Introduction
Primary pulmonary lymphoma (PPL) is defined as a
clonal lymphoid proliferation affecting one or both lungs in
patients with no detectable extrapulmonary involvement or bone
marrow disease at the time of diagnosis and during the subsequent 3
months (1). This disease is a rare
entity that accounts for 1% of malignant lymphomas (2) and 3.6% of extranodal lymphomas
worldwide (3). The rarity of PPL may
be attributed to the relatively low levels of lymphoid tissue in
pulmonary tissue compared with the levels at other sites (4). The most frequent subtypes are MALT-type
lymphoma, and other subtypes are commonly identified in
immunocompromised patients (5).
Pulmonary MALT lymphoma is referred to as nodal marginal-zone
B-cell lymphoma, with similar cytopathological features to other
MALT lymphomas, especially gastric lymphoma, 70 to 90% of PPL cases
are classified as mucosa-associated lymphoid tissue (MALT)-type
non-Hodgkin's lymphomas (NHLs) (5).
The male to female ratio of patients with PPL varies from 1:1 to
1:2 (6), and the mean age of
patients with NHL is 53±12 years (6). In general, NHL can be divided into two
types according to World Health Organization (WHO) criteria: i)
Indolent-type lymphomas, which include follicular lymphoma (FL)
grade I–II, marginal zone B-cell lymphoma, small lymphocytic
lymphoma and hairy cell leukemia, and ii) aggressive-type
lymphomas, which include diffuse large B-cell lymphoma (DLBCL),
anaplastic large cell lymphoma (ALCL), peripheral T-cell lymphoma
unspecified (PTCL-U), mantle cell lymphoma (MCL) and natural
killer/T-cell lymphoma (NK/T-L) (7).
To the best of our knowledge, few PP-NHL cases have
been reported in China. Therefore, a 16-year retrospective study
was conducted that focused on the clinical features and prognostic
factors of 29 patients with PP-NHL at a single
institution-Guangdong Provincial People's Hospital/Guangdong
Academy of Medical Sciences, and the majority of patients were from
Southern and Central China Region, to provide awareness and useful
diagnostic and prognostic indicators of PP-NHL. It was indicated
that minimally invasive procedures, including bronchoscopy and
computed tomography (CT)-guided percutaneous needle lung biopsy,
serve an important role in obtaining a definitive diagnosis for the
majority of patients with PP-NHL, as aggressive PP-NHL was highly
associated with a poor 5-year OS rate and poor prognosis.
Patients and methods
Patients
The present retrospective study included a total of
29 patients with PP-NHL diagnosed between January 2001 and June
2017 in Guangdong Provincial People's Hospital/Guangdong Academy of
Medical Sciences who met the following criteria (8): Unilateral or bilateral pulmonary
involvement, no previous diagnosis of extrathoracic lymphoma, no
evidence of extrathoracic disease by clinical staging workup,
including thorough physical examination, CT scans of the chest,
abdomen or pelvis, positron emission tomography-computed tomography
(PET-CT) scans of the whole body or bone marrow biopsy, and no
evidence of extrathoracic disease up to 3 months after the initial
diagnosis. In accordance with previous reports, 12 patients with
hilar or mediastinal lymphadenopathy were included (9,10).
Primary clinical data were collected from the medical records of
all patients.
Definitive diagnosis and
histopathology
All patients were diagnosed by pathological analysis
of samples collected through various invasive procedures, including
bronchoscopic biopsy, CT-guided percutaneous needle lung biopsy,
video-assisted thoracic surgery (VATS), open lung biopsy and
pleural membrane biopsy. Histological specimens were evaluated and
classified by hematopathologists using the updated WHO guidelines
(7). In addition to morphology and
growth pattern analysis, based on hematoxylin-eosin (HE) staining,
immunohistochemistry or in situ hybridization (ISH) staining was
performed to support the diagnosis of suspected subtypes based on
unique markers. The tissue samples were fixed in 10% buffered
formalin for 6 h at room temperature and embedded in paraffin,
processed routinely, 4-µm sectioned and stained with hematoxylin
(for 5 min at room temperature) and eosin (for 1 min at room
temperature). Then, sections were deparaffinized and subsequently
rehydrated in a descending alcohol series (100% alcohol for 5 min,
95% alcohol for 4 min, 85% alcohol for 2 min). Antigens were
heat-retrieved at 98°C in EDTA solution. Following cooling to room
temperature, the tissue sections were quenched with 3% hydrogen
peroxidase, and non-specific binding sites were blocked with 5%
goat serum (Thermo Fisher Scientific, Inc.) at 37°C for 30 min.
Immunohistochemical staining was performed on 4-µm sections using a
Real Envision Kit (K5007; Dako, Carpinteria, CA, USA) on an
automated immunostaining module (Leica Bond III), according to the
manufacturer's protocol. All primary antibodies were diluted to the
manufacturer's recommendations or to previously optimized dilution
and were incubated at room temperature for 30 min. The
immunohistochemical staining was observed under the Olympus light
microscope at ×40, ×100 and ×400 magnification after dehydrating
and stabilizing with mounting medium. Immunohistochemical staining
was performed using commercially available antibodies (mouse
anti-human monoclonal, Dako, Glostrup, Denmark) to the following
antigens: CD3, CD5, CD10, CD19, CD20, CD30, CD45, CD56, CD79a,
Ki-67. CD3 (clone F7.2.38, dilution, 1:200; M725401-2), CD5 (clone
4C7, dilution, 1;100; M3641), CD10 (clone 56C6, dilution, 1:100;
IS64830-2), CD19 (clone LE-CD19, dilution, 1:200; M729629-2), CD20
(clone L26, dilution, 1:800; M0755), CD45 (clones 2B11+PD7/26,
dilution, 1:200; IS75130-2), CD56 (clone 123C3, dilution, dilution,
1:100; M730429-2) and CD79a (clone JCB117, dilution, 1:200; M7050),
Ki-67 (clone MIB1, dilution, 1:100; F726801-8). In-situ
hybridization (ISH) for EBER was detected in all cases, according
to the manufacturer's protocol. Tumor cells that only appeared to
have distinct nuclear staining were recorded as positive. The
paraffin sections were detected for EBER by a peptide nucleic acid
probe (Dako-Y5200) labeled with fluorescein isothiocyanate,
followed by anti-rabbit immunoglobulin (Ig)G with horseradish
peroxidase. 3,30-Diaminobenzidine was used to detect the
hybridization signal of chromogen detection. EBV positive
nasopharyngeal carcinoma paraffin specimens were used for positive
controls. The entire pathological tissue slices of immunostaining
of Ki-67 were observed under a light microscope (magnification,
×100; Olympus BX51; Olympus Corporation, Tokyo, Japan), regions of
active growth of tumor cells were chosen and observed
(magnification, ×400; Olympus BX51; Olympus Corporation), at least
5 high power fields were further randomly selected, then the
percentage of ki-67 positive tumor cells (ki-67 index) was recorded
as a proliferation fraction.
Staging, treatment and treatment
response
Disease stages were defined according to the Ann
Arbor system (11). Updated National
Comprehensive Cancer Network guidelines were consulted for patient
treatments (12). Treatment
responses were defined according to previously reported criteria
(13). Overall survival (OS) was
measured from the time of diagnosis to the date the patient
succumbed from any cause or to the date of the last follow-up.
Statistical analysis
For statistical analyses, the patients were divided
into indolent and aggressive lymphoma groups based on their
pathological results. The clinical characteristics of all PP-NHL
cases were summarized using the median and percentages, and were
compared using Fisher's exact test. Survival curves were analyzed
using the Kaplan-Meier method. Univariate analyses were performed
to examine the survival of patients with different prognostic
factors, including age, sex, symptoms, comorbidities, treatment,
stage, hilar or mediastinal lymphadenopathy, histology, lactate
dehydrogenase (LDH) levels, and unilateral or bilateral lesions.
Cox proportional hazards regression analysis with a forward
stepwise selection procedure was used to estimate OS and was
adjusted for a number of independent factors with P<0.1 by
univariate analysis. Two-tailed tests with P<0.05 were
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS 13.0 (SPSS,
Inc.).
Results
Demographic and clinical
characteristics at initial clinical diagnosis
As presented in Table
I, this study included a total of 29 patients with PP-NHL (10
female and 19 male; mean, 50.3 years; range, 19–87 years),
including 15 patients with indolent disease and 14 with aggressive
disease. Of these, 4 patients (13.8%) were asymptomatic and were
incidentally diagnosed during radiographic examination. Patients
with aggressive lymphoma were approximately 3–4 times more likely
to exhibit elevated LDH levels and systemic symptoms (B symptoms)
at diagnosis compared with patients with indolent lymphoma (71.4%
vs. 20.0%; P=0.01; and 71.4% vs. 13.3%; P=0.02, respectively).
There were no significant differences in respiratory symptoms, age
or stage between the two groups. The rate of initial clinical
misdiagnosis was 100% prior to biopsy or surgery, including false
diagnoses of lung cancer (48.3%; 14/29), pneumonia (31.0%; 9/29),
tuberculosis (10.3%; 3/29), and pulmonary abscess (10.3%;
3/29).
 | Table I.Demographics and clinical
characteristics of the 29 subjects. |
Table I.
Demographics and clinical
characteristics of the 29 subjects.
| Characteristics | Indolent
lymphoma | Aggressive
lymphoma | Total | P-value |
|---|
| Total patients,
n | 15 | 14 | 29 | – |
| Age, years |
|
|
| – |
| Mean
(range) | 51.73 (31–72) | 47.93 (19–87) | 50.3 (19–87) | 0.45 |
| >60,
n (%) | 7 (46.7) | 5 (35.7) | 12 (41.4) | 0.31 |
| Sex, n |
|
|
| – |
|
Male | 8 (53.3) | 11 (78.6) | 19 (65.5) | 0.63 |
|
Female | 7 (46.7) | 3 (21.4) | 10 (34.5) | 0.57 |
| Symptoms, n
(%) |
|
|
| – |
|
Asymptomatic | 4 (26.7) | 0 (0.0) | 4 (13.8) | – |
| B
symptoms | 2
(13.3)a | 10
(71.4)a | 12 (41.4) | 0.02 |
|
Respiratory | 11 (73.3) | 14 (100.0) | 25 (86.2) | 0.73 |
| High serum LDH
level, n (%) | 3
(20.0)a | 10
(71.4)a | 13 (44.8) | 0.01 |
| Bone marrow
involvement, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| EBERs, n (%) | 0 (0.0) | 6 (42.9) | 6 (20.7) | – |
|
Comorbidityb, n (%) | 1 (6.7) | 3 (21.4) | 4 (13.8) | – |
| Ann Arbor stage, n
(%) |
|
|
| – |
| IE | 11 (73.3) | 6 (42.9) | 17 (58.6) | 0.50 |
| II
(1E-2E) | 4 (26.7) | 8 (57.1) | 12 (41.4) | 0.44 |
| Initial clinical
diagnosis, n (%) |
|
|
|
|
|
Pneumonia | 4 (26.7) | 5 (35.7) | 9 (31.0) | – |
|
Tuberculosis | 1 (6.7) | 2 (14.3) | 3 (10.3) | – |
| Pulmonary
abscess | 2 (13.3) | 1 (7.1) | 3 (10.3) | – |
| Lung
cancer | 8 (53.3) | 6 (42.9) | 14 (48.3) | – |
Chest radiographic findings
As presented in Table
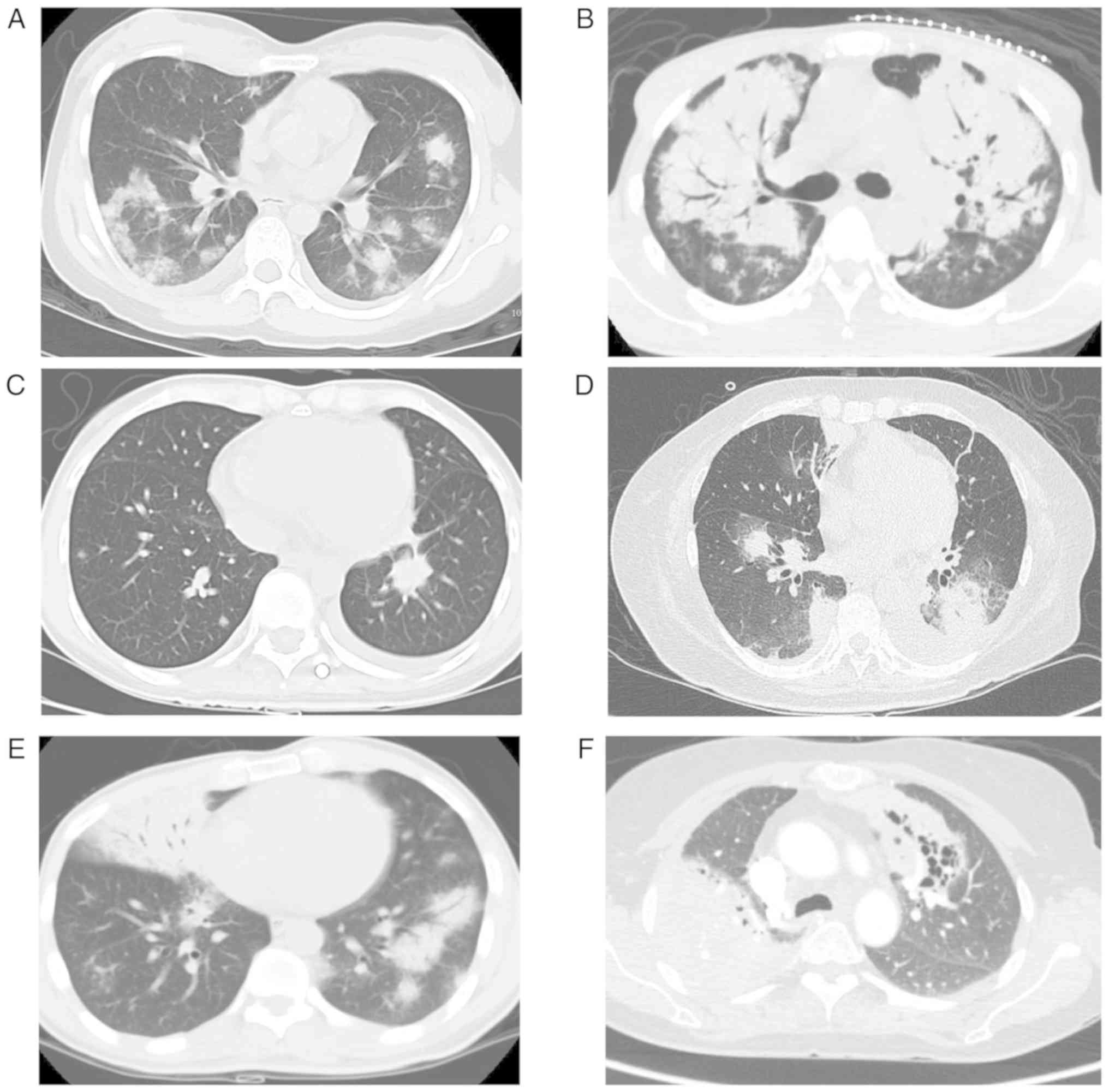
II and Fig. 1, the radiographic
findings were both diverse and non-specific. The most common
radiological finding was pulmonary nodules/masses (55.2%; 16/29),
followed by pulmonary consolidation (41.4%; 12/29),
mediastinal/hilar lymph node involvement (41.4%; 12/29), pleural
effusion (24.1%; 7/29), air bronchogram (17.2%; 5/29) and
cavitation (6.9%; 2/29).
 | Table II.Chest radiographic findings,
diagnostic procedures and pathological types of the 29
subjects. |
Table II.
Chest radiographic findings,
diagnostic procedures and pathological types of the 29
subjects.
| Group | Indolent lymphoma,
n (%) | Aggressive
lymphoma, n (%) | Total, n (%) |
|---|
| Total patients | 15 (51.7) | 14 (48.3) | 29 (100.0) |
| Radiographic
findings |
|
|
|
|
Unilateral | 9 (60.0) | 5 (35.7) | 14 (48.3) |
|
Bilateral | 6 (40.0) | 9 (64.3) | 15 (51.7) |
|
Nodule/mass | 9 (60.0) | 7 (50.0) | 16 (55.2) |
|
Consolidation | 5 (33.3) | 7 (50.0) | 12 (41.4) |
| Air
bronchogram | 1 (6.7) | 4 (28.6) | 5 (17.2) |
| Pleural
effusion | 5 (33.3) | 2 (14.3) | 7 (24.1) |
|
Cavitation | 2 (13.3) | 0 (0.0) | 2 (6.9) |
|
Mediastinal/hilar
lymphadenopathy | 4 (26.7) | 8 (57.1) | 12 (41.4) |
| Definitive
diagnostic procedures |
|
|
|
|
Endobronchial or
transbronchial biopsy | 7 (46.6) | 5 (35.7) | 12 (41.4) |
|
CT-guided percutaneous needle
biopsy | 2 (13.3) | 9 (64.3) | 11 (37.9) |
| Open
lung biopsy | 3 (20.0) | 0 (0) | 3 (10.3) |
|
VATS | 4 (26.7) | 1 (7.1) | 5 (17.2) |
| Pleural
biopsy | 1 (6.7) | 0 (0) | 1 (3.4) |
| Pathological
types |
|
|
|
|
MALT | 13 (86.7) | 0 (0) | 13 (44.8) |
| FL | 2 (13.3) | 0 (0) | 9 (31.0) |
|
DLBCL | 0 (0) | 6 (42.9) | 6 (20.7) |
|
ALCL | 0 (0) | 4 (28.6) | 4 (13.8) |
|
PTCL-U | 0 (0) | 1 (7.1) | 1 (3.4) |
|
Extranodal nasal NK/T | 0 (0) | 3 (21.4) | 3 (10.3) |
Diagnostic procedures
As presented in Table
II, while endobronchial biopsy and transbronchial lung biopsy
(TBLB) resulted in diagnostic yields of 80% (12/15), CT-guided
percutaneous needle lung biopsy (11/11) and surgery (8/8) obtained
diagnostic yields of 100%. In addition, 3 patients received the
same final diagnosis from two different procedures, with 2 patients
receiving the same diagnosis by bronchoscopic biopsy and CT-guided
percutaneous needle lung biopsy, and 1 patient receiving the same
diagnosis by TBLB and pleural biopsy. The diagnoses of the
remaining 8 patients were validated by either open lung biopsy
(n=3) or VATS (n=5).
Histopathological outcomes
As presented in Table
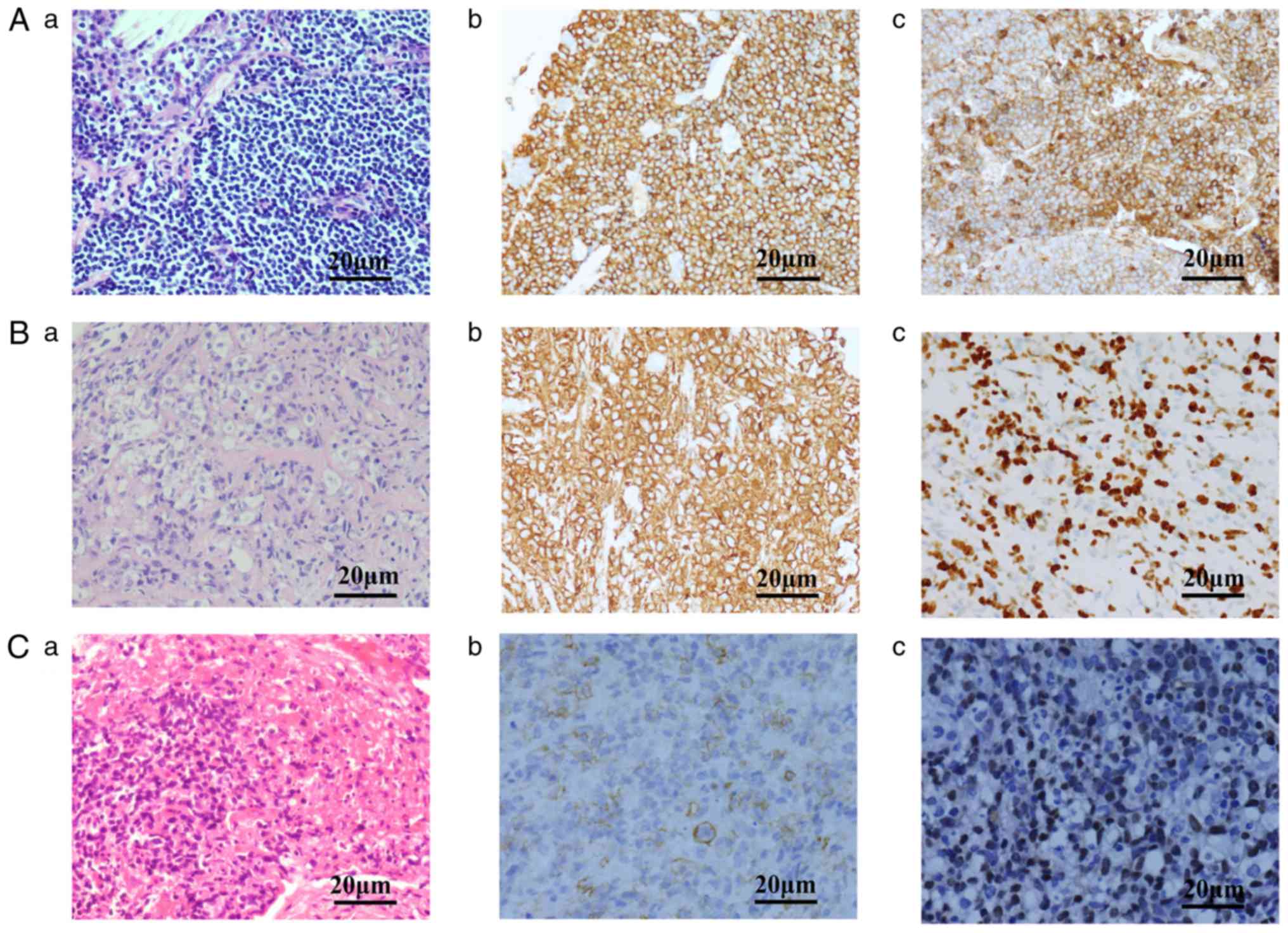
II and Fig. 2, the present study
consisted of 15 cases of indolent lymphoma (MALT lymphoma, n=13;
FL, n=2) and 14 cases of aggressive lymphoma (DLBCL, n=6; ALCL,
n=4; PTCL-U, n=1; extranodal nasal NK/T-L, n=3). No lymphoma cells
were identified in any patient by bone marrow biopsy or aspiration.
The diagnosis of NHL was first confirmed by morphological and
growth pattern characterization based on HE staining. For example,
diffuse small and medium-sized lymphoid cells were observed to have
infiltrated the area surrounding the bronchus, lymphoepithelial
lesions were observed and mitoses were rare in primary pulmonary
MALT lymphoma; neoplastic cells were medium to large in size, with
abundant pale cytoplasm and ovoid or irregular nuclei in primary
pulmonary diffuse large B-cell lymphoma; diffuse lymphoid
infiltration in the bronchial mucosa was observed, the neoplastic
cells appeared homogeneous and were medium-sized with irregular
nuclei, coagulative necrosis and apoptotic bodies were common in
primary pulmonary NK/T-cell lymphoma, nasal type. The diagnosis was
further differentiated by immunohistochemistry or ISH staining to
establish the lymphoma subtype. Normal IHC results were as follows:
i) MALT lymphoma: CD20+, CD3−,
CD5−, CD10−, CD23−,
CyclinD1+ and λ light-chain restriction+; ii)
FL: CD20+, CD10+, BCL2+/−,
BCL6+, CD5−, CD23−,
CyclinD1−; iii) DLBCL: CD20+, high Ki-67
index; iv) ALCL: Positive for T-cell markers, strong punctate
membranous and paranuclear CD30 staining, strong cytoplasmic
anaplastic lymphoma kinase staining; v) PTCL: Positive for T-cell
markers, with no other characteristic positive markers; and vi)
NK/T: CD2+, cytoplasmic CD3ε+,
CD56+ and EBV-encoded mRNA positivity required for
diagnostic confirmation.
Treatments and responses
The majority of patients were treated with
first-line chemotherapy, either cyclophosphamide, doxorubicin,
vincristine and prednisone (CHOP) or CHOP-like, including
etoposide, doxorubicin, vincristine, prednisone and
cyclophosphamide (EPOCH), regimens. A total of 8 patients underwent
partial or complete surgical resection of lung lesions followed by
chemotherapy, while 2 patients received radiotherapy with
subsequent chemotherapy and 10 patients received chemotherapy
alone. Overall, 4 patients received rituximab combined with CHOP
therapy (median, 5 cycles; range, 2–9 cycles) and 2 patients were
treated with rituximab alone. The objective response rate to
chemotherapy was higher in the indolent lymphoma group than in the
aggressive lymphoma group (67% vs. 7.1%, respectively; P<0.05).
A total of 7 patients diagnosed with primary pulmonary MALT
lymphoma were under watchful waiting and clinical observation
only.
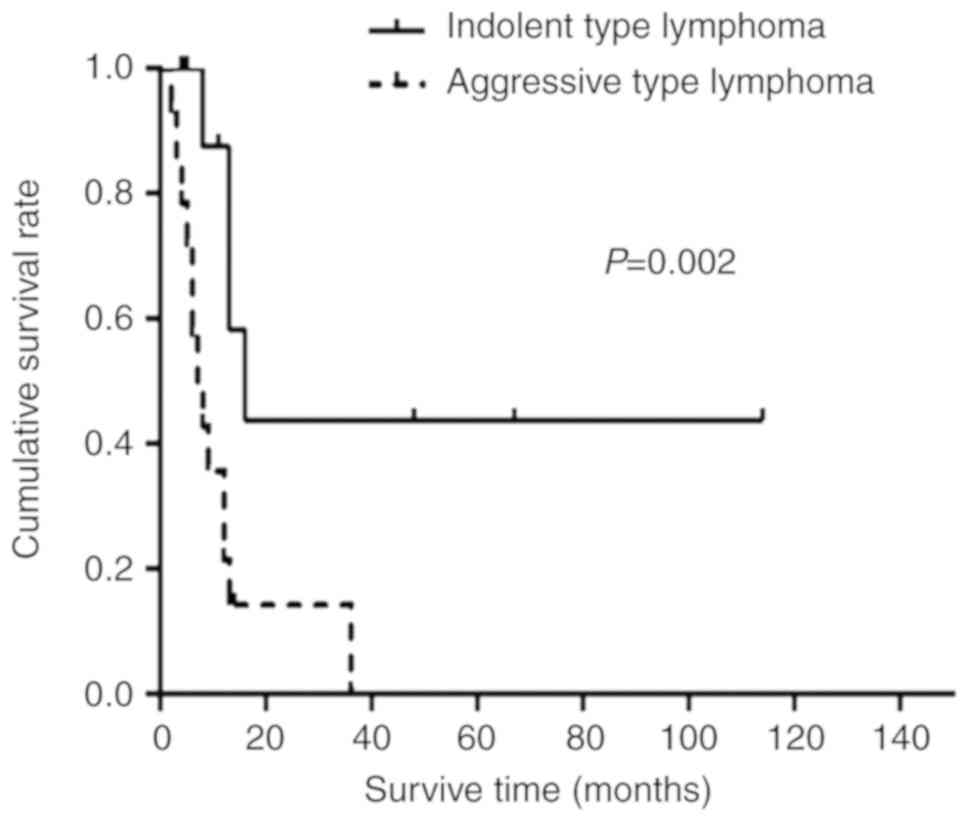
Survival time
At the time of final contact (June 30, 2017), 7
patients with primary pulmonary MALT lymphoma remained alive. The
respective 1-, 3- and 5-year OS rates were 42, 31 and 21% for all
patients, 72, 57 and 43% for patients with indolent lymphoma, and
13, 6 and 0% for patients with aggressive lymphoma. The median OS
time for all patients was 12.0 months (95% confidence interval,
4.1–19.9 months). The median OS of patients with aggressive
lymphoma was significantly shorter compared with that of patients
with indolent lymphoma (7.1 months vs. 16.6 months; P=0.002;
Fig. 3). OS times were compared
across the indolent lymphoma group to assess therapeutic efficacy,
and it was indicated that surgery and subsequent chemotherapy was
not superior to other treatment regimens (14.2 months vs. 9.3
months; P=0.412). These other treatments regimens included
chemotherapy alone, chemoradiotherapy and observation. In the
aggressive lymphoma group, the OS time was not significantly
increased for patients receiving chemotherapy alone compared with
that for patients receiving other therapies, including surgery and
subsequent chemotherapy or chemoradiotherapy (4.0 months vs. 2.6
months; P=0.856).
Prognostic factors
As presented in Table
III, univariate analyses were used to evaluate a number of
factors that contributed to OS. A poor 5-year OS rate was indicated
to be significantly associated with aggressive lymphomas (P=0.005),
but it was not significantly associated with bilateral pulmonary
lesions (P=0.05). Due to the relatively small number of cases, only
factors, including pathological type, sex and pulmonary lesions,
with P<0.1 were included in the multivariate Cox regression
analysis. Aggressive pathological type was an independent
prognostic factor for 5-year survival (hazard ratio, 5.98;
P=0.014), suggesting poor prognoses for patients with aggressive
lymphoma compared with patients with indolent lymphoma.
 | Table III.Five-year survival rates of the 29
subjects in terms of various prognostic factors. |
Table III.
Five-year survival rates of the 29
subjects in terms of various prognostic factors.
| Factors | 5-year survival
rate, % | Univariate analyses
P-value | Multivariate
analyses P-value |
|---|
| Age |
|
|
|
| ≥60
years vs. <60 years | 19 vs. 19 | 0.54 | – |
| Sex |
|
|
|
| Male
vs. female | 11 vs. 29 | 0.09 | 0.26 |
| Asymptomatic at
diagnosis |
|
|
|
| Yes vs.
no | 0 vs. 19 | 0.56 | – |
| B symptoms |
|
|
|
| Yes vs.
no | 21 vs. 20 | 0.66 | – |
| Histology |
|
|
|
|
Indolent vs. aggressive | 43 vs. 0 | 0.005 | 0.014 |
| Elevated serum
LDH |
|
|
|
| Yes vs.
no | 0 vs. 22 | 0.87 | – |
| Hilar or hilar
lymphadenopathy |
|
|
|
| Yes vs.
no | 15 vs. 20 | 0.66 | – |
| Lesions |
|
|
|
|
Bilateral vs. unilateral | 19 vs. 21 | 0.05 | 0.39 |
| Comorbidity |
|
|
|
| Yes vs.
no | 16 vs. 25 | 0.49 | – |
| EBERs |
|
|
|
|
Positive vs. negative | 0 vs. 21 | 0.35 | – |
| Stage |
|
|
|
| I E vs.
≥II (1E-2E) | 27 vs. 12 |
0.12 | – |
| Surgery followed by
chemotherapy |
|
|
|
| Yes vs.
no | 20 vs. 18 | 0.22 | – |
| Chemotherapy
alone |
|
|
|
| Yes vs.
no | 20 vs. 17 | 0.52 | – |
Discussion
The low early diagnosis rate of PP-NHL remains a
serious issue and presents a challenge for clinicians. Three
factors may be responsible for poor early diagnosis rates. First,
patients with PP-NHL often have non-specific clinical
manifestations. In the present study, 31% (4/13) of patients with
primary pulmonary MALT lymphoma were asymptomatic at diagnosis.
This rate was lower compared with the rates recorded in other
reports (36 and 88%, respectively) (14,15).
However, similar to previous reports, it was indicated that
patients with aggressive lymphomas, including primary pulmonary
DLBCL and ALCL, presented with elevated LDH levels and systemic
symptoms more frequently compared with patients with indolent
lymphoma (16–18).
Second, radiographic findings in patients with
PP-NHL are generally non-specific (19). CT findings in pulmonary lesions may
co-exist in a number of manifestations. The most common CT findings
in the present patients were nodules/masses, followed by
mediastinal/hilar lymph node enlargement, consolidation, pleural
effusion, air bronchogram and cavitation. In the present study, the
initial diagnoses of all patients were misleading. Although the
radiographic findings were atypical, there were number of hints
suggesting PP-NHL, including a fuzzy shadow at the edge of the lung
mass with air bronchogram, long-term stability of the lung mass
shadow and a pneumonia-like presentation without infectious
clinical or lab manifestations. These radiographic findings
resembled a spectrum of other pulmonary diseases, including lung
cancer, metastatic tumors, pneumonia, pulmonary tuberculosis and
pulmonary abscesses (20). We
believe that PP-NHL should be considered in the differential
diagnosis of a pulmonary lesion, in particular for patients with
long-lasting pulmonary shadows, systemic symptoms, including fever,
weight loss and night sweats, elevated LDH levels or poor responses
to antibiotic treatments.
Third, it remains challenging to definitively
pathologically diagnose certain patients. In the present study, the
diagnostic yields of the bronchoscopy and CT-guided percutaneous
needle lung biopsies were considerably higher compared with a
number of previously reported yields (2,9). Based
on our experience, the following is suggested: i) A thin spiral
thoracic CT scan and bronchoscopy should be performed to determine
the lesion location and size, as well as pathological changes in
the bronchial mucous; ii) based on the results of the CT scan and
bronchoscopy, endobronchial biopsy or TBLB is the optimal approach
for central pulmonary lesions or bronchial mucosal lesions, whereas
CT-guided percutaneous needle lung biopsy is more suitable for
peripheral pulmonary lesions, particularly for lesions close to the
chest wall; iii) ≥2 TBLB or CT-guided percutaneous needle lung
biopsies may improve the diagnostic yield; iv) a combination of
bronchoscopy and CT-guided percutaneous needle lung biopsies may
also increase the diagnostic yield; and v) surgical procedures,
including lobectomy under VATS and conventional surgery, are more
invasive and have a higher surgical risk compared with other
procedures. Therefore, strict patient screening should be
considered if only using these procedures for diagnosis. However, a
large sample size and high diagnostic yield can be obtained by
surgery. If positive specimens cannot be obtained, VATS should be
selected, since it is safer and less invasive compared with
conventional surgery. Clonal analysis of alveolar lymphocytes using
bronchoalveolar lavage fluid (BALF) has been recently suggested to
be helpful in diagnosing PP-NHL (21,22),
although BALF alone may not be sufficient for morphological
analysis (23). PET-CT may be
helpful for initial staging and evaluation of the response to
chemotherapy (24). In our opinion,
without tissue pathology analysis, the initial false-positive rate
can be as high as 100%; therefore, it is critical to determine the
optimal procedure resulting in a definitive pathological diagnosis.
Minimally invasive procedures, confirmed by flexible fiberoptic
bronchoscopy and CT-guided percutaneous needle lung biopsy, were
found to be helpful and reliable procedures, and should be
preferentially considered to obtain pathological diagnoses in
patients with PP-NHL. Additionally, diagnostic yields will increase
with repeated biopsies. Each specimen collection method has its own
advantages and disadvantages and therefore requires comprehensive
consideration prior to use.
The optimal modality for managing PP-NHL has not yet
been determined (10). For MALT, the
most common indolent type of PP-NHL, the following should be
considered: Complete surgical resection should be utilized for
localized tumors (25,26), chemotherapy should be utilized for
patients exhibiting diffuse involvement of one or both lungs
(9), and a combination of
radiotherapy and alkylating drug-based chemotherapy should be
utilized to preserve lung function and reduce the risks associated
with surgery (25). In addition,
adjuvant chemotherapy followed by radical resection may not provide
additional survival benefits (27).
In the present study, the 6 patients with MALT lymphomas, who
underwent surgery followed by chemotherapy, exhibited increased OS
times compared with patients receiving other treatments; however,
this difference was not significant. Furthermore, the role of
rituximab remains controversial (15,16).
Only 4 patients were treated with rituximab, and its efficacy could
not be adequately evaluated in the present study due to the small
sample size. Clinical observations of early-stage or indolent
lymphomas are also advisable (26);
therefore, a total of 7 patients diagnosed with primary pulmonary
MALT lymphoma were under watchful waiting and clinical observation
only in the present study. Regarding aggressive lymphomas,
including DLBCL, combination chemotherapy regimens are often
administered following attempts at curative surgical resection
(23). CHOP or rituximab-CHOP
combined with radiotherapy has previously been recommended for
patients with aggressive lymphomas, radiotherapy was used to treat
those patients who exhibited chemotherapeutic resistance, and
dose-adjusted EPOCH was recommended as an alternative regimen
(28). Although chemotherapy or
radiotherapy should be considered for unfavorable primary pulmonary
non-MALT lymphomas (2), in the
present study, the objective response rate (7.1%) to the treatment
of primary pulmonary aggressive lymphomas was inadequate. It was
speculated that a combinatorial therapeutic regimen, including
surgical resection, chemotherapy and radiotherapy, may be more
efficacious for patients with a more aggressive form of the
disease. The following two unusual phenomena were also observed in
the present study: The complete response rate among the patients
with aggressive PP-NHL was considerably lower compared with that
observed in patients with systemic aggressive lymphoma, and ALCL
cases constituted 14% of PP-NHL cases, which was considerably
higher compared with the 2% ALCL cases observed among systemic NHL
cases. It was also proposed that PP-NHL exhibits biological traits
that are distinct from those of systemic NHL, and that a number of
NHL pathological types tend to involve specific organs, including
ALCLs, which tend to primarily involve the lung. A total of 75%
(3/4) patients with ALCL in the present study were <30 years
old, which is consistent with the fact that ALCL usually occurs in
young people or the elderly (29);
thus, the small sample size of the present study may not truly
reflect the real situation.
The survival times of patients with PP-NHL varied
due to the heterogeneous patient groups. Overall, the median time
to mortality was 7 years, the 3-year OS rate was 86% and the 5-year
OS rate was 57–75% (2,30). For patients with indolent lymphomas,
including MALT lymphomas, the survival rates were 68% at 5-year and
53% at 10-year (6). By contrast,
aggressive lymphomas, including DLBCL, tended to be more diffuse
and destructive, with 5-year survival rates ranging from 0–65%
(31). In the present study, the
respective 3-year and 5-year OS rates were 31 and 21% for all
patients, 57 and 43% for patients with indolent lymphomas, and 6
and 0% for patients with aggressive lymphomas. Although the median
survival time, 3-year OS rate and 5-year OS rate were poor in the
present study compared with those in previous studies, the present
study demonstrated that patients with indolent lymphomas exhibited
an improved 5-year OS rate (43% vs. 0, P=0, 0.005) compared with
patients with aggressive lymphomas, which is consistent with a
previous report (8). The small
sample size, the differences between Chinese and Caucasian
patients, and the poor economic conditions may have contributed to
the observed differences in disease control and survival.
Previous studies have not adequately defined the
PP-NHL prognostic factors affecting patient survival. These studies
reported that lymphoma stage, extent of resection and presence of
mediastinal lymphadenopathy were not associated with poor prognosis
(27). Another relevant report
indicated that elevated serum LDH levels and hilar/mediastinal
lymphadenopathy were independently associated with poor OS rate
(18). In the present study of 29
cases, univariate analyses revealed that the 5-year OS rate of
patients with aggressive lymphomas was significantly worse compared
with that of patients with indolent lymphomas. Furthermore, the
multivariate Cox regression analysis demonstrated that histological
type was the only factor influencing OS and prognosis, i.e.,
patients with aggressive lymphomas presented with worse outcomes
compared with patients with indolent lymphomas. These results are
similar to the results of a French multicenter retrospective study
(8). Therefore, attempts to procure
adequate pathological tissue samples are critical in order to
ensure accurate early diagnoses are made and to avoid misdiagnoses,
thus directly affecting the therapeutic outcomes and prognosis of
patients with PP-NHL.
In conclusion, PP-NHL is difficult to diagnose due
to its rarity, non-specific clinical manifestations and atypical
radiographic findings. Obtaining a suitable tissue sample is vital
to making a definitive diagnosis. Endobronchial biopsy or TBLB and
CT-guided percutaneous needle lung biopsy should be performed to
facilitate a definitive diagnosis for the majority of patients with
PPL. However, the optimal modality for managing PP-NHL has not yet
been established. The present study is limited by its retrospective
nature and small sample size. The study systematically assessed the
clinical features, laboratory and imaging data, pathological
characteristics, therapeutic outcomes and prognostic factors of
PP-NHL, and indicated that pathological lymphoma type was highly
associated with patient prognosis. Lymphoma aggressiveness was
indicated to be an independent prognostic factor for 5-year OS, as
patients with aggressive lymphomas had shorter 5-year OS rates and
worse prognoses compared with patients with indolent lymphomas.
Therefore, although further investigation is required, the present
study may provide clinicians, pathologists and radiologists with
useful guidance for the diagnosis and treatment of patients with
PP-NHL.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and
Technology Program of Guangzhou (grant no. 2014J4100040), the
Special Foundation for Health Care Collaborative Innovation Major
Projects of Guangzhou (grant no. 201400000002), the Science and
Technology Program of Guangdong (grant no. 2013B031800026) and
Shanghai Wu Mengchao Medical Science Foundation (Grant no.
JJHXM-2019004).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, XG, XF, JD, HA, QL and WL collected the clinical
records of all patients and participated in the design of the
study. DL performed the pathological diagnosis of all cases. JZ
analyzed the roentgenographic findings for all patients. JQ and JW
summarized and analyzed the clinical data from all patients, and
drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the clinical
research Ethics committee of Guangdong Provincial People's
Hospital/Guangdong Academy of Medical Sciences (Guangzhou,
Guangdong, China). Informed consent was obtained from all subjects
prior to participation in the study.
Patient consent for publication
Patient consent for publication was obtained for the
thoracic computed tomography scans.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cadranel J, Wislez M and Antoine M:
Primary pulmonary lymphoma. Eur Respir J. 20:750–762. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parissis H: Forty years literature review
of primary lung lymphoma. J Cardiothorac Surg. 6:232011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delgado Torralbo JA, García Gómez LC and
Sánchez Varilla JM: Unilateral lung infiltrate: A rare form of
presentation of primary pulmonary lymphoma. Arch Bronconeumol.
54:103–104. 2018.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong Y, Zeng M, Zhang B, Han L, Liu E,
Lian Z, Liu J, Liang C and Zhang S: Significance of imaging and
clinical features in the differentiation between primary and
secondary pulmonary lymphoma. Oncol Lett. 14:6224–6230.
2017.PubMed/NCBI
|
|
5
|
Nicholson AG and Harris NL: Marginal zone
diffuse B-cell lymphoma of the mucosa-associated lymphoid tissue
(MALT) type. World Health Organization Classification of Tumoours.
Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and
Heart. Travis WB, Brambilla A, Muller-Hermelinck HK and Harris CC:
Lyon, France: IARC Press; pp. 88–90. 2004
|
|
6
|
Graham BB, Mathisen DJ, Mark EJ and
Takvorian RW: Primary pulmonary lymphoma. Ann Thorac Surg.
80:1248–1253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cordier JF, Chailleux E, Lauque D,
Reynaud-Gaubert M, Dietemann-Molard A, Dalphin JC, Blanc-Jouvan F
and Loire R: Primary pulmonary lymphomas. A clinical study of 70
cases in nonimmunocompromised patients. Chest. 103:201–208. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vanden Eynden F, Fadel E, de Perrot M, de
Montpreville V, Mussot S and Dartevelle P: Role of surgery in the
treatment of primary pulmonary B-cell lymphoma. Ann Thorac Surg.
83:236–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JH, Lee SH, Park J, Kim HY, Lee SI,
Park JO, Kim K, Kim WS, Jung CW, Park YS, et al: Primary pulmonary
non-Hodgkin's lymphoma. Jpn J Clin Oncol. 34:510–514. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lister TA, Crowther D, Sutcliffe SB,
Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA and
Tubiana M: Report of a committee convened to discuss the evaluation
and staging of patients with Hodgkin's disease: Cotswolds meeting.
J Clin Oncol. 7:1630–1636. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zelenetz AD, Wierda WG, Abramson JS,
Advani RH, Andreadis CB, Bartlett N, Bellam N, Byrd JC, Czuczman
MS, Fayad LE, et al: Non-Hodgkin's lymphomas, version 1.2013. J
Natl Compr Canc Netw. 11:257–272; quiz 273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheson BD, Horning SJ, Coiffier B, Shipp
MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A,
Hagenbeek A, et al: Report of an international workshop to
standardize response criteria for non-Hodgkin's lymphomas. NCI
sponsored international working group. J Clin Oncol. 17:12441999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borie R, Wislez M, Thabut G, Antoine M,
Rabbat A, Couderc LJ, Monnet I, Nunes H, Blanc FX, Mal H, et al:
Clinical characteristics and prognostic factors of pulmonary MALT
lymphoma. Eur Respir J. 34:1408–1416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ogusa E, Tomita N, Ishii Y, Takasaki H,
Hattori Y, Matsumoto C and Ishigatsubo Y: Clinical manifestations
of primary pulmonary extranodal marginal zone lymphoma of
mucosa-associated lymphoid tissue in Japanese population. Hematol
Oncol. 31:18–21. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang J, Lin T, Li ZM, Xu R, Huang H and
Jiang W: Primary pulmonary non-Hodgkin's lymphoma: A retrospective
analysis of 29 cases in a Chinese population. Am J Hematol.
85:523–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neri N, Jesus Nambo M and Aviles A:
Diffuse large B-cell lymphoma primary of lung. Hematology.
16:110–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu YH, Hsiao LT, Yang CF, Chiou TJ, Liu
JH, Gau JP, Yen CC, Chou TY, Hsu WH, Chen PM and Tzeng CH:
Prognostic factors of Chinese patients with primary pulmonary
non-Hodgkin's lymphoma: The single-institute experience in Taiwan.
Ann Hematol. 88:839–846. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sirajuddin A, Raparia K, Lewis VA, Franks
TJ, Dhand S, Galvin JR and White CS: Primary pulmonary lymphoid
lesions: Radiologic and pathologic findings. Radiographics.
36:53–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao D, Zhang L, Wu PL, Gu XL, Chen YF,
Wang LX and Huang XY: Clinical and misdiagnosed analysis of primary
pulmonary lymphoma: A retrospective study. BMC Cancer. 18:2812018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borie R, Wislez M, Antoine M, Fleury-Feith
J, Thabut G, Crestani B, Monnet I, Nunes H, Delfau-Larue MH and
Cadranel J: Clonality and phenotyping analysis of alveolar
lymphocytes is suggestive of pulmonary MALT lymphoma. Respir Med.
105:1231–1237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cardenas-Garcia J, Talwar A, Shah R and
Fein A: Update in primary pulmonary lymphomas. Curr Opin Pulm Med.
21:333–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsumoto T, Otsuka K, Funayama Y, Imai Y
and Tomii K: Primary pulmonary lymphoma mimicking a refractory lung
abscess: A case report. Oncol Lett. 9:1575–1578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwal KK, Dhanapathi H, Nazar AH and
Kumar R: Primary pulmonary lymphoma-role of fluoro-deoxy-glucose
positron emission tomography-computed tomography in the initial
staging and evaluating response to treatment-case reports and
review of literature. Indian J Nucl Med. 31:194–197. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Xia ZJ, Zhang YJ, Huang HQ, Lin TY
and Lu Y: Radical surgery may be not an optimal treatment approach
for pulmonary MALT lymphoma. Tumour Biol. 36:6409–6416. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sammassimo S, Pruneri G, Andreola G,
Montoro J, Steffanoni S, Nowakowski GS, Gandini S, Negri M,
Habermann TM, Raderer M, et al: A retrospective international study
on primary extranodal marginal zone lymphoma of the lung (BALT
lymphoma) on behalf of International Extranodal Lymphoma Study
Group (IELSG). Hematol Oncol. 34:177–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferraro P, Trastek VF, Adlakha H,
Deschamps C, Allen MS and Pairolero PC: Primary non-Hodgkin's
lymphoma of the lung. Ann Thorac Surg. 69:993–997. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang AG, Gao XY and Lu HY: Diagnosis and
management of a patient with primary pulmonary diffuse large B-cell
lymphoma: A case report and review of the literature. Exp Ther Med.
8:797–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Medeiros LJ and Elenitoba-Johnson KS:
Anaplastic large cell lymphoma. Am J Clin Pathol. 127:702–722.
2007. View Article : Google Scholar
|
|
30
|
Kocaturk CI, Seyhan EC, Gunluoglu MZ, Urer
N, Kaynak K, Dincer SI and Bedirhan MA: Primary pulmonary
non-Hodgkin's lymphoma: Ten cases with a review of the literature.
Tuberk Toraks. 60:246–253. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hare SS, Souza CA, Bain G, Seely JM, Frcp
c, Gomes MM and Quigley M: The radiological spectrum of pulmonary
lymphoproliferative disease. Br J Radiol. 85:848–864. 2012.
View Article : Google Scholar : PubMed/NCBI
|