 0.340 | 0.956 | 0.872 | 1.048 |
| Metastasis
stage |
|
|
|
|
|
|
|
|
| M0 |
| 1.000 |
|
|
| 1.000 |
|
|
| M1 | <0.001 | 11.147 | 9.472 | 13.121 | <0.001 | 2.419 | 1.979 | 2.957 |
| Radiation
therapy |
|
|
|
|
|
|
|
|
| No |
| 1.000 |
|
|
| 1.000 |
|
|
|
Yes | <0.001 | 0.361 | 0.297 | 0.439 | <0.001 | 0.337 | 0.281 | 0.405 |
| Prostatectomy
surgery |
|
|
|
|
|
|
|
|
| No |
| 1.000 |
|
|
| 1.000 |
|
|
|
Yes | <0.001 | 0.041 | 0.031 | 0.055 | <0.001 | 0.049 | 0.038 | 0.0620 |
| PSA level,
ng/ml |
|
|
|
|
|
|
|
|
|
≤20 |
| 1.000 |
|
|
| 1.000 |
|
|
|
>20 | 0.056 | 1.241 | 0.994 | 1.569 | 0.019 | 1.271 | 1.032 | 1.565 |
| Distant lymph node
metastasis |
|
|
|
|
|
|
|
|
|
Yes |
| 1.000 |
|
|
| 1.000 |
|
|
| No | <0.001 | 0.627 | 0.569 | 0.692 | <0.001 | 0.61 | 0.558 | 0.666 |
| Bone
metastasis |
|
|
|
|
|
|
|
|
|
Yes |
| 1.000 |
|
|
| 1.000 |
|
|
| No | <0.001 | 0.167 | 0.149 | 0.188 | <0.001 | 0.192 | 0.173 | 0.212 |
| Brain
metastasis |
|
|
|
|
|
|
|
|
|
Yes |
| 1.000 |
|
|
| 1.000 |
|
|
| No | <0.001 | 0.303 | 0.223 | 0.413 | <0.001 | 0.327 | 0.245 | 0.437 |
| Liver
metastasis |
|
|
|
|
|
|
|
|
|
Yes |
| 1.000 |
|
|
| 1.000 |
|
|
| No | <0.001 | 0.233 | 0.197 | 0.276 | <0.001 | 0.256 | 0.219 | 0.300 |
| Lung
metastasis |
|
|
|
|
|
|
|
|
|
Yes |
| 1.000 |
|
|
| 1.000 |
|
|
| No | <0.001 | 0.360 | 0.312 | 0.416 | <0.001 | 0.384 | 0.336 | 0.438 |
 | Table III.Multivariate analysis of CSS and OS
in 10,777 patients with advanced prostate cancer. |
Table III.
Multivariate analysis of CSS and OS
in 10,777 patients with advanced prostate cancer.
|
| CSS | OS |
|---|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Marital status |
|
|
|
|
|
|
|
|
|
Married |
| 1.000 |
|
|
| 1.000 |
|
|
|
Single | <0.001 | 1.164 | 1.069 | 1.269 | <0.001 | 1.211 | 1.121 | 1.309 |
| Age, years |
|
|
|
|
|
|
|
|
|
<50 |
| 1.000 |
|
|
| 1.000 |
|
|
|
≥50 | 0.034 | 1.309 | 1.021 | 1.682 | 0.003 | 1.421 | 1.124 | 1.798 |
| Race |
|
|
|
|
|
|
|
|
|
White |
| 1.000 |
|
|
| 1.000 |
|
|
|
Black | 0.009 | 1.151 | 1.024 | 1.262 | 0.527 | 0.971 | 0.881 | 1.067 |
|
Othera | 0.005 | 0.750 | 0.618 | 0.921 | 0.004 | 0.774 | 0.649 | 0.922 |
| Grade |
|
|
|
|
|
|
|
|
| Well
differentiated |
| 1.000 |
|
|
| 1.000 |
|
|
|
Moderately differentiated | 0.146 | 0.419 | 0.111 | 1.352 | 0.004 | 0.296 | 0.128 | 0.685 |
| Poor
differentiated | 0.653 | 0.771 | 0.248 | 2.397 | 0.084 | 0.493 | 0.221 | 1.101 |
|
Undifferentiated | 0.477 | 1.537 | 0.470 | 5.031 | 0.801 | 0.895 | 0.376 | 2.127 |
| Tumor stage |
|
|
|
|
|
|
|
|
| T0 |
| 1.000 |
|
|
| 1.000 |
|
|
| T1 | 0.065 | 0.643 | 0.402 | 1.028 | 0.266 | 0.773 | 0.491 | 1.217 |
| T2 | 0.072 | 0.653 | 0.410 | 1.039 | 0.279 | 0.780 | 0.497 | 1.224 |
| T3 | 0.071 | 0.641 | 0.396 | 1.037 | 0.179 | 0.727 | 0.456 | 1.158 |
| T4 | 0.742 | 1.081 | 0.678 | 1.725 | 0.358 | 1.236 | 0.786 | 1.944 |
| Node stage |
|
|
|
|
|
|
|
|
| N0 |
| 1.000 |
|
|
| 1.000 |
|
|
| N1 | 0.950 | 1.003 | 0.906 | 1.111 | 0.340 | 0.956 | 0.872 | 1.048 |
| Metastasis
stage |
|
|
|
|
|
|
|
|
| M0 |
| 1.000 |
|
|
| 1.000 |
|
|
| M1 | <0.001 | 3.096 | 2.443 | 3.923 | <0.001 | 2.419 | 1.979 | 2.957 |
| Radiation
therapy |
|
|
|
|
|
|
|
|
| No |
| 1.000 |
|
|
| 1.000 |
|
|
|
Yes | 0.066 | 0.829 | 0.679 | 1.012 | 0.003 | 0.756 | 0.628 | 0.911 |
| Prostatectomy
surgery |
|
|
|
|
|
|
|
|
| No |
| 1.000 |
|
|
| 1.000 |
|
|
|
Yes | <0.001 | 0.147 | 0.105 | 0.206 | <0.001 | 0.143 | 0.108 | 0.189 |
| PSA level,
ng/ml |
|
|
|
|
|
|
|
|
|
≤20 |
| 1.000 |
|
|
| 1.000 |
|
|
|
>20 | 0.069 | 1.236 | 0.984 | 1.553 | 0.035 | 1.251 | 1.016 | 1.541 |
| Distant lymph node
metastasis |
|
|
|
|
|
|
|
|
|
Yes |
| 1.000 |
|
|
| 1.000 |
|
|
| No | 0.722 | 1.019 | 0.92 | 1.128 | 0.384 | 0.96 | 0.875 | 1.053 |
| Bone
metastasis |
|
|
|
|
|
|
|
|
|
Yes |
| 1.000 |
|
|
| 1.000 |
|
|
| No | <0.001 | 0.739 | 0.633 | 0.863 | <0.001 | 0.775 | 0.674 | 0.891 |
| Brain
metastasis |
|
|
|
|
|
|
|
|
|
Yes |
| 1.000 |
|
|
| 1.000 |
|
|
| No | 0.115 | 0.774 | 0.562 | 1.064 | 0.102 | 0.779 | 0.579 | 1.049 |
| Liver
metastasis |
|
|
|
|
|
|
|
|
|
Yes |
| 1.000 |
|
|
| 1.000 |
|
|
| No | <0.001 | 0.472 | 0.396 | 0.563 | <0.001 | 0.501 | 0.424 | 0.589 |
| Lung
metastasis |
|
|
|
|
|
|
|
|
|
Yes |
| 1.000 |
|
|
| 1.000 |
|
|
| No | <0.001 | 0.776 | 0.667 | 0.902 | 0.001 | 0.794 | 0.691 | 0.912 |
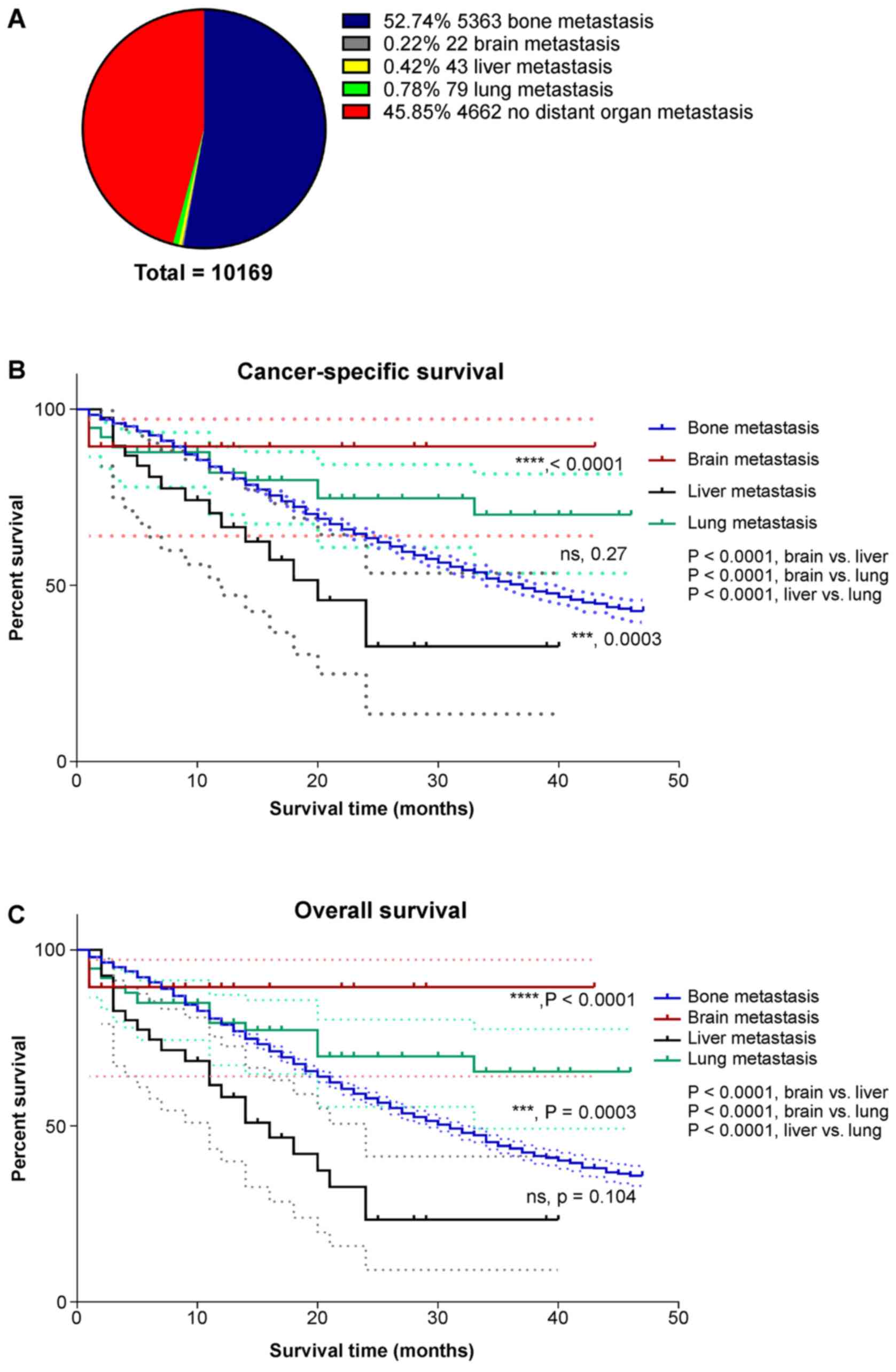
Impact of site-specific metastasis on
survival outcomes
As it was found that metastasis to different organs
may induce different survival outcomes in stage IV PCa patients,
Kaplan-Meier survival analysis was performed to compare OS in
advanced PCa patients with metastasis to the bone, brain, liver and
lung. A total of 608 patients with metastatic lesions in multiple
organs were excluded, and 10,169 patients were included in the
analysis. Of these patients, 52.74% had bone metastasis, 0.22% had
brain metastasis, 0.42% had liver metastasis, and 0.78% had lung
metastasis (Fig. 3A). It was
demonstrated that PCa patients with only liver metastasis had the
worst 3-year CSS and OS rates (31.9 and 22.8%, respectively). The
3-year CSS and OS rates of patients with only bone metastasis were
49.6 and 41.6%, respectively. Patients with only lung metastasis
(3-year CSS and OS, 79.9 and 63.7%) had improved OS compared with
those with only bone or liver metastasis (Fig. 3B and C).
Identification of risk factors for
bone metastasis in patients with stage IV PCa
As the present results indicated that stage IV PCa
was most prone to distant metastasis to the bone, multivariate
binary logistic regression analysis was performed to identify risk
factors of bone metastasis in 10,777 patients with advanced PCa.
The results suggested that single/unmarried status, black race, NX
stage and grade IV (undifferentiated adenocarcinoma) were risk
factors for bone metastasis. T3 and T4 stage patients, as well as
N1 stage patients, were less likely to have bone metastasis
(Table IV).
 | Table IV.Multivariate binary logistic
regression analysis of patient characteristics classified by bone
metastasis at diagnosis (n=10,777). |
Table IV.
Multivariate binary logistic
regression analysis of patient characteristics classified by bone
metastasis at diagnosis (n=10,777).
|
| Bone
metastasis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Variable | No | Yes | Pearson
χ2 P-value | Odds ratio | 95% CI | P-value |
|---|
| Total patients | 4,814 (44.7) | 5,963 (55.3) |
|
|
|
|
|
| Marital status |
|
| <0.001 |
|
|
|
|
|
Married | 3,457 (49.8) | 3,484 (50.2) |
| 1.000 |
|
|
|
|
Single | 1,357 (35.4) | 2,479 (64.6) |
| 1.671 | 1.505 | 1.856 | <0.001 |
| Age, years |
|
| 0.001 |
|
|
|
|
|
<50 | 224 (52.6) | 202 (47.4) |
| 1.000 |
|
|
|
|
≥50 | 4,590 (44.3) | 5761 (55.7) |
| 1.044 | 0.818 | 1.334 | 0.729 |
| Race |
|
| <0.001 |
|
|
|
|
|
White | 3,783 (46.3) | 4396 (53.7) |
| 1.000 |
|
|
|
|
Black | 735 (38.1) | 1194 (61.9) |
| 1.155 | 1.013 | 1.317 | 0.032 |
|
Othera | 296 (44.2) | 373 (55.8) |
| 1.093 | 0.893 | 1.338 | 0.387 |
| Grade |
|
| <0.001 |
|
|
|
|
| Well
differentiated | 10 (47.6) | 11 (52.4) |
| 1.000 |
|
|
|
|
Moderately differentiated | 306 (62.7) | 182 (37.3) |
| 0.488 | 0.168 | 1.411 | 0.907 |
| Poorly
differentiated | 4,122 (50.2) | 4094 (49.8) |
| 1.064 | 0.376 | 3.01 | 0.581 |
|
Undifferentiated | 32 (39.5) | 49 (60.5) |
| 1.391 | 1.01 | 8.26 | 0.048 |
|
Unknown | 344 (17.5) | 1627 (82.5) |
| 4.301 | 1.812 | 10.204 | <0.001 |
| Tumor stage |
|
| <0.001 |
|
|
|
|
| T0 | 10 (18.9) | 43 (81.1) |
| 1.000 |
|
|
|
| T1 | 422 (21.4) | 1,550 (78.6) |
| 1.11 | 0.523 | 2.357 | 0.758 |
| T2 | 750 (29.4) | 1,804 (70.6) |
| 0.815 | 0.385 | 1.723 | 0.59 |
| T3 | 1,445 (72.5) | 548 (27.5) |
| 0.211 | 0.1 | 0.448 | <0.001 |
| T4 | 1,996 (73.9) | 705 (26.1) |
| 0.069 | 0.033 | 0.146 | <0.001 |
| TX | 191 (12.7) | 1313 (87.3) |
| 1.499 | 0.790 | 3.233 | 0.192 |
| Node stage |
|
| <0.001 |
|
|
|
|
| N0 | 1,817 (34.6) | 3,433 (65.4) |
| 1.000 |
|
|
|
| N1 | 2,868 (67.4) | 1,390 (32.6) |
| 0.166 | 0.148 | 0.186 | <0.001 |
| NX | 129 (10.2) | 1,140 (89.8) |
| 2.342 | 1.882 | 2.914 | <0.001 |
Identification of ‘risk’ factors and
‘protective’ factors for CSS and OS in patients with advanced PCa
with only bone metastasis
Univariate and multivariate COX hazards regression
analyses were conducted in 5,363 patients with stage IV PCa with
only bone metastasis to identify the contribution of different
variates to CSS and OS. According to the results, single/unmarried
status was deemed as risk factor for CSS and OS; age ≥50 years was
deemed as risk factor for OS (Tables
V and VI). On the other hand,
‘other’ race (American Indian/Alaska native and Asian/Pacific
Islander), T1 stage, T3 stage and prostatectomy were regarded as
‘protective’ factors in stage IV patients with only bone
metastasis, moreover, T2 stage was additionally regarded as a
‘protective’ factor for CSS in stage IV PCa patients but not OS
(Tables V and VI).
 | Table V.Univariate COX analysis of CSS and OS
in patients with advanced prostate cancer and only bone
metastasis. |
Table V.
Univariate COX analysis of CSS and OS
in patients with advanced prostate cancer and only bone
metastasis.
|
| CSS | OS |
|---|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Marital status |
|
|
|
|
|
|
|
|
|
Married |
| 1.000 |
|
|
| 1.000 |
|
|
|
Single | <0.001 | 1.244 | 1.128 | 1.373 | <0.001 | 1.29 | 1.182 | 1.410 |
| Age, years |
|
|
|
|
|
|
|
|
|
<50 |
| 1.000 |
|
|
| 1.000 |
|
|
|
≥50 | 0.089 | 1.277 | 0.963 | 1.692 | 0.016 | 1.386 | 1.062 | 1.808 |
| Race |
|
|
|
|
|
|
|
|
|
White |
| 1.000 |
|
|
| 1.000 |
|
|
|
Black | 0.779 | 1.018 | 0.901 | 1.15 | 0.551 | 1.034 | 0.926 | 1.155 |
|
Othersa | 0.001 | 0.675 | 0.532 | 0.856 | 0.003 | 0.731 | 0.593 | 0.9 |
| Grade |
|
|
|
|
|
|
|
|
| Well
differentiated |
| 1.000 |
|
|
| 1.000 |
|
|
|
Moderately differentiated | 0.228 | 0.410 | 0.096 | 1.746 | 0.027 | 0.310 | 0.110 | 0.876 |
| Poorly
differentiated | 0.889 | 1.103 | 0.276 | 4.419 | 0.427 | 0.672 | 0.252 | 1.793 |
|
Undifferentiated | 0.465 | 1.727 | 0.399 | 7.477 | 0.878 | 0.918 | 0.311 | 2.714 |
| Tumor stage |
|
|
|
|
|
|
|
|
| T0 |
| 1.000 |
|
|
| 1.000 |
|
|
| T1 | <0.001 | 0.332 | 0.194 | 0.566 | <0.001 | 0.393 | 0.235 | 0.657 |
| T2 | <0.001 | 0.374 | 0.219 | 0.636 | 0.002 |
| 0.262 | 0.730 |
| T3 | <0.001 | 0.320 | 0.184 | 0.556 | <0.001 | 0.357 | 0.210 | 0.608 |
| T4 | 0.052 | 0.585 | 0.341 | 1.004 | 0.076 |
| 0.371 | 1.051 |
| Node stage |
|
|
|
|
|
|
|
|
| N0 |
| 1.000 |
|
|
| 1.000 |
|
|
| N1 | 0.004 | 1.196 | 1.059 | 1.351 | 0.086 | 1.103 | 0.986 | 1.234 |
| Radiation
therapy |
|
|
|
|
|
|
|
|
| No |
| 1.000 |
|
|
| 1.000 |
|
|
|
Yes | 0.918 | 0.987 | 0.772 | 1.263 | 0.725 | 0.960 | 0.765 | 1.204 |
| Prostatectomy
surgery |
|
|
|
|
|
|
|
|
| No |
| 1.000 |
|
|
| 1.000 |
|
|
|
Yes | <0.001 | 0.216 | 0.103 | 0.454 | <0.001 | 0.255 | 0.137 | 0.475 |
 | Table VI.Multivariate COX analysis of CSS and
OS in patients with stage IV prostate cancer with only bone
metastasis. |
Table VI.
Multivariate COX analysis of CSS and
OS in patients with stage IV prostate cancer with only bone
metastasis.
|
| CSS | OS |
|---|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Marital status |
|
|
|
|
|
|
|
|
|
Married |
| 1.000 |
|
|
| 1.000 |
|
|
|
Single | 0.002 | 1.172 | 1.058 | 1.293 | <0.001 | 1.221 | 1.114 | 1.336 |
| Age, years |
|
|
|
|
|
|
|
|
|
<50 |
| 1.000 |
|
|
| 1.000 |
|
|
|
≥50 | 0.104 | 1.265 | 0.953 | 1.678 | 0.025 | 1.357 | 1.039 | 1.772 |
| Race |
|
|
|
|
|
|
|
|
|
White |
| 1.000 |
|
|
| 1.000 |
|
|
|
Black | 0.909 | 0.993 | 0.877 | 1.124 | 0.991 | 0.999 | 0.893 | 1.118 |
|
Othera | 0.002 | 0.685 | 0.540 | 0.869 | 0.006 | 0.746 | 0.605 | 0.919 |
| Grade |
|
|
|
|
|
|
|
|
| Well
differentiated |
| 1.000 |
|
|
| 1.000 |
|
|
|
Moderately differentiated | 0.171 | 0.363 | 0.085 | 1.546 | 0.016 | 0.279 | 0.099 | 0.791 |
| Poorly
differentiated | 0.933 | 0.942 | 0.235 | 3.781 | 0.295 | 0.591 | 0.221 | 1.581 |
|
Undifferentiated | 0.599 | 1.483 | 0.342 | 6.438 | 0.736 | 0.829 | 0.280 | 2.459 |
| T stage |
|
|
|
|
|
|
|
|
| T0 |
| 1.000 |
|
|
| 1.000 |
|
|
| T1 | 0.011 | 0.495 | 0.287 | 0.854 | 0.044 | 0.584 | 0.346 | 0.986 |
| T2 | 0.019 | 0.523 | 0.305 | 0.897 | 0.063 | 0.611 | 0.363 | 1.027 |
| T3 | 0.011 | 0.480 | 0.273 | 0.844 | 0.025 | 0.539 | 0.314 | 0.926 |
| T4 | 0.349 | 0.771 | 0.445 | 1.331 | 0.493 | 0.831 | 0.491 | 1.409 |
| N stage |
|
|
|
|
|
|
|
|
| N0 |
| 1.000 |
|
|
| 1.000 |
|
|
| N1 | 0.099 | 1.111 | 0.98 | 1.258 | 0.498 | 1.041 | 0.928 | 1.167 |
| Radiation
therapy |
|
|
|
|
|
|
|
|
| No |
| 1.000 |
|
|
| 1.000 |
|
|
|
Yes | 0.641 | 0.942 | 0.735 | 1.209 | 0.484 | 0.922 | 0.733 | 1.159 |
| Prostatectomy
surgery |
|
|
|
|
|
|
|
|
| No |
| 1.000 |
|
|
| 1.000 |
|
|
|
Yes | 0.001 | 0.291 | 0.138 | 0.614 | 0.001 | 0.346 | 0.185 | 0.648 |
Prostatectomy is effective in
improving survival outcomes in patients with advanced PCa with only
bone metastasis
Kaplan-Meier analysis was conducted using
significant factors from Cox regression analysis to determine their
impact on survival in patients with stage IV PCa with only bone
metastasis. According to the results, married patients had better
3-year survival rates than single/unmarried patients (CSS, 51.9 vs.
46.2%; OS, 45.6 vs. 38.5%; Fig. 4A).
‘Other’ race patients (American Indian/Alaska native and
Asian/Pacific Islander) also had better CSS and OS than white
patients (Fig. 4B). T1 and T3 stage
patients had better CSS and OS than T0 stage patients, although
this difference was not statistically significant (Fig. 4C). Radiation therapy did not
significantly improve patient's survival (Fig. 4D). Moreover, it was found that
prostatectomy could potently improve the 3-year CSS and OS rates
(vs. no prostatectomy; CSS, 85.1 vs. 49.0%; OS, 81.5 vs. 42.1%;
Fig. 4E). The HR of CSS in patients
with T2 stage bone metastases was 0.523; suggesting that patients
with stage T2 cancer had improved specific survival outcomes
compared with T0 patients (Table
VI). However, as the effect of T2 stage did not significantly
affect OS, it is possible that T2 stage would be a protective
factor for survival outcomes in single bone metastasis PCa
patients.
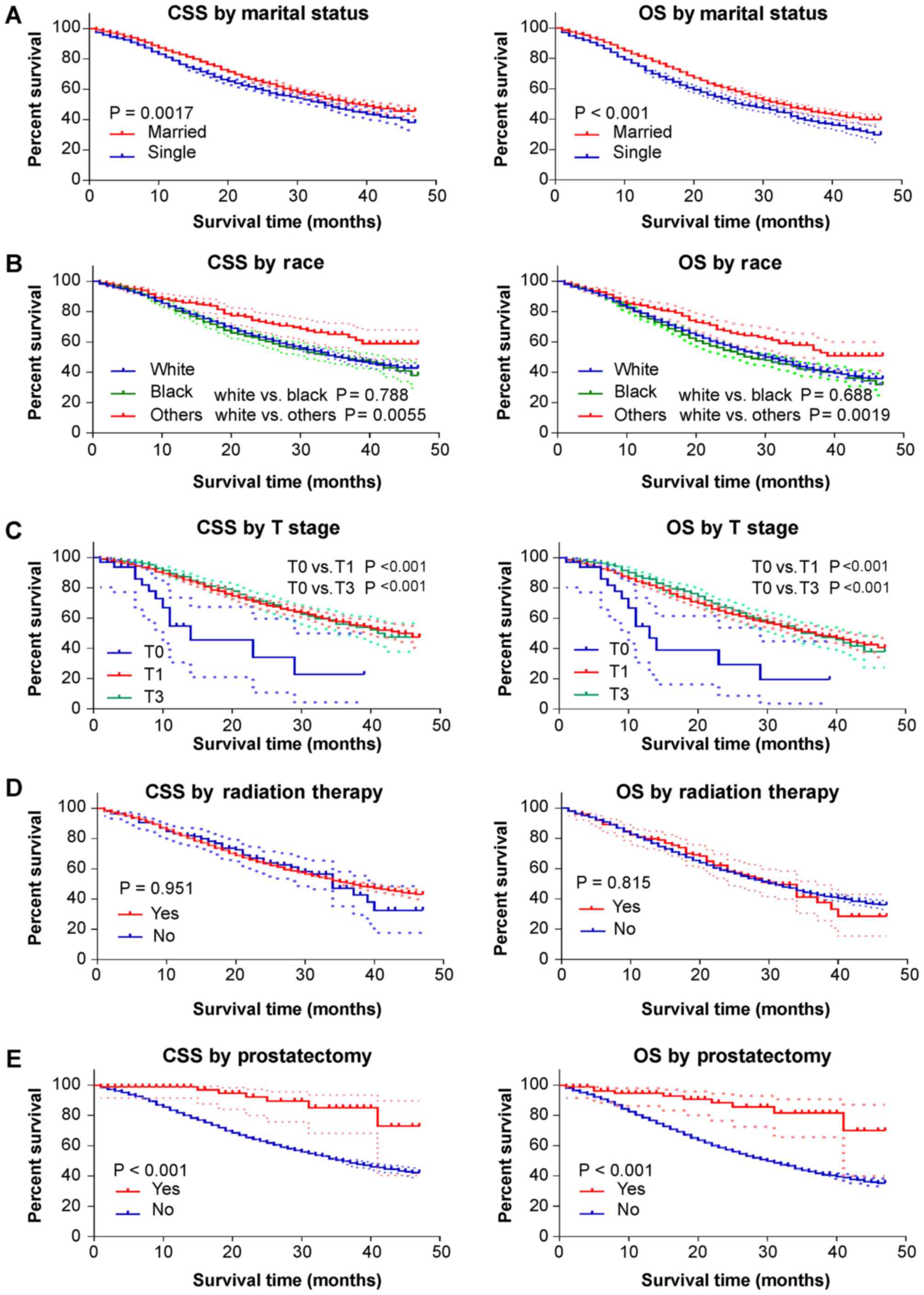 | Figure 4.Kaplan-Meier curves for CSS and OS by
different study variates. The dotted lines indicate the 95%
confidence interval of each point on the Kaplan-Meier curve. The
marital status ‘single’ includes divorced, single (never married),
separated and widowed. ‘Others’ race includes American
Indian/Alaska native and Asian/Pacific Islander. Prostatectomy
includes: Radical prostatectomy, NOS; total prostatectomy, NOS;
Excised prostate, prostatic capsule, ejaculatory ducts, seminal
vesicle and including a narrow cuff of bladder neck; Prostatectomy,
NOS. NOS, no other specification; CSS, cancer-specific survival;
OS, overall survival. |
Discussion
The main findings of the present study were: i)
Prostate adenocarcinoma patients with only liver metastasis had
worse prognostic outcomes than those with only bone or only lung
metastasis; ii) prostatectomy potently improved the CSS and OS of
stage IV PCa patients with only bone metastasis; and iii) unmarried
status, age ≥50 years, M1 stage, bone metastasis, liver metastasis
and lung metastasis were risk factors for survival in patients with
stage IV PCa.
The Cox regression model is widely used in survival
analysis with censoring data and different covariates (11). When analyzing survival data of the
patients, the HR generated by Cox regression model represents the
probability of death at a particular time. The Kaplan-Meier curve
can efficiently use all data, including the censored data, to
estimate the time-to-event curve. Comparisons of different groups
is assessed by log-rank test, which is able to estimate the
long-term prognosis of patients (11). Therefore, survival analysis of stage
IV PCa patients and single bone metastasis advanced PCa patients
was performed using the Cox regression model and Kaplan-Meier
analysis methods.
Using COX regression models, M1 stage, bone
metastasis, liver metastasis and lung metastasis were first
identified to be significantly associated with impaired CSS and OS
(Tables II and III) among stage IV PCa patients.
Radiation therapy and prostatectomy were also identified to be
effective therapeutic methods to improve patient CSS and OS in
advanced PCa.
The bone was revealed to be the most common
metastatic site for stage IV PCa, and patients with bone metastases
had significantly impaired CSS and OS rates. Therefore, it was
necessary to find risk factors that were associated with bone
metastasis in stage IV PCa. Multivariate binary logistic regression
analysis suggested that single/unmarried patients, black patients
and patients with grade IV (undifferentiated adenocarcinoma) were
more likely to have bone metastasis (Table VI). However, T3 stage, T4 stage and
N1 stage were ‘protective’ factors. One possible explanation for
this result is that certain groups of patients were included that
were diagnosed as having stage IV PCa according to the AJCC 7th
edition, but did not have metastatic disease: Patients with i) any
T stage, N1, M0; ii) T4,any N stage, M0; and iii) T3, N1, M0.
As bone metastasis may potently impair survival
outcomes for patients with stage IV PCa, finding effective
treatment methods to improve the CSS and OS of patients with bone
metastasis is necessary. Historically, RP has not been recommended
for patients with advanced PCa presumed to have extra-prostatic
disease; instead, patients with advanced PCa were counseled to
undergo radiation therapy or hormonal therapy (12). However, local resection of the
primary site for metastatic solid tumors has been demonstrated to
be helpful in various cancer types, including metastatic renal cell
carcinoma, hepatocellular carcinoma, pancreatic cancer and
metastatic breast cancer (13–21).
Culp et al (8) demonstrated
that metastatic PCa patients undergoing definitive local treatment
had higher 5-year OS and CSS rates than those not undergoing local
therapy. For patients with PCa, there is still no consensus about
the benefit of primary site surgery in the presence of metastatic
disease. The current findings demonstrate that, for patients with
bone metastasis, prostatectomy may significantly improve both CSS
and OS (Fig. 4). Although radiation
therapy provided obvious improvements in both CSS and OS in stage
IV PCa patients (Fig. 2), the
present results indicated that there was no significant improvement
in CSS and OS with the administration of radiation therapy to
patients with only bone metastasis (Fig.
4).
Mechanisms underlying the survival benefit of
primary tumor resection remain unknown. According to the
‘self-seeding’ hypothesis, cancer cells may seed distant sites, as
well as the primary tumor site (22,23).
Eliminating the primary source of the metastatic tumor cells by
removing the prostate may reduce the number of circulating tumor
cells (24). Therefore, it is
reasonable to believe that prostatectomy may be beneficial for
patients with metastatic PCa.
Cooperberg et al (25) showed that PCa patients aged ≥50 years
may show higher CAPRA scores (the CAPRA score was developed using
the Cancer of the Prostate Strategic Urologic Research Endeavor
registry data) (26), implying that
older age increases the risk of metastasis in PCa patients.
Similarly, in the present study, it was demonstrated that age ≥50
years was a risk factor for both CSS and OS in patients with only
bone metastasis (Tables V and
VI). The current study also
revealed that patients with grade IV PCa (undifferentiated) had a
higher risk of bone metastases than grade I patients (Table IV). Moreover, Brawley et al
(27) suggested that mortality rates
for patients with PCa are higher for black Americans than for white
Americans. The findings of the current study also suggested that
black patients with stage IV PCa had worse CSS and OS compared with
white patients (Fig. 2). A recent
study by Guo et al (28)
suggested that liver, lung or brain metastasis resulted in a poorer
prognosis in prostate cancer patients diagnosed with bone
metastasis (28). By contrast, the
present study assessed the effects of four specific single
metastasis sites on the survival of patients with advanced PCa.
Moreover, prostatectomy was identified as an effective treatment
for PCa patients with single bone metastases instead of radiation
therapy.
Analysis of the association between disease
prognosis and metastatic site may help in optimizing disease
management and devising systemic therapy strategies for PCa. The
current findings suggested that patients with only liver metastasis
had worse CSS and OS rates compared with those with only bone or
liver metastasis (Fig. 3).
The following limitations of the present study
should be considered. First, information about smoking, obesity,
Gleason scores and other risk factors for PCa were not registered
in the SEER database. The current analysis only evaluated
prostatectomy (RP; total prostatectomy and cystoprostatectomy were
included) for metastatic PCa patients, but specific surgical
information was not included (e.g., the use of laparoscopic or
robotic-assisted surgeries). Resection of metastatic lesions can
also affect the survival outcomes of patients with metastatic PCa,
but such information cannot be retrieved from the SEER database. In
addition, information on androgen deprivation therapy and
neoadjuvant chemotherapy, are not included. The dataset used was
representative only of the United States, so the applicability of
the results to a wider population is uncertain. As the current
study was based on patient data from 2010-2013, 5-year survival
rate, which is deemed as an effective indicator for predicting the
long-term prognosis of patients, was not available. Instead, 3-year
survival rate was used as an indicator to estimate the prognosis of
patients with advanced PCa. Studies based on updated SEER PCa data
that include >5 years of records of ‘specific site metastases’
can provide 5 year-survival rate, as a long-term prognosis
indicator, to further validate the current results. Although the
prognosis of PCa patients can be changed with improvements of
treatment, we still hypothesize that prostatectomy is beneficial
for prognosis outcomes of patients with stage IV PCa.
In conclusion, based on the results of SEER
analysis, patients with advanced prostatic adenocarcinoma with only
liver metastasis have worse outcomes than those with only bone or
lung metastasis. Despite the limitations of the SEER database, the
current results suggest that prostatectomy confers a survival
advantage in PCa patients with only bone metastasis.
Acknowledgements
Not applicable.
Funding
This study was supported in part by funding from the
National Natural Science Foundation of China (grant nos. 81772183
and 5177030177).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the SEER repository (https://seer.cancer.gov/).
Authors' contributions
YD, RB and CW designed the study. YD and ZZ
extracted the data. ZZ, BX, SL and WAR assisted with the data
processing and statistical analysis. YD and WAR wrote the article.
CW funded the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamada Y, Naruse K, Nakamura K, Taki T,
Tobiume M, Zennami K, Nishikawa G, Itoh Y, Muramatsu Y, Nanaura H,
et al: Investigation of risk factors for prostate cancer patients
with bone metastasis based on clinical data. Exp Ther Med.
1:635–639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heidenreich A, Bellmunt J, Bolla M, Joniau
S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel
T, et al: EAU guidelines on prostate cancer. Part 1: Screening,
diagnosis, and treatment of clinically localised disease. Eur Urol.
59:61–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang L, Drake BF and Colditz GA: Obesity
and other cancers. J Clin Oncol. 34:4231–4237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Humphrey PA: Histopathology of prostate
cancer. Cold Spring Harb Perspect Med. 7:a0304112017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bill-Axelson A, Holmberg L, Filén F, Ruutu
M, Garmo H, Busch C, Nordling S, Häggman M, Andersson SO, Bratell
S, et al: Radical prostatectomy versus watchful waiting in
localized prostate cancer: The Scandinavian prostate cancer group-4
randomized trial. J Natl Cancer Inst. 100:1144–1154. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Veeratterapillay R, Goonewardene SS,
Barclay J, Persad R and Bach C: Radical prostatectomy for locally
advanced and metastatic prostate cancer. Ann R Coll Surg Engl.
99:259–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Culp SH, Schellhammer PF and Williams MB:
Might men diagnosed with metastatic prostate cancer benefit from
definitive treatment of the primary tumor? A SEER-based study. Eur
Urol. 65:1058–1066. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stephen B, David R, Carolyn C and April G:
AJCC cancer staging manual seventh edition. Am Joint Committee
Cancer. 2010, https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspxAugust
29–2017
|
|
10
|
Adamo M, Dickie L and Ruhl J: SEER program
coding and staging manual 2016. National Cancer Institute;
Bethesda, MD 20850-9765, U.S.: Department of Health and Human
Services National Institutes of Health National Cancer Institute, .
2016, https://seer.cancer.gov/tools/codingmanuals/historical.htmlAugust
29–2017
|
|
11
|
Fisher LD and Lin DY: Time-dependent
covariates in the Cox proportional-hazards regression model. Annu
Rev Public Health. 20:145–157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Freedland SJ, Partin AW, Humphreys EB,
Mangold LA and Walsh PC: Radical prostatectomy for clinical stage
T3a disease. Cancer. 109:1273–1278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heng DY, Wells JC, Rini BI, Beuselinck B,
Lee JL, Knox JJ, Bjarnason GA, Pal SK, Kollmannsberger CK, Yuasa T,
et al: Cytoreductive nephrectomy in patients with synchronous
metastases from renal cell carcinoma: Results from the
international metastatic renal cell carcinoma database consortium.
Eur Urol. 66:704–710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keutgen XM, Nilubol N, Glanville J,
Sadowski SM, Liewehr DJ, Venzon DJ, Steinberg SM and Kebebew E:
Resection of primary tumor site is associated with prolonged
survival in metastatic nonfunctioning pancreatic neuroendocrine
tumors. Surgery. 159:311–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hüttner FJ, Schneider L, Tarantino I,
Warschkow R, Schmied BM, Hackert T, Diener MK, Büchler MW and
Ulrich A: Palliative resection of the primary tumor in 442
metastasized neuroendocrine tumors of the pancreas: A
population-based, propensity score-matched survival analysis.
Langenbecks Arch Surg. 400:715–723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abdel-Rahman O: Role of liver-directed
local tumor therapy in the management of hepatocellular carcinoma
with extrahepatic metastases: A SEER database analysis. Expert Rev
Gastroenterol Hepatol. 11:183–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abou-Alfa GK and Venook AP: The impact of
new data in the treatment of advanced hepatocellular carcinoma.
Curr Oncol Rep. 10:199–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oweira H, Petrausch U, Helbling D, Schmidt
J, Mannhart M, Mehrabi A, Schöb O, Giryes A, Decker M and
Abdel-Rahman O: Prognostic value of site-specific metastases in
pancreatic adenocarcinoma: A surveillance epidemiology and end
results database analysis. World J Gastroenterol. 23:1872–1880.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shien T, Nakamura K, Shibata T, Kinoshita
T, Aogi K, Fujisawa T, Masuda N, Inoue K, Fukuda H and Iwata H: A
randomized controlled trial comparing primary tumour resection plus
systemic therapy with systemic therapy alone in metastatic breast
cancer (PRIM-BC): Japan Clinical Oncology Group Study JCOG1017. Jpn
J Clin Oncol. 42:970–973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruiterkamp J, Voogd AC, Tjan-Heijnen VC,
Bosscha K, van der Linden YM, Rutgers EJ, Boven E, van der Sangen
MJ and Ernst MF; Dutch Breast Cancer Trialists' Group (BOOG), :
SUBMIT: Systemic therapy with or without up front surgery of the
primary tumor in breast cancer patients with distant metastases at
initial presentation. BMC Surg. 12:52012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fields RC, Jeffe DB, Trinkaus K, Zhang Q,
Arthur C, Aft R, Dietz JR, Eberlein TJ, Gillanders WE and
Margenthaler JA: Surgical resection of the primary tumor is
associated with increased long-term survival in patients with stage
IV breast cancer after controlling for site of metastasis. Ann Surg
Oncol. 14:3345–3351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Comen E, Norton L and Massagué J: Clinical
implications of cancer self-seeding. Nat Rev Clin Oncol. 8:369–377.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Norton L: Cancer stem cells, self-seeding,
and decremented exponential growth: Theoretical and clinical
implications. Breast Dis. 29:27–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Resel Folkersma L, San José Manso L,
Galante Romo I, Moreno Sierra J and Olivier Gómez C: Prognostic
significance of circulating tumor cell count in patients with
metastatic hormone-sensitive prostate cancer. Urology.
80:1328–1332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cooperberg MR, Broering JM and Carroll PR:
Risk assessment for prostate cancer metastasis and mortality at the
time of diagnosis. J Natl Cancer Inst. 101:878–887. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cook MM, Rosenberg PS, McCarty FA, Wu M,
King J, Eheman C and Anderson WF: Racial disparities in prostate
cancer incidence rates by census division in the United States,
1999–2008. Prostate. 75:758–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brawley OW, Jani AB and Master V: Prostate
cancer and race. Curr Probl Cancer. 31:211–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo X, Zhang C, Guo Q, Xu Y, Feng G, Li L,
Han X, Lu F, Ma Y, Wang X and Wang G: The homogeneous and
heterogeneous risk factors for the morbidity and prognosis of bone
metastasis in patients with prostate cancer. Cancer Manag Res.
10:1639–1646. 2018. View Article : Google Scholar : PubMed/NCBI
|