Introduction
Breast cancer accounts for ~25% of all cancer cases
and ~15% of cancer-associated mortalities in females worldwide
(1). Triple-negative breast cancer
(TNBC) refers to breast cancer that is negative for the expression
of the estrogen receptor (ER), progesterone receptor (PR) and
hormone epidermal growth factor receptor 2 (HER2) (2). TNBC accounts for 15–20% of all breast
cancer pathological types (2).
Treatment for breast cancer includes surgery, chemotherapy,
radiation, endocrine and targeted therapy (3). As TNBC tumor tissue lacks expression of
ER, PR and HER-2, patients with TNBC cannot benefit from endocrine
therapy or targeted therapy against HER-2, unlike patients with
other subtypes of breast cancer (4–7).
Therefore, TNBC is a biologically aggressive subtype of breast
cancer and has a poor prognosis (8,9). There
is currently no specific targeted therapy for TNBC (10).
The ecotropic virus integration site 1 (EVI-1)
protein is an oncogenic dual domain zinc finger transcription
factor that was first identified to be abnormally expressed in
myeloid tumors in mice (11). EVI-1
has since been revealed to be highly expressed in several solid
tumors, including pancreatic, colorectal and prostate cancer,
glioblastoma, infratentorial ependymoma and hepatocellular
carcinoma, and to negatively correlate with prognosis (12–18). A
common EVI-1 polymorphism (rs6774494 A>G) targeted by microRNA
(miR)-206/133b was suggested to predict adverse outcomes in
patients with postmenopausal breast carcinoma (19). In breast cancer, EVI-1 expression was
associated with poor outcome in patients with ER-negative breast
cancer (20,21). Calreticulin (CRT) is a
multifunctional calcium-binding molecular chaperone. It serves an
important role in promoting tumor cell proliferation, metastasis
and adhesion, as well as inducing apoptosis resistance (22). CRT is highly expressed in malignant
tumors, including breast cancer, and is associated with a poor
prognosis (21–27).
Several predictive biomarkers in TNBC, including the
epidermal growth factor receptor (EGFR), androgen receptor and the
adhesion molecules CD24 and CD44, have been investigated in recent
years (28–31). To the best of the authors' knowledge,
the prognostic value of the expression levels of CRT and EVI-1 in
TNBC is unknown. Therefore, the present study investigated the
associations between the expression levels of EVI-1 and CRT and the
clinical characteristics, disease-free survival (DFS) and overall
survival (OS) of patients with TNBC.
Materials and methods
Study population
The current retrospective cohort study was approved
by the Ethical Review Committee of the First Affiliated Hospital of
Jinzhou Medical University (no. 2018-0006). All patients provided
written informed consent prior to enrollment.
The medical records of The First Affiliated Hospital
and the Third Affiliated Hospital of Jinzhou Medical University
between January 2010 and June 2015 were reviewed, and patients who
met the following eligibility criteria were included in the current
study: i) ≥18 years; ii) received surgical resection following
breast cancer diagnosis in the aforementioned time period; iii)
defined as TNBC following immunohistochemical analysis (negative
for ER, PR and HER-2 expression); and iv) positive for EGFR
expression. The exclusion criteria were as follows: i) Male
patients; ii) treatment with neo-adjuvant chemotherapy,
radiotherapy or endocrine therapy prior to resection; iii)
confirmed distant metastasis; and iv) refusal to provide written
consent.
Patient clinical information, including age, tumor
size, TNM stage according to the 7th American Joint Committee on
Cancer staging system (32), lymph
node metastasis, histological type and treatment received, were
obtained from the medical records. A paraffin-embedded specimen of
TNBC tumor tissue and a matched paracancerous tissue specimen
(defined as tissues beyond 5 cm from the edge of the tumor tissue)
from each patient. All patients with TNBC were followed-up until
August 2016.
Immunohistochemical analysis
TNBC tissue samples were processed and subjected to
immunohistochemical staining as follows. Tissue specimens were
fixed with 10% formalin and kept away from light at room
temperature. Paraffin-embedded tissue sections (4 µm) were
deparaffinized in xylene, rehydrated with a gradient of ethanol and
washed in distilled water. A pressure cooker was used to perform
the antigen retrieval step using citrate buffer at 108°C for 1–2
min. A 3% hydrogen peroxide/methanol solution was used to block the
endogenous peroxidase activity for 15 min at room temperature.
Non-specific antibody binding was subsequently blocked by
incubation with 1% diluted normal horse serum (Beijing Solarbio
Science & Technology Co., Ltd.) for 30 min at room temperature.
The sections were incubated at room temperature for 60 min with
antibodies against EVI-1 (ab-28457, 1:800; Abcam) and CRT (ab4,
1:2,000; Abcam). The sections were visualized using the SP-9001 kit
and DAB kit (Rabbits SP kit; cat. no. SP-9001; OriGene
Technologies, Inc.) following the manufacturer's instructions and
counterstained with hematoxylin at room temperature for 1 min.
Sections incubated with PBS and without a primary antibody served
as a negative control. Five high-power fields of view
(magnification, ×400) were randomly selected in each section using
a light microscope.
EVI-1 and CRT immunoreactivity was evaluated
independently by CSX and YJJ using a manual histopathology scoring
method (33). For evaluation of
EVI-1 and CRT staining, light yellow to brown particles in the
cytoplasm and/or nucleus were defined as positive. A
semi-quantitative scoring was used to judge the overall score based
on tissue staining intensity and the percentage of positive cells
according to the standard reported by Wang et al (34). Image-pro plus software (version 6.0;
Media Cybernetics, Inc.) was used to conduct image analysis. Three
sections were selected from each group (cancerous tissue and
paracancerous tissue) according to the random number method, and
five visual fields at ×400 magnification were randomly chosen from
each section for measurement of the average optical density of
positive staining (integrated optical density (IOD)/area).
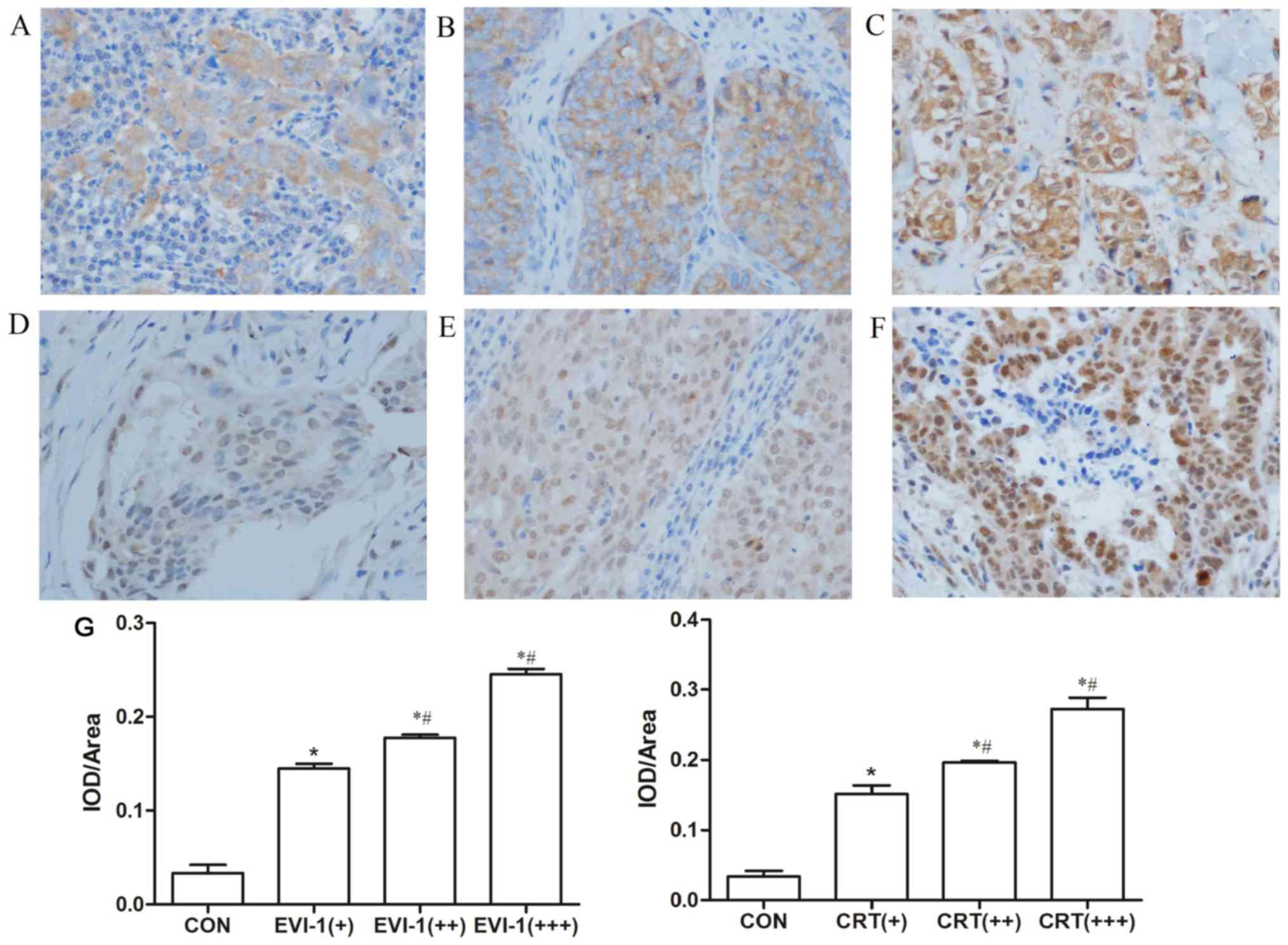
Quantitative results are presented in Fig. 1, which represents the difference of
IOD among the different groups. The staining intensity was
characterized as 0 points for no coloring, 1 point for weak
staining, 2 points for medium staining and 3 points for strong
staining (19,35,36). The
percentage of positive cells was categorized as follows: 0 points
for no positive cells, 1 point for ≤25% positive area, 2 points for
26–50% positive area, 3 points for 51–75% positive area and 4
points for >75% positive area. To obtain the overall score, the
aforementioned two scores were multiplied, and a result of 0–4 was
defined as low expression (negative) and 6–12 as high expression
(positive).
Statistical analysis
A Chi-square test was performed to assess the
associations between EVI-1 and CRT expression and various
clinicopathological variables. The Spearman's correlation test was
used to determine if the expression levels of EVI-1 and CRT
correlated with TNBC. Comparison among multiple groups were
performed by one-way analysis of variance followed by the
least-significant difference test. The main outcomes of the current
study were OS and DFS. DFS referred to the period from the date of
primary surgery to the date of diagnosis of distant or local
recurrence, and OS was defined as the period from the date of
primary surgery to the date of mortality from any cause. The
Kaplan-Meier method to evaluate the median DFS and OS times and the
log-rank test was used to test the significance of differences in
DFS and OS. Multivariable analysis of predictors of DFS and OS was
performed using the Cox proportional hazards regression model.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using SPSS
statistical software (version 17; SPSS, Inc, Chicago, IL, USA).
Results
Patient information
A total of 88 patients with TNBC who met the
eligibility criteria were included in the final analysis. The
patient characteristics are presented in Table I. The mean age of the patients was 54
years (range, 30–77 years). The median follow-up time was 43.5
months (range, 12–75 months). No patient data were lost during the
follow-up. At the end of the study, 62.5% (55/88) of the patients
had experienced local/distant recurrence, and 51.13% (45/88) had
succumbed.
 | Table I.Patient clinicopathological
characteristics (N=88). |
Table I.
Patient clinicopathological
characteristics (N=88).
| Characteristic | Category | N (%) |
|---|
| Age (years) | 30–45 | 21 (23.9) |
|
| 46–61 | 48 (54.5) |
|
| 62–77 | 19 (21.6) |
| Sex | Female | 88 (100) |
| Body mass index
(kg/m2) | ≤18.49 | 2 (2.3) |
|
| 18.5–24.99 | 49 (55.7) |
|
| 25-27.99 | 23 (26.1) |
|
| 28–32 | 14 (15.9) |
| Histopathological
type | Invasive
ductal/lobular carcinoma | 76 (86.4) |
|
| Apocrine
carcinoma | 5 (5.7) |
|
| Others | 7 (8.0) |
|
T-stagea | T1 | 28 (31.8) |
|
| T2 | 58 (65.9) |
|
| T3-T4 | 2 (2.3) |
| Number of lymph
nodes with metastasis | 0 | 53 (60.2) |
|
| ≤4 | 25 (28.4) |
|
| >4 | 10 (11.4) |
| Cancer-associated
thrombosis | Yes | 72 (81.8) |
|
| No | 16 (18.2) |
| Histological
differentiation gradeb | Low/moderate | 35 (39.8) |
|
| High | 38 (43.2) |
|
| Moderate/high | 15 (17.0) |
| Pathological
stagea | I | 30 (34.1) |
|
| II | 41 (46.6) |
|
| III | 17 (19.3) |
| Ki-67 staining | Low (≤14%) | 9 (10.2) |
|
| High (>14%) | 79 (89.8) |
| p53 staining | Negative | 40 (45.5) |
|
| Positive | 48 (54.5) |
| Therapy following
surgery | Similar standard
treatment | 88 (100) |
EVI-1 and CRT expression in TNBC and
paracancerous tissues
Among the 88 patients with TNBC, 59.1% exhibited
high expression levels of EVI-1. EVI-1 immunostaining was mainly
localized in the cytoplasm, but staining was also present in the
nuclei of tumor cells (Fig. 1). High
expression of CRT was observed in 72.7% of the patients, and its
expression was localized in the nucleus and cytoplasm (Fig. 1). Staining for EVI-1 and CRT revealed
low expression levels in all adjacent normal tissues. Statistically
significant differences in the expression of EVI-1 and CRT were
observed between the TNBC tumor tissues and adjacent normal tissues
(P<0.05; Table II). The positive
rates of EVI-1 and CRT staining in TNBC tissues were significantly
higher than those in adjacent healthy tissues (P<0.05).
 | Table II.EVI-1 and CRT expression in TNBC
tissues and paracancerous tissues (n=88). |
Table II.
EVI-1 and CRT expression in TNBC
tissues and paracancerous tissues (n=88).
|
|
| Status of EVI-1
protein expression, n (%) |
| Status of CRT
expression, n (%) |
|
|---|
|
|
|
|
|
|
|
|---|
| Tissue | N | High | Low | P-value | High | Low | P-value |
|---|
| TNBC | 88 | 52 (59.1) | 36 (40.9) | 0.003a | 64 (72.7) | 24 (27.3) | 0.003a |
| Adjacent
normal | 88 | 10 (11.4) | 78 (88.6) |
| 0 | 88 (100) |
|
EVI-1 and CRT expression in TNBC
Among the 88 cases of TNBC, 44 revealed high
expression levels of both EVI-1 and CRT, and 16 cases had low
expression of both. The Spearman's correlation test demonstrated
that the expression levels of EVI-1 and CRT were significantly
positively correlated with TNBC (r2=0.321; P=0.002; data
not shown).
Association of EVI-1 and CRT
expression levels with other clinicopathological variables
The expression levels of EVI-1 and CRT in TNBC was
associated with clinicopathological variables (Table III). High expression of EVI-1 was
closely associated with the histopathological type,
cancer-associated thrombosis, lymph node metastasis, pathological
stage and elevated Ki-67 expression (P<0.05). Chi-square
analysis revealed that the expression of CRT was closely associated
with age and elevated Ki-67 expression (P<0.05). Younger age and
higher Ki-67 expression were associated with increased expression
of CRT.
 | Table III.Correlations between EVI-1 and CRT
expression in cancer tissues and clinicopathological variables in
patients with triple-negative breast cancer (n=88). |
Table III.
Correlations between EVI-1 and CRT
expression in cancer tissues and clinicopathological variables in
patients with triple-negative breast cancer (n=88).
|
|
| EVI-1 |
| CRT |
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | Category | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Age (years) | 30–45 | 11 | 10 | 0.168a | 1 | 20 | 0.020a |
|
| 46–61 | 16 | 32 |
| 15 | 33 |
|
|
| 62–77 | 10 | 9 |
| 8 | 11 |
|
| BMI
(kg/m2) | <18.5 | 1 | 1 | 0.692 | 1 | 1 | 0.427 |
|
| 18.5–24.99 | 22 | 27 |
| 16 | 33 |
|
|
| 25–28 | 7 | 16 |
| 5 | 18 |
|
|
| 28–32 | 6 | 8 |
| 2 | 12 |
|
| Histopathological
type | Invasive
ductal/lobular carcinoma | 29 | 47 | 0.031a | 19 | 57 | 0.481 |
|
| Apocrine
carcinoma | 1 | 4 |
| 2 | 3 |
|
|
| Others | 6 | 1 |
| 3 | 4 |
|
| Histological
differentiation | Low/moderate | 13 | 22 | 0.251 | 9 | 26 | 0.952 |
| gradeb | High | 19 | 19 |
| 11 | 27 |
|
|
| Moderate/high | 4 | 11 |
| 4 | 11 |
|
|
Cancer-associated | No | 34 | 38 | 0.012a | 21 | 51 | 0.397 |
| thrombosis | Yes | 2 | 14 |
| 3 | 13 |
|
|
T-stagec | T1 | 13 | 15 | 0.728 | 11 | 17 | 0.183 |
|
| T2 | 22 | 36 |
| 13 | 45 |
|
|
| T3-T4 | 1 | 1 |
| 1 | 1 |
|
| Number of
lymph-node | 0 | 27 | 26 | 0.030a | 16 | 37 | 0.419 |
| metastases | ≤4 | 8 | 17 |
| 7 | 18 |
|
|
| >4 | 1 | 9 |
| 1 | 9 |
|
| Pathological
stagec | I | 15 | 15 | 0.024a | 11 | 19 | 0.183 |
|
| II | 19 | 22 |
| 11 | 30 |
|
|
| III | 2 | 15 |
| 2 | 15 |
|
| p53 | Negative | 17 | 23 | 0.830 | 12 | 28 | 0.637 |
|
| Positive | 19 | 29 |
| 12 | 36 |
|
| Ki-67 | ≤14% | 7 | 2 | 0.029a | 7 | 2 |
<0.001a |
|
| >14% | 29 | 50 |
| 17 | 62 |
|
Clinicopathological variables
associated with poor prognosis in TNBC
COX risk regression models revealed that age, BMI,
Ki-67 expression, EVI-1 expression and CRT expression were
independent risk factors for a poor prognosis of TNBC (Table IV).
 | Table IV.Multivariate analysis of predictive
factors for disease-free survival and overall survival in patients
with triple-negative breast cancer (n=88). |
Table IV.
Multivariate analysis of predictive
factors for disease-free survival and overall survival in patients
with triple-negative breast cancer (n=88).
| A, Overall
survival |
|---|
|
|---|
| Variable | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age (46–61
years) | 4.175 | 1.06–16.44 | 0.041a |
| Age (62–77
years) | 4.369 | 1.43–13.25 | 0.010a |
| BMI (18.5–24.99
kg/m2) | 55.793 | 2.739–1136.489 | 0.009a |
| Pathological stage
(II) | 0.236 | 0.059–0.950 | 0.042a |
| CRT | 1.506 | 0.516–4.394 | 0.453 |
| EVI-1 | 0.114 | 0.034–0.380 |
<0.001a |
| p53 | 2.688 | 1.153–6.266 | 0.022a |
| Ki-67 | 0.066 | 0.007–0.578 | 0.014a |
|
| B, Disease-free
survival | Odds
ratio | 95% confidence
interval | P-value |
|
| BMI (18.5–24.99
kg/m2) | 76.399 | 4.347–1342.653 | 0.003a |
| Pathological stage
(II) | 0.219 | 0.051–0.937 | 0.041a |
| Pathological stage
(III) | 0.297 | 0.089–0.996 | 0.049a |
| CRT | 3.667 | 1.255–10.715 | 0.018a |
| EVI-1 | 0.097 | 0.027–0.344 |
<0.001a |
| p53 | 1.978 | 0.881–4.443 | 0.098a |
| Ki-67 | 0.064 | 0.007–0.571 | 0.014a |
EVI-1/CRT expression and prognosis of
patients with TNBC
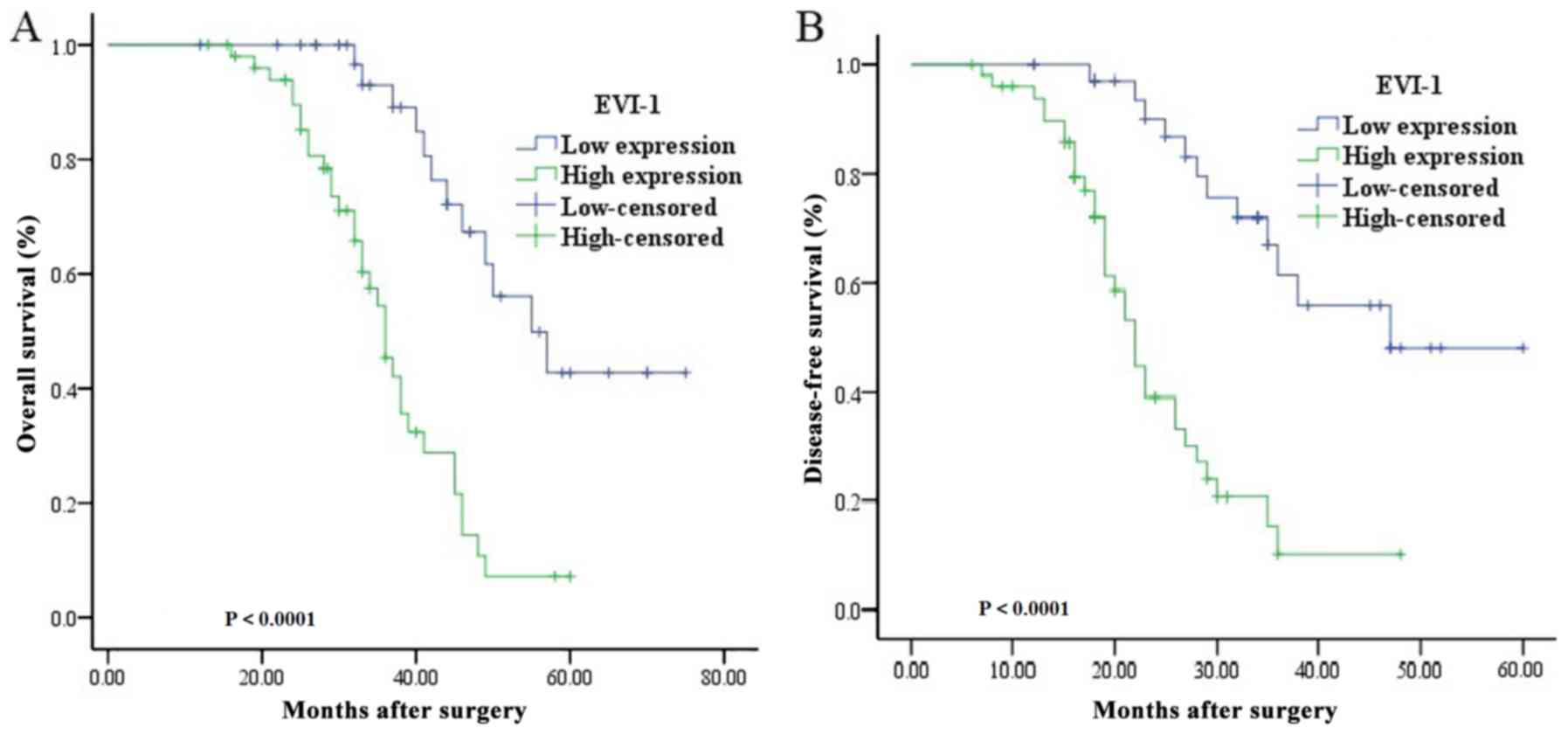
The survival curves revealed that the OS (mean ±
standard deviation) for patients with a high expression level of
EVI-1 was significantly lower compared with patients with a low
expression level (36.79±1.67 months vs. 58.11±3.42 months;
P<0.001; Fig. 2). Similar results
were obtained for the DFS (24.62±1.72 months vs. 45.57±3.09 months;
P<0.001; Fig. 2).
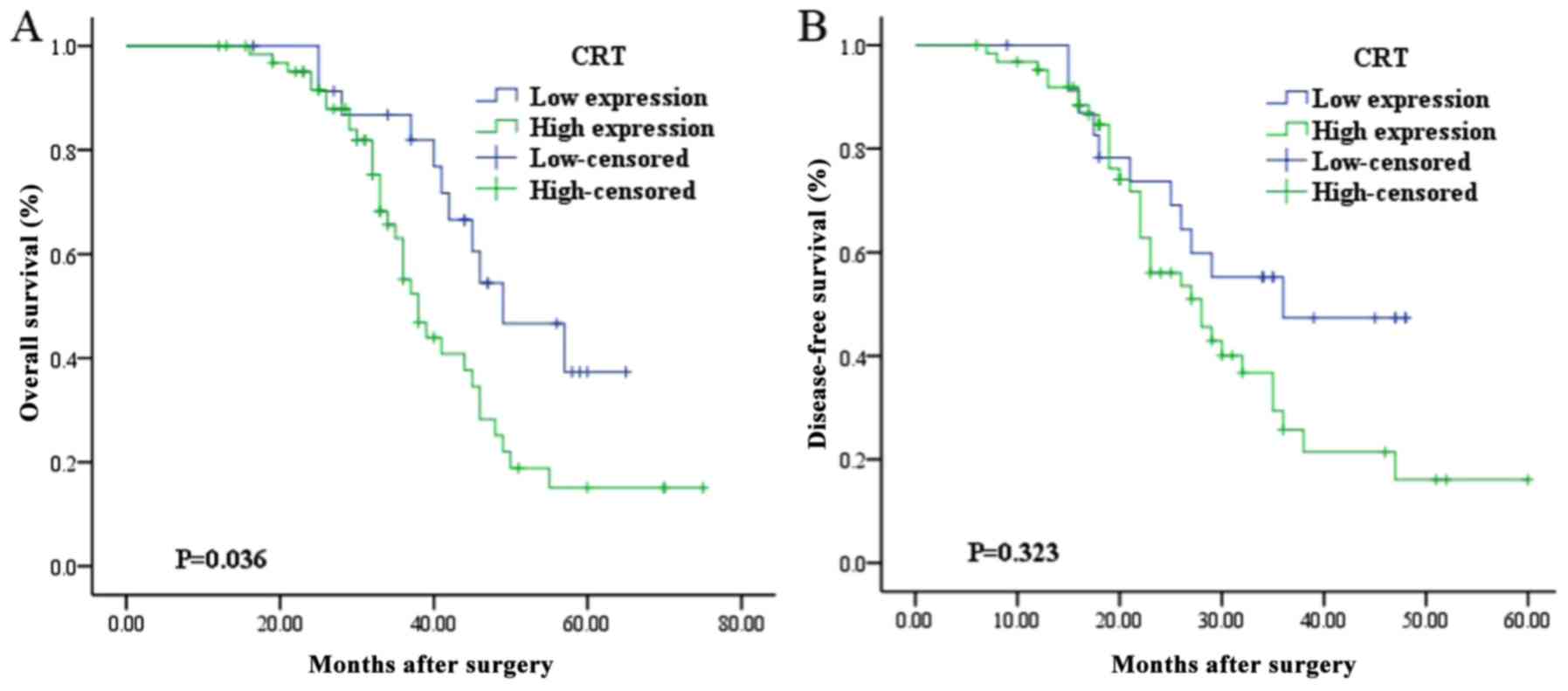
The OS of patients with high CRT expression was
significantly lower compared with that of patients with low CRT
expression (42.76±2.54 months vs. 50.44±3.12 months; P=0.036;
Fig. 3). However, the level of CRT
expression did not result in a significant difference in PFS (high
expression, 31.35±2.44 months vs. low expression, 35.00±2.88
months; P=0.323; Fig. 3).
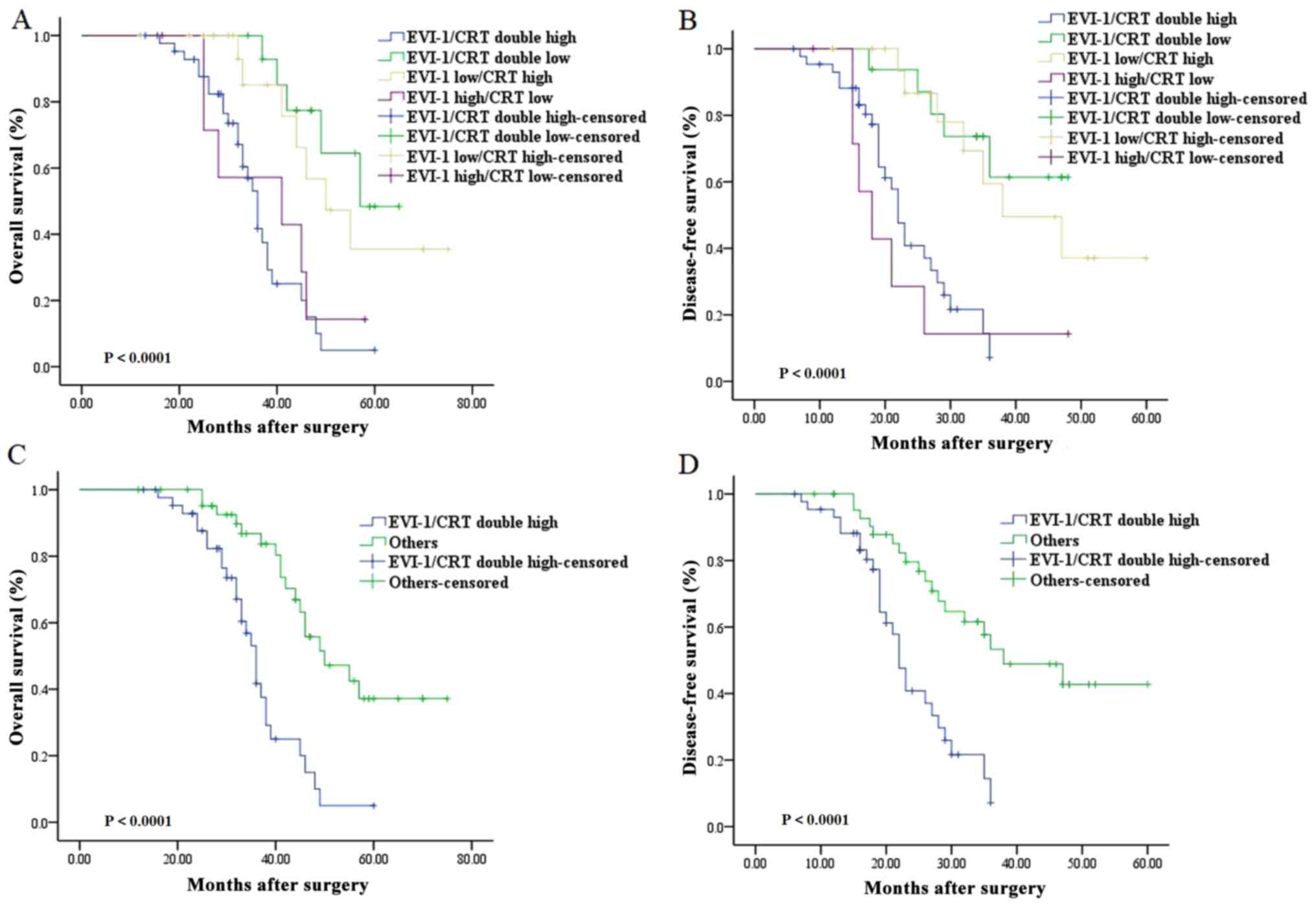
Combination of EVI-1 and CRT
expression as a prognostic biomarker in TNBC
The present study further explored whether combining
EVI-1 and CRT expression statuses may be used as a prognostic
biomarker for patients with TNBC. The patients were sub-categorized
into four groups according to EVI-1 and CRT expression status: i)
Low EVI-1/low CRT expression (N=16); ii) low EVI-1/high CRT
expression (n=20); iii) high EVI-1/low CRT expression (N=8), and
iv) high EVI-1/high CRT expression (N=44). Among the four groups,
Kaplan-Meier analyses revealed significant differences in DFS
(P<0.001) and OS (P<0.001). Additionally, this difference was
greatest between the high EVI-1/high CRT group and all other groups
(Fig. 4). Subsequent analysis
revealed that patients in the high EVI-1/high CRT group (N=44) had
significantly reduced DFS (P<0.001, log-rank test) and OS
(P<0.001, log-rank test) compared with patients in all other
groups (N=44; Fig. 4).
Discussion
The current retrospective cohort study evaluated the
effects of EVI-1 and CRT expression on the clinicopathological
features and prognosis of patients with TNBC. The expression levels
of EVI-1 and CRT in TNBC and paracancerous tissues were analyzed.
EVI-1 and CRT expression was low or absent in paracancerous tissues
and increased in TNBC tissues, which is consistent with previous
studies (21,24). No association between EVI-1
expression in TNBC tissues and age, BMI, histological grade, tumor
size and p53 expression was observed. However, EVI-1 expression was
significantly associated with pathological type, presence of
vascular tumor embolus, lymph node metastasis, pathological stage
and a hyperproliferative Ki-67 index (P<0.05). The expression of
CRT was closely associated with age and Ki-67 expression. Younger
age and higher Ki-67 expression were associated with increased
expression of CRT.
As the TNBC cell proliferation activity is increased
with elevated expression of Ki-67, the degree of malignancy of TNBC
is also increased (37). The results
of the present study suggested that the prognosis of patients with
TNBC with low EVI-1 expression is improved. Wang et al
(14) revealed that increased
expression of EVI-1 is associated with the proliferation of ER and
HER-2 negative breast cancer cells, providing a theoretical basis
for the current study. The present study revealed that the
increased expression of EVI-1 and CRT was associated with an
increase in Ki-67 expression, suggesting that EVI-1 and CRT in TNBC
tissues may be closely associated with the proliferation of cancer
cells. Therefore, it may be speculated that EVI-1 and CRT may be
related to invasion and metastasis of cancer cells, thereby
affecting patient PFS and OS. Chen et al (38) demonstrated that the increased
expression of CRT in gastric cancer was positively correlated with
vascular invasion and lymph node metastasis and was associated with
poor prognosis. The overexpression of CRT promoted proliferation of
pancreatic cancer cells in vitro, whereas knockdown of CRT
inhibited the proliferation of pancreatic cancer cells (25). CRT is considered to inhibit
downstream signaling in the MAPK and p53 signaling pathways
(39). However, the mechanisms
underlying the effect of EVI-1 expression on the prognosis of
patients with TNBC remains unclear. One hypothesis is that EVI-1
may promote the activation of the PI3K/AKT signaling pathway,
antagonize the transforming growth factor-β-signaling pathway, and
regulate long-chain non-coding RNA to promote tumor cell
proliferation.
The increase expression of EVI-1 and CRT may lead to
increased proliferation, invasion and metastasis of TNBC, which in
turn affects the PFS and OS of patients with TNBC. By combining the
CRT status and EVI-1 status for predicting prognosis in TNBC, the
present study demonstrated that patients with high expression of
both EVI-1 and CRT had significantly decreased OS and DFS compared
with the patients in the other subgroups. Quan et al
(35) revealed that the level of
miR-206 expression in breast cancer tissue was significantly higher
compared with that in adjacent tissues, and that the 3-year
survival rate of patients with high miR-206 expression was
decreased compared with patients with low miR-206 expression. Our
previous studies suggested that miR-206 negatively regulated the
expression of transcription factor EVI-1 (40), which indicates that EVI-1 may
regulate its expression and have an impact on biological behaviors
of tumor cells through specific miRNAs. In addition, recent studies
revealed that low expression of suppressor of cytokine signaling 1
and high expression of DNA polymerase Δ1, catalytic subunit are
associated with the occurrence and development of breast cancer and
may have prognostic value in breast cancer (29,30).
Therefore, testing for both EVI-1 and CRT may be useful to evaluate
the prognosis of patients with breast cancer and prevent
recurrence. In addition, the identification of novel biomarkers may
aid clinical practice and improve patient outcomes.
The present study had a number of limitations. Cases
with missing data were excluded from the final analysis,
potentially leading to information bias. The effect of therapy on
patient outcome was not assessed as all the patients included in
the current study received a similar standard treatment strategy.
The small sample size limited the statistical power to some degree
and more cases are required for future analysis. In conclusion,
EVI-1 and CRT may serve important roles in the progression of TNBC.
The detection of the expression levels of EVI-1 and CRT may aid the
diagnosis of TNBC and serve as a prognosis indicator for patients
with TNBC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372812).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DNH designed the study, conducted the literature
search and patient data collection, appraised all potential studies
and wrote and revised the draft manuscript and subsequent
manuscripts. LW revised the draft manuscript, data analysis, and
subsequent manuscripts. XXL and XDL assisted with the presentation
of findings and assisted with drafting and revising the manuscript.
XXL collected and followed up the cases. XDL analyzed and
interpreted the experimental data. PM and YHJ conceived and
designed the study, assisted with searches, appraised relevant
studies, and assisted with drafting and revising the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current retrospective study was approved by The
Ethical Review Committee of the First Affiliated Hospital of
Jinzhou Medical University (no. 2018-0006). All participants
provided written informed consent prior to enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beiki O, Hall P, Ekbom A and Moradi T:
Breast cancer incidence and case fatality among 4.7 million women
in relation to social and ethnic background: A population-based
cohort study. Breast cancer Res. 14:R52012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Breast cancer version 2.2015. J Natl Compr Canc
Netw. 13:448–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carey LA: Directed therapy of subtypes of
triple-negative breast cancer. Oncologist. 16 (Suppl 1):S71–S78.
2011. View Article : Google Scholar
|
|
6
|
Irvin WJ Jr and Carey LA: What is
triple-negative breast cancer? Eur J Cancer. 44:2799–2805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adamo V, Ricciardi GR, De Placido S,
Colucci G, Conte P, Giuffrida D, Gebbia N, Masci G, Cognetti F,
Dondi D and Venturini M: Management and treatment of
triple-negative breast cancer patients from the NEMESI study: An
Italian experience. Eur J Cancer. 48:642–647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hudis CA and Gianni L: Triple-negative
breast cancer: An unmet medical need. Oncologist. 16 (Suppl
1):S1–S11. 2011. View Article : Google Scholar
|
|
10
|
Hon JD, Singh B, Sahin A, Du G, Wang J,
Wang VY, Deng FM, Zhang DY, Monaco ME and Lee P: Breast cancer
molecular subtypes: From TNBC to QNBC. Am J Cancer Res.
6:1864–1872. 2016.PubMed/NCBI
|
|
11
|
Buonamici S, Chakraborty S, Senyuk V and
Nucifora G: The role of EVI1 in normal and leukemic cells. Blood
Cells Mol Dis. 31:206–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka M, Suzuki HI, Shibahara J, Kunita
A, Isagawa T, Yoshimi A, Kurokawa M, Miyazono K, Aburatani H,
Ishikawa S and Fukayama M: EVI1 oncogene promotes KRAS pathway
through suppression of microRNA-96 in pancreatic carcinogenesis.
Oncogene. 33:2454–2463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng X, Cao Y, Liu Y, Li F, Sambandam K,
Rajaraman S, Perkins AS, Fields AP, Hellmich MR, Townsend CM Jr, et
al: Overexpression of Evi-1 oncoprotein represses TGF-β signaling
in colorectal cancer. Mol Carcinog. 52:255–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Schaefer T, Konantz M, Braun M,
Varga Z, Paczulla AM, Reich S, Jacob F, Perner S, Moch H, et al:
Prominent oncogenic roles of EVI1 in breast carcinoma. Cancer Res.
77:2148–2160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hou A, Zhao L, Zhao F, Wang W, Niu J, Li
B, Zhou Z and Zhu D: Expression of MECOM is associated with
unfavorable prognosis in glioblastoma multiforme. Onco Targets
Ther. 9:315–320. 2016.PubMed/NCBI
|
|
16
|
Koos B, Bender S, Witt H, Mertsch S,
Felsberg J, Beschorner R, Korshunov A, Riesmeier B, Pfister S,
Paulus W and Hasselblatt M: The transcription factor evi-1 is
overexpressed, promotes proliferation, and is prognostically
unfavorable in infratentorial ependymomas. Clin Cancer Res.
17:3631–3637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Queisser A, Hagedorn S, Wang H, Schaefer
T, Konantz M, Alavi S, Deng M, Vogel W, von Mässenhausen A,
Kristiansen G, et al: Ecotropic viral integration site 1, a novel
oncogene in prostate cancer. Oncogene. 36:1573–1584. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yasui K, Konishi C, Gen Y, Endo M, Dohi O,
Tomie A, Kitaichi T, Yamada N, Iwai N, Nishikawa T, et al: EVI1, a
target gene for amplification at 3q26, antagonizes transforming
growth factor-β-mediated growth inhibition in hepatocellular
carcinoma. Cancer Sci. 106:929–937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang TY, Huang YP and Ma P: Correlations
of common polymorphism of EVI-1 gene targeted by miRNA-206/133b
with the pathogenesis of breast cancer. Tumour Biol. 35:9255–9262.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patel JB, Appaiah HN, Burnett RM,
Bhat-Nakshatri P, Wang G, Mehta R, Badve S, Thomson MJ, Hammond S,
Steeg P, et al: Control of EVI-1 oncogene expression in metastatic
breast cancer cells through microRNA miR-22. Oncogene.
30:1290–1301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brooks DJ, Woodward S, Thompson FH, Dos
Santos B, Russell M, Yang JM, Guan XY, Trent J, Alberts DS and
Taetle R: Expression of the zinc finger gene EVI-1 in ovarian and
other cancers. Br J Cancer. 74:1518–1525. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu YC, Chen CN, Wang B, Hsu WM, Chen ST,
Chang KJ, Chang CC and Lee H: Changes in tumor growth and
metastatic capacities of J82 human bladder cancer cells suppressed
by down-regulation of calreticulin expression. Am J Pathol.
179:1425–1433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lwin ZM, Guo C, Salim A, Yip GW, Chew FT,
Nan J, Thike AA, Tan PH and Bay BH: Clinicopathological
significance of calreticulin in breast invasive ductal carcinoma.
Mod Pathol. 23:1559–1566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Erić-Nikolić A, Milovanović Z, Sánchez D,
Pekáriková A, Dzodić R, Matić IZ, Tučková L, Jevrić M, Buta M,
Rašković S and Juranić Z: Overexpression of calreticulin in
malignant and benign breast tumors: Relationship with humoral
immunity. Oncology. 82:48–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheng W, Chen C, Dong M, Zhou J, Liu Q,
Dong Q and Li F: Overexpression of calreticulin contributes to the
development and progression of pancreatic cancer. J Cell Physiol.
229:887–897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiang WF, Hwang TZ, Hour TC, Wang LH,
Chiu CC, Chen HR, Wu YJ, Wang CC, Wang LF, Chien CY, et al:
Calreticulin, an endoplasmic reticulum-resident protein, is highly
expressed and essential for cell proliferation and migration in
oral squamous cell carcinoma. Oral Oncol. 49:534–541. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng RQ, Chen YB, Ding Y, Zhang R, Zhang
X, Yu XJ, Zhou ZW, Zeng YX and Zhang XS: Expression of calreticulin
is associated with infiltration of T-cells in stage IIIB colon
cancer. World J Gastroenterol. 16:2428–2434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kallel I, Rebai M, Khabir A and Rebaï A:
What common biomarkers characterize a triple-negative profile in
breast cancer? Pathol Biol (Paris). 63:224–229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ali AM, Ansari JAK, El-Aziz NMA, Abozeed
WN, Warith AMA, Alsaleh K and Nabholtz JM: Triple negative breast
cancer: A tale of two decades. Anticancer Agents Med Chem.
17:491–499. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu N, Zhang J, Zhao J, Mu K, Zhang J, Jin
Z, Yu J and Liu J: Precision medicine based on tumorigenic
signaling pathways for triple-negative breast cancer. Oncol Lett.
16:4984–4996. 2018.PubMed/NCBI
|
|
31
|
Israel BB, Tilghman SL, Parker-Lemieux K
and Payton-Stewart F: Phytochemicals: Current strategies for
treating breast cancer. Oncol Lett. 15:7471–7478. 2018.PubMed/NCBI
|
|
32
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aeffner F, Wilson K, Martin NT, Black JC,
Hendriks CL, Bolon B, Rudmann DG, Gianani R, Koegler SR, Krueger J
and Young GD: The gold standard paradox in digital image analysis:
Manual versus automated scoring as ground truth. Arch Pathol Lab
Med. 141:1267–1275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang CJ, Zhou ZG, Holmqvist A, Zhang H, Li
Y, Adell G and Sun XF: Survivin expression quantified by Image
Pro-Plus compared with visual assessment. Appl Immunohistochem Mol
Morphol. 17:530–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quan Y, Huang X and Quan X: Expression of
miRNA-206 and miRNA-145 in breast cancer and correlation with
prognosis. Oncol Lett. 16:6638–6642. 2018.PubMed/NCBI
|
|
36
|
Lv Y, Song G and Li P: Correlation of
SOCS-1 gene with onset and prognosis of breast cancer. Oncol Lett.
16:383–387. 2018.PubMed/NCBI
|
|
37
|
Synnestvedt M, Borgen E, Russnes HG, Kumar
NT, Schlichting E, Giercksky KE, Kåresen R, Nesland JM and Naume B:
Combined analysis of vascular invasion, grade, HER2 and Ki67
expression identifies early breast cancer patients with
questionable benefit of systemic adjuvant therapy. Acta Oncol.
52:91–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen CN, Chang CC, Su TE, Hsu WM, Jeng YM,
Ho MC, Hsieh FJ, Lee PH, Kuo ML, Lee H and Chang KJ: Identification
of calreticulin as a prognosis marker and angiogenic regulator in
human gastric cancer. Ann Surg Oncol. 16:524–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zamanian M, Qader Hamadneh LA,
Veerakumarasivam A, Abdul Rahman S, Shohaimi S and Rosli R:
Calreticulin mediates an invasive breast cancer phenotype through
the transcriptional dysregulation of p53 and MAPK pathways. Cancer
Cell Int. 16:562016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meng L, Wang TY, Li XX and Ma P: Effects
of miR-206/miR-1 on breast cancer stem cell proliferation and the
mechanism. J China Med Univ. 44:394–399. 2015.
|
|
41
|
Frank GA, Danilova NV, Andreeva IuIu and
Nefedova NA: WHO classification of tumors of the breast, 2012. Arkh
Patol. 75:53–63. 2013.(In Russian). PubMed/NCBI
|