Introduction
Immune checkpoint inhibitors (ICIs), specifically
those targeting the programmed cell death protein-1 (PD-1) pathway,
have improved outcomes in the treatment of several advanced cancers
such as non-small cell lung cancer (NSCLC), malignant melanoma
(MM), renal cell carcinoma, urothelial cancer, head and neck
cancer, gastric cancer, and Hodgkin's lymphoma (1–6). PD-1 is
highly expressed on activated T and B cells and PD-1 ligands have
been identified as programmed death-ligand 1 (PD-L1) and PD-L2.
PD-L1 and PD-L2 have been shown to down-regulate T cell activation
upon binding to PD-1. The PD-1 blockade suppresses its negative
signal and potentiates T cell responses, thereby activates tumor
immunity (7). Based on this
mechanism, PD-1 blockade therapy, which includes nivolumab and
pembrolizumab, has evoked persistent antitumor responses and long
term remissions in a subset of patients with a broad spectrum of
cancers. Generally, this treatment is better tolerated than
conventional chemotherapy. However, it occasionally causes
inflammatory side effects, which are called immune-related adverse
events (irAEs), likely owing to the enhanced autoimmunity (8,9). Various
regions, such as the skin, lungs, liver, intestinal tract, thyroid,
and other endocrine glands have been reported to be affected by
this treatment. The mechanisms that result in irAEs are still under
research. Some potential mechanisms include increasing T cell
activity against antigens that are present in tumors and healthy
tissue, increasing levels of preexisting autoantibodies, an
increase in the level of inflammatory cytokines, and enhanced
complement-mediated inflammation due to direct binding of an
antibody against cytotoxic T-lymphocyte antigen 4 (CTLA-4) with
CTLA-4 expressed on normal tissue (10).
In addition, the development of irAEs has been found
to be associated with durable responses and better prognoses in MM
and NSCLC (11–14). However, contradictory reports exist,
and no consensus has been reached yet.
Thyroid dysfunction is one of the most frequent
irAEs induced by PD-1 blockade. In one study, it was observed in
8.6% of patients with MM who were treated with nivolumab (15), and in another, it was reported in 21%
of patients with NSCLC in a phase 1 clinical trial for
pembrolizumab (16). Some studies
indicated that thyroid disorders are likely to occur in patients
with preexisting antithyroid antibodies with the implication that
PD-1 blockade modulates humoral immunity, enhancing these
antibodies (16,17).
The aim of the present study was to clarify the
clinical characteristics of thyroid dysfunction mediated by PD-1
blockade, and its association with the therapeutic effect of the
treatment in advanced cancers.
Patients and methods
Patients
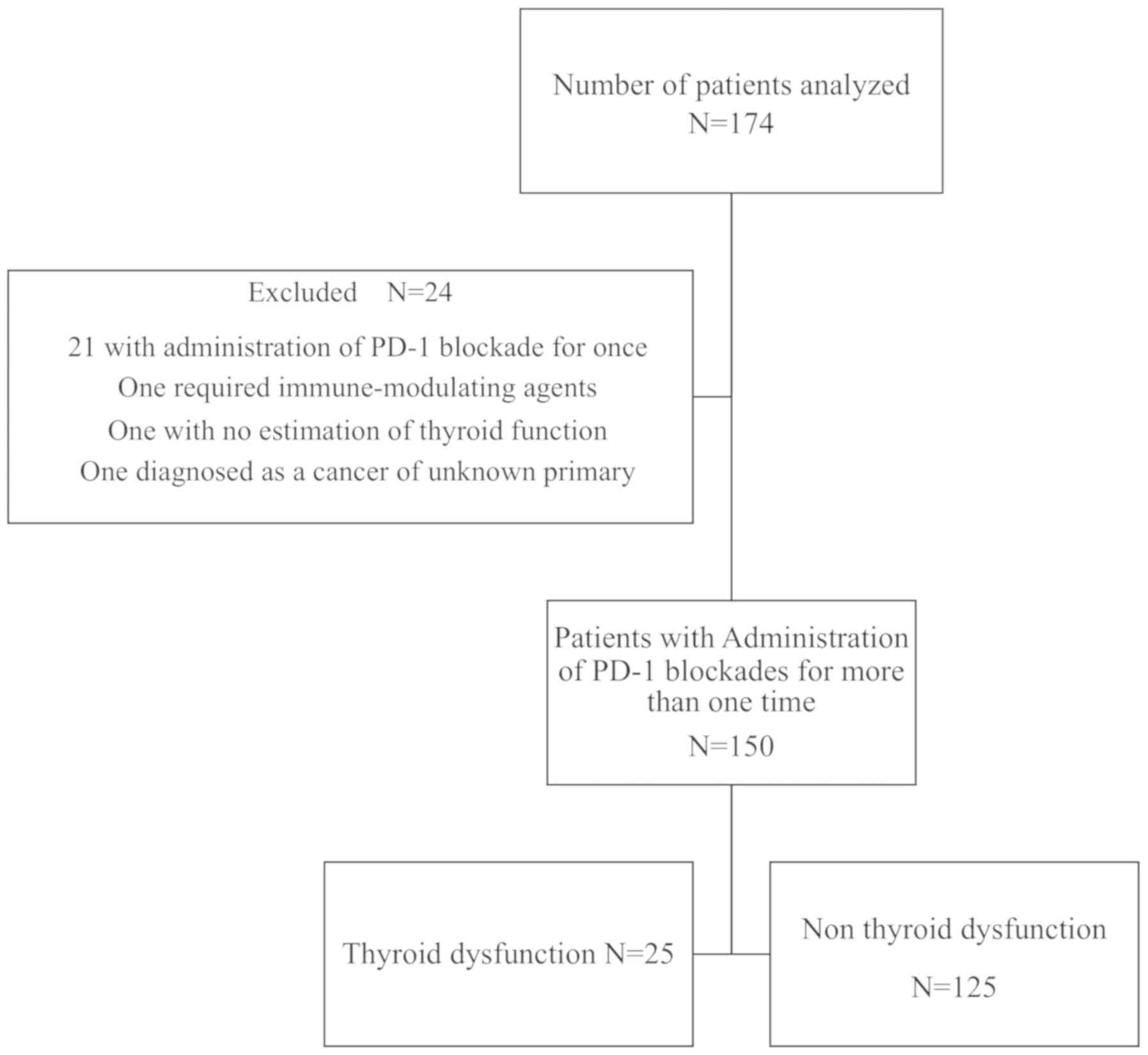
We performed a retrospective review of electronic
medical records of all 174 patients who received nivolumab or
pembrolizumab for metastatic or unresectable advanced cancers from
September 2014 to July 2018 at Kyoto Prefecture University of
Medicine. Among these patients, 24 were excluded for the following
reasons: 21 patients were administered a PD-1 blockade once, 1
patient required immune-modulating agents for the initiation of
PD-1 blockade therapy, thyroid function estimation data was
unavailable for 1, and 1 had cancer of an unknown primary origin.
None of the patients had a history of pretreatment with other ICIs
such as ipilimumab, which is an anti-cytotoxic T-lymphocyte
associated protein 4 (CTLA-4) antibody. The treatment was
administered until disease progression or unacceptable toxicity was
noted.
Assessments
All enrolled patients received PD-1 blockade
intravenously, according to a schedule of 3 mg/kg every 2 weeks for
nivolumab, and 2 mg/kg every 3 weeks for pembrolizumab. Screening
with thyroid function tests, including those for serum
thyroid-stimulating hormone (TSH), free thyroxine (FT4), and free
triiodothyronine (FT3), were conducted at baseline and during
treatment. Additionally, a majority of the patients were tested for
the presence of anti-thyroglobulin antibodies (TgAbs) and
anti-thyroid peroxidase antibodies (TPOAbs) within one month before
the induction of PD-1 blockade. Serum levels of FT3, FT4, TSH,
TgAbs and TPOAbs were measured using electro chemiluminescence
immunoassays. The normal ranges of FT3, FT4, TSH, TgAbs and TPOAbs
were 2.30 to 4.00 pg/ml, 0.90 to 1.70 ng/dl, 0.500 to 5.000 µIU/ml,
<28 and <16 IU/ml, respectively.
Based on the development of thyroid dysfunction, we
divided the patients into two groups: The thyroid dysfunction group
(patients with at least two consecutive abnormal TSH level
measurements) and the euthyroid group (patients with normal TSH
levels or with fewer than two abnormal TSH level measurements).
Patients with hypothyroidism at baseline (hypothyroidism which was
graded one or more according to CTCAE 4.0 criteria) were
categorized into the thyroid dysfunction group, wherein the
initiation of levothyroxine or a dose increase was required, and
the others to the euthyroid group. Overt hypothyroidism was defined
by elevated serum TSH and low FT4 levels, and subclinical
hypothyroidism was defined by elevated TSH with normal FT4 levels.
The severity of thyroid dysfunctions was graded according to CTCAE
4.0 criteria. The patients' characteristics such as gender, age,
performance status (PS), type of tumor, prior therapy lines, and
development of other irAEs were also retrieved from medical
records. PS was based on the Eastern Cooperative Oncology Group
(ECOG) scale (18). The detailed
description of the scaling system is shown in Table S1. Prior therapy lines were defined
by the number of chemotherapy regimens used before PD-1
treatment.
Furthermore, progression-free survival (PFS) and
overall survival (OS) were also evaluated in the study. PFS was
defined as the time from the start of treatment to the date of
documented disease progression or death due to any cause, whereas
OS was defined as the time from the start of treatment to death due
to any cause. Those without progression or death were censored at
the time of the last imaging assessment before the data-lock on
July 31, 2018. The evaluation of clinical responses was based on
the Response Evaluation Criteria in Solid Tumors (RECIST) and
laboratory findings.
Statistical analysis
Mann-Whitney U tests were used to compare continuous
variables between the thyroid dysfunction and euthyroid groups. The
associations between the development of thyroid dysfunction,
anti-thyroid antibodies, other (non-thyroid) irAEs, and clinical
features in response to PD-1 blockade were examined using the
Chi-square test (or Fisher's exact test). Kaplan-Meier
cumulative-event curves and the log-rank test were used to compare
OS and PFS between the two groups. Furthermore, Cox proportional
hazard regression models were used to estimate hazard ratios (HRs)
with 95% confidence intervals (CIs) for OS and PFS according to
thyroid dysfunction, age, gender, PS, and line of treatment. All
statistical tests were two sided and P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using JMP® 13 (SAS Institute
Inc., Cary, NC, USA).
Results
Baseline characteristics
The group of 150 patients in this study was composed
of 59 patients with non-small cell lung carcinoma (NSCLC), 26 with
malignant melanoma (MM), 24 with renal cell carcinoma, 19 with head
and neck cancer, 16 with gastric cancer, 5 with urothelial cancer,
and 1 with Hodgkin's lymphoma. Of these, 117 received nivolumab and
33 received pembrolizumab. At the time of analysis, the median
follow-up duration was 29 weeks (range 4–203 weeks). The median
number of administrations for PD-1 blockade was 8 (range 2–68). The
baseline clinical characteristics of the patients in the thyroid
dysfunction and euthyroid groups are shown in Table I. There were no significant
differences in age, gender, performance status (PS), tumor type,
prior therapy lines, thyroid dysfunction at baseline, and type of
PD-1 blockade administered between the two groups. On the other
hand, among the 122 patients whose TPOAbs and/or TgAbs were
examined, 13 of 22 (59%) in the thyroid dysfunction group tested
positive for TPOAbs and/or TgAbs at baseline, whereas in the
euthyroid group, only 18 of 100 patients did; this difference was
statistically significant. Moreover, two patients initially tested
negative for thyroid antibodies, but eventually showed the presence
of TPOAbs at the onset of hypothyroidism.
 | Table I.Baseline characteristics. |
Table I.
Baseline characteristics.
|
Characteristics | Thyroid
dysfunction, n=25 | Non- thyroid
dysfunction, n=125 | P-value |
|---|
| Age (median) | 72 (56–83) | 69 (38–87) | 0.08 |
| Sex,
male/female | 16/9 | 85/40 | 0.70 |
| PS |
|
| 0.14 |
| 0 | 17 | 68 |
|
| 1 | 8 | 41 |
|
| ≥2 | 0 | 16 |
|
| Tumor type |
|
| 0.19 |
|
NSCLC | 11 | 48 |
|
| MM | 6 | 20 |
|
|
RCC | 4 | 20 |
|
| Head
and neck | 2 | 17 |
|
|
Gastric | 1 | 15 |
|
|
Urothelial cancer | 0 | 5 |
|
| HL | 1 | 0 |
|
| Thyroid dysfunction
at baseline | 4
(16%) | 31
(24.8%) | 0.34 |
| Prior therapy lines
≤1/≥2 | 12/13 | 64/61 | 0.77 |
|
Nivolumab/Pembrolizumab | 21/4 | 96/29 | 0.43 |
| Preexisting
anti-thyroid Abs | 13
(59%)a | 18
(18%)b | 0.0002 |
Clinical features of thyroid
dysfunction induced by PD-1 blockade
Thyroid dysfunction was observed in 25 of 150
patients (16.7%) (Fig. 1), which is
a similar ratio to that reported previously (19). Twenty-one out of 115 patients
developed thyroid dysfunction newly and 4 out of 35 patients showed
worsening hypothyroidism. Subsequently, among the 21 patients who
newly developed thyroid dysfunction, 9 patients underwent an
initial period of transient hyperthyroidism. They were all
asymptomatic and did not require any medication. Among these 9
patients, 5 developed continuous hypothyroidism, whereas the status
normalized in the rest. The median time to onset of hyperthyroidism
was 10 weeks (range 4–15 weeks), which is rather long as compared
with previous reports (16,20). Furthermore, hypothyroidism occurred
in 17 patients, with a median time to onset of 17.8 weeks (range
5–56), which is similar to that reported previously (16,20). In
the 4 patients who had worsening hypothyroidism, no patient
underwent transient hyperthyroidism, and the median time to onset
of worsening hypothyroidism was 9.5 weeks (range 5–12). The results
also showed that the severity of hypothyroidism was mild and
controllable; initially, we noted grade 1 in 5 (24%), and grade 2
(that is, demanding levothyroxine replacement) in 16 (76%) of 21
patients. Overt and subclinical hypothyroidism were noted in 12 and
9 patients, respectively. No patient was required to discontinue or
delay PD-1 blockade administration due to thyroid dysfunction.
Moreover, none of the patients were diagnosed with secondary
hypothyroidism caused by hypopituitarism. Furthermore, there was no
difference in the development of irAEs, with the exception of
thyroid dysfunction, between these two groups (data not shown).
Association between the development of
thyroid dysfunction and the efficacy of treatment
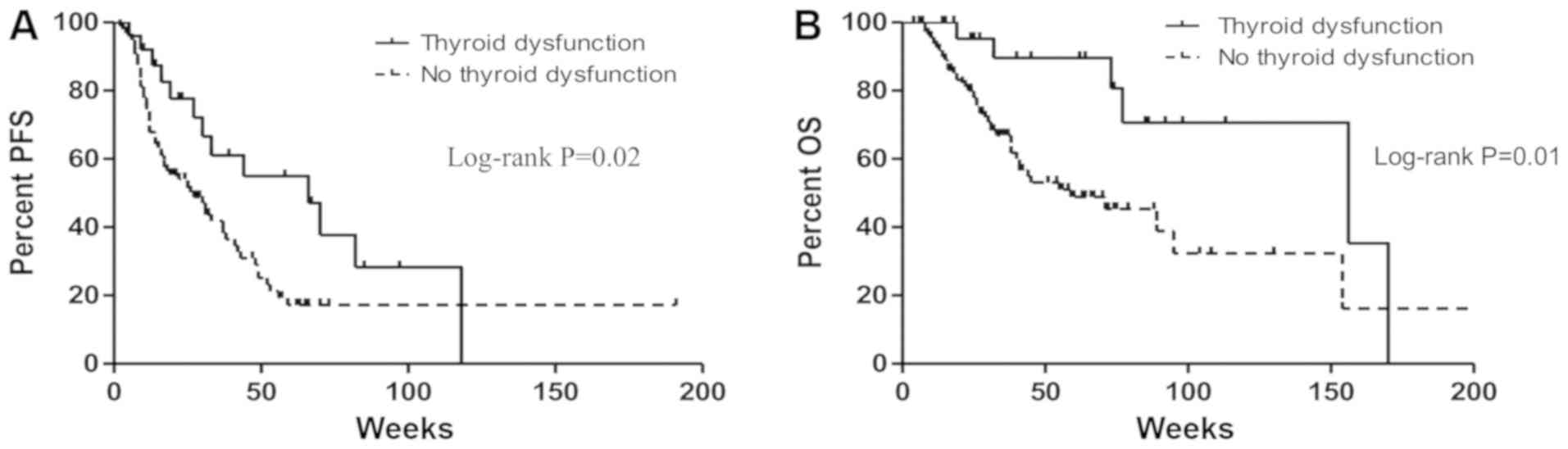
OS and PFS were estimated among the 150 patients.
The PFS of the thyroid dysfunction group was significantly longer
than that of the euthyroid group (median: 66 vs. 27 weeks, HR:
0.50, 95% CI: 0.26–0.89, P=0.02; Fig.
2A). A statistically significant improvement in OS was also
noted in patients with thyroid dysfunction, when compared to those
who had none (median: 156 vs. 59 weeks, HR: 0.34, 95% CI:
0.13–0.75, P=0.01; Fig. 2B).
Furthermore, the effects of the development of thyroid dysfunction
on PFS and OS were examined based on a hazard ratio using
time-dependent Cox regression analyses (Table II). In the multivariate analysis,
statistically significant improvement in OS was found to be
associated with PS (P<0.0001, HR: 0.12, 95% CI: 0.06–0.23) and
the development of thyroid dysfunction (P=0.04, HR: 0.42, 95% CI:
0.16–0.97). Moreover, the association between the development of
thyroid dysfunction and the improvement in PFS was numerically but
not statistically significant (P=0.058, HR: 0.56, 95% CI:
0.29–1.02); PS was the only independent predictive factor (P=0.038,
HR: 0.41, 95% CI: 0.20–0.95). Moreover, age, gender, and therapy
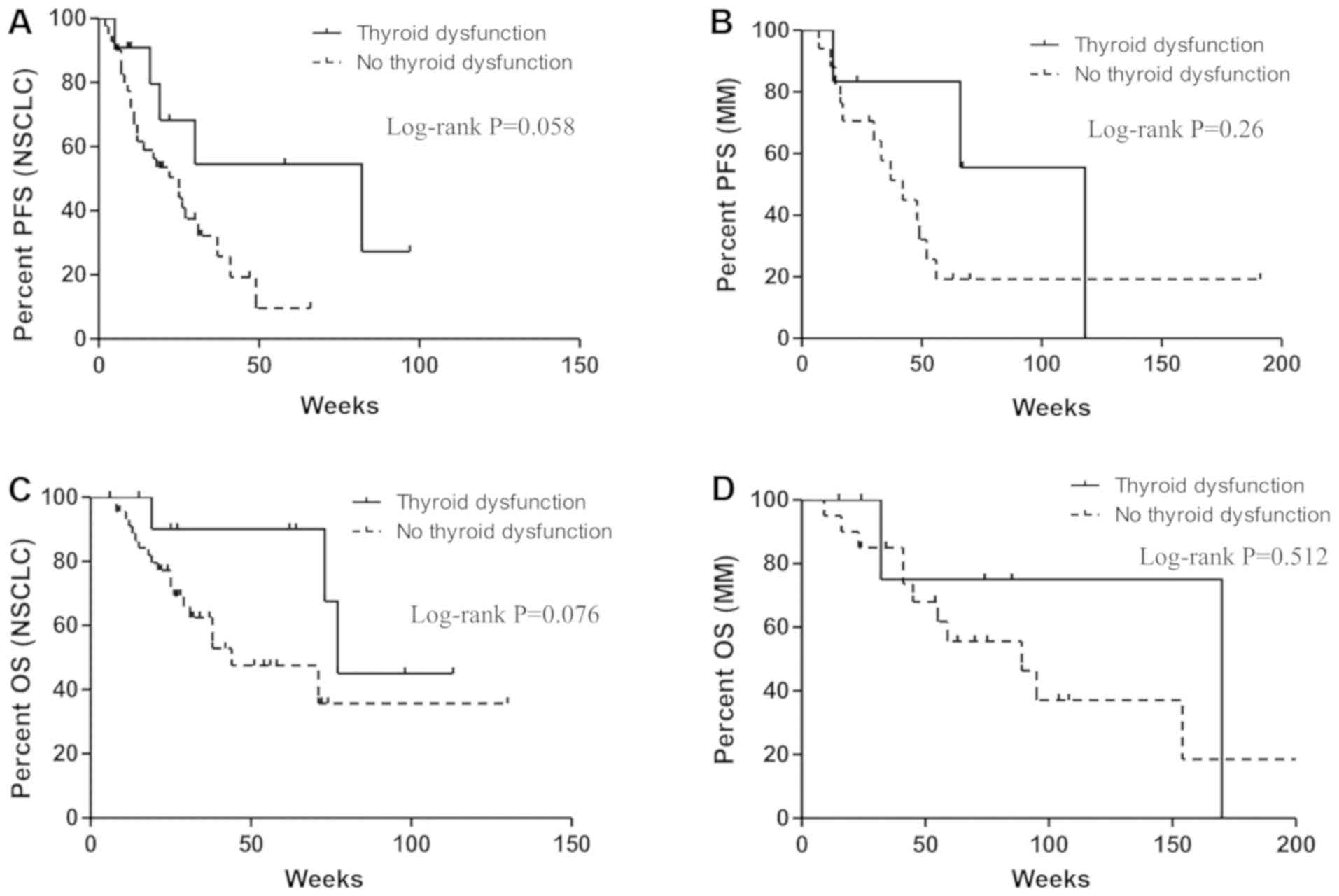
were not associated with PFS nor OS. Interestingly, when we focused
on patients with NSCLC and MM individually, the correlation between
thyroid dysfunction and improvement in PFS and OS almost reached
statistical significance (P=0.058, P=0.076 respectively) in
patients with NSCLC, whereas no significance effects were noted in
patients with MM (P=0.261, P=0.512 respectively; Fig. 3A-D).
 | Table II.Cox proportional hazard regression
analysis of PFS and OS, according to thyroid dysfunction and other
factors. |
Table II.
Cox proportional hazard regression
analysis of PFS and OS, according to thyroid dysfunction and other
factors.
| A, Univariate |
|---|
|
|---|
|
| OS | PFS |
|---|
|
|
|
|
|---|
| Factor | Hazard Ratio (95%
CI) | P-value | Hazard Ratio (95%
CI) | P-value |
|---|
| Age (≤69) | 1.36
(0.79–2.38) | 0.27 | 1.43
(0.91–2.26) | 0.12 |
| Sex (Male) | 1.12
(0.65–2.01) | 0.68 | 1.32
(0.83–2.18) | 0.24 |
| Prior therapy lines
(≤1) | 1.20
(0.69–2.11) | 0.52 | 1.27
(0.80–2.02) | 0.31 |
| PS (≤1) | 0.10
(0.05–0.20) | <0.0001 | 0.38
(0.19–0.88) | 0.026 |
| Thyroid
Dysfunction | 0.34
(0.13–0.75) | 0.006 | 0.50
(0.26–0.89) | 0.016 |
|
| B,
Multivariate |
|
|
| OS | PFS |
|
|
|
|
| Factor | Hazard Ratio
(95% CI) | P-value | Hazard Ratio
(95% CI) | P-value |
|
| Age (≤69) | – | – | 1.38
(0.87–2.20) | 0.168 |
| Sex (Male) | – | – | – | – |
| Prior therapy lines
(≤1) | – | – | – | – |
| PS (≤1) | 0.12
(0.06–0.23) | <0.0001 | 0.41
(0.20–0.95) | 0.038 |
| Thyroid
Dysfunction | 0.42
(0.16–0.97) | 0.04 | 0.56
(0.29–1.02) | 0.058 |
Discussion
In the present study, we described the clinical
features of thyroid dysfunction in patients with metastatic and/or
unresectable malignancies treated with PD-1 blockade. Some previous
related studies on NSCLC and MM patients have been conducted
(16,19); however, to our knowledge, ours is the
first study to evaluate these specific irAEs in multiple advanced
cancers. Data showed that 16.7% of the patients treated with
nivolumab or pembrolizumab developed thyroid dysfunction.
Nevertheless, the incidence of thyroid dysfunction was not related
to the type of tumor nor the type of PD-1 blockade, but was
strongly correlated with the presence of anti-thyroid antibodies.
Evidently, most cases had occurred in a fixed and relatively early
time period, with a similar clinical course. Many of these patients
ultimately developed hypothyroidism, and in some cases, a preceding
transient period of hyperthyroidism was observed. However, the
severity of dysthyroidism was low and no discontinuation of PD-1
blockade was required. Furthermore, the development of thyroid
dysfunction showed a statistically significant effect on the
improvement of PFS and OS, and it was also an independent
prognostic factor for OS after adjustment for other factors. When
we focused on NSCLC and MM individually, there was a correlation
between the development of thyroid dysfunction and the improvement
in PFS and OS in patients with NSCLC; however, no relationship was
noted in MM, suggesting that this trend may vary based on the type
of tumor.
There was a uniform pattern of immune-related
thyroid dysfunction among the patients, namely, a transient period
of hyperthyroidism, followed by hypothyroidism and then destructive
thyroiditis. The onset of hyperthyroidism in this study was seen in
the later stage; this contrasts with the results of the previous
studies, which reported a median of 3–5 weeks (16,20).
Thus, perhaps, the estimation of thyroid function was unjustified
and was dependent on the attending doctor, leading to a lag in
detection. In addition, no symptomatic hyperthyroidism was
observed, which also might contribute to the delay. In the present
study, hypothyroidism persisted and no patient experienced
spontaneous recovery. Clearly, a larger number of patients and a
longer period of observation are needed to validate this trend.
The mechanism of thyroid dysfunction is consistent
with the onset of acute inflammation and destruction of thyroid
glands. As is indicated in several studies (16,17),
anti-thyroid antibodies are highly correlated to thyroid
dysfunction. This suggests that immune responses to thyroglobulin
or thyroid peroxidase may be responsible for thyroiditis, although
the precise immunological pathogenesis remains unclear.
The management of thyroid dysfunction induced by
PD-1 blockade needs more investigation and is still controversial.
Levothyroxine replacements are recommended in patients with overt
hypothyroidism, whereas subclinical hypothyroidism can be followed
up biologically without treatment; however, principles for
medication administration have not yet been determined.
Additionally, guidelines based on other clinical scenarios for
subclinical hypothyroidism suggest that patients with symptoms or
TSH levels ≥10
mlU/l should receive levothyroxine replacement (21,22). In
our study, every patient with overt hypothyroidism, and 3 out of 10
patients with subclinical hypothyroidism, received levothyroxine
replacement. Furthermore, no patient was required to discontinue
the use of PD-1 blockade due to the clinical influence of thyroid
disorders.
Hyperthyroidism induced by antitumor immunotherapy
is self-limited and frequently asymptomatic. Hence, observation
rather than additional diagnostic testing or anti-thyroid drug
administration is recommended (23).
For patients with symptomatic hyperthyroidism, beta-blockers can be
considered along with a frequent assessment of thyroid
function.
It is worthy to note that there is a close
association between the development of thyroid dysfunction and an
improvement in survival in patients with advanced cancers. The
correlations between all irAEs and clinical outcomes in NSCLC have
been described (14,24), and a report of meta-analysis has
shown that cutaneous irAEs have a significant association with
increased survival in malignant melanoma (13). Some reports have recently
demonstrated that the presence of thyroid dysfunction is related to
the improvement of survival in NSCLC, but not in other malignancies
(16,25,26). Our
study also showed this trend, suggesting that the correlation may
vary by tumor type. However, differences in clinical conditions and
immunological backgrounds, depending on the tumor type, may
influence the results. This implies that further investigation with
larger patient sample sizes and longer observation periods is
needed for arriving at a strong conclusion.
This study has some limitations. First, this was a
single center retrospective study; therefore, the nature of
analysis and the limited comprehensive evaluations of thyroid
function and tumor response may be insufficient for precise
diagnosis. Moreover, the patient sample size for each advanced
cancer was relatively small. Second, we did not perform a landmark
analysis, which is more appropriate for estimating the association
between adverse events and treatment efficacy. However, we noted
that this association was not simply related to the fact that
patients with longer treatment periods were at a greater risk of
developing adverse events, as thyroid dysfunction was observed
early in the clinical course.
In conclusion, thyroid dysfunction induced by PD-1
blockade occurs frequently in the early period, but with mild
symptoms. An examination of anti-thyroid antibodies can facilitate
the prediction of thyroid dysfunction. Therefore, a close
estimation of thyroid function is recommended before and during
PD-1 treatment for relevant patient care and optimizing the
application of antitumor immunotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Author's contributions
TS, TI, YI and KT were responsible for the design of
the study, and the collection, analysis and interpretation of the
data. TS, TI, YI and KT critically revised the manuscript for
important intellectual content. JU, YC, SK, JA, TN, AA, TsK, HT,
ToK, HK, FH, MI, SH, OU and TT were responsible for the acquisition
and clinical interpretation of the data. All authors contributed to
the writing of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Review Committee of the Kyoto Prefectural University of Medicine
(approval no. ERB-C-867-1). Given the retrospective nature of this
work, informed consent was waived for the individual participants
included in the study in accordance with the standards of the Kyoto
Prefectural University of Medicine Institutional Medical Ethics
Review Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ICI
|
immune checkpoint inhibitors
|
|
PD-1
|
programmed cell death protein 1
|
|
NSCLC
|
non-small cell lung cancer
|
|
MM
|
malignant melanoma
|
|
irAEs
|
immune-related adverse events
|
|
CTLA-4
|
cytotoxic T-lymphocyte associated
protein 4
|
|
TSH
|
thyroid-stimulating hormone
|
|
FT4
|
free thyroxine
|
|
FT3
|
free triiodothyronine
|
|
TgAbs
|
anti-thyroglobulin antibodies
|
|
TPOAbs
|
anti-thyroid peroxidase antibodies
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
PS
|
performance status
|
References
|
1
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al:
Mutational landscape determines sensitivity to PD-1 blockade in
non-small cell lung cancer. Science. 348:124–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boutros C, Tarhini A, Routier E, Lambotte
O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S,
Berdelou A, et al: Safety profiles of anti-CTLA-4 and anti-PD-1
antibodies alone and in combination. Nat Rev Clin Oncol.
13:473–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Friedman CF, Proverbs-Singh TA and Postow
MA: Treatment of the immune-related adverse effects of immune
checkpoint inhibitors: A review. JAMA Oncol. 2:1346–1353. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanlorenzo M, Vujic I, Daud A, Algazi A,
Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K and
Ortiz-Urda S: Pembrolizumab cutaneous adverse events and their
association with disease progression. JAMA Dermatol. 151:1206–1212.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura Y, Tanaka R, Asami Y, Teramoto Y,
Imamura T, Sato S, Maruyama H, Fujisawa Y, Matsuya T, Fujimoto M
and Yamamoto A: Correlation between vitiligo occurrence and
clinical benefit in advanced melanoma patients treated with
nivolumab: A multi-institutional retrospective study. J Dermatol.
44:117–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teulings H-E, Limpens J, Jansen SN,
Zwinderman AH, Reitsma JB, Spuls PI and Luiten RM: Vitiligo-like
depigmentation in patients with stage III–IV melanoma receiving
immunotherapy and its association with survival: A systematic
review and meta-analysis. J Clin Oncol. 33:773–781. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haratani K, Hayashi H, Chiba Y, Kudo K,
Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M and
Nakagawa K: Association of immune-related adverse events with
nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol.
4:374–378. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osorio JC, Ni A, Chaft JE, Pollina R,
Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok
JD, et al: Antibody-mediated thyroid dysfunction during T-cell
checkpoint blockade in patients with non-small-cell lung cancer.
Ann Oncol. 28:583–589. 2017.PubMed/NCBI
|
|
17
|
Kobayashi T, Iwama S, Yasuda Y, Okada N,
Tsunekawa T, Onoue T, Takagi H, Hagiwara D, Ito Y, Morishita Y, et
al: Patients with antithyroid antibodies are prone to develop
destructive thyroiditis by nivolumab: A prospective study. J Endocr
Soc. 2:241–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Filette J, Jansen Y, Schreuer M,
Everaert H, Velkeniers B, Neyns B and Bravenboer B: Incidence of
thyroid-related adverse events in melanoma patients treated with
pembrolizumab. J Clin Endocrinol Metab. 101:4431–4439. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jaafar J, Fernandez E, Alwan H and
Philippe J: Programmed cell death-1 and programmed cell death
ligand-1 antibodies-induced dysthyroidism. Endocr Connect.
7:R196–R211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pearce SHS, Brabant G, Duntas LH, Monzani
F, Peeters RP, Razvi S and Wemeau JL: 2013 ETA guideline:
Management of subclinical hypothyroidism. Eur Thyroid J. 2:215–228.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garber JR, Cobin RH, Gharib H, Hennessey
JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA and Woeber
KA: Clinical practice guidelines for hypothyroidism in adults:
Cosponsored by the american association of clinical
endocrinologists and the american thyroid association. Endocr
Pract. 18:988–1028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morganstein DL, Lai Z, Spain L, Diem S,
Levine D, Mace C, Gore M and Larkin J: Thyroid abnormalities
following the use of cytotoxic T-lymphocyte antigen-4 and
programmed death receptor protein-1 inhibitors in the treatment of
melanoma. Clin Endocrinol (Oxf). 86:614–620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato K, Akamatsu H, Murakami E, Sasaki S,
Kanai K, Hayata A, Tokudome N, Akamatsu K, Koh Y, Ueda H, et al:
Correlation between immune-related adverse events and efficacy in
non-small cell lung cancer treated with nivolumab. Lung Cancer.
115:71–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Freeman-Keller M, Kim Y, Cronin H,
Richards A, Gibney G and Weber JS: Nivolumab in resected and
unresectable metastatic melanoma: Characteristics of immune-related
adverse events and association with outcomes. Clin Cancer Res.
22:886–894. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HI, Kim M, Lee SH, Park SY, Kim YN,
Kim H, Jeon MJ, Kim TY, Kim SW, Kim WB, et al: Development of
thyroid dysfunction is associated with clinical response to PD-1
blockade treatment in patients with advanced non-small cell lung
cancer. Oncoimmunology. 7:e13756422017. View Article : Google Scholar : PubMed/NCBI
|